Multiple S-Layer Proteins of Brevibacillus laterosporus as Virulence Factors against Insects
Abstract
1. Introduction
2. Results
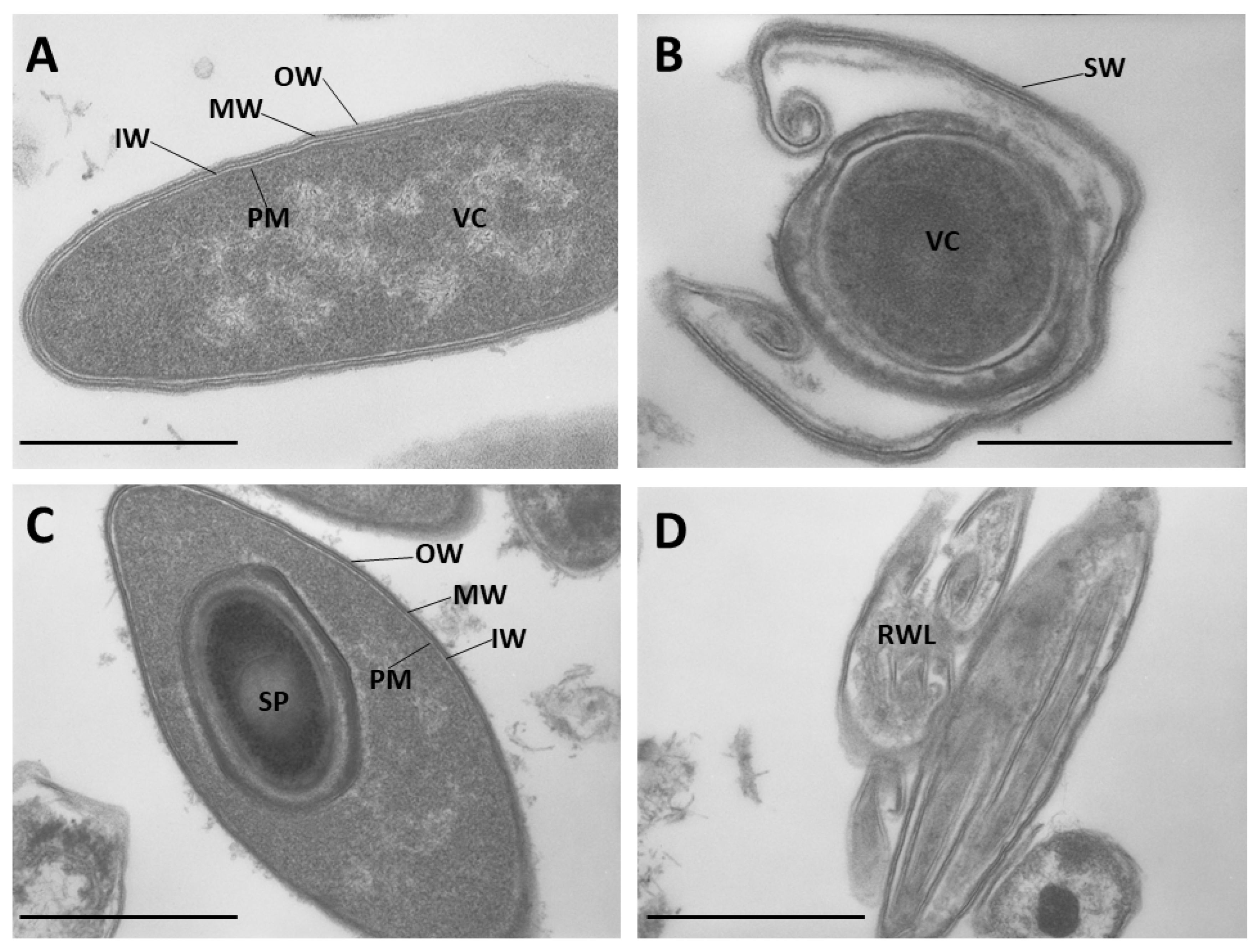
2.1. Multilayer Cell Wall Structure and Shedding
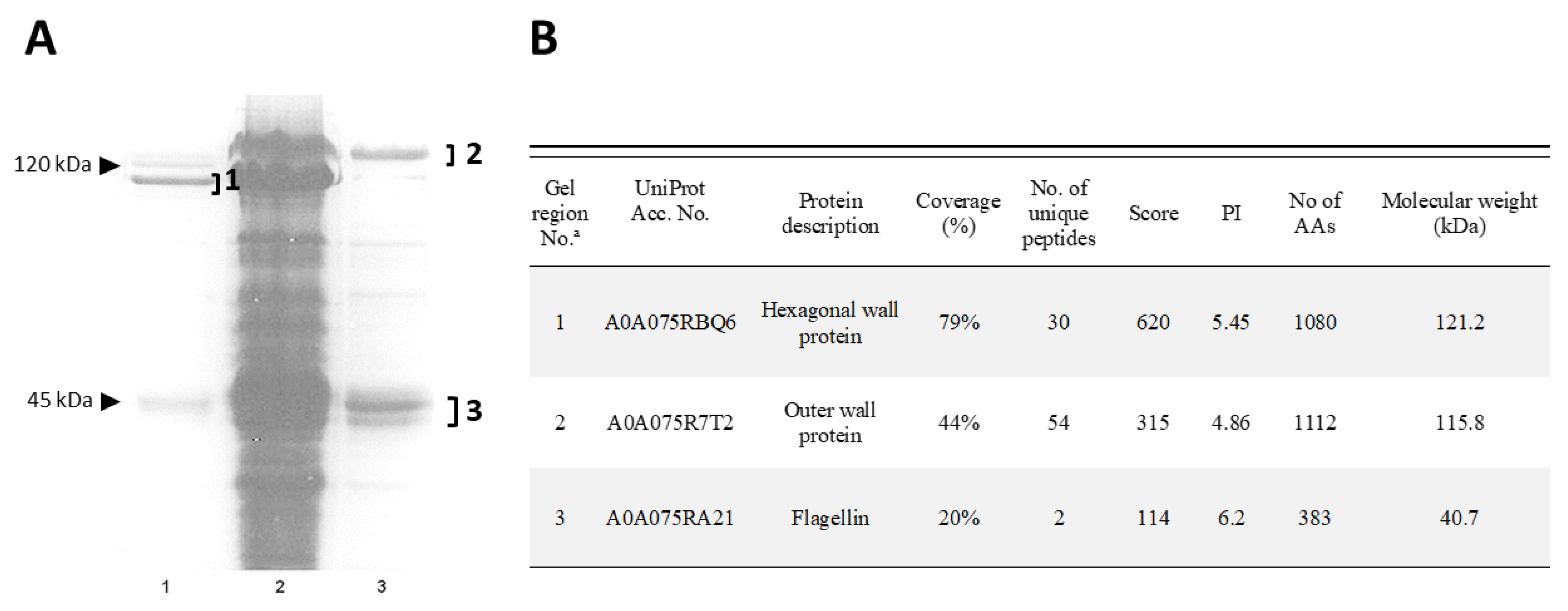
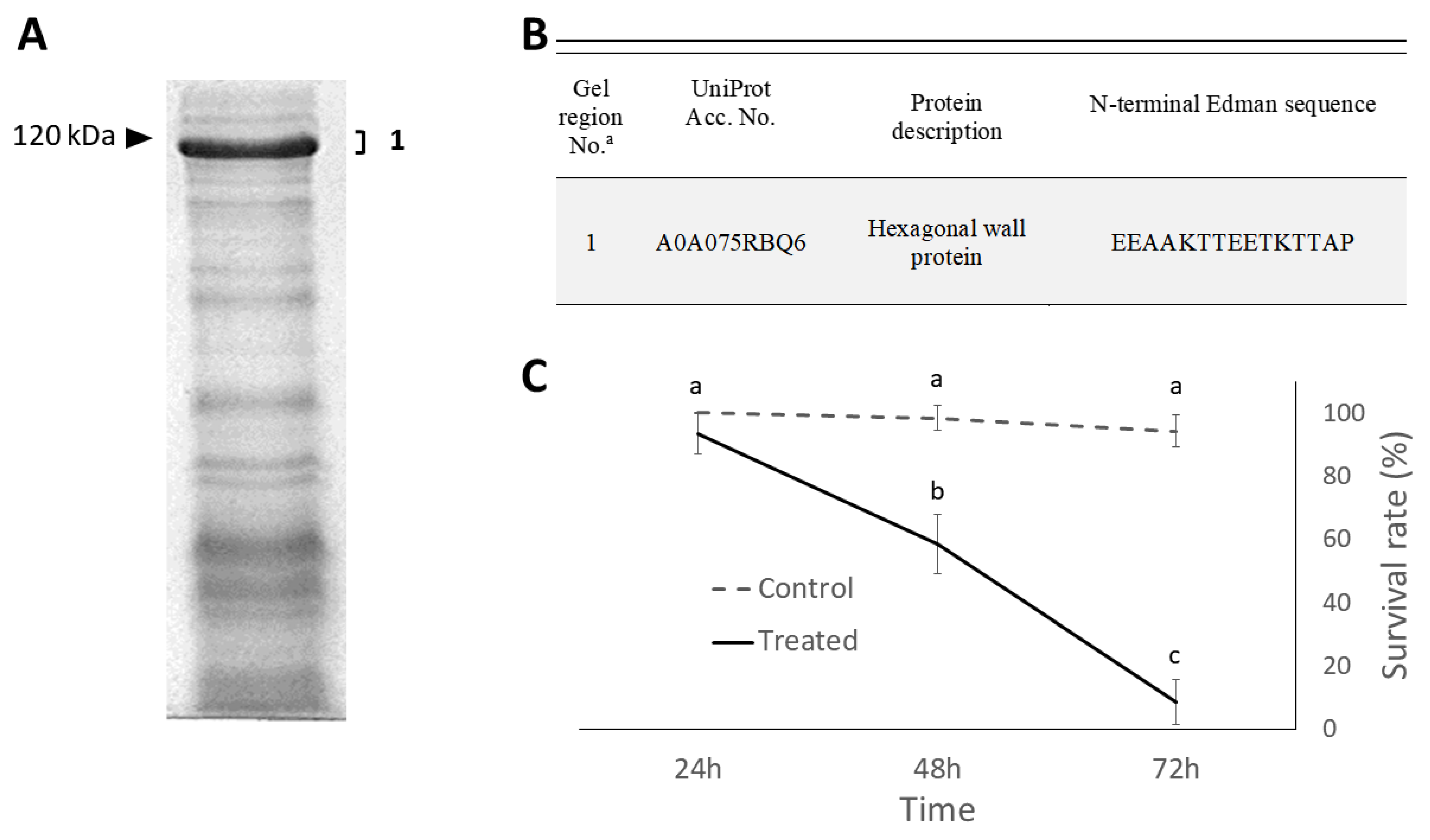
2.2. Isolation and Identification of Cell Wall Proteins
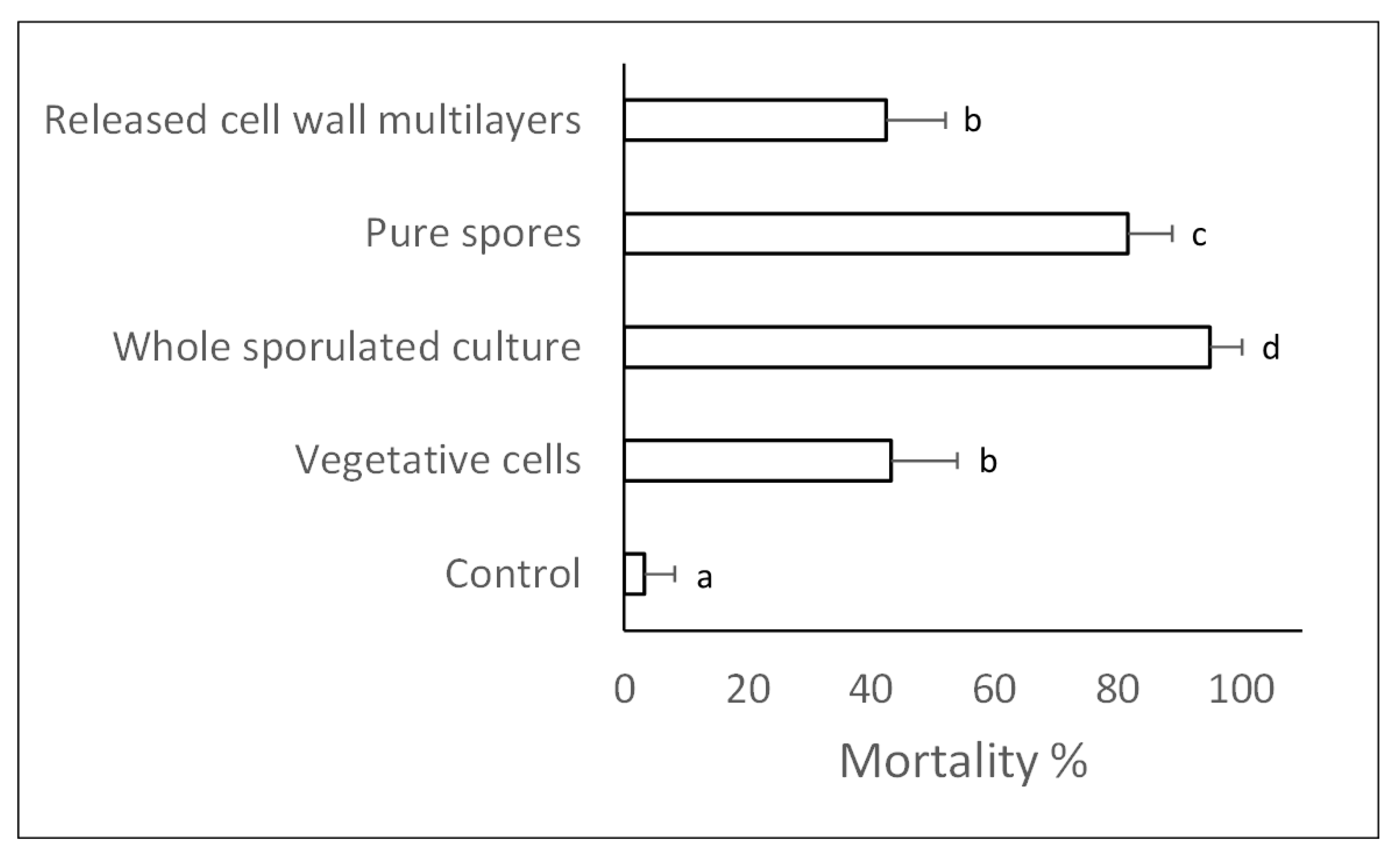
2.3. Insecticidal Effects
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain and Growth Conditions
4.2. Cell Wall Observation by Transmission Electron Microscopy
4.3. Protein Analyses
4.4. Insect Bioassays
4.5. Statistical Analysis
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerbino, E.; Carasi, P.; Mobili, P.; Serradell, M.A.; Gómez-Zavaglia, A. Role of S-layer proteins in bacteria. World J. Microbiol. Biotechnol. 2015, 31, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Sára, M.; Sleytr, U.B. S-layer proteins. J. Bacteriol. 2000, 182, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Fagan, R.P.; Fairweather, N.F. Biogenesis and functions of bacterial S-layers. Nat. Rev. Microbiol. 2014, 12, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Zhang, L.; Zhou, Z.; Ma, Q.; Liu, J.; Zhu, C.; Sun, M. A new group of parasporal inclusions encoded by the S-layer gene of Bacillus thuringiensis. FEMS Microbiol. Lett. 2008, 282, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pena, G.; Miranda-Rios, J.; de la Riva, G.; Pardo-López, L.; Soberón, M.; Bravo, A. A Bacillus thuringiensis S-layer protein involved in toxicity against Epilachna varivestis (Coleoptera: Coccinellidae). App. Environ. Microbiol. 2006, 72, 353–360. [Google Scholar] [CrossRef]
- Allievi, M.C.; Palomino, M.M.; Prado Acosta, M.; Lanati, L.; Ruzal, S.M.; Sánchez-Rivas, C. Contribution of S-layer proteins to the mosquitocidal activity of Lysinibacillus sphaericus. PLoS ONE 2014, 9, e111114. [Google Scholar]
- Yamada, H.; Tsukagoshi, N.; Udaka, S. Morphological alterations of cell wall concomitant with protein release in a protein-producing bacterium, Bacillus brevis 47. J. Bacteriol. 1981, 148, 322–332. [Google Scholar] [CrossRef]
- Djukic, M.; Poehlein, A.; Thürmer, A.; Daniel, R. Genome sequence of Brevibacillus laterosporus LMG 15441, a pathogen of invertebrates. J. Bacteriol. 2011, 193, 5535–5536. [Google Scholar] [CrossRef]
- Favret, E.M.; Yousten, A.A. Insecticidal activity of Bacillus laterosporus. J. Invertebr. Pathol. 1985, 45, 195–203. [Google Scholar] [CrossRef]
- Rivers, D.B.; Vann, C.N.; Zimmack, H.L.; Dean, D.H. Mosquitocidal activity of Bacillus laterosporus. J. Invertebr. Pathol. 1991, 58, 444–447. [Google Scholar] [CrossRef]
- De Oliveira, E.J.; Rabinovitch, L.; Monnerat, R.G.; Passos, L.K.J.; Zahner, V. Molecular characterization of Brevibacillus laterosporus and its potential use in biological control. Appl. Environ. Microbiol. 2004, 70, 6657–6664. [Google Scholar] [CrossRef]
- Marche, M.G.; Mura, M.E.; Falchi, G.; Ruiu, L. Spore surface proteins of Brevibacillus laterosporus are involved in insect pathogenesis. Sci. Rep. 2017, 7, 43805. [Google Scholar] [CrossRef]
- Marche, M.G.; Camiolo, S.; Porceddu, A.; Ruiu, L. Survey of Brevibacillus laterosporus insecticidal protein genes and virulence factors. J. Invertebr. Pathol. 2018, 155, 38–43. [Google Scholar] [CrossRef]
- Glare, T.R.; Durrant, A.; Berry, C.; Palma, L.; Ormskirk, M.M.; Cox, M.P. Phylogenetic determinants of toxin gene distribution in genomes of Brevibacillus laterosporus. Genomics 2020, 112, 1042–1053. [Google Scholar] [CrossRef]
- Crickmore, N.; Berry, C.; Panneerselvam, S.; Mishra, R.; Connor, T.R.; Bonning, B.C. A structure-based nomenclature for Bacillus thuringiensis and other bacteria-derived pesticidal proteins. J. Invertebr. Pathol. 2021, 186, 107438. [Google Scholar] [CrossRef]
- Ebisu, S.; Tsuboi, A.; Takagi, H.; Naruse, Y.; Yamagata, H.; Tsukagoshi, N.; Udaka, S. Conserved structures of cell wall protein genes among protein-producing Bacillus brevis strains. J. Bacteriol. 1990, 172, 1312–1320. [Google Scholar] [CrossRef]
- Tsuboi, A.; Uchihi, R.; Tabata, R.; Takahashi, Y.; Hashiba, H.; Sasaki, T.; Yamagata, H.; Tsukagoshi, N.; Udaka, S. Characterization of the genes coding for two major cell wall proteins from protein-producing Bacillus brevis 47: Complete nucleotide sequence of the outer wall protein gene. J. Bacteriol. 1986, 168, 365–373. [Google Scholar] [CrossRef]
- Henriques, A.O.; Moran, C.P., Jr. Structure, assembly, and function of the spore surface layers. Annu. Rev. Microbiol. 2007, 61, 555–588. [Google Scholar] [CrossRef]
- Mura, M.E.; Ruiu, L. Brevibacillus laterosporus pathogenesis and local immune response regulation in the house fly midgut. J. Invertebr. Pathol. 2017, 145, 55–61. [Google Scholar] [CrossRef]
- de Andrade Pereira, L.; de Carvalho Queiroz, M.M.; Faria, S.C.R.; Zahner, V. Ultrastructural and pathogenicity of Brevibacillus laterosporus against sinantropic muscoid dipterans. Microsc. Res. Tech. 2022, 85, 149–155. [Google Scholar] [CrossRef]
- Auger, S.; Ramarao, N.; Faille, C.; Fouet, A.; Aymerich, S.; Gohar, M. Biofilm formation and cell surface properties among pathogenic and nonpathogenic strains of the Bacillus cereus group. Appl. Environ. Microbiol. 2009, 75, 6616–6618. [Google Scholar] [CrossRef] [PubMed]
- Poppinga, L.; Janesch, B.; Fünfhaus, A.; Sekot, G.; Garcia-Gonzalez, E.; Hertlein, G.; Hedtke, K.; Schäffer, C.; Genersch, E. Identification and functional analysis of the S-Layer protein SplA of Paenibacillus larvae, the causative agent of american foulbrood of honey bees. PLoS Pathog. 2012, 8, e1002716. [Google Scholar] [CrossRef] [PubMed]
- Taverniti, V.; Stuknyte, M.; Minuzzo, M.; Arioli, S.; De Noni, I.; Scabiosi, C.; Cordova, Z.M.; Junttila, I.; Hämäläinen, S.; Turpeinen, H.; et al. S-layer protein mediates the stimulatory effect of Lactobacillus helveticus MIMLh5 on innate immunity. Appl. Environ. Microbiol. 2013, 79, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Brady, T.S.; Fajardo, C.P.; Merrill, B.D.; Hilton, J.A.; Graves, K.A.; Eggett, D.L.; Hope, S. Bystander phage therapy: Inducing host-associated bacteria to produce antimicrobial toxins against the pathogen using phages. Antibiotics 2018, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Bagherzadeh Kasmani, F.; Torshizi, K.; Mehri, M. Effect of Brevibacillus laterosporus Probiotic on Hematology, Internal Organs, Meat Peroxidation and Ileal Microflora in Japanese Quails Fed Aflatoxin B1. J. Agric. Sci. Technol. 2018, 20, 459–468. [Google Scholar]
- Khaled, J.M.; Al-Mekhlafi, F.A.; Mothana, R.A.; Alharbi, N.S.; Alzaharni, K.E.; Sharafaddin, A.H.; Kadaikunnan, S.; Alobaidi, A.S.; Bayaqoob, N.I.; Govindarajan, M.; et al. Brevibacillus laterosporus isolated from the digestive tract of honeybees has high antimicrobial activity and promotes growth and productivity of honeybee’s colonies. Environ. Sci. Pollut. Res. 2018, 25, 10447–10455. [Google Scholar] [CrossRef]
- Alippi, A.M.; Reynaldi, F.J. Inhibition of the growth of Paenibacillus larvae, the causal agent of American foulbrood of honeybees, by selected strains of aerobic spore-forming bacteria isolated from apiarian sources. J. Invertebr. Pathol. 2006, 91, 141–146. [Google Scholar] [CrossRef]
- Marche, M.G.; Mura, M.E.; Ruiu, L. Brevibacillus laterosporus inside the insect body: Beneficial resident or pathogenic outsider? J. Invertebr. Pathol. 2016, 137, 58–61. [Google Scholar] [CrossRef]
- Bedini, S.; Muniz, E.R.; Tani, C.; Conti, B.; Ruiu, L. Insecticidal potential of Brevibacillus laterosporus against dipteran pest species in a wide ecological range. J. Invertebr. Pathol. 2020, 177, 107493. [Google Scholar] [CrossRef]
- Luckevich, M.D.; Beveridge, T.J. Characterization of a dynamic S layer on Bacillus thuringiensis. J. Bacteriol. 1989, 171, 6656–6667. [Google Scholar] [CrossRef]
- Mesnage, S.; Haustant, M.; Fouet, A. A general strategy for identification of S-layer genes in the Bacillus cereus group: Molecular characterization of such a gene in Bacillus thuringiensis subsp. galleriae NRRL 4045The GenBank accession number for the slpA sequence is AJ249446. Microbiology 2001, 147, 1343–1351. [Google Scholar] [CrossRef]
- Laemmli, U.K.; Favre, M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J. Mol. Biol. 1973, 229, 319–327. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Camiolo, S.; Porceddu, A.; Ruiu, L. Genome sequence of Brevibacillus laterosporus UNISS 18, a pathogen of mosquitoes and flies. Genome Announc. 2017, 5, e00419-17. [Google Scholar] [CrossRef]
- Ruiu, L.; Satta, A.; Floris, I. Comparative applications of azadirachtin-and Brevibacillus laterosporus-based formulations for house fly management experiments in dairy farms. J. Med. Entomol. 2011, 48, 345–350. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2013; Available online: https://www.R-project.org (accessed on 1 October 2022).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiu, L. Multiple S-Layer Proteins of Brevibacillus laterosporus as Virulence Factors against Insects. Int. J. Mol. Sci. 2023, 24, 1781. https://doi.org/10.3390/ijms24021781
Ruiu L. Multiple S-Layer Proteins of Brevibacillus laterosporus as Virulence Factors against Insects. International Journal of Molecular Sciences. 2023; 24(2):1781. https://doi.org/10.3390/ijms24021781
Chicago/Turabian StyleRuiu, Luca. 2023. "Multiple S-Layer Proteins of Brevibacillus laterosporus as Virulence Factors against Insects" International Journal of Molecular Sciences 24, no. 2: 1781. https://doi.org/10.3390/ijms24021781
APA StyleRuiu, L. (2023). Multiple S-Layer Proteins of Brevibacillus laterosporus as Virulence Factors against Insects. International Journal of Molecular Sciences, 24(2), 1781. https://doi.org/10.3390/ijms24021781





