Pharmacokinetic and Permeation Studies in Rat Brain of Natural Compounds Led to Investigate Eugenol as Direct Activator of Dopamine Release in PC12 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Formulations and Doses for the Administration of Eugenol, Cinnmaldehyde and D-Limonene
2.2. Intravenous Administration of Eugenol, Cinnamaldehyde and D-Limonene
2.3. Oral Administration of Eugenol, Cinnamaldehyde and D-Limonene
2.4. Effect of Eugenol on Cell Viability in PC12 Cells
2.5. Dopamine Release evoked by Elevated Extracellular K+ and Eugenol in PC12 Neuronal Cell Model
3. Materials and Methods
3.1. Materials
3.2. In Vivo Administration of Eugenol, Cinnamaldehyde and D-Limonene
3.2.1. Intravenous Administration
3.2.2. Oral Administration
3.2.3. Pharmacokinetic Calculations
3.3. HPLC Analysis
3.4. PC12 Cells Culture and Treatments
3.5. Cell Viability Assay
3.6. Dopamine Release
3.7. Enzyme-Linked Immunosorbent Assay (ELISA)
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Donati Sarti, M.; Campieri, M.; Valerii, M.C. Antioxidant, anti-inflammatory, and microbial-modulating activities of essential oils: Implications in colonic pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I. An overview of the biological effects of some mediterranean essential oils on human health. Biomed. Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef] [PubMed]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid. Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef] [PubMed]
- Hajinejad, M.; Ghaddaripouri, M.; Dabzadeh, M.; Forouzanfar, F.; Sahab-Negah, S. Natural cinnamaldehyde and its derivatives ameliorate neuroinflammatory pathways in neurodegenerative diseases. Biomed. Res. Int. 2020, 2020, 1034325. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wu, X.; Tang, S.; Yin, J.; Song, Z.; He, X.; Yin, Y. Eugenol alleviates dextran sulfate sodium-induced colitis independent of intestinal microbiota in mice. J. Agric. Food Chem. 2021, 69, 10506–10514. [Google Scholar] [CrossRef]
- Zhu, L.; Andersen-Civil, A.I.S.; Myhill, L.J.; Thamsborg, S.M.; Kot, W.; Krych, L.; Nielsen, D.S.; Blanchard, A.; Williams, A.R. The phytonutrient cinnamaldehyde limits intestinal inflammation and enteric parasite infection. J. Nutr. Biochem. 2022, 100, 108887. [Google Scholar] [CrossRef]
- Valerii, M.C.; Turroni, S.; Ferreri, C.; Zaro, M.; Sansone, A.; Dalpiaz, A.; Botti, G.; Ferraro, L.; Spigarelli, R.; Bellocchio, I.; et al. Effect of a Fiber D-Limonene-enriched food supplement on intestinal microbiota and metabolic parameters of mice on a high-fat diet. Pharmaceutics 2021, 13, 1753. [Google Scholar] [CrossRef]
- Feng, W.; Jin, L.; Xie, Q.; Huang, L.; Jiang, Z.; Ji, Y.; Li, C.; Yang, L.; Wang, D. Eugenol protects the transplanted heart against ischemia/reperfusion injury in rats by inhibiting the inflammatory response and apoptosis. Exp. Ther. Med. 2018, 16, 3464–3470. [Google Scholar] [CrossRef] [Green Version]
- Song, F.; Li, H.; Sun, J.; Wang, S. Protective effects of cinnamic acid and cinnamic aldehyde on isoproterenol-induced acute myocardial ischemia in rats. J. Ethnopharmacol. 2013, 150, 125–130. [Google Scholar] [CrossRef]
- Rhana, P.; Barros, G.M.; Santos, V.C.O.; Costa, A.D.; Santos, D.M.D.; Fernandes-Braga, W.; Durço, A.O.; Santos, M.R.V.; Roman-Campos, D.; Vasconcelos, C.M.L.; et al. S-limonene protects the heart in an experimental model of myocardial infarction induced by isoproterenol: Possible involvement of mitochondrial reactive oxygen species. Eur. J. Pharmacol. 2022, 930, 175134. [Google Scholar] [CrossRef]
- Ma, L.; Mu, Y.; Zhang, Z.; Sun, Q. Eugenol promotes functional recovery and alleviates inflammation, oxidative stress, and neural apoptosis in a rat model of spinal cord injury. Restor. Neurol. Neurosci. 2018, 36, 659–668. [Google Scholar] [CrossRef]
- Piccialli, I.; Tedeschi, V.; Caputo, L.; Amato, G.; De Martino, L.; De Feo, V.; Secondo, A.; Pannaccione, A. The antioxidant activity of limonene counteracts neurotoxicity triggered by Aβ1-42 oligomers in primary cortical neurons. Antioxidants 2021, 10, 937. [Google Scholar] [CrossRef]
- Chen, M.; Ruan, G.; Chen, L.; Ying, S.; Li, G.; Xu, F.; Xiao, Z.; Tian, Y.; Lv, L.; Ping, Y.; et al. Neurotransmitter and intestinal interactions: Focus on the microbiota-gut-brain axis in irritable bowel syndrome. Front. Endocrinol. 2022, 13, 817100. [Google Scholar] [CrossRef]
- Ricci, C.; Rizzello, F.; Valerii, M.C.; Spisni, E.; Gionchetti, P.; Turroni, S.; Candela, M.; D’Amico, F.; Spigarelli, R.; Bellocchio, I.; et al. Geraniol treatment for irritable bowel syndrome: A double-blind randomized clinical trial. Nutrients 2022, 14, 4208. [Google Scholar] [CrossRef]
- Moreira Vasconcelos, C.F.; da Cunha Ferreira, N.M.; Hardy Lima Pontes, N.; de Sousa Dos Reis, T.D.; Basto Souza, R.; Aragão Catunda Junior, F.E.; Vasconcelos Aguiar, L.M.; Maranguape Silva da Cunha, R. Eugenol and its association with levodopa in 6-hydroxydopamine-induced hemiparkinsonian rats: Behavioural and neurochemical alterations. Basic Clin. Pharmacol. Toxicol. 2020, 127, 287–302. [Google Scholar] [CrossRef]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell line: Cell types, coating of culture vessels, differentiation and other culture conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef]
- Guénette, S.A.; Beaudry, F.; Marier, J.F.; Vachon, P. Pharmacokinetics and anesthetic activity of eugenol in male Sprague–Dawley rats. J. Vet. Pharmacol. Therap. 2006, 29, 265–270. [Google Scholar] [CrossRef]
- Pang, S.N.J. Final report on the safety assessment of PEG-30,-33,-35,-36, and-40 castor oil and PEG-30 and-40 hydrogenated castor oil. Int. J. Toxicol. 1997, 16, 269–306. [Google Scholar] [CrossRef]
- Sellamuthu, R. Eugenol. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: London, UK, 2014; Volume 2, pp. 539–541. [Google Scholar] [CrossRef]
- Lehman-McKeeman, L.D.; Rodriguez, P.A.; Takigiku, R.; Caudill, D.; Fey, M.L. d-Limonene-induced male rat-specific nephrotoxicity: Evaluation of the association between d-limonene and alpha 2u-globulin. Toxicol. Appl. Pharmacol. 1989, 99, 250–259. [Google Scholar] [CrossRef]
- Yuan, J.H.; Dieter, M.P.; Bucher, J.R.; Jameson, C.W. Toxicokinetics of cinnamaldehyde in F344 rats. Fd. Chem. Toxic. 1992, 30, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Nikfar, S.; Behboudi, A.F. Limonene. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: London, UK, 2014; Volume 3, pp. 78–82. [Google Scholar] [CrossRef]
- Chen, H.; Chan, K.K.; Budd, T. Pharmacokinetics of d-limonene in the rat by GC–MS assay. J. Pharm. Biomed. Anal. 1998, 17, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Sun, J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar] [PubMed]
- Zhao, H.; Xie, Y.; Yang, Q.; Cao, Y.; Tu, H.; Cao, H.; Wang, S. Pharmacokinetic study of cinnamaldehyde in rats by GC-MS after oral and intravenous administration. J. Pharm. Biomed. Anal. 2014, 89, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yuan, J.; Yang, Q.; Xie, Y.; Cao, W.; Wang, S. Cinnamaldehyde in a novel intravenous submicrometer emulsion: Pharmacokinetics, tissue distribution, antitumor efficacy, and toxicity. J. Agric. Food Chem. 2015, 63, 6386–6392. [Google Scholar] [CrossRef]
- Weibel, H.; Hansen, J. Interaction of cinnamaldehyde (a sensitizer in fragrance) with protein. Contact Dermat. 1989, 20, 161–166. [Google Scholar] [CrossRef]
- Yuan, J.; Bucher, J.R.; Goehl, T.J.; Dieter, M.P.; Jameson, C.W. Quantitation of cinnamaldehyde and cinnamic acid in blood by HPLC. J. Anal. Toxicol. 1992, 16, 359–362. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Gao, Z.; Xiong, M.; Zhong, Z.; Ye, L. Gas chromatographic-mass spectrometric analysis of d-limonene in human plasma. J. Pharm. Biomed. Anal. 2007, 44, 1095–1099. [Google Scholar] [CrossRef]
- Guénette, S.A.; Ross, A.; Marier, J.F.; Beaudry, F.; Vachon, P. Pharmacokinetics of eugenol and its effects on thermal hypersensitivity in rats. Eur. J. Pharmacol. 2007, 562, 60–67. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, W.; Zhang, J.; Liao, Y.; Firempong, C.K.; Adu-Frimpong, M.; Deng, W.; Zhang, H.; Yu, J.; Xu, X. Self-microemulsifying drug delivery system for improved oral delivery of limonene: Preparation, characterization, in vitro and in vivo evaluation. AAPS Pharm Sci Tech. 2019, 20, 153. [Google Scholar] [CrossRef]
- Pavan, B.; Dalpiaz, A.; Marani, L.; Beggiato, S.; Ferraro, L.; Canistro, D.; Paolini, M.; Vivarelli, F.; Valerii, M.C.; Comparone, A.; et al. Geraniol pharmacokinetics, bioavailability and its multiple effects on the liver antioxidant and xenobiotic-metabolizing enzymes. Front Pharmacol. 2018, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Lazar, V.; Holban, A.M.; Curutiu, C.; Ditu, L.M. Modulation of gut microbiota by essential oils and inorganic nanoparticles: Impact in nutrition and health. Front. Nutr. 2022, 9, 920413. [Google Scholar] [CrossRef]
- Ma, L.; Liu, J.; Lin, Q.; Gu, Y.; Yu, W. Eugenol protects cells against oxidative stress via Nrf2. Exp. Ther. Med. 2021, 21, 107. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, W.; Wang, H.; Bao, L.; Li, G.; Li, T.; Song, S.; Li, H.; Hao, J.; Sun, J. Resveratrol protects PC12 cell against 6-OHDA damage via CXCR4 signaling pathway. Evid. Based Complement. Altern. Med. 2015, 2015, 730121. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.P.; Chen, Y.J.; Lee, Y.L.; Wang, J.S.; Chang, M.C.; Lan, W.H.; Chang, H.H.; Chao, W.M.; Tai, T.F.; Lee, M.Y.; et al. Effects of root-end filling materials and eugenol on mitochondrial dehydrogenase activity and cytotoxicity to human periodontal ligament fibroblasts. J. Biomed. Mater. Res. B. Appl. Biomater. 2004, 71, 429–440. [Google Scholar] [CrossRef]
- Zhang, G.; Buchler, I.P.; De Pasquale, M.; Wormald, M.; Liao, G.; Wei, H.; Barrow, J.C.; Carr, G.V. Development of a PC12 cell based assay for screening catechol-O-methyltransferase inhibitors. ACS Chem. Neurosci. 2019, 10, 4221–4226. [Google Scholar] [CrossRef]
- Westerink, R.H.; Ewing, A.G. The PC12 cell as model for neurosecretion. Acta. Physiol. 2008, 192, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Mount, H.; Welner, S.; Quirion, R.; Boksa, P. Glutamate stimulation of [3H]dopamine release from dissociated cell cultures of rat ventral mesencephalon. J. Neurochem. 1989, 52, 1300–1310. [Google Scholar] [CrossRef]
- Sutou, S.; Koeda, A.; Komatsu, K.; Shiragiku, T.; Seki, H.; Kudo, T. Collaborative study of thresholds for mutagens: Hormetic responses in cell proliferation tests using human and murine lymphoid cells. Dose Response 2021, 19, 8473. [Google Scholar] [CrossRef]
- van den Berg, M.P.; Romeijn, S.G.; Verhoef, J.C.; Merkus, F.W. Serial cerebrospinal fluid sampling in a rat model to study drug uptake from the nasal cavity. J. Neurosci. Methods 2002, 116, 99–107. [Google Scholar] [CrossRef]
- Dalpiaz, A.; Ferraro, L.; Perrone, D.; Leo, E.; Iannuccelli, V.; Pavan, B.; Paganetto, G.; Beggiato, S.; Scalia, S. Brain uptake of a zidovudine prodrug after nasal administration of solid lipid microparticles. Mol. Pharm. 2014, 11, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Junior, E.R.; Truzzi, E.; Ferraro, L.; Fogagnolo, M.; Pavan, B.; Beggiato, S.; Rustichelli, C.; Maretti, E.; Lima, E.M.; Leo, E.; et al. Nasal administration of nanoencapsulated geraniol/ursodeoxycholic acid conjugate: Towards a new approach for the management of Parkinson’s disease. J. Control. Release 2020, 321, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Bourne, D.W.A.; Dittert, L.W. Pharmacokinetics. In Modern Pharmaceutics; Banker, G.S., Rhodes, C.T., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1990; pp. 91–142. [Google Scholar]
- Simovic, S.; Heard, P.; Hui, H.; Song, Y.; Peddie, F.; Davey, A.K.; Lewis, A.; Rades, T.; Prestidge, C.A. Dry hybrid lipid−silica microcapsules engineered from submicron lipid droplets and nanoparticles as a novel delivery system for poorly soluble drugs. Mol. Pharm. 2009, 6, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Felgenhauer, K. Protein size and cerebrospinal fluid composition. Klin. Wochenschr. 1974, 52, 1158–1164. [Google Scholar] [CrossRef]
- Madu, A.; Cioffe, C.; Mian, U.; Burroughs, M.; Tuomanen, E.; Mayers, M.; Schwartz, E.; Miller, M. Pharmacokinetics of fluconazole in cerebrospinal fluid and serum of rabbits: Validation of an animal model used to measure drug concentrations in cerebrospinal fluid. Antimicrob. Agents Chemother. 1994, 38, 2111–2115. [Google Scholar] [CrossRef] [Green Version]
- Pavan, B.; Frigato, E.; Pozzati, S.; Prasad, P.D.; Bertolucci, C.; Biondi, C. Circadian clocks regulate adenylyl cyclase activity rhythms in human RPE cells. Biochem. Biophys. Res. Commun. 2006, 350, 169–173. [Google Scholar] [CrossRef]
- Ndikung, J.; Storm, D.; Violet, N.; Kramer, A.; Schönfelder, G.; Ertych, N.; Oelgeschläger, M. Restoring circadian synchrony in vitro facilitates physiological responses to environmental chemicals. Environ. Int. 2020, 134, 105265. [Google Scholar] [CrossRef]
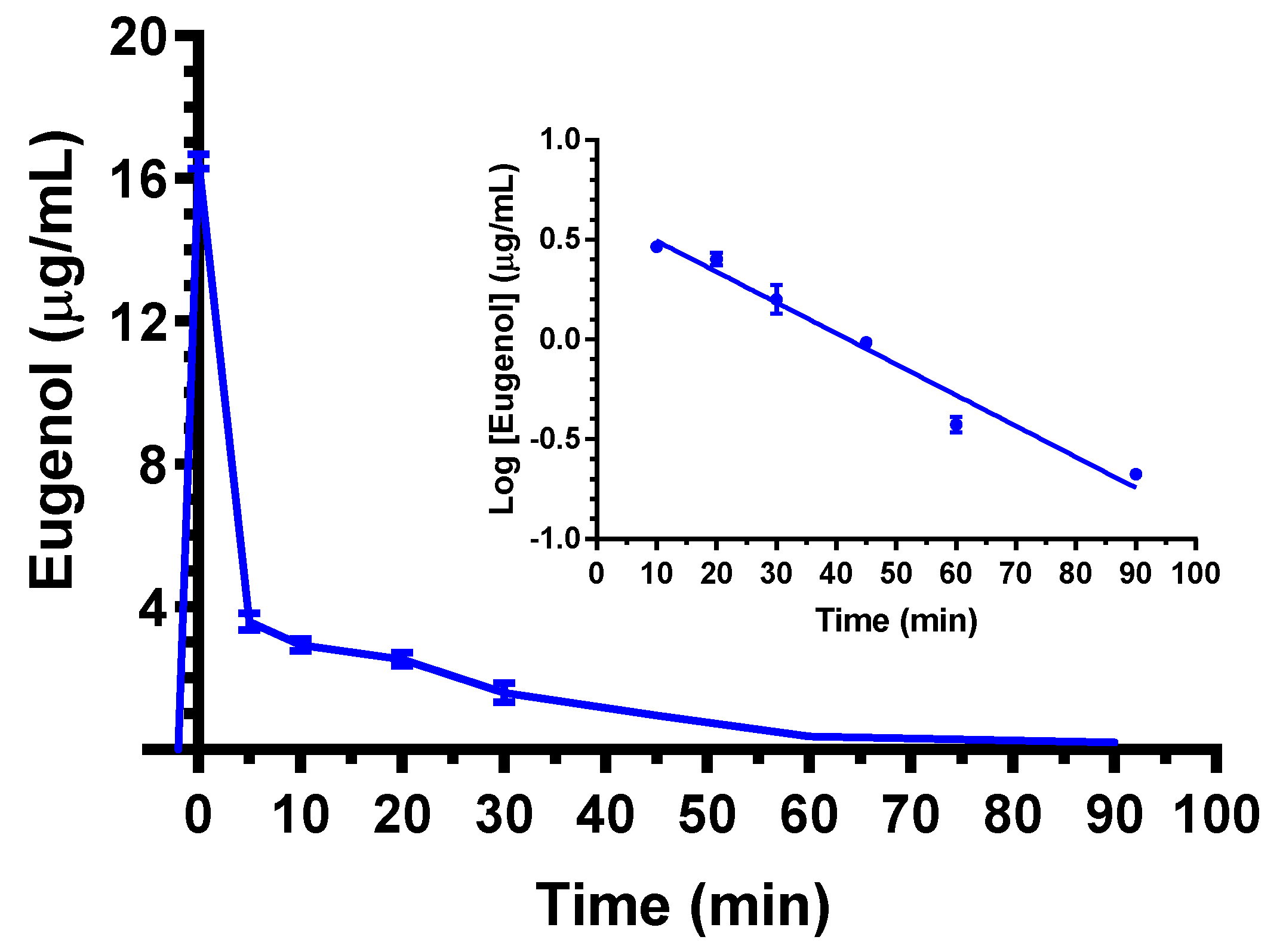
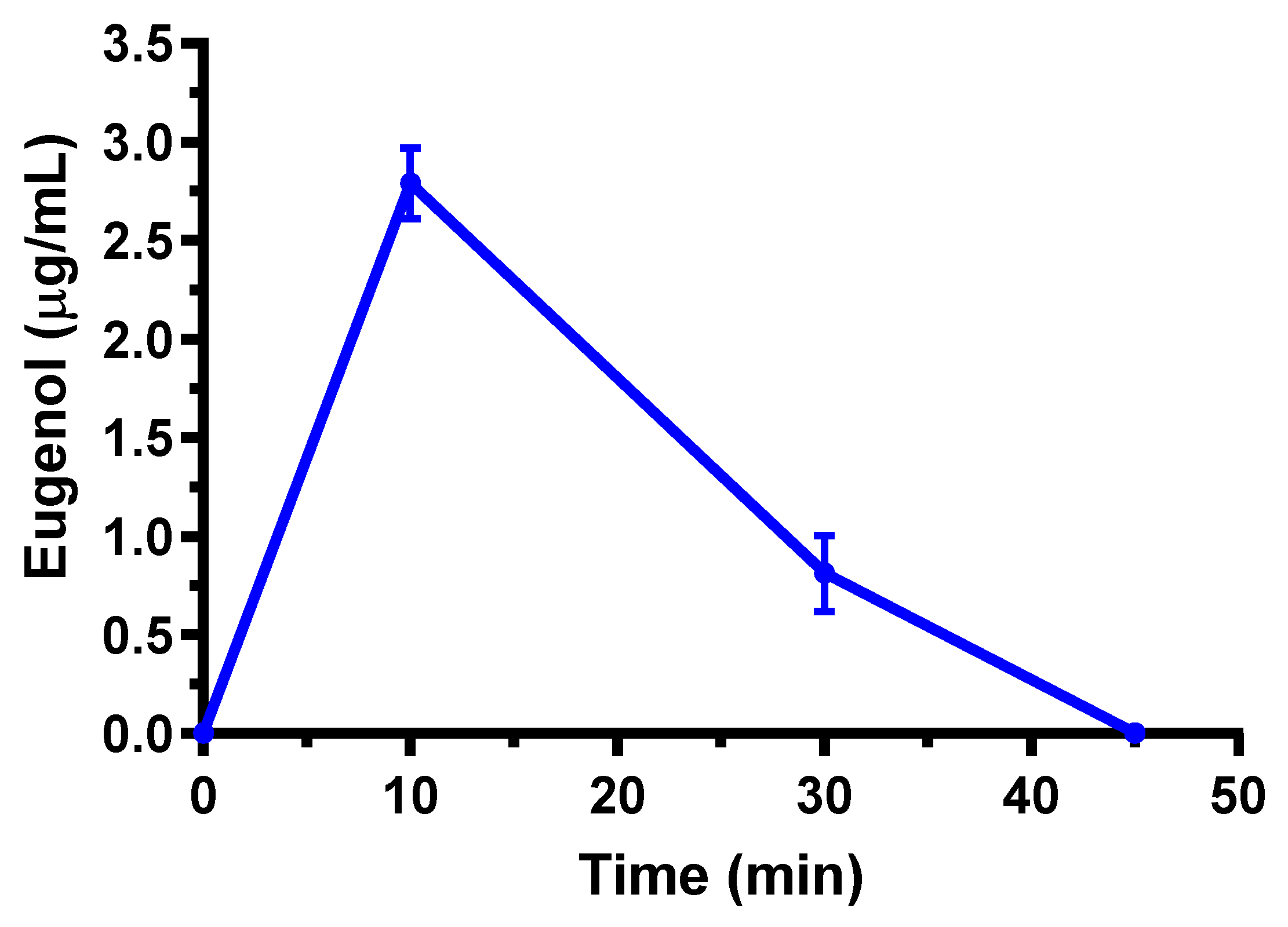
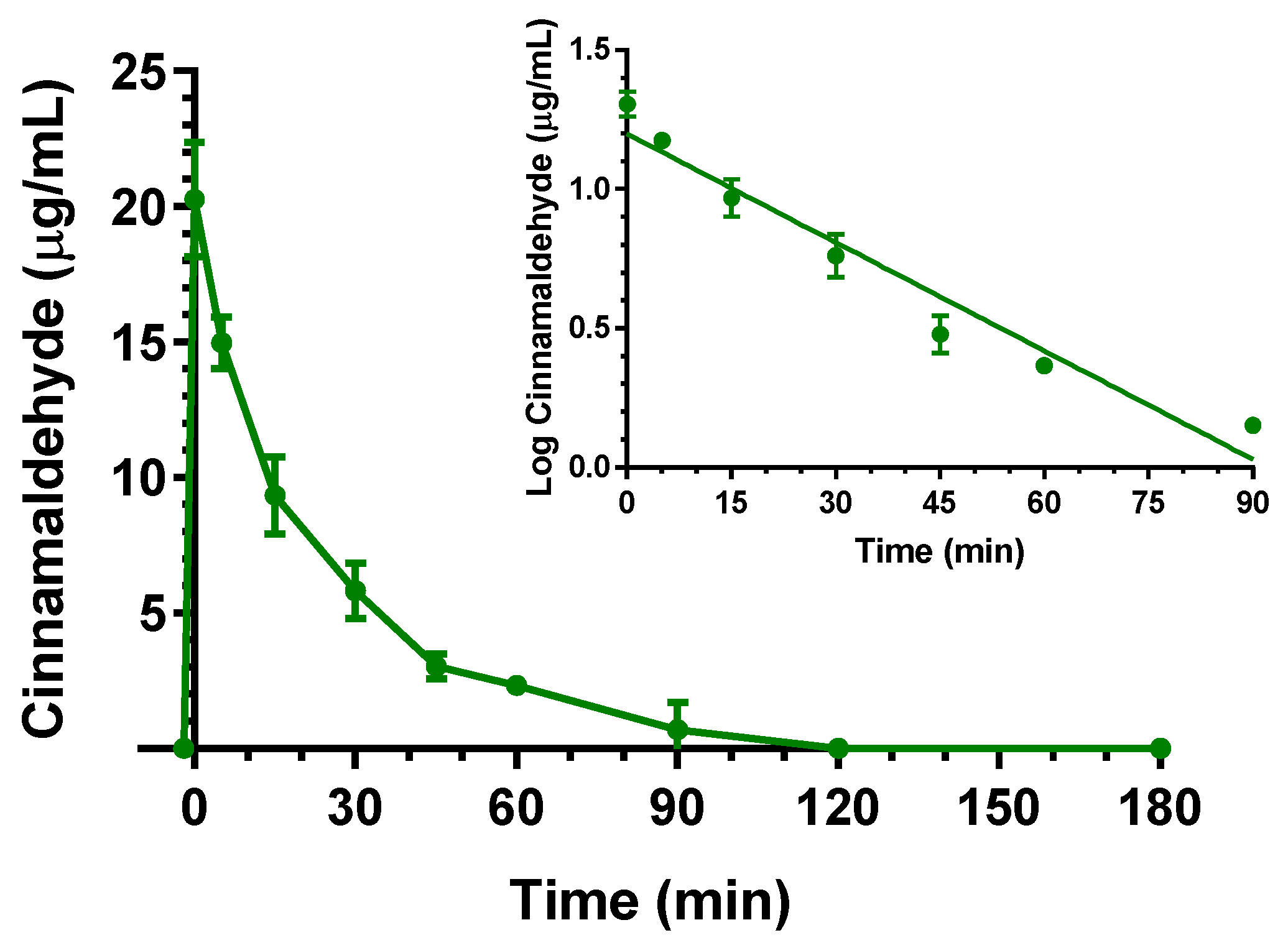
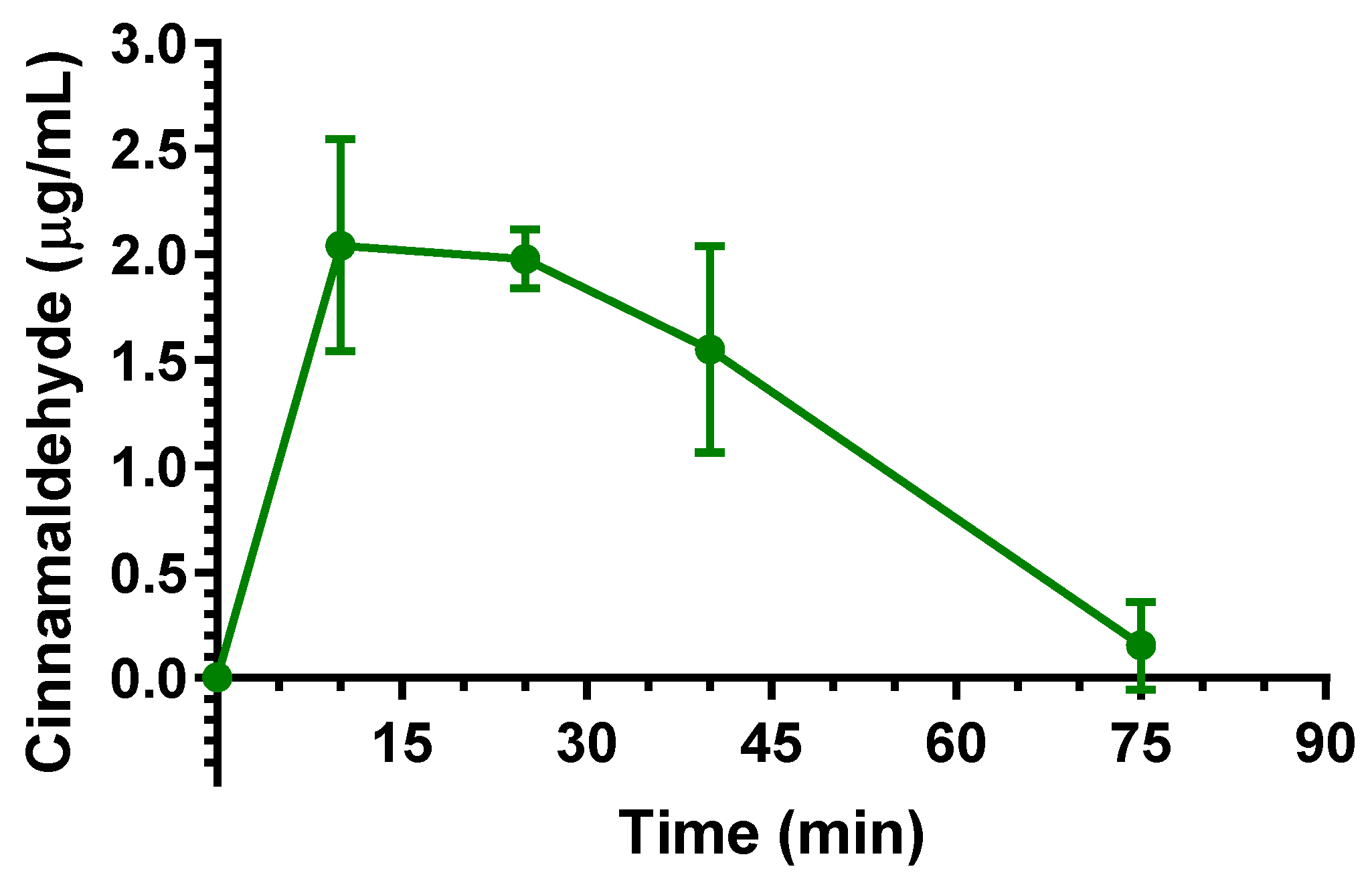
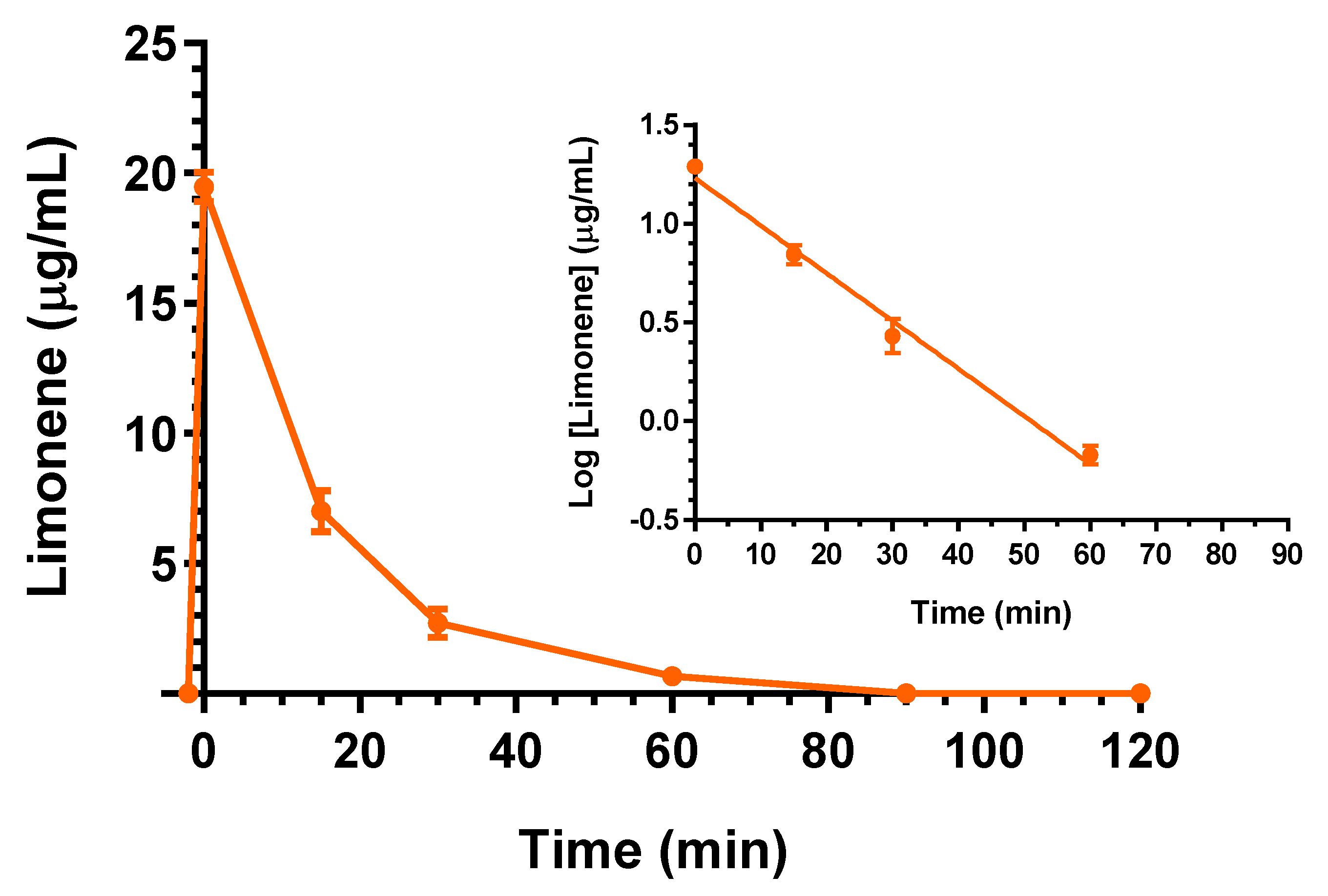
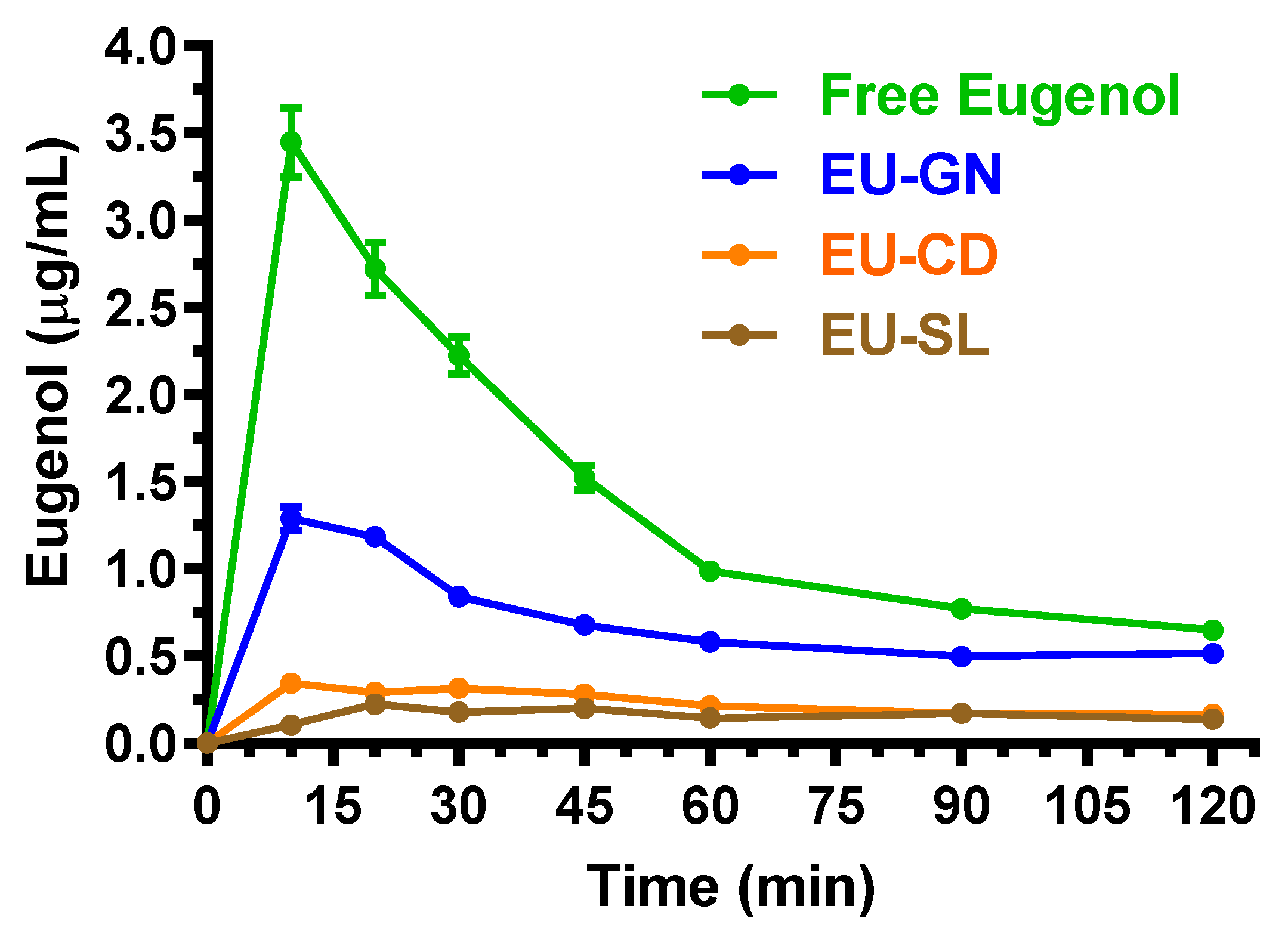
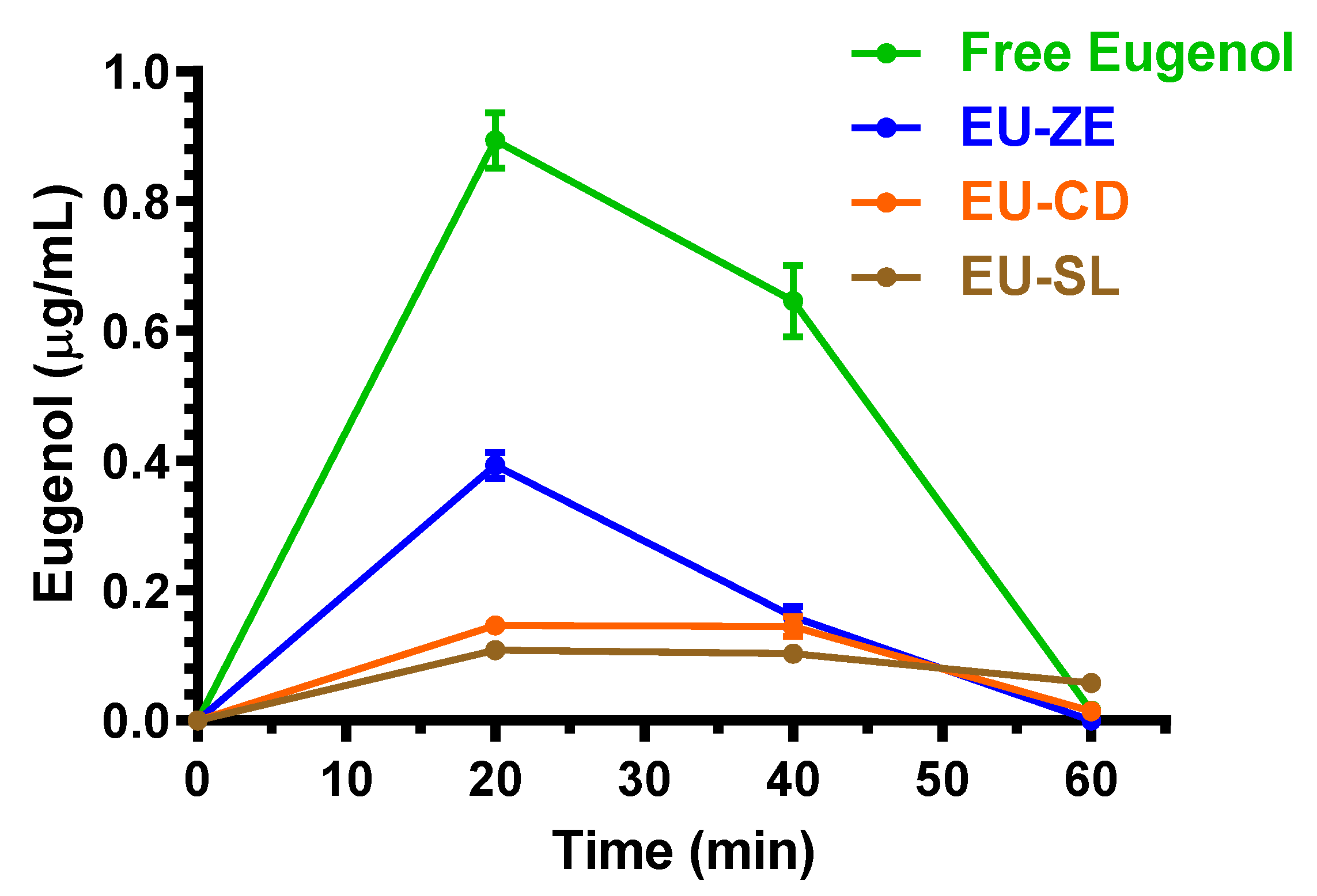
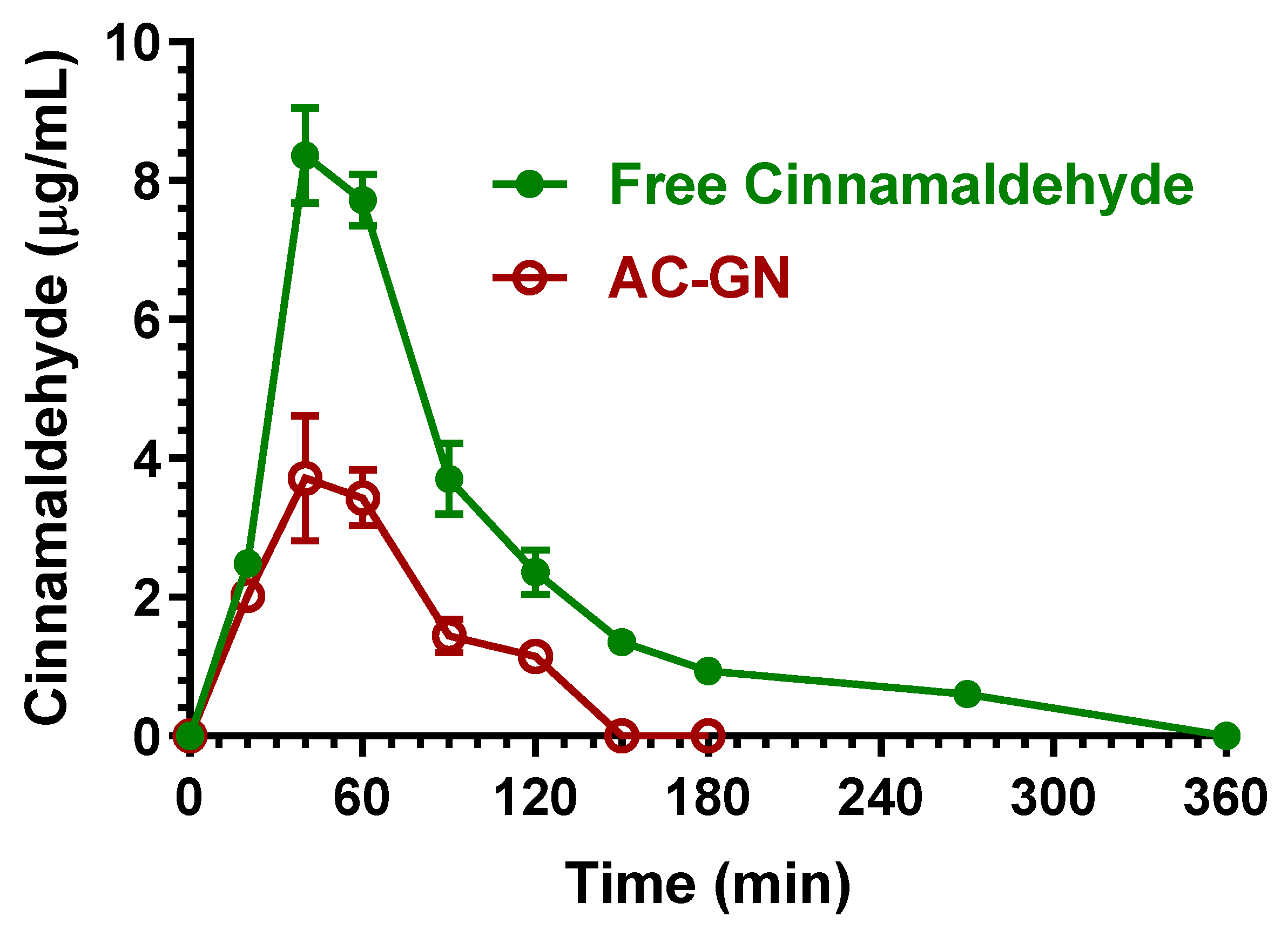
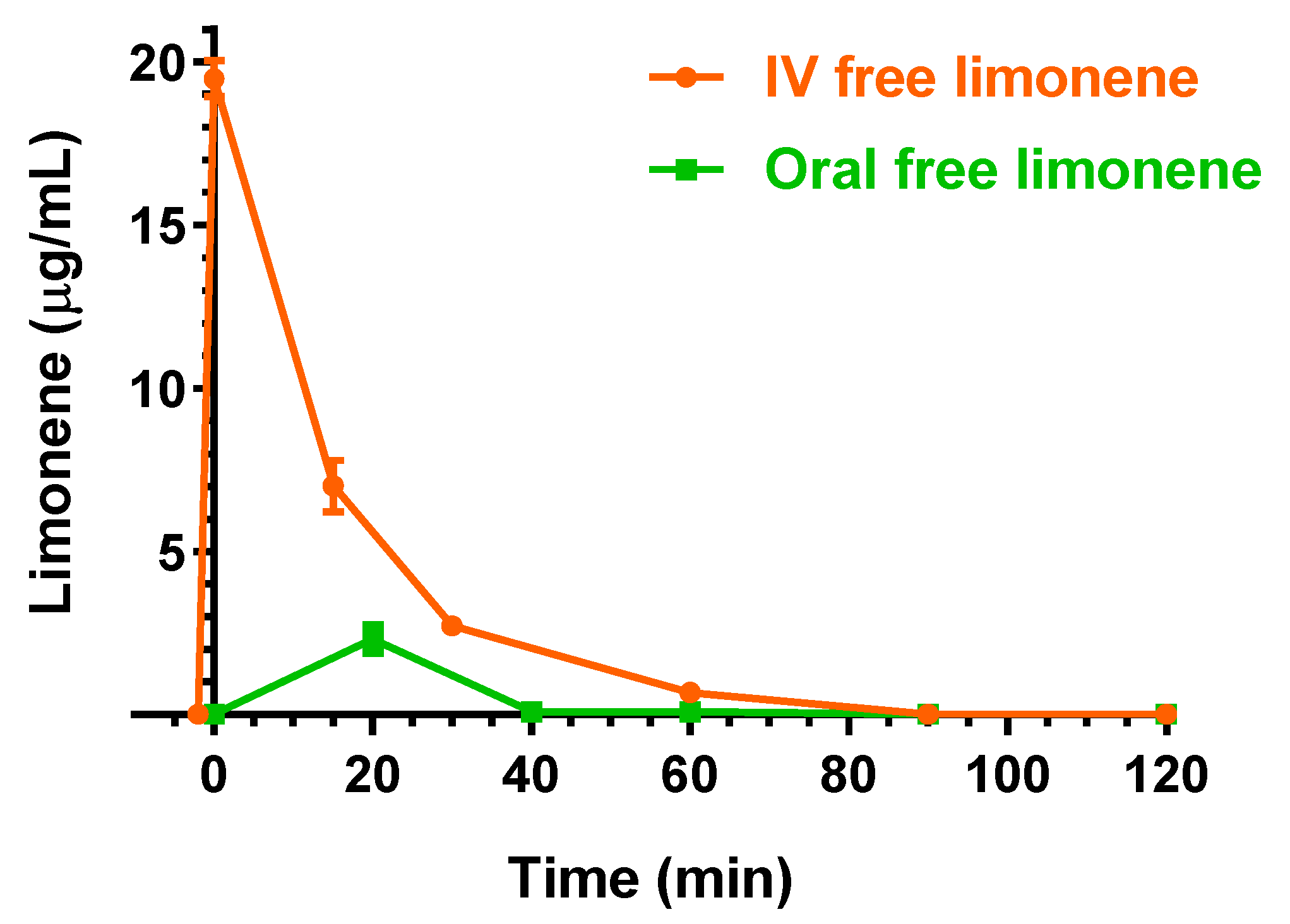

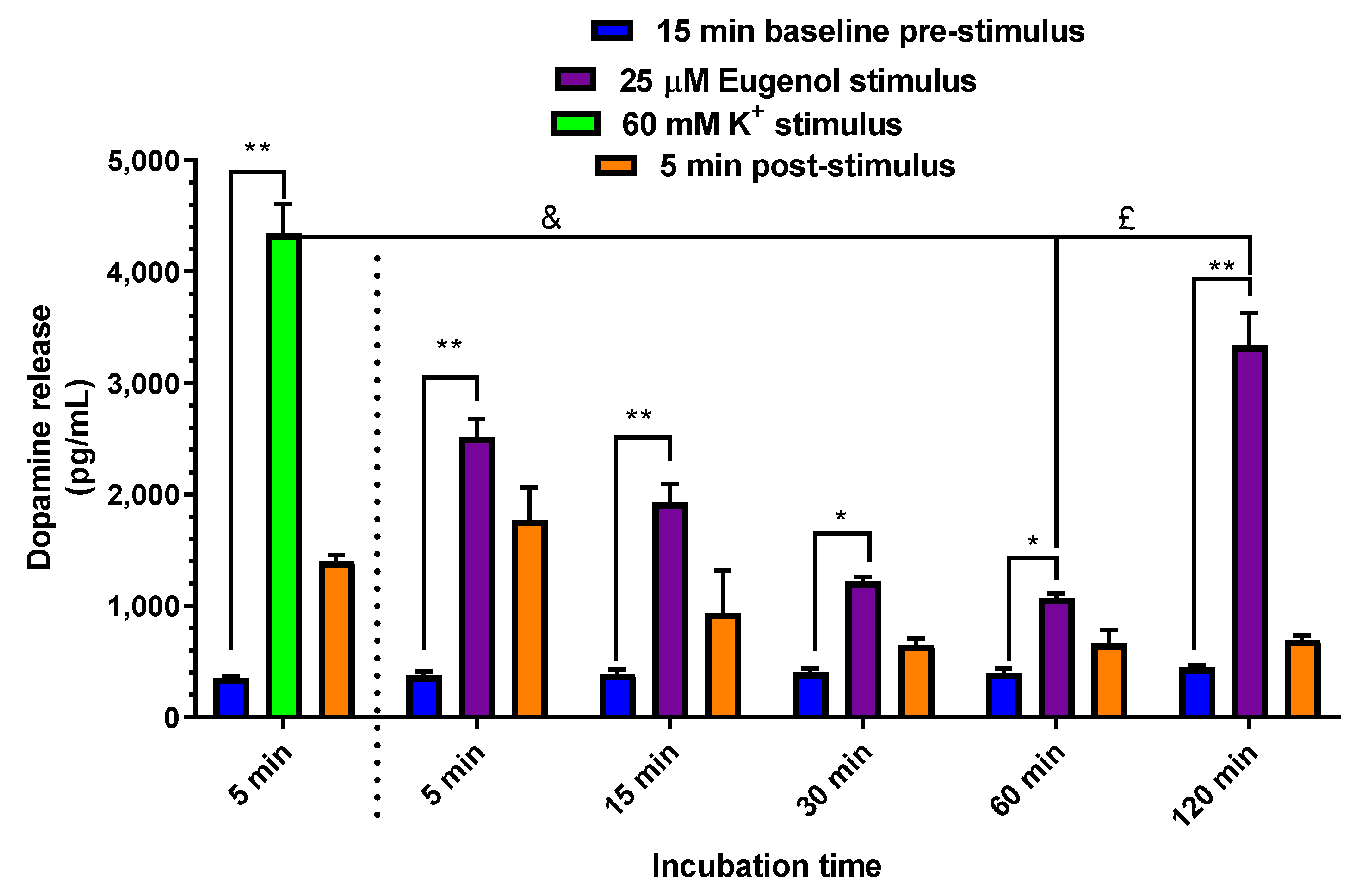
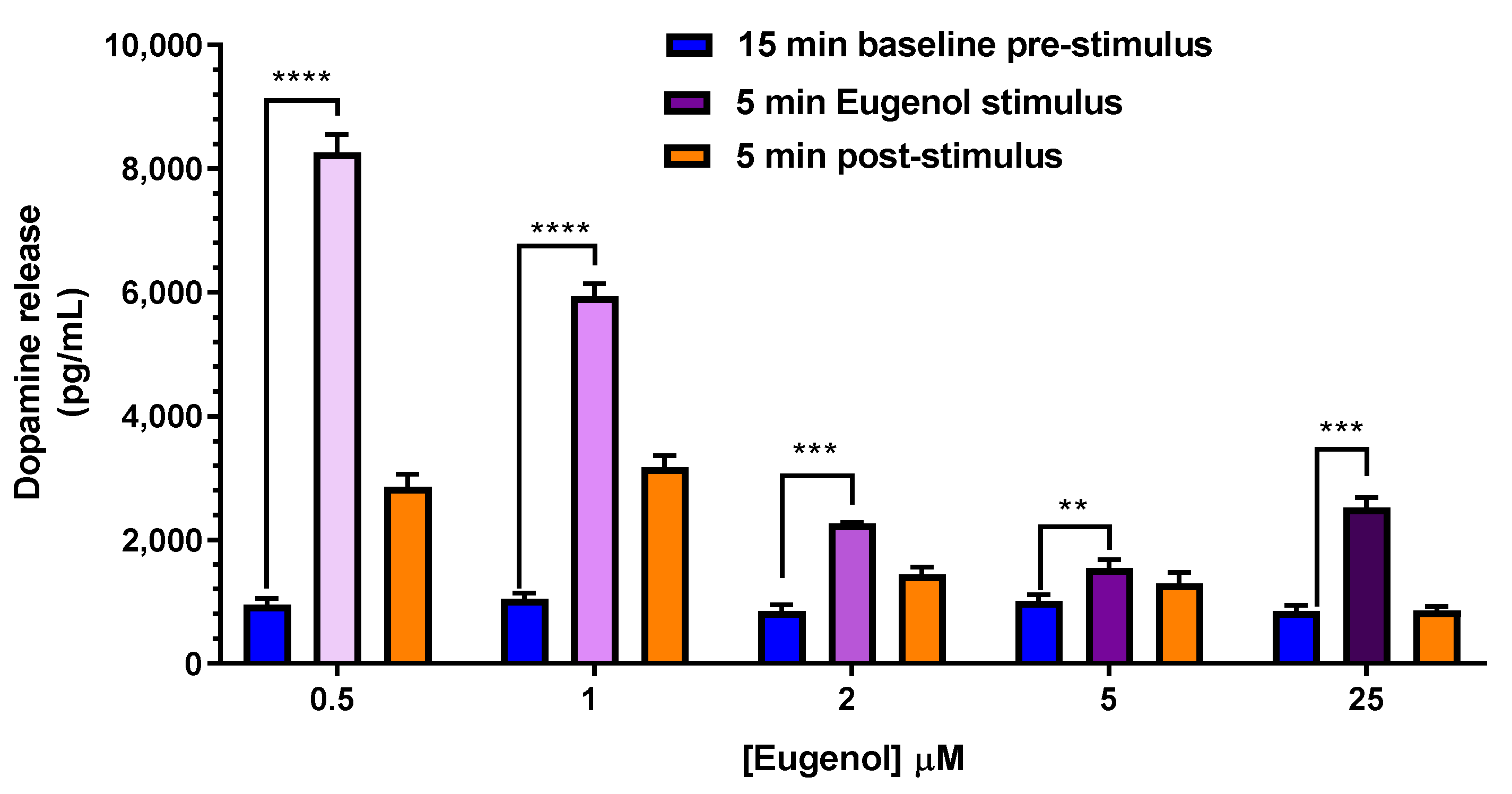
| Compound | Dose | C0 (μg/mL) | kel (min−1) | t1/2 (min) | AUC (μg mL−1·min) | Cl (mL·min·kg−1) | Vd (mL·kg−1) |
|---|---|---|---|---|---|---|---|
| Eugenol | 5 mg (20 mg/kg) | 16.5 ± 0.2 | 0.036 ± 0.002 a | 19.4 ± 2.1 a | 174.8 ± 3.1 | 114 ± 2 b | 3212 ± 247 b |
| Cinnamaldehyde | 5 mg (20 mg/kg) | 20.3 ± 1.5 | 0.030 ± 0.001 | 23.1 ± 1.6 | 506.0 ± 22.3 | 39.5 ± 1.7 | 1320 ± 190 |
| D-Limonene | 25 mg (100 mg/kg) | 19.5 ± 0.4 | 0.056 ± 0.004 | 12.4 ± 0.9 | 352.2 ± 13.1 | 284 ± 10 | 5516 ± 558 |
| Compound | Dose | Cmax (μg/mL) | Tmax (min) | AUC (μg·mL−1·min) | R |
|---|---|---|---|---|---|
| Eugenol | 5 mg (20 mg/kg) | 2.79 ± 0.18 | 10 | 56.1 ± 4.2 | 0.96 ± 0.08 |
| Cinnamaldehyde | 5 mg (20 mg/kg) | 2.04 ± 0.36 | 10 | 96.7 ± 11.0 | 0.16 ± 0.04 |
| D-Limonene | 25 mg (100 mg/kg) | 0 | 0 | 0 | 0 |
| Formulation | Dose | Cmax (μg/mL) | Tmax (min) | AUC (μg·mL−1·min) | F (%) | RB (%) |
|---|---|---|---|---|---|---|
| Free eugenol | 125 mg (500 mg/kg) | 3.4 ± 0.2 | 10 | 185.7 ± 3.1 | 4.25 ± 0.11 | 100 |
| EU-GN | 125 mg (500 mg/kg) | 1.29 ± 0.10 | 10 | 95.6 ± 1.5 a | 2.19 ± 0.05 | 51.5 ± 1.1 c |
| EU-CD | 125 mg (500 mg/kg) | 0.35 ± 0.05 | 10 | 31.6 ± 1.7 a | 0.72 ± 0.04 | 17.0 ± 1.0 c |
| EU-SL | 125 mg (500 mg/kg) | 0.23 ± 0.01 | 20 | 29.2 ± 0.7 a | 0.67 ± 0. 02 | 15.7 ± 0.5 c |
| Free cinnamaldehyde | 100 mg (400 mg/kg) | 8.36 ± 0.49 | 40 | 742.7 ± 18.4 | 7.33 ± 0.37 | 100 |
| AC-GN | 100 mg (400 mg/kg) | 3.71 ± 0.64 | 40 | 278.3 ± 15.9 b | 2.75 ± 0.19 | 37.5 ± 3.2 d |
| Free D-limonene | 50 mg (200 mg/kg) | 2.31 ± 0.44 | 30 | 49.6 ± 6.7 | 7.04 ± 0.96 | 100 |
| LM-CA | 50 mg (200 mg/kg) | 0 | 0 | 0 | 0 | 0 |
| Compound | Dose | Cmax (μg/mL) | Tmax (min) | AUC (μg·mL−1·min) |
|---|---|---|---|---|
| Free eugenol | 125 mg (500 mg/kg) | 0.89 ± 0.06 | 20 | 30.97 ± 2.18 |
| EU-GN | 125 mg (500 mg/kg) | 0.39 ± 0.03 | 20 | 11.05 ± 0.69 a |
| EU-CD | 125 mg (500 mg/kg) | 0.146 ± 0.002 | 20 | 5.96 ± 0.31 a |
| EU-SL | 125 mg (500 mg/kg) | 0.110 ± 0.006 | 20 | 5.69 ± 0.09 a |
| Free cinnamaldehyde | 100 mg (400 mg/kg) | 0 | 0 | 0 |
| AC-GN | 100 mg (400 mg/kg) | 0 | 0 | 0 |
| Free D-limonene | 50 mg (200 mg/kg) | 0 | 0 | 0 |
| LM-CA | 50 mg (200 mg/kg) | 0 | 0 | 0 |
| Intravenous administration | ||||
| Compound | Dose | Formulation | Administered volume | Infusion time |
| Eugenol | 5 mg (20 mg/kg) | 12.5 mg/mL eugenol emulsion | 0.4 mL | 2 min |
| Cinnamaldehyde | 5 mg (20 mg/kg) | 12.5 mg/mL cinnamaldehyde emulsion | 0.4 mL | 2 min |
| D-Limonene | 25 mg (100 mg/kg) | Pure compound | 30 µL | 2 min |
| Oral administration | ||||
| Compound | Dose | Formulation | Administration method | Administered amounts |
| Eugenol (free) | 125 mg (500 mg/kg) | Corn oil solution (125 mg/mL) | Gavage | 1 mL |
| Eugenol | 125 mg (500 mg/kg) | EU-GN (20% w/w) | Palatable food | 625 mg |
| Eugenol | 125 mg (500 mg/kg) | EU-CD (30 mg/mL) | Gavage | 4.2 mL |
| Eugenol | 125 mg (500 mg/kg) | EU-SL (390 mg/mL) | Gavage | 390 μL |
| Cinnamaldehyde (free) | 100 mg (400 mg/kg) | Corn oil solution (100 mg/mL) | Gavage | 1 mL |
| Cinnamaldehyde | 100 mg (400 mg/kg) | AC-GN (14% w/w) | Palatable food | 714 mg |
| D-Limonene (free) | 50 mg (200 mg/kg) | Corn oil solution (100 mg/mL) | Gavage | 500 μL |
| D-Limonene | 50 mg (200 mg/kg) | LM-CA (14% w/w) | Palatable formulation | 300 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavan, B.; Bianchi, A.; Botti, G.; Ferraro, L.; Valerii, M.C.; Spisni, E.; Dalpiaz, A. Pharmacokinetic and Permeation Studies in Rat Brain of Natural Compounds Led to Investigate Eugenol as Direct Activator of Dopamine Release in PC12 Cells. Int. J. Mol. Sci. 2023, 24, 1800. https://doi.org/10.3390/ijms24021800
Pavan B, Bianchi A, Botti G, Ferraro L, Valerii MC, Spisni E, Dalpiaz A. Pharmacokinetic and Permeation Studies in Rat Brain of Natural Compounds Led to Investigate Eugenol as Direct Activator of Dopamine Release in PC12 Cells. International Journal of Molecular Sciences. 2023; 24(2):1800. https://doi.org/10.3390/ijms24021800
Chicago/Turabian StylePavan, Barbara, Anna Bianchi, Giada Botti, Luca Ferraro, Maria Chiara Valerii, Enzo Spisni, and Alessandro Dalpiaz. 2023. "Pharmacokinetic and Permeation Studies in Rat Brain of Natural Compounds Led to Investigate Eugenol as Direct Activator of Dopamine Release in PC12 Cells" International Journal of Molecular Sciences 24, no. 2: 1800. https://doi.org/10.3390/ijms24021800







