Understanding the Role of Oxidative Stress, Neuroinflammation and Abnormal Myelination in Excessive Aggression Associated with Depression: Recent Input from Mechanistic Studies
Abstract
1. Major Depressive Disorder and Excessive Aggression
2. Animal Models of Excessive Aggression
| Animal Model | Strains | Core Characteristics | References |
|---|---|---|---|
| Genetic Models | |||
| Knock-out dopamine D2 receptor | D2R−/− mice | Elevated aggression in males, reduced hypothalamic orexin precursor expression, increased serum prolactin levels | [21,48] |
| Knock-out α-isoform of the oestrogen receptor | ERα−/− mice | Elevated aggression in males, compromised neuroplasticity | [23,24] |
| Knock-out arginine vasopressin V1b receptor | V1bR−/− mice | Elevated aggression in males, compromised neuroplasticity, decreased neurogenesis | [49] |
| Knock-out dopamine β-hydroxylase | DBH−/− mice | Elevated aggression in males, compromised neuroplasticity, decreased insulin receptor substrate-1 (IRS-1) and insulin receptor substrate-2 (IRS-2) signalling | [26,48] |
| Knock-out tryptophan hydroxylase 2 | Tph2−/− male mice | Elevated aggression in males, decreased 5-HT level, compromised neuroplasticity | [27,28] |
| Knock-out 5-HT1B receptor | 5-HT1B−/− mice | Elevated aggression in males, deficient neuroplasticity | [29] |
| Knock-out dopamine transporter | DAT−/− mice | Elevated aggression in males, deficient synaptic plasticity | [30] |
| Knock-out nitric oxide synthase | NOS−/− mice | Elevated aggression in males, compromised neuroplasticity, antioxidant system disbalance | [31] |
| Knock-out MAOA | MAOA−/− mice | Elevated aggression in males, deficient synaptic plasticity and pruning, disbalance of brain monoamine levels | [32] |
| Bred for short attack latency (SAL) | SAL mice | Elevated aggression, low brain 5-HT level, reduced 5-HT reuptake transporter activity | [33,34] |
| Turku Aggressive mice | Turku Aggressive mice | Elevated aggression in males | [35] |
| North Carolina 900 mice | NC900 mice | Elevated aggression in males, reduced GABA-ergic neurotransmission | [36] |
| North Carolina 100 mice | NC100 mice | Elevated aggression in males, lower dopamine concentrations | [36] |
| Wistar rats with low anxiety-like behaviour (LAB) | LAB rats | Elevated aggression in males, compromised neuroplasticity | [37,38] |
| Environment Stress Models | |||
| Social isolation | CD1, C57BL/6J mice | Excessive aggressive behaviour in males, alterations in the function of the HPA axis | [41,42,43,44] |
| Maternal separation | C57BL/6J mice | Rats: increased intermale aggression at 14–16 weeks of age, lowered maternal aggression. Mice: females are more aggressive towards male intruders; males are less aggressive towards male intruders | [45,46] |
| Chronic mild stress | BALB/C, CD1, C57BL/6J mice | Increased offensive and aggressive behaviours in males; GSK3-β overexpression; microglial activation, reduced neuroplasticity | [18,50,51,52,53,54,55] |
| Rat exposure | C57BL/6 mice | Increased aggressive behaviour in males, aberrant neurogenesis, reduced neuroplasticity; oxidative stress | [52,56] |
| Social defeat | C57BL/6 mice | Excessive aggression in dominant males, microglial activation, reduced neuroplasticity and synaptic pruning, deficient neurogenesis, GSK3-β overexpression, oxidative stress | [57,58,59,60,61] |
| Ultrasound stress | BALB/C, CD1, C57BL/6J mice; Wistar, Sprague-Dawley rats | Increased aggressive behaviours in males; microglial activation, reduced neuroplasticity, GSK3-β overexpression, oxidative stress | [62,63,64,65,66,67] |
| Maternal Models | |||
| Stimuli from pups | BALB/C, CD1, C57BL/6J mice; Wistar, Sprague-Dawley rats; Syrian hamsters; | Increased aggressive behaviours in females, deficient neuroplasticity and reduced neurogenesis | [68,69,70,71] |
| Gene × Environment Interaction Models | |||
| Deficiency of tryptophane hydroxylase and 5-day predation stress | Tph2+/− male mice | Increased aggressive behaviours in males; reduced brain serotonin content, reduced expression of 5-HT6 receptor, GSK3-β overexpression | [72] |
| Tph2+/− female mice | Increased aggressive behaviours in females; reduced brain serotonin content, GSK3-β and myelin basic protein overexpression; deficient neuroplasticity, downregulation of synaptophysin | [73] | |
3. Neuroanatomical Basis of Aggression in Humans and Rodents in the Context of Depressive Disorder
4. Neuroinflammatory Mechanisms of Depression and Excessive Aggression
5. Oxidative Stress Markers, Insulin Receptor Signalling and Excessive Aggression
6. Role of Disrupted Myelination and Connectivity in Excessive Aggression and Impulsivity Associated with Depressive Syndrome
7. Challenges in the Management of Excessive Aggression
8. New Strategies in Pharmacological Management of Pathological Aggression
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berrios, G.E. Melancholia and Depression During the 19th Century: A Conceptual History. Br. J. Psychiatry 1988, 153, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Ormel, J.; Cuijpers, P.; Jorm, A.F.; Schoevers, R. Prevention of Depression Will Only Succeed When It Is Structurally Embedded and Targets Big Determinants. World Psychiatry 2019, 18, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sundquist, K.; Rastkhani, H.; Palmér, K.; Memon, A.A.; Sundquist, J. Association of Mitochondrial DNA in Peripheral Blood with Depression, Anxiety and Stress- and adjustment Disorders in Primary Health Care Patients. Eur. Neuropsychopharmacol. 2017, 27, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.; Weltens, I.; Bervoets, C.; de Fruyt, J.; Samochowiec, J.; Fiorillo, A.; Sampogna, G.; Bienkowski, P.; Preuss, W.U.; Misiak, B.; et al. The Pharmacological Management of Agitated and Aggressive Behaviour: A Systematic Review and Meta-Analysis. Eur. Psychiatry 2019, 57, 78–100. [Google Scholar] [CrossRef] [PubMed]
- Pratt, L.A.; Brody, D.J. Depression in the United States Household Population, 2005–2006. NCHS Data Brief 2008, 1–8. [Google Scholar]
- Shelton, D. Emotional Disorders in Young Offenders. J. Nurs. Scholarsh. 2001, 33, 259–263. [Google Scholar] [CrossRef]
- Swanson, J.W.; Holzer, C.E.; Ganju, V.K.; Jono, R.T. Violence and Psychiatric Disorder in the Community: Evidence from the Epidemiologic Catchment Area Surveys. Psychiatr. Serv. 1990, 41, 761–770. [Google Scholar] [CrossRef]
- Korn, M.L.; Plutchik, R.; van Praag, H.M. Panic-Associated Suicidal and Aggressive Ideation and Behavior. J. Psychiatr. Res. 1997, 31, 481–487. [Google Scholar] [CrossRef]
- Satyanarayana, V.A.; Chandra, P.S.; Vaddiparti, K. Mental Health Consequences of Violence against Women and Girls. Curr. Opin. Psychiatry 2015, 28, 350–356. [Google Scholar] [CrossRef]
- Palma, A.; Ansoleaga, E.; Ahumada, M. Workplace Violence among Health Care Workers. Rev. Med. Chile 2018, 146, 213–222. [Google Scholar] [CrossRef]
- van Praag, H.M. Anxiety/Aggression—Driven Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 893–924. [Google Scholar] [CrossRef]
- Fava, M. Psychopharmacologic Treatment of Pathologic Aggression. Psychiatr. Clin. N. Am. 1997, 20, 427–451. [Google Scholar] [CrossRef]
- Nierenberg, A.A. Strategies for Achieving Full Remission When First-Line Antidepressants Are Not Enough. J. Clin. Psychiatry 2013, 74, e26. [Google Scholar] [CrossRef]
- Sharma, T.; Guski, L.S.; Freund, N.; Gøtzsche, P.C. Suicidality and Aggression during Antidepressant Treatment: Systematic Review and Meta-Analyses Based on Clinical Study Reports. BMJ 2016, 352, i65. [Google Scholar] [CrossRef]
- Wise, J. Antidepressants May Double Risk of Suicide and Aggression in Children, Study Finds. BMJ 2016, 352, i545. [Google Scholar] [CrossRef]
- Stone, M.H. Long-Term Course of Borderline Personality Disorder. Psychodyn. Psychiatry 2016, 44, 449–474. [Google Scholar] [CrossRef]
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular Pathways of Major Depressive Disorder Converge on the Synapse. Mol. Psychiatry 2022, 1–14. [Google Scholar] [CrossRef]
- Strekalova, T.; Liu, Y.; Kiselev, D.; Khairuddin, S.; Chiu, J.L.Y.; Lam, J.; Chan, Y.-S.; Pavlov, D.; Proshin, A.; Lesch, K.-P.; et al. Chronic Mild Stress Paradigm as a Rat Model of Depression: Facts, Artifacts, and Future Perspectives. Psychopharmacology 2022, 239, 663–693. [Google Scholar] [CrossRef]
- Bortolato, M.; Pivac, N.; Muck Seler, D.; Nikolac Perkovic, M.; Pessia, M.; di Giovanni, G. The Role of the Serotonergic System at the Interface of Aggression and Suicide. Neuroscience 2013, 236, 160–185. [Google Scholar] [CrossRef]
- Haller, J.; Kruk, M.R. Normal and Abnormal Aggression: Human Disorders and Novel Laboratory Models. Neurosci. Biobehav. Rev. 2006, 30, 292–303. [Google Scholar] [CrossRef]
- Vukhac, K.L.; Sankoorikal, E.B.; Wang, Y. Dopamine D2L Receptor- and Age-Related Reduction in Offensive Aggression. Neuroreport 2001, 12, 1035–1038. [Google Scholar] [CrossRef]
- Takahashi, A.; Miczek, K.A. Neurogenetics of Aggressive Behavior: Studies in Rodents. Curr. Top. Behav. Neurosci. 2014, 17, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Scordalakes, E.M.; Rissman, E.F. Aggression in Male Mice Lacking Functional Estrogen Receptor Alpha. Behav. Neurosci. 2003, 117, 38–45. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, V.E.M.; Bakker, J. Neuroendocrine Regulation of Female Aggression. Front. Endocrinol. 2022, 13, 1853. [Google Scholar] [CrossRef]
- Wersinger, S.R.; Caldwell, H.K.; Christiansen, M.; Young, W.S. Disruption of the Vasopressin 1b Receptor Gene Impairs the Attack Component of Aggressive Behavior in Mice. Genes Brain Behav. 2007, 6, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.D.; Bourdélat-Parks, B.N.; Cameron Liles, L.; Weinshenker, D. Genetic Reduction of Noradrenergic Function Alters Social Memory and Reduces Aggression in Mice. Behav. Brain Res. 2005, 161, 197–203. [Google Scholar] [CrossRef]
- Mosienko, V.; Bader, M.; Alenina, N. The Serotonin-Free brain: Behavioral consequences of Tph2 deficiency in animal models. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 601–607. ISBN 9780444641250. [Google Scholar]
- Mosienko, V.; Bert, B.; Beis, D.; Matthes, S.; Fink, H.; Bader, M.; Alenina, N. Exaggerated Aggression and Decreased Anxiety in Mice Deficient in Brain Serotonin. Transl. Psychiatry 2012, 2, e122-9. [Google Scholar] [CrossRef]
- Saudou, F.; Amara, D.A.; Dierich, A.; LeMeur, M.; Ramboz, S.; Segu, L.; Buhot, M.C.; Hen, R. Enhanced Aggressive Behavior in Mice Lacking 5-HT1B Receptor. Science 1994, 265, 1875–1878. [Google Scholar] [CrossRef]
- Rodriguiz, R.M.; Chu, R.; Caron, M.G.; Wetsel, W.C. Aberrant Responses in Social Interaction of Dopamine Transporter Knockout Mice. Behav. Brain Res. 2004, 148, 185–198. [Google Scholar] [CrossRef]
- Nelson, R.J.; Demas, G.E.; Huang, P.L.; Fishman, M.C.; Dawson, V.L.; Dawson, T.M.; Snyder, S.H. Behavioural Abnormalities in Male Mice Lacking Neuronal Nitric Oxide Synthase. Nature 1995, 378, 383–386. [Google Scholar] [CrossRef]
- Cases, O.; Seif, I.; Grimsby, J.; Gaspar, P.; Chen, K.; Pournin, S.; Müller, U.; Aguet, M.; Babinet, C.; Shih, J.C.; et al. Aggressive Behavior and Altered Amounts of Brain Serotonin and Norepinephrine in Mice Lacking MAOA. Science 1995, 268, 1763–1766. [Google Scholar] [CrossRef]
- Benus, R.F.; Bohus, B.; Koolhaas, J.M.; van Oortmerssen, G.A. Heritable Variation for Aggression as a Reflection of Individual Coping Strategies. Experientia 1991, 47, 1008–1019. [Google Scholar] [CrossRef]
- Veenema, A.H.; Cremers, T.I.F.H.; Jongsma, M.E.; Steenbergen, P.J.; de Boer, S.F.; Koolhaas, J.M. Differences in the Effects of 5-HT1A Receptor Agonists on Forced Swimming Behavior and Brain 5-HT Metabolism between Low and High Aggressive Mice. Psychopharmacology 2005, 178, 151–160. [Google Scholar] [CrossRef]
- Sluyter, F.; Arseneault, L.; Moffitt, T.E.; Veenema, A.H.; de Boer, S.; Koolhaas, J.M. Toward an Animal Model for Antisocial Behavior: Parallels between Mice and Humans. Behav. Genet. 2003, 33, 563–574. [Google Scholar] [CrossRef]
- Natarajan, D.; de Vries, H.; de Boer, S.F.; Koolhaas, J.M. Violent Phenotype in SAL Mice Is Inflexible and Fixed in Adulthood. Aggress. Behav. 2009, 35, 430–436. [Google Scholar] [CrossRef]
- Beiderbeck, D.I.; Reber, S.O.; Havasi, A.; Bredewold, R.; Veenema, A.H.; Neumann, I.D. High and Abnormal Forms of Aggression in Rats with Extremes in Trait Anxiety—Involvement of the Dopamine System in the Nucleus Accumbens. Psychoneuroendocrinology 2012, 37, 1969–1980. [Google Scholar] [CrossRef]
- Landgraf, R.; Kessler, M.S.; Bunck, M.; Murgatroyd, C.; Spengler, D.; Zimbelmann, M.; Nussbaumer, M.; Czibere, L.; Turck, C.W.; Singewald, N.; et al. Candidate Genes of Anxiety-Related Behavior in HAB/LAB Rats and Mice: Focus on Vasopressin and Glyoxalase-I. Neurosci. Biobehav. Rev. 2007, 31, 89–102. [Google Scholar] [CrossRef]
- Gobrogge, K.L. Sex, drugs, and violence: Neuromodulation of attachment and conflict in voles. In Brain Imaging in Behavioral Neuroscience; Springer: Berlin/Heidelberg, Germany, 2013; pp. 229–264. ISBN 9783642209246. [Google Scholar]
- Takahashi, A. Social Stress and Aggression in Murine Models. Curr. Top. Behav. Neurosci. 2022, 54, 181–208. [Google Scholar] [CrossRef]
- Ago, Y.; Takuma, K.; Matsuda, T. Potential Role of Serotonin1A Receptors in Post-Weaning Social Isolation-Induced Abnormal Behaviors in Rodents. J. Pharmacol. Sci. 2014, 125, 237–241. [Google Scholar] [CrossRef]
- Brain, P.F.; Nowell, N.W.; Wouters, A. Some Relationships between Adrenal Function and the Effectiveness of a Period of Isolation in Inducing Intermale Aggression in Albino Mice. Physiol. Behav. 1971, 6, 27–29. [Google Scholar] [CrossRef]
- Pinna, G. In a Mouse Model Relevant for Post-Traumatic Stress Disorder, Selective Brain Steroidogenic Stimulants (SBSS) Improve Behavioral Deficits by Normalizing Allopregnanolone Biosynthesis. Behav. Pharmacol. 2010, 21, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Ieraci, A.; Mallei, A.; Popoli, M. Social Isolation Stress Induces Anxious-Depressive-Like Behavior and Alterations of Neuroplasticity-Related Genes in Adult Male Mice. Neural Plast. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Veenema, A.H.; Blume, A.; Niederle, D.; Buwalda, B.; Neumann, I.D. Effects of Early Life Stress on Adult Male Aggression and Hypothalamic Vasopressin and Serotonin. Eur. J. Neurosci. 2006, 24, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Veenema, A.H.; Bredewold, R.; Neumann, I.D. Opposite Effects of Maternal Separation on Intermale and Maternal Aggression in C57BL/6 Mice: Link to Hypothalamic Vasopressin and Oxytocin Immunoreactivity. Psychoneuroendocrinology 2007, 32, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Veenema, A.H. Early Life Stress, the Development of Aggression and Neuroendocrine and Neurobiological Correlates: What Can We Learn from Animal Models? Front. Neuroendocrinol. 2009, 30, 497–518. [Google Scholar] [CrossRef]
- Grinevich, V.P.; Zakirov, A.N.; Berseneva, U.V.; Gerasimova, E.V.; Gainetdinov, R.R.; Budygin, E.A. Applying a Fast-Scan Cyclic Voltammetry to Explore Dopamine Dynamics in Animal Models of Neuropsychiatric Disorders. Cells 2022, 11, 1533. [Google Scholar] [CrossRef]
- Wersinger, S.R.; Ginns, E.I.; O’Carroll, A.M.; Lolait, S.J.; Young, W.S. Vasopressin V1b Receptor Knockout Reduces Aggressive Behavior in Male Mice. Mol. Psychiatry 2002, 7, 975–984. [Google Scholar] [CrossRef]
- Strekalova, T.; Steinbusch, H.W.M. Measuring Behavior in Mice with Chronic Stress Depression Paradigm. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 348–361. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Prasol, D.J.; Belzung, C.; Crusio, W.E. Agonistic Behavior and Unpredictable Chronic Mild Stress in Mice. Behav. Genet. 2003, 33, 513–519. [Google Scholar] [CrossRef]
- Costa-Nunes, J.; Zubareva, O.; Araújo-Correia, M.; Valença, A.; Schroeter, C.A.; Pawluski, J.L.; Vignisse, J.; Steinbusch, H.; Hermes, D.; Phillipines, M.; et al. Altered Emotionality, Hippocampus-Dependent Performance and Expression of NMDA Receptor Subunit MRNAs in Chronically Stressed Mice. Stress 2014, 17, 108–116. [Google Scholar] [CrossRef]
- Strekalova, T.; Spanagel, R.; Bartsch, D.; Henn, F.A.; Gass, P. Stress-Induced Anhedonia in Mice Is Associated with Deficits in Forced Swimming and Exploration. Neuropsychopharmacology 2004, 29, 2007–2017. [Google Scholar] [CrossRef]
- Mutlu, O.; Gumuslu, E.; Ulak, G.; Celikyurt, I.K.; Kokturk, S.; Kir, H.M.; Akar, F.; Erden, F. Effects of Fluoxetine, Tianeptine and Olanzapine on Unpredictable Chronic Mild Stress-Induced Depression-like Behavior in Mice. Life Sci. 2012, 91, 1252–1262. [Google Scholar] [CrossRef]
- Malki, K.; Tosto, M.G.; Pain, O.; Sluyter, F.; Mineur, Y.S.; Crusio, W.E.; de Boer, S.; Sandnabba, K.N.; Kesserwani, J.; Robinson, E.; et al. Comparative MRNA Analysis of Behavioral and Genetic Mouse Models of Aggression. Am. J. Med. Genet. Part B: Neuropsychiatr. Genet. 2016, 171, 427–436. [Google Scholar] [CrossRef]
- Vignisse, J.; Sambon, M.; Gorlova, A.; Pavlov, D.; Caron, N.; Malgrange, B.; Shevtsova, E.; Svistunov, A.; Anthony, D.C.; Markova, N.; et al. Thiamine and Benfotiamine Prevent Stress-Induced Suppression of Hippocampal Neurogenesis in Mice Exposed to Predation without Affecting Brain Thiamine Diphosphate Levels. Mol. Cell. Neurosci. 2017, 82, 126–136. [Google Scholar] [CrossRef]
- Bouvier, E.; Brouillard, F.; Molet, J.; Claverie, D.; Cabungcal, J.H.; Cresto, N.; Doligez, N.; Rivat, C.; Do, K.Q.; Bernard, C.; et al. Nrf2-Dependent Persistent Oxidative Stress Results in Stress-Induced Vulnerability to Depression. Mol. Psychiatry 2017, 22, 1701–1713. [Google Scholar] [CrossRef]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; Leclair, K.B.; et al. Social Stress Induces Neurovascular Pathology Promoting Depression. Nat. Neurosci. 2017, 20, 1752–1760. [Google Scholar] [CrossRef]
- Elkhatib, S.K.; Moshfegh, C.M.; Watson, G.F.; Case, A.J. Peripheral Inflammation Is Strongly Linked to Elevated Zero Maze Behavior in Repeated Social Defeat Stress. Brain Behav. Immun. 2020, 90, 279–285. [Google Scholar] [CrossRef]
- Heshmati, M.; Christoffel, D.J.; LeClair, K.; Cathomas, F.; Golden, S.A.; Aleyasin, H.; Turecki, G.; Friedman, A.K.; Han, M.H.; Menard, C.; et al. Depression and Social Defeat Stress Are Associated with Inhibitory Synaptic Changes in the Nucleus Accumbens. J. Neurosci. 2020, 40, 6228–6233. [Google Scholar] [CrossRef]
- Golden, S.A.; Covington, H.E.; Berton, O.; Russo, S.J. A Standardized Protocol for Repeated Social Defeat Stress in Mice. Nat. Protoc. 2011, 6, 1183–1191. [Google Scholar] [CrossRef]
- Costa-Nunes, J.; Gorlova, A.; Pavlov, D.; Cespuglio, R.; Gorovaya, A.; Proshin, A.; Umriukhin, A.; Ponomarev, E.D.; Kalueff, A.V.; Strekalova, T.; et al. Ultrasound Stress Compromises the Correlates of Emotional-like States and Brain AMPAR Expression in Mice: Effects of Antioxidant and Anti-Inflammatory Herbal Treatment. Stress 2020, 23, 481–495. [Google Scholar] [CrossRef]
- de Munter, J.; Pavlov, D.; Gorlova, A.; Sicker, M.; Proshin, A.; Kalueff, A.v.; Svistunov, A.; Kiselev, D.; Nedorubov, A.; Morozov, S.; et al. Increased Oxidative Stress in the Prefrontal Cortex as a Shared Feature of Depressive- and PTSD-Like Syndromes: Effects of a Standardized Herbal Antioxidant. Front. Nutr. 2021, 8, 661455. [Google Scholar] [CrossRef] [PubMed]
- Morozova, A.; Zubkov, E.; Strekalova, T.; Kekelidze, Z.; Storozeva, Z.; Schroeter, C.A.; Bazhenova, N.; Lesch, K.-P.; Cline, B.H.; Chekhonin, V. Ultrasound of Alternating Frequencies and Variable Emotional Impact Evokes Depressive Syndrome in Mice and Rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 68, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Bahzenova, N.; Trofimov, A.; Schmitt-Böhrer, A.G.; Markova, N.; Grigoriev, V.; Zamoyski, V.; Serkova, T.; Redkozubova, O.; Vinogradova, D.; et al. Pro-Neurogenic, Memory-Enhancing and Anti-Stress Effects of DF302, a Novel Fluorine Gamma-Carboline Derivative with Multi-Target Mechanism of Action. Mol. Neurobiol. 2018, 55, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Gorlova, A.; Pavlov, D.; Anthony, D.C.; Ponomarev, E.D.; Sambon, M.; Proshin, A.; Shafarevich, I.; Babaevskaya, D.; Lesch, K.P.; Bettendorff, L.; et al. Thiamine and Benfotiamine Counteract Ultrasound-Induced Aggression, Normalize AMPA Receptor Expression and Plasticity Markers, and Reduce Oxidative Stress in Mice. Neuropharmacology 2019, 156, 107543. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, D.; Bettendorff, L.; Gorlova, A.; Olkhovik, A.; Kalueff, A.V.; Ponomarev, E.D.; Inozemtsev, A.; Chekhonin, V.; Lesch, K.P.; Anthony, D.C.; et al. Neuroinflammation and Aberrant Hippocampal Plasticity in a Mouse Model of Emotional Stress Evoked by Exposure to Ultrasound of Alternating Frequencies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 90, 104–116. [Google Scholar] [CrossRef]
- Bosch, O.J.; Meddle, S.L.; Beiderbeck, D.I.; Douglas, A.J.; Neumann, I.D. Brain Oxytocin Correlates with Maternal Aggression: Link to Anxiety. J. Neurosci. 2005, 25, 6807–6815. [Google Scholar] [CrossRef]
- Bosch, O.J. Maternal Aggression in Rodents: Brain Oxytocin and Vasopressin Mediate Pup Defence. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130085. [Google Scholar] [CrossRef]
- Gutzler, S.J.; Karom, M.; Erwin, W.D.; Albers, H.E. Arginine-Vasopressin and the Regulation of Aggression in Female Syrian Hamsters (Mesocricetus Auratus). Eur. J. Neurosci. 2010, 31, 1655–1663. [Google Scholar] [CrossRef]
- Been, L.E.; Gibbons, A.B.; Meisel, R.L. Towards a Neurobiology of Female Aggression. Neuropharmacology 2019, 156, 107451. [Google Scholar] [CrossRef]
- Gorlova, A.; Ortega, G.; Waider, J.; Bazhenova, N.; Veniaminova, E.; Proshin, A.; Kalueff, A.v.; Anthony, D.C.; Lesch, K.P.; Strekalova, T. Stress-Induced Aggression in Heterozygous TPH2 Mutant Mice Is Associated with Alterations in Serotonin Turnover and Expression of 5-HT6 and AMPA Subunit 2A Receptors. J. Affect. Disord. 2020, 272, 440–451. [Google Scholar] [CrossRef]
- Svirin, E.; Veniaminova, E.; Costa-Nunes, J.P.; Gorlova, A.; Umriukhin, A.; Kalueff, A.V.; Proshin, A.; Anthony, D.; Nedorubov, A.; Tse, A.C.K.; et al. Predation Stress Causes Excessive Aggression in Female Mice with Partial Genetic Inactivation of Tryptophan Hydroxylase-2: Evidence for Altered Myelination-Related Processes. Cells 2022, 11, 1036. [Google Scholar] [CrossRef]
- Strekalova, T.; Steinbusch, H. Factors of reproducibility of anhedonia induction in a chronic stress depression model in mice. In Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests; Gould, T.D., Ed.; Neuromethods; Humana Press: Totowa, NJ, USA, 2009; Volume 42, pp. 153–176. ISBN 978-1-60761-302-2. [Google Scholar]
- Yang, C.R.; Bai, Y.Y.; Ruan, C.S.; Zhou, H.F.; Liu, D.; Wang, X.F.; Shen, L.J.; Zheng, H.Y.; Zhou, X.F. Enhanced Aggressive Behaviour in a Mouse Model of Depression. Neurotox. Res. 2015, 27, 129–142. [Google Scholar] [CrossRef]
- Barnow, S.; Lucht, M.; Freyberger, H.J. Influence of Punishment, Emotional Rejection, Child Abuse, and Broken Home on Aggression in Adolescence: An Examination of Aggressive Adolescents in Germany. Psychopathology 2001, 34, 167–173. [Google Scholar] [CrossRef]
- Heim, C.; Nemeroff, C.B. The Role of Childhood Trauma in the Neurobiology of Mood and Anxiety Disorders: Preclinical and Clinical Studies. Biol. Psychiatry 2001, 49, 1023–1039. [Google Scholar] [CrossRef]
- Fontes, M.A.P.; Xavier, C.H.; Marins, F.R.; Limborço-Filho, M.; Vaz, G.C.; Müller-Ribeiro, F.C.; Nalivaiko, E. Emotional Stress and Sympathetic Activity: Contribution of Dorsomedial Hypothalamus to Cardiac Arrhythmias. Brain Res. 2014, 1554, 49–58. [Google Scholar] [CrossRef]
- Angkaw, A.C.; Ross, B.S.; Pittman, J.O.E.; Kelada, A.M.Y.; Valencerina, M.A.M.; Baker, D.G. Post-Traumatic Stress Disorder, Depression, and Aggression in OEF/OIF Veterans. Mil. Med. 2013, 178, 1044–1050. [Google Scholar] [CrossRef]
- Fanning, J.R.; Keedy, S.; Berman, M.E.; Lee, R.; Coccaro, E.F. Neural Correlates of Aggressive Behavior in Real Time: A Review of FMRI Studies of Laboratory Reactive Aggression. Curr. Behav. Neurosci. Rep. 2017, 4, 138–150. [Google Scholar] [CrossRef]
- Constantini, F.; D’Amato, F.R. Ultrasonic Vocalizations in Mice and Rats: Social Contexts and Functions. Dong Wu Xue Bao 2006, 52, 619–633. [Google Scholar]
- Pavlov, D.A.; Gorlova, A.v.; Ushakova, V.M.; Zubkov, E.A.; Morozova, A.Y.; Inozemtsev, A.N.; Chekhonin, V.P. Effects of Chronic Exposure to Ultrasound of Alternating Frequencies on the Levels of Aggression and Anxiety in CBA and BALB/c Mice. Bull. Exp. Biol. Med. 2017, 163, 409–411. [Google Scholar] [CrossRef]
- Haller, J.; van de Schraaf, J.; Kruk, M.R. Deviant Forms of Aggression in Glucocorticoid Hyporeactive Rats: A Model for ‘Pathological’ Aggression? J. Neuroendocrinol. 2001, 13, 102–107. [Google Scholar] [CrossRef]
- Halász, J.; Liposits, Z.; Kruk, M.R.; Haller, J. Neural Background of Glucocorticoid Dysfunction-Induced Abnormal Aggression in Rats: Involvement of Fear- and Stress-Related Structures. Eur. J. Neurosci. 2002, 15, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Faccidomo, S.; Bannai, M.; Miczek, K.A. Escalated Aggression after Alcohol Drinking in Male Mice: Dorsal Raphé and Prefrontal Cortex Serotonin and 5-HT(1B) Receptors. Neuropsychopharmacology 2008, 33, 2888–2899. [Google Scholar] [CrossRef] [PubMed]
- Sontate, K.V.; Rahim Kamaluddin, M.; Naina Mohamed, I.; Mohamed, R.M.P.; Shaikh, M.F.; Kamal, H.; Kumar, J. Alcohol, Aggression, and Violence: From Public Health to Neuroscience. Front. Psychol. 2021, 12, 699726. [Google Scholar] [CrossRef] [PubMed]
- Boden, J.M.; Fergusson, D.M. Alcohol and Depression. Addiction 2011, 106, 906–914. [Google Scholar] [CrossRef] [PubMed]
- McHugh, R.K.; Weiss, R.D. Alcohol Use Disorder and Depressive Disorders. Alcohol Res. 2019, 40, 1. [Google Scholar] [CrossRef]
- WHO. WHO Expert Committee on Problems Related to Alcohol Consumption; Second Report; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 2007; pp. 1–53, pp. 55–57, back cover. [Google Scholar]
- Neumann, I.D.; Veenema, A.H.; Beiderbeck, D.I. Aggression and Anxiety: Social Context and Neurobiological Links. Front. Behav. Neurosci. 2010, 4, 12. [Google Scholar] [CrossRef]
- Thapar, A.; Harold, G.; Rice, F.; Langley, K.; O’donovan, M. The Contribution of Gene-Environment Interaction to Psychopathology. Dev. Psychopathol. 2007, 19, 989–1004. [Google Scholar] [CrossRef]
- Tuvblad, C.; Baker, L.A. Human Aggression across the Lifespan. Genetic Propensities and Environmental Moderators, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 75, ISBN 9780123808585. [Google Scholar]
- Svirin, E.; Gorlova, A.; Lim, L.W.; Veniaminova, E.; Costa-Nunes, J.; Anthony, D.; Lesch, K.-P.; Strekalova, T. Sexual bias in the altered expression of myelination factors in mice with partial genetic deficiency of tryptophan hydroxylase 2 and pro-aggressive effects of predation stress. In Proceedings of the IBNS 30th Annual Meeting, Puerto Vallarta, Mexico, 1–5 June 2021. [Google Scholar]
- Angoa-Pérez, M.; Kane, M.J.; Briggs, D.I.; Sykes, C.E.; Shah, M.M.; Francescutti, D.M.; Rosenberg, D.R.; Thomas, D.M.; Kuhn, D.M. Genetic Depletion of Brain 5HT Reveals a Common Molecular Pathway Mediating Compulsivity and Impulsivity. J. Neurochem. 2012, 121, 974–984. [Google Scholar] [CrossRef]
- Strekalova, T.; Svirin, E.; Waider, J.; Gorlova, A.; Cespuglio, R.; Kalueff, A.; Pomytkin, I.; Schmitt-Boehrer, A.G.; Lesch, K.-P.; Anthony, D.C. Altered Behaviour, Dopamine and Norepinephrine Regulation in Stressed Mice Heterozygous in TPH2 Gene. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 108, 110155. [Google Scholar] [CrossRef]
- Svirin, E.; de Munter, J.; Umriukhin, A.; Sheveleva, E.; Kalueff, A.v.; Svistunov, A.; Morozov, S.; Walitza, S.; Strekalova, T. Aberrant Ganglioside Functions to Underpin Dysregulated Myelination, Insulin Signalling, and Cytokine Expression: Is There a Link and a Room for Therapy? Biomolecules 2022, 12, 1434. [Google Scholar] [CrossRef]
- Tóth, M.; Halász, J.; Mikics, É.; Barsy, B.; Haller, J. Early Social Deprivation Induces Disturbed Social Communication and Violent Aggression in Adulthood. Behav. Neurosci. 2008, 122, 849–854. [Google Scholar] [CrossRef]
- Drevets, W.C. Functional Neuroimaging Studies of Depression: The Anatomy of Melancholia. Annu. Rev. Med. 1998, 49, 341–361. [Google Scholar] [CrossRef][Green Version]
- Haller, J.; Tóth, M.; Halasz, J.; de Boer, S.F. Patterns of Violent Aggression-Induced Brain c-Fos Expression in Male Mice Selected for Aggressiveness. Physiol. Behav. 2006, 88, 173–182. [Google Scholar] [CrossRef]
- Herman, J.P.; Figueiredo, H.; Mueller, N.K.; Ulrich-Lai, Y.; Ostrander, M.M.; Choi, D.C.; Cullinan, W.E. Central Mechanisms of Stress Integration: Hierarchical Circuitry Controlling Hypothalamo-Pituitary-Adrenocortical Responsiveness. Front. Neuroendocrinol. 2003, 24, 151–180. [Google Scholar] [CrossRef]
- Haller, J. Aggression, Aggression-Related Psychopathologies and Their Models. Front. Behav. Neurosci. 2022, 16, 936105. [Google Scholar] [CrossRef]
- Rosell, D.R.; Siever, L.J. The Neurobiology of Aggression and Violence. CNS Spectr. 2015, 20, 254–279. [Google Scholar] [CrossRef]
- Fairchild, G.; Hawes, D.J.; Frick, P.J.; Copeland, W.E.; Odgers, C.L.; Franke, B.; Freitag, C.M.; de Brito, S.A. Conduct Disorder. Nat. Rev. Dis. Prim. 2019, 5, 43. [Google Scholar] [CrossRef]
- Cardinal, R.N.; Parkinson, J.A.; Hall, J.; Everitt, B.J. The Contribution of the Amygdala, Nucleus Accumbens, and Prefrontal Cortex to Emotion and Motivated Behaviour. Int. Congr. Ser. 2003, 1250, 347–370. [Google Scholar] [CrossRef]
- Malin, E.L.; Ibrahim, D.Y.; Tu, J.W.; McGaugh, J.L. Involvement of the Rostral Anterior Cingulate Cortex in Consolidation of Inhibitory Avoidance Memory: Interaction with the Basolateral Amygdala. Neurobiol. Learn. Mem. 2007, 87, 295–302. [Google Scholar] [CrossRef][Green Version]
- Adolphs, R.; Tranel, D.; Damasio, H.; Damasio, A. Impaired Recognition of Emotion in Facial Expressions Following Bilateral Damage to the Human Amygdala. Nature 1994, 372, 669–672. [Google Scholar] [CrossRef]
- Fahim, C.; He, Y.; Yoon, U.; Chen, J.; Evans, A.; Pérusse, D. Neuroanatomy of Childhood Disruptive Behavior Disorders. Aggress. Behav. 2011, 37, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Z.; Zhao, C.; Li, L. Medial Amygdala Lesions Modify Aggressive Behavior and Immediate Early Gene Expression in Oxytocin and Vasopressin Neurons during Intermale Exposure. Behav. Brain Res. 2013, 245, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Kim, D.-W.; Anderson, D.J. Antagonistic Control of Social versus Repetitive Self-Grooming Behaviors by Separable Amygdala Neuronal Subsets. Cell 2014, 158, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Schoenbaum, G.; Setlow, B.; Saddoris, M.P.; Gallagher, M. Encoding Predicted Outcome and Acquired Value in Orbitofrontal Cortex during Cue Sampling Depends upon Input from Basolateral Amygdala. Neuron 2003, 39, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Haller, J. The Role of Central and Medial Amygdala in Normal and Abnormal Aggression: A Review of Classical Approaches. Neurosci. Biobehav. Rev. 2018, 85, 34–43. [Google Scholar] [CrossRef]
- Zhang, Y. Aggression Priming by Potentiation of Medial Amygdala Circuits. J. Neurosci. 2021, 41, 28–30. [Google Scholar] [CrossRef]
- da Cunha-Bang, S.; Fisher, P.M.; Hjordt, L.V.; Perfalk, E.; Persson Skibsted, A.; Bock, C.; Ohlhues Baandrup, A.; Deen, M.; Thomsen, C.; Sestoft, D.M.; et al. Violent Offenders Respond to Provocations with High Amygdala and Striatal Reactivity. Soc. Cogn. Affect. Neurosci. 2017, 12, 802–810. [Google Scholar] [CrossRef]
- Siever, L.J. Neurobiology of Aggression and Violence. Am. J. Psychiatry 2008, 165, 429–442. [Google Scholar] [CrossRef]
- Jackman, S.L.; Chen, C.H.; Offermann, H.L.; Drew, I.R.; Harrison, B.M.; Bowman, A.M.; Flick, K.M.; Flaquer, I.; Regehr, W.G. Title: Cerebellar Purkinje Cell Activity Modulates Aggressive Behavior. eLife 2020, 9, e53229. [Google Scholar] [CrossRef]
- Barbano, M.F.; Wang, H.-L.; Zhang, S.; Miranda-Barrientos, J.; Estrin, D.J.; Figueroa-González, A.; Liu, B.; Barker, D.J.; Morales, M. VTA Glutamatergic Neurons Mediate Innate Defensive Behaviors. Neuron 2020, 107, 368–382.e8. [Google Scholar] [CrossRef]
- Grafman, J.; Schwab, K.; Warden, D.; Pridgen, A.; Brown, H.R.; Salazar, A.M. Frontal Lobe Injuries, Violence, and Aggression: A Report of the Vietnam Head Injury Study. Neurology 1996, 46, 1231–1238. [Google Scholar] [CrossRef]
- Raine, A.; Lencz, T.; Bihrle, S.; LaCasse, L.; Colletti, P. Reduced Prefrontal Gray Matter Volume and Reduced Autonomic Activity in Antisocial Personality Disorder. Arch. Gen. Psychiatry 2000, 57, 119–127; discussion 128–129. [Google Scholar] [CrossRef]
- Gregory, S.; Ffytche, D.; Simmons, A.; Kumari, V.; Howard, M.; Hodgins, S.; Blackwood, N. The Antisocial Brain: Psychopathy Matters. Arch. Gen. Psychiatry 2012, 69, 962–972. [Google Scholar] [CrossRef]
- Yang, Y.; Glenn, A.L.; Raine, A. Brain Abnormalities in Antisocial Individuals: Implications for the Law. Behav. Sci. Law 2008, 26, 65–83. [Google Scholar] [CrossRef]
- Chang, C.H.; Gean, P.W. The Ventral Hippocampus Controls Stress-Provoked Impulsive Aggression through the Ventromedial Hypothalamus in Post-Weaning Social Isolation Mice. Cell Rep. 2019, 28, 1195–1205.e3. [Google Scholar] [CrossRef]
- Matrisciano, F.; Pinna, G. Ppar-α Hypermethylation in the Hippocampus of Mice Exposed to Social Isolation Stress Is Associated with Enhanced Neuroinflammation and Aggressive Behavior. Int. J. Mol. Sci. 2021, 22, 678. [Google Scholar] [CrossRef]
- Leroy, F.; Park, J.; Asok, A.; Brann, D.H.; Meira, T.; Boyle, L.M.; Buss, E.W.; Kandel, E.R.; Siegelbaum, S.A. A Circuit from Hippocampal CA2 to Lateral Septum Disinhibits Social Aggression. Nature 2018, 564, 213–218. [Google Scholar] [CrossRef]
- Yang, C.F.; Chiang, M.C.; Gray, D.C.; Prabhakaran, M.; Alvarado, M.; Juntti, S.A.; Unger, E.K.; Wells, J.A.; Shah, N.M. Sexually Dimorphic Neurons in the Ventromedial Hypothalamus Govern Mating in Both Sexes and Aggression in Males. Cell 2013, 153, 896–909. [Google Scholar] [CrossRef]
- Rizzi, M.; Gambini, O.; Marras, C.E. Posterior Hypothalamus as a Target in the Treatment of Aggression: From Lesioning to Deep Brain Stimulation. Handb. Clin. Neurol. 2021, 182, 95–106. [Google Scholar] [CrossRef]
- Raine, A.; Ishikawa, S.S.; Arce, E.; Lencz, T.; Knuth, K.H.; Bihrle, S.; LaCasse, L.; Colletti, P. Hippocampal Structural Asymmetry in Unsuccessful Psychopaths. Biol. Psychiatry 2004, 55, 185–191. [Google Scholar] [CrossRef]
- Siegel, A.; Brutus, M. Neural Substrates of Aggression and Rage in the Cat. Prog. Psychobiol. Physiol. Psychol. 1990, 14, 135–233. [Google Scholar]
- Todd, W.D.; Fenselau, H.; Wang, J.L.; Zhang, R.; Machado, N.L.; Venner, A.; Broadhurst, R.Y.; Kaur, S.; Lynagh, T.; Olson, D.P.; et al. A Hypothalamic Circuit for the Circadian Control of Aggression. Nat. Neurosci. 2018, 21, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.W.; Nagel, J. First-Order Projections Activated by Stimulation of Hypothalamic Sites Eliciting Attack and Flight in Rats. Behav. Neurosci. 1996, 110, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Hashikawa, Y.; Hashikawa, K.; Falkner, A.L.; Lin, D. Ventromedial Hypothalamus and the Generation of Aggression. Front. Syst. Neurosci. 2017, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Falkner, A.L.; Lin, D. Recent Advances in Understanding the Role of the Hypothalamic Circuit during Aggression. Front. Syst. Neurosci. 2014, 8, 168. [Google Scholar] [CrossRef]
- Gregg, T.R.; Siegel, A. Brain Structures and Neurotansmitters Regulating Aggression in Cats: Implications for Human Aggression. Prog Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 91–140. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Wei, D.; Song, S.C.; Lim, B.; Tritsch, N.X.; Lin, D. Posterior Amygdala Regulates Sexual and Aggressive Behaviors in Male Mice. Nat. Neurosci. 2020, 23, 1111–1124. [Google Scholar] [CrossRef]
- Ramamurthi, B. Stereotactic operation in behaviour disorders. Amygdalotomy and hypothalamotomy. In Personality and Neurosurgery; Springer: Vienna, Austria, 1988; Volume 44, pp. 152–157. [Google Scholar]
- Caria, A.; Dall’Ò, G.M. Functional Neuroimaging of Human Hypothalamus in Socioemotional Behavior: A Systematic Review. Brain Sci. 2022, 12, 707. [Google Scholar] [CrossRef]
- George, D.T.; Rawlings, R.R.; Williams, W.A.; Phillips, M.J.; Fong, G.; Kerich, M.; Momenan, R.; Umhau, J.C.; Hommer, D. A Select Group of Perpetrators of Domestic Violence: Evidence of Decreased Metabolism in the Right Hypothalamus and Reduced Relationships between Cortical/Subcortical Brain Structures in Position Emission Tomography. Psychiatry Res. Neuroimaging 2004, 130, 11–25. [Google Scholar] [CrossRef]
- Spellman, T.; Liston, C. Toward Circuit Mechanisms of Pathophysiology in Depression. Am. J. Psychiatry 2020, 177, 381–390. [Google Scholar] [CrossRef]
- Hirschfeld, R.M. History and Evolution of the Monoamine Hypothesis of Depression. J. Clin. Psychiatry 2000, 61 (Suppl. 6), 4–6. [Google Scholar] [PubMed]
- Seo, D.; Patrick, C.J.; Kennealy, P.J. Role of Serotonin and Dopamine System Interactions in the Neurobiology of Impulsive Aggression and Its Comorbidity with Other Clinical Disorders. Aggress. Violent Behav. 2008, 13, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R.; Cuthbert, B.N. Medicine. Brain Disorders? Precisely. Science 2015, 348, 499–500. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Flanigan, M.E.; McEwen, B.S.; Russo, S.J. Aggression, Social Stress, and the Immune System in Humans and Animal Models. Front. Behav. Neurosci. 2018, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, F.; Caggiano, M.; Schiavo, L.; Savarese, G.; Carpinelli, L.; Amato, A.; Iandolo, A. Chronic Stress and Depression in Periodontitis and Peri-Implantitis: A Narrative Review on Neurobiological, Neurobehavioral and Immune-Microbiome Interplays and Clinical Management Implications. Dent. J 2022, 10, 49. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J.; Moulana, M.; Deloach, P.H.; Rajkowska, G. Chronic Unpredictable Stress Reduces Immunostaining for Connexins 43 and 30 and Myelin Basic Protein in the Rat Prelimbic and Orbitofrontal Cortices. Chronic Stress 2018, 2, 247054701881418. [Google Scholar] [CrossRef]
- Jia, X.; Gao, Z.; Hu, H. Microglia in Depression: Current Perspectives. Sci. China Life Sci. 2021, 64, 911–925. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Sun, Z.; Ren, S.; Liu, M.; Wang, G.; Yang, J. Microglia in Depression: An Overview of Microglia in the Pathogenesis and Treatment of Depression. J. Neuroinflamm. 2022, 19, 132. [Google Scholar] [CrossRef]
- Bierhaus, A.; Wolf, J.; Andrassy, M.; Rohleder, N.; Humpert, P.M.; Petrov, D.; Ferstl, R.; von Eynatten, M.; Wendt, T.; Rudofsky, G.; et al. A Mechanism Converting Psychosocial Stress into Mononuclear Cell Activation. Proc. Natl. Acad. Sci. USA 2003, 100, 1920–1925. [Google Scholar] [CrossRef]
- Dean, J.; Keshavan, M. The Neurobiology of Depression: An Integrated View. Asian J. Psychiatry 2017, 27, 101–111. [Google Scholar] [CrossRef]
- Siegel, J.L. Acute Bacterial Meningitis and Stroke. Neurol. Neurochir. Pol. 2019, 53, 242–250. [Google Scholar] [CrossRef]
- Biouss, G.; Antounians, L.; Li, B.; O’Connell, J.S.; Seo, S.; Catania, V.D.; Guadagno, J.; Rahman, A.; Zani-Ruttenstock, E.; Svergun, N.; et al. Experimental Necrotizing Enterocolitis Induces Neuroinflammation in the Neonatal Brain. J. Neuroinflamm. 2019, 16, 97. [Google Scholar] [CrossRef]
- Lyons, J.L. Viral Meningitis and Encephalitis. Contin. Lifelong Learn. Neurol. 2018, 24, 1284–1297. [Google Scholar] [CrossRef]
- Benatti, C.; Blom, J.M.C.; Rigillo, G.; Alboni, S.; Zizzi, F.; Torta, R.; Brunello, N.; Tascedda, F. Disease-Induced Neuroinflammation and Depression. CNS Neurol. Disord. Drug Targets 2016, 15, 414–433. [Google Scholar] [CrossRef]
- Rom, S.; Zuluaga-Ramirez, V.; Gajghate, S.; Seliga, A.; Winfield, M.; Heldt, N.A.; Kolpakov, M.A.; Bashkirova, Y.v.; Sabri, A.K.; Persidsky, Y. Hyperglycemia-Driven Neuroinflammation Compromises BBB Leading to Memory Loss in Both Diabetes Mellitus (DM) Type 1 and Type 2 Mouse Models. Mol. Neurobiol. 2019, 56, 1883–1896. [Google Scholar] [CrossRef]
- Veniaminova, E.; Oplatchikova, M.; Bettendorff, L.; Kotenkova, E.; Lysko, A.; Vasilevskaya, E.; Kalueff, A.v.; Fedulova, L.; Umriukhin, A.; Lesch, K.-P.; et al. Prefrontal Cortex Inflammation and Liver Pathologies Accompany Cognitive and Motor Deficits Following Western Diet Consumption in Non-Obese Female Mice. Life Sci. 2020, 241, 117163. [Google Scholar] [CrossRef]
- Więckowska-Gacek, A.; Mietelska-Porowska, A.; Wydrych, M.; Wojda, U. Western Diet as a Trigger of Alzheimer’s Disease: From Metabolic Syndrome and Systemic Inflammation to Neuroinflammation and Neurodegeneration. Ageing Res. Rev. 2021, 70, 101397. [Google Scholar] [CrossRef]
- Huat, T.J.; Camats-Perna, J.; Newcombe, E.A.; Valmas, N.; Kitazawa, M.; Medeiros, R. Metal Toxicity Links to Alzheimer’s Disease and Neuroinflammation. J. Mol. Biol. 2019, 431, 1843–1868. [Google Scholar] [CrossRef]
- Suarez, E.C.; Lewis, J.G.; Kuhn, C. The Relation of Aggression, Hostility, and Anger to Lipopolysaccharide-Stimulated Tumor Necrosis Factor (TNF)-Alpha by Blood Monocytes from Normal Men. Brain Behav. Immun. 2002, 16, 675–684. [Google Scholar] [CrossRef]
- Coccaro, E.F. Association of C-Reactive Protein Elevation with Trait Aggression and Hostility in Personality Disordered Subjects: A Pilot Study. J. Psychiatr. Res. 2006, 40, 460–465. [Google Scholar] [CrossRef]
- Martone, G. Can Taming Inflammation Help Reduce Aggression? Curr. Psychiatry 2019, 18, 49–50. [Google Scholar]
- Lotrich, F.E.; Sears, B.; McNamara, R.K. Anger Induced by Interferon-Alpha Is Moderated by Ratio of Arachidonic Acid to Omega-3 Fatty Acids. J. Psychosom. Res. 2013, 75, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Mommersteeg, P.M.C.; Vermetten, E.; Kavelaars, A.; Geuze, E.; Heijnen, C.J. Hostility Is Related to Clusters of T-Cell Cytokines and Chemokines in Healthy Men. Psychoneuroendocrinology 2008, 33, 1041–1050. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Loving, T.J.; Stowell, J.R.; Malarkey, W.B.; Lemeshow, S.; Dickinson, S.L.; Glaser, R. Hostile Marital Interactions, Proinflammatory Cytokine Production, and Wound Healing. Arch. Gen. Psychiatry 2005, 62, 1377. [Google Scholar] [CrossRef]
- Coccaro, E.F.; Lee, R.; Coussons-Read, M. Elevated Plasma Inflammatory Markers in Individuals with Intermittent Explosive Disorder and Correlation with Aggression in Humans. JAMA Psychiatry 2014, 71, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Siegel, A.; Zalcman, S.S. Lack of Aggression and Anxiolytic-like Behavior in TNF Receptor (TNF-R1 and TNF-R2) Deficient Mice. Brain Behav. Immun. 2010, 24, 1276–1280. [Google Scholar] [CrossRef]
- Beaulieu, J.-M.; Zhang, X.; Rodriguiz, R.M.; Sotnikova, T.D.; Cools, M.J.; Wetsel, W.C.; Gainetdinov, R.R.; Caron, M.G. Role of GSK3 Beta in Behavioral Abnormalities Induced by Serotonin Deficiency. Proc. Natl. Acad. Sci. USA 2008, 105, 1333–1338. [Google Scholar] [CrossRef]
- Ng, F.; Berk, M.; Dean, O.; Bush, A.I. Oxidative Stress in Psychiatric Disorders: Evidence Base and Therapeutic Implications. Int. J. Neuropsychopharmacol. 2008, 11, 851–876. [Google Scholar] [CrossRef]
- Gvozdjáková, A.; Kucharská, J.; Ostatníková, D.; Babinská, K.; Nakládal, D.; Crane, F.L. Ubiquinol Improves Symptoms in Children with Autism. Oxidative Med. Cell. Longev. 2014, 2014, 798957. [Google Scholar] [CrossRef]
- Kuloglu, M.; Atmaca, M.; Tezcan, E.; Ustundag, B.; Bulut, S. Antioxidant Enzyme and Malondialdehyde Levels in Patients with Panic Disorder. Neuropsychobiology 2002, 46, 186–189. [Google Scholar] [CrossRef]
- Okazawa, H.; Ikawa, M.; Tsujikawa, T.; Kiyono, Y.; Yoneda, M. Brain Imaging for Oxidative Stress and Mitochondrial Dysfunction in Neurodegenerative Diseases. Q. J. Nucl. Med. Mol. Imaging Off. Publ. Ital. Assoc. Nucl. Med. (AIMN) Int. Assoc. Radiopharmacol. (IAR) Sect. Soc. 2014, 58, 387–397. [Google Scholar]
- Tobore, T.O. On the Neurobiological Role of Oxidative Stress in Alcohol-Induced Impulsive, Aggressive and Suicidal Behavior. Subst. Use Misuse 2019, 54, 2290–2303. [Google Scholar] [CrossRef]
- Coccaro, E.F.; Lee, R.; Gozal, D. Elevated Plasma Oxidative Stress Markers in Individuals with Intermittent Explosive Disorder and Correlation with Aggression in Humans. Biol. Psychiatry 2016, 79, 127–135. [Google Scholar] [CrossRef]
- Fanning, J.R.; Lee, R.; Gozal, D.; Coussons-Read, M.; Coccaro, E.F. Childhood Trauma and Parental Style: Relationship with Markers of Inflammation, Oxidative Stress, and Aggression in Healthy and Personality Disordered Subjects. Biol. Psychol. 2015, 112, 56–65. [Google Scholar] [CrossRef]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W.J.H. Is Depression Associated with Increased Oxidative Stress? A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef]
- Verma, A.; Bajpai, A.; Keshari, A.; Srivastava, M.; Srivastava, S.; Srivastava, R. Association of Major Depression with Serum Prolidase Activity and Oxidative Stress. Br. J. Med. Med. Res. 2017, 20, 1–8. [Google Scholar] [CrossRef]
- Patki, G.; Atrooz, F.; Alkadhi, I.; Solanki, N.; Salim, S. High Aggression in Rats Is Associated with Elevated Stress, Anxiety-like Behavior, and Altered Catecholamine Content in the Brain. Neurosci. Lett. 2015, 584, 308–313. [Google Scholar] [CrossRef]
- Rammal, H.; Bouayed, J.; Soulimani, R. A Direct Relationship between Aggressive Behavior in the Resident/Intruder Test and Cell Oxidative Status in Adult Male Mice. Eur. J. Pharmacol. 2010, 627, 173–176. [Google Scholar] [CrossRef]
- Garratt, M.; Brooks, R.C. A Genetic Reduction in Antioxidant Function Causes Elevated Aggression in Mice. J. Exp. Biol. 2015, 218, 223–227. [Google Scholar] [CrossRef]
- Seo, J.-S.; Park, J.-Y.; Choi, J.; Kim, T.-K.; Shin, J.-H.; Lee, J.-K.; Han, P.-L. NADPH Oxidase Mediates Depressive Behavior Induced by Chronic Stress in Mice. J. Neurosci. 2012, 32, 9690–9699. [Google Scholar] [CrossRef]
- Lopes, I.S.; Oliveira, I.C.M.; Capibaribe, V.C.C.; Valentim, J.T.; da Silva, D.M.A.; de Souza, A.G.; de Araújo, M.A.; de Chaves, R.C.; Gutierrez, S.J.C.; Barbosa Filho, J.M.; et al. Riparin II Ameliorates Corticosterone-Induced Depressive-like Behavior in Mice: Role of Antioxidant and Neurotrophic Mechanisms. Neurochem. Int. 2018, 120, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Diehl, L.A.; Alvares, L.O.; Noschang, C.; Engelke, D.; Andreazza, A.C.; Gonçalves, C.A.S.; Quillfeldt, J.A.; Dalmaz, C. Long-Lasting Effects of Maternal Separation on an Animal Model of Post-Traumatic Stress Disorder: Effects on Memory and Hippocampal Oxidative Stress. Neurochem. Res. 2012, 37, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Boufleur, N.; Antoniazzi, C.T.D.; Pase, C.S.; Benvegnú, D.M.; Dias, V.T.; Segat, H.J.; Roversi, K.; Roversi, K.; Nora, M.D.; Koakoskia, G.; et al. Neonatal Handling Prevents Anxiety-like Symptoms in Rats Exposed to Chronic Mild Stress: Behavioral and Oxidative Parameters. Stress 2013, 16, 321–330. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.A.; Velasco-Estevez, M.; Dev, K.K. Demyelination Induced by Oxidative Stress Is Regulated by Sphingosine 1-Phosphate Receptors. Glia 2017, 65, 1119–1136. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.V.; Murray, K.A.; Marsh, E.D.; Golden, J.A.; Simmons, R.A.; Grinspan, J.B. Delayed Myelination in an Intrauterine Growth Retardation Model Is Mediated by Oxidative Stress Upregulating Bone Morphogenetic Protein 4. J. Neuropathol. Exp. Neurol. 2012, 71, 640–653. [Google Scholar] [CrossRef]
- Maas, D.A.; Vallès, A.; Martens, G.J.M. Oxidative Stress, Prefrontal Cortex Hypomyelination and Cognitive Symptoms in Schizophrenia. Transl. Psychiatry 2017, 7, e1171. [Google Scholar] [CrossRef]
- Miyamoto, N.; Maki, T.; Pham, L.-D.D.; Hayakawa, K.; Seo, J.H.; Mandeville, E.T.; Mandeville, J.B.; Kim, K.-W.; Lo, E.H.; Arai, K. Oxidative Stress Interferes with White Matter Renewal after Prolonged Cerebral Hypoperfusion in Mice. Stroke 2013, 44, 3516–3521. [Google Scholar] [CrossRef]
- Power, J.H.T.; Asad, S.; Chataway, T.K.; Chegini, F.; Manavis, J.; Temlett, J.A.; Jensen, P.H.; Blumbergs, P.C.; Gai, W.-P. Peroxiredoxin 6 in Human Brain: Molecular Forms, Cellular Distribution and Association with Alzheimer’s Disease Pathology. Acta Neuropathol. 2008, 115, 611–622. [Google Scholar] [CrossRef]
- Pomytkin, I.A.; Cline, B.H.; Anthony, D.C.; Steinbusch, H.W.; Lesch, K.P.; Strekalova, T. Endotoxaemia Resulting from Decreased Serotonin Tranporter (5-HTT) Function: A Reciprocal Risk Factor for Depression and Insulin Resistance? Behav. Brain Res. 2015, 276, 111–117. [Google Scholar] [CrossRef]
- Pomytkin, I.; Costa-Nunes, J.P.; Kasatkin, V.; Veniaminova, E.; Demchenko, A.; Lyundup, A.; Lesch, K.-P.; Ponomarev, E.D.; Strekalova, T. Insulin Receptor in the Brain: Mechanisms of Activation and the Role in the CNS Pathology and Treatment. CNS Neurosci. 2018, 24, 763–774. [Google Scholar] [CrossRef]
- Cline, B.H.; Steinbusch, H.W.M.; Malin, D.; Revishchin, A.v.; Pavlova, G.v.; Cespuglio, R.; Strekalova, T. The Neuronal Insulin Sensitizer Dicholine Succinate Reduces Stress-Induced Depressive Traits and Memory Deficit: Possible Role of Insulin-like Growth Factor 2. BMC Neurosci. 2012, 13, 1. [Google Scholar] [CrossRef]
- Cline, B.H.; Anthony, D.C.; Lysko, A.; Dolgov, O.; Anokhin, K.; Schroeter, C.; Malin, D.; Kubatiev, A.; Steinbusch, H.W.; Lesch, K.P.; et al. Lasting Downregulation of the Lipid Peroxidation Enzymes in the Prefrontal Cortex of Mice Susceptible to Stress-Induced Anhedonia. Behav. Brain Res. 2015, 276, 118–129. [Google Scholar] [CrossRef]
- Schüle, C.; Nothdurfter, C.; Rupprecht, R. The Role of Allopregnanolone in Depression and Anxiety. Prog. Neurobiol. 2014, 113, 79–87. [Google Scholar] [CrossRef]
- Nave, K.-A.; Werner, H.B. Myelination of the Nervous System: Mechanisms and Functions. Annu. Rev. Cell Dev. Biol. 2014, 30, 503–533. [Google Scholar] [CrossRef]
- Wang, X.-X.; Zhang, B.; Xia, R.; Jia, Q.-Y. Inflammation, Apoptosis and Autophagy as Critical Players in Vascular Dementia. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9601–9614. [Google Scholar] [CrossRef]
- Stadelmann, C.; Timmler, S.; Barrantes-Freer, A.; Simons, M. Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol. Rev. 2019, 99, 1381–1431. [Google Scholar] [CrossRef]
- Saab, A.S.; Tzvetanova, I.D.; Nave, K.A. The Role of Myelin and Oligodendrocytes in Axonal Energy Metabolism. Curr. Opin. Neurobiol. 2013, 23, 1065–1072. [Google Scholar] [CrossRef]
- Garbern, J.Y.; Yool, D.A.; Moore, G.J.; Wilds, I.B.; Faulk, M.W.; Klugmann, M.; Nave, K.A.; Sistermans, E.A.; van der Knaap, M.S.; Bird, T.D.; et al. Patients Lacking the Major CNS Myelin Protein, Proteolipid Protein 1, Develop Length-Dependent Axonal Degeneration in the Absence of Demyelination and Inflammation. Brain 2002, 125, 551–561. [Google Scholar] [CrossRef]
- Ye, P.; Carson, J.; D’Ercole, A.J. Insulin-like Growth Factor-I Influences the Initiation of Myelination: Studies of the Anterior Commissure of Transgenic Mice. Neurosci. Lett. 1995, 201, 235–238. [Google Scholar] [CrossRef]
- Fields, R.D.; Bukalo, O. Myelin Makes Memories. Nat. Neurosci. 2020, 23, 469–470. [Google Scholar] [CrossRef]
- Pan, S.; Mayoral, S.R.; Choi, H.S.; Chan, J.R.; Kheirbek, M.A. Preservation of a Remote Fear Memory Requires New Myelin Formation. Nat. Neurosci. 2020, 23, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Gozzi, M.; Nielson, D.M.; Lenroot, R.K.; Ostuni, J.L.; Luckenbaugh, D.A.; Thurm, A.E.; Giedd, J.N.; Swedo, S.E. A Magnetization Transfer Imaging Study of Corpus Callosum Myelination in Young Children with Autism. Biol. Psychiatry 2012, 72, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.P. Editorial: Can Dysregulated Myelination Be Linked to ADHD Pathogenesis and Persistence? J. Child Psychol. Psychiatry 2019, 60, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Onnink, A.M.H.; Zwiers, M.P.; Hoogman, M.; Mostert, J.C.; Dammers, J.; Kan, C.C.; Vasquez, A.A.; Schene, A.H.; Buitelaar, J.; Franke, B. Deviant White Matter Structure in Adults with Attention-Deficit/Hyperactivity Disorder Points to Aberrant Myelination and Affects Neuropsychological Performance. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 63, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Sakurai, T.; Davis, K.L.; Buxbaum, J.D. Linking Oligodendrocyte and Myelin Dysfunction to Neurocircuitry Abnormalities in Schizophrenia. Prog. Neurobiol. 2011, 93, 13–24. [Google Scholar] [CrossRef]
- Jha, S.C.; Meltzer-Brody, S.; Steiner, R.J.; Cornea, E.; Woolson, S.; Ahn, M.; Verde, A.R.; Hamer, R.M.; Zhu, H.; Styner, M.; et al. Antenatal Depression, Treatment with Selective Serotonin Reuptake Inhibitors, and Neonatal Brain Structure: A Propensity-Matched Cohort Study. Psychiatry Res. Neuroimaging 2016, 253, 43–53. [Google Scholar] [CrossRef]
- Lake, E.M.R.; Steffler, E.A.; Rowley, C.D.; Sehmbi, M.; Minuzzi, L.; Frey, B.N.; Bock, N.A. Altered Intracortical Myelin Staining in the Dorsolateral Prefrontal Cortex in Severe Mental Illness. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 369–376. [Google Scholar] [CrossRef]
- Gos, T.; Schroeter, M.L.; Lessel, W.; Bernstein, H.-G.; Dobrowolny, H.; Schiltz, K.; Bogerts, B.; Steiner, J. S100B-Immunopositive Astrocytes and Oligodendrocytes in the Hippocampus Are Differentially Afflicted in Unipolar and Bipolar Depression: A Postmortem Study. J. Psychiatr. Res. 2013, 47, 1694–1699. [Google Scholar] [CrossRef]
- Vostrikov, V.M.; Uranova, N.A. Reduced Density of Oligodendrocytes and Oligodendrocyte Clusters in the Caudate Nucleus in Major Psychiatric Illnesses. Schizophr. Res. 2020, 215, 211–216. [Google Scholar] [CrossRef]
- Makinodan, M.; Ikawa, D.; Yamamuro, K.; Yamashita, Y.; Toritsuka, M.; Kimoto, S.; Yamauchi, T.; Okumura, K.; Komori, T.; Fukami, S.; et al. Effects of the Mode of Re-Socialization after Juvenile Social Isolation on Medial Prefrontal Cortex Myelination and Function. Sci. Rep. 2017, 7, 5481. [Google Scholar] [CrossRef]
- Laine, M.A.; Trontti, K.; Misiewicz, Z.; Sokolowska, E.; Kulesskaya, N.; Heikkinen, A.; Saarnio, S.; Balcells, I.; Ameslon, P.; Greco, D.; et al. Genetic Control of Myelin Plasticity after Chronic Psychosocial Stress. eNeuro 2018, 5. [Google Scholar] [CrossRef]
- Bonnefil, V.; Dietz, K.; Amatruda, M.; Wentling, M.; Aubry, A.v.; Dupree, J.L.; Temple, G.; Park, H.J.; Burghardt, N.S.; Casaccia, P.; et al. Region-Specific Myelin Differences Define Behavioral Consequences of Chronic Social Defeat Stress in Mice. eLife 2019, 8, e40855. [Google Scholar] [CrossRef]
- Tang, J.; Liang, X.; Zhang, Y.; Chen, L.; Wang, F.; Tan, C.; Luo, Y.; Xiao, Q.; Chao, F.; Zhang, L.; et al. The Effects of Running Exercise on Oligodendrocytes in the Hippocampus of Rats with Depression Induced by Chronic Unpredictable Stress. Brain Res. Bull. 2019, 149, 1–10. [Google Scholar] [CrossRef]
- Miyata, S.; Taniguchi, M.; Koyama, Y.; Shimizu, S.; Tanaka, T.; Yasuno, F.; Yamamoto, A.; Iida, H.; Kudo, T.; Katayama, T.; et al. Association between Chronic Stress-Induced Structural Abnormalities in Ranvier Nodes and Reduced Oligodendrocyte Activity in Major Depression. Sci. Rep. 2016, 6, 23084. [Google Scholar] [CrossRef]
- Blair, R.J.R. The Neurobiology of Impulsive Aggression. J. Child Adolesc. Psychopharmacol. 2016, 26, 4–9. [Google Scholar] [CrossRef]
- Strekalova, T.; Svirin, E.; Veniaminova, E.; Kopeikina, E.; Veremeyko, T.; Yung, A.W.Y.; Proshin, A.; Walitza, S.; Anthony, D.C.; Lim, L.W.; et al. ASD-like Behaviors, a Dysregulated Inflammatory Response and Decreased Expression of PLP1 Characterize Mice Deficient for Sialyltransferase ST3GAL5. Brain Behav. Immun. Health 2021, 16, 100306. [Google Scholar] [CrossRef]
- Martinez-Rachadell, L.; Aguilera, A.; Perez-Domper, P.; Pignatelli, J.; Fernandez, A.M.; Torres-Aleman, I. Cell-Specific Expression of Insulin/Insulin-like Growth Factor-I Receptor Hybrids in the Mouse Brain. Growth Horm. IGF Res. 2019, 45, 25–30. [Google Scholar] [CrossRef]
- Hackett, A.R.; Strickland, A.; Milbrandt, J. Disrupting Insulin Signaling in Schwann Cells Impairs Myelination and Induces a Sensory Neuropathy. Glia 2020, 68, 963–978. [Google Scholar] [CrossRef]
- Ye, P.; Li, L.; Lund, P.K.; D’Ercole, A.J. Deficient Expression of Insulin Receptor Substrate-1 (IRS-1) Fails to Block Insulin-like Growth Factor-I (IGF-I) Stimulation of Brain Growth and Myelination. Brain Res. Dev. Brain Res. 2002, 136, 111–121. [Google Scholar] [CrossRef]
- Gorman, D.A.; Gardner, D.M.; Murphy, A.L.; Feldman, M.; Bélanger, S.A.; Steele, M.M.; Boylan, K.; Cochrane-Brink, K.; Goldade, R.; Soper, P.R.; et al. Canadian Guidelines on Pharmacotherapy for Disruptive and Aggressive Behaviour in Children and Adolescents with Attention-Deficit Hyperactivity Disorder, Oppositional Defiant Disorder, or Conduct Disorder. Can. J. Psychiatry 2015, 60, 62–76. [Google Scholar] [CrossRef]
- Felthous, A.R.; Stanford, M.S. A Proposed Algorithm for the Pharmacotherapy of Impulsive Aggression. J. Am. Acad. Psychiatry Law 2015, 43, 456–467. [Google Scholar] [PubMed]
- Rinne, T.; van den Brink, W.; Wouters, L.; van Dyck, R. SSRI Treatment of Borderline Personality Disorder: A Randomized, Placebo-Controlled Clinical Trial for Female Patients with Borderline Personality Disorder. Am. J. Psychiatry 2002, 159, 2048–2054. [Google Scholar] [CrossRef] [PubMed]
- Kamarck, T.W.; Haskett, R.F.; Muldoon, M.; Flory, J.D.; Anderson, B.; Bies, R.; Pollock, B.; Manuck, S.B. Citalopram Intervention for Hostility: Results of a Randomized Clinical Trial. J. Consult. Clin. Psychol. 2009, 77, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.; Iturra, P.; Solari, A.; Villarroel, J.; Jerez, S.; Jiménez, M.; Galleguillos, F.; Bustamante, M.L. Fluoxetine Response in Impulsive-Aggressive Behavior and Serotonin Transporter Polymorphism in Personality Disorder. Psychiatr. Genet. 2010, 20, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Sagar-Ouriaghli, I.; Lievesley, K.; Santosh, P.J. Propranolol for Treating Emotional, Behavioural, Autonomic Dysregulation in Children and Adolescents with Autism Spectrum Disorders. J. Psychopharmacol. 2018, 32, 641–653. [Google Scholar] [CrossRef]
- O’Connor, W.; Earley, B.; Leonard, B. Antidepressant Properties of the Triazolobenzodiazepines Alprazolam and Adinazolam: Studies on the Olfactory Bulbectomized Rat Model of Depression. Br. J. Clin. Pharmacol. 1985, 19, 49S–56S. [Google Scholar] [CrossRef]
- Strekalova, T.; Spanagel, R.; Dolgov, O.; Bartsch, D. Stress-Induced Hyperlocomotion as a Confounding Factor in Anxiety and Depression Models in Mice. Behav. Pharmacol. 2005, 16, 171–180. [Google Scholar] [CrossRef]
- Pandey, D.K.; Mahesh, R.; kumar, A.A.; Rao, V.S.; Arjun, M.; Rajkumar, R. A Novel 5-HT2A Receptor Antagonist Exhibits Antidepressant-like Effects in a Battery of Rodent Behavioural Assays: Approaching Early-Onset Antidepressants. Pharmacol. Biochem. Behav. 2010, 94, 363–373. [Google Scholar] [CrossRef]
- Bessa, J.M.; Morais, M.; Marques, F.; Pinto, L.; Palha, J.A.; Almeida, O.F.X.; Sousa, N. Stress-Induced Anhedonia Is Associated with Hypertrophy of Medium Spiny Neurons of the Nucleus Accumbens. Transl. Psychiatry 2013, 3. [Google Scholar] [CrossRef]
- Bessa, J.M.; Ferreira, D.; Melo, I.; Marques, F.; Cerqueira, J.J.; Palha, J.A.; Almeida, O.F.X.; Sousa, N. The Mood-Improving Actions of Antidepressants Do Not Depend on Neurogenesis but Are Associated with Neuronal Remodeling. Mol. Psychiatry 2009, 14, 764–773. [Google Scholar] [CrossRef]
- Connor, T.J.; Harkin, A.; Kelly, J.P.; Leonard, B.E. Olfactory Bulbectomy Provokes a Suppression of Interleukin-1β and Tumour Necrosis Factor-α Production in Response to an in Vivo Challenge with Lipopolysaccharide: Effect of Chronic Desipramine Treatment. Neuroimmunomodulation 2000, 7, 27–35. [Google Scholar] [CrossRef]
- Roche, M.; Harkin, A.; Kelly, J.P. Chronic Fluoxetine Treatment Attenuates Stressor-Induced Changes in Temperature, Heart Rate, and Neuronal Activation in the Olfactory Bulbectomized Rat. Neuropsychopharmacology 2007, 32, 1312–1320. [Google Scholar] [CrossRef]
- Strekalova, T.; Gorenkova, N.; Schunk, E.; Dolgov, O.; Bartsch, D. Selective Effects of Citalopram in a Mouse Model of Stress-Induced Anhedonia with a Control for Chronic Stress. Behav. Pharmacol. 2006, 17, 271–287. [Google Scholar] [CrossRef]
- Aswar, U.M.; Kalshetti, P.P.; Shelke, S.M.; Bhosale, S.H.; Bodhankar, S.L. Effect of Newly Synthesized 1,2,4-Triazino[5,6-b]Indole-3-Thione Derivatives on Olfactory Bulbectomy Induced Depression in Rats. Asian Pac. J. Trop. Biomed. 2012, 2, 992–998. [Google Scholar] [CrossRef]
- Holubova, K.; Kleteckova, L.; Skurlova, M.; Ricny, J.; Stuchlik, A.; Vales, K. Rapamycin Blocks the Antidepressant Effect of Ketamine in Task-Dependent Manner. Psychopharmacology 2016, 233, 2077–2097. [Google Scholar] [CrossRef]
- Redmond, A. Behavioural and Neurochemical Effects of Dizocilpine in the Olfactory Bulbectomized Rat Model of Depression. Pharmacol. Biochem. Behav. 1997, 58, 355–359. [Google Scholar] [CrossRef]
- Ho, Y.-J.; Chen, K.-H.; Tai, M.-Y.; Tsai, Y.-F. MK-801 Suppresses Muricidal Behavior but Not Locomotion in Olfactory Bulbectomized Rats: Involvement of NMDA Receptors. Pharmacol. Biochem. Behav. 2004, 77, 641–646. [Google Scholar] [CrossRef]
- Sun, J.-D.; Liu, Y.; Yuan, Y.-H.; Li, J.; Chen, N.-H. Gap Junction Dysfunction in the Prefrontal Cortex Induces Depressive-Like Behaviors in Rats. Neuropsychopharmacology 2012, 37, 1305–1320. [Google Scholar] [CrossRef]
- Rygula, R.; Abumaria, N.; Havemann-Reinecke, U.; Rüther, E.; Hiemke, C.; Zernig, G.; Fuchs, E.; Flügge, G. Pharmacological Validation of a Chronic Social Stress Model of Depression in Rats: Effects of Reboxetine, Haloperidol and Diazepam. Behav. Pharmacol. 2008, 19, 183–196. [Google Scholar] [CrossRef]
- Papp, M. Pharmacological Validation of the Chronic Mild Stress Model of Depression. Eur. Neuropsychopharmacol. 2001, 11, S166–S167. [Google Scholar] [CrossRef]
- Pavlov, D.; Markova, N.; Bettendorff, L.; Chekhonin, V.; Pomytkin, I.; Lioudyno, V.; Svistunov, A.; Ponomarev, E.; Lesch, K.P.; Strekalova, T. Elucidating the Functions of Brain GSK3α: Possible Synergy with GSK3β Upregulation and Reversal by Antidepressant Treatment in a Mouse Model of Depressive-like Behaviour. Behav. Brain Res. 2017, 335, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, D.; Gorlova, A.; Bettendorff, L.; Kalueff, A.A.; Umriukhin, A.; Proshin, A.; Lysko, A.; Landgraf, R.; Anthony, D.C.; Strekalova, T. Enhanced Conditioning of Adverse Memories in the Mouse Modified Swim Test Is Associated with Neuroinflammatory Changes—Effects That Are Susceptible to Antidepressants. Neurobiol. Learn. Mem. 2020, 172, 107227. [Google Scholar] [CrossRef] [PubMed]
- Sambon, M.; Gorlova, A.; Demelenne, A.; Alhama-Riba, J.; Coumans, B.; Lakaye, B.; Wins, P.; Fillet, M.; Anthony, D.C.; Strekalova, T.; et al. Dibenzoylthiamine Has Powerful Antioxidant and Anti-Inflammatory Properties in Cultured Cells and in Mouse Models of Stress and Neurodegeneration. Biomedicines 2020, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.W.; Wroolie, T.E.; Robakis, T.; Rasgon, N.L. Adjuvant Pioglitazone for Unremitted Depression: Clinical Correlates of Treatment Response. Psychiatry Res. 2015, 230, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Colle, R.; de Larminat, D.; Rotenberg, S.; Hozer, F.; Hardy, P.; Verstuyft, C.; Fève, B.; Corruble, E. PPAR-γ Agonists for the Treatment of Major Depression: A Review. Pharmacopsychiatry 2017, 50, 49–55. [Google Scholar] [CrossRef]
- Ballard, C.G.; Gauthier, S.; Cummings, J.L.; Brodaty, H.; Grossberg, G.T.; Robert, P.; Lyketsos, C.G. Management of Agitation and Aggression Associated with Alzheimer Disease. Nat. Rev. Neurol. 2009, 5, 245–255. [Google Scholar] [CrossRef]
- Ostinelli, E.G.; Hussein, M.; Ahmed, U.; Rehman, F.-U.; Miramontes, K.; Adams, C.E. Risperidone for Psychosis-Induced Aggression or Agitation (Rapid Tranquillisation). Cochrane Database Syst. Rev. 2018, 4, CD009412. [Google Scholar] [CrossRef]
- Albrecht, B.; Staiger, P.K.; Hall, K.; Miller, P.; Best, D.; Lubman, D.I. Benzodiazepine Use and Aggressive Behaviour: A Systematic Review. Aust. N. Z. J. Psychiatry 2014, 48, 1096–1114. [Google Scholar] [CrossRef]
- Gillies, D.; Beck, A.; McCloud, A. Benzodiazepines alone or in combination with antipsychotic drugs for acute psychosis. In The Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Comai, S.; Tau, M.; Gobbi, G. The Psychopharmacology of Aggressive Behavior: A Translational Approach: Part 1: Neurobiology. J. Clin. Psychopharmacol. 2012, 32, 83–94. [Google Scholar] [CrossRef]
- Varghese, B.S.; Rajeev, A.; Norrish, M.; Khusaiby, S.B.M. Topiramate for Anger Control: A Systematic Review. Indian J. Pharmacol. 2010, 42, 135–141. [Google Scholar] [CrossRef]
- Lane, S.D.; Kjome, K.L.; Moeller, F.G. Neuropsychiatry of Aggression. Neurol. Clin. 2011, 29, 49–64. [Google Scholar] [CrossRef]
- Gowin, J.L.; Green, C.E.; Alcorn, J.L.; Swann, A.C.; Moeller, F.G.; Lane, S.D. Chronic Tiagabine Administration and Aggressive Responding in Individuals with a History of Substance Abuse and Antisocial Behavior. J. Psychopharmacol. 2012, 26, 982–993. [Google Scholar] [CrossRef]
- Cooney, G.M.; Dwan, K.; Greig, C.A.; Lawlor, D.A.; Rimer, J.; Waugh, F.R.; McMurdo, M.; Mead, G.E. Exercise for Depression. Cochrane Database Syst. Rev. 2013, 34, CD004366. [Google Scholar] [CrossRef]
- Aleman, A.; Kahn, R.S. Effects of the Atypical Antipsychotic Risperidone on Hostility and Aggression in Schizophrenia: A Meta-Analysis of Controlled Trials. Eur. Neuropsychopharmacol. 2001, 11, 289–293. [Google Scholar] [CrossRef]
- Donovan, S.J.; Aybar, B.J. Pharmacological interventions: Anticonvulsants. In Aggression; Coccaro, E., Ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 369–383. [Google Scholar]
- Stanford, M.S.; Helfritz, L.E.; Conklin, S.M.; Villemarette-Pittman, N.R.; Greve, K.W.; Adams, D.; Houston, R.J. A Comparison of Anticonvulsants in the Treatment of Impulsive Aggression. Exp. Clin. Psychopharmacol. 2005, 13, 72–77. [Google Scholar] [CrossRef]
- Stuckelman, Z.D.; Mulqueen, J.M.; Ferracioli-Oda, E.; Cohen, S.C.; Coughlin, C.G.; Leckman, J.F.; Bloch, M.H. Risk of Irritability with Psychostimulant Treatment in Children With ADHD: A Meta-Analysis. J. Clin. Psychiatry 2017, 78, e648–e655. [Google Scholar] [CrossRef]
- Masood, A.; Nadeem, A.; Mustafa, S.J.; O’Donnell, J.M. Reversal of Oxidative Stress-Induced Anxiety by Inhibition of Phosphodiesterase-2 in Mice. J. Pharmacol. Exp. 2008, 326, 369–379. [Google Scholar] [CrossRef]
- McDermott, R.; Tingley, D.; Cowden, J.; Frazzetto, G.; Johnson, D.D.P. Monoamine Oxidase A Gene (MAOA) Predicts Behavioral Aggression Following Provocation. Proc. Natl. Acad. Sci. USA 2009, 106, 2118–2123. [Google Scholar] [CrossRef]
- Pesarico, A.P.; Birmann, P.T.; Pinto, R.; Padilha, N.B.; Lenardão, E.J.; Savegnago, L. Short- and Long-Term Repeated Forced Swim Stress Induce Depressive-Like Phenotype in Mice: Effectiveness of 3-[(4-Chlorophenyl)Selanyl]-1-Methyl-1H-Indole. Front. Behav. Neurosci. 2020, 14, 140. [Google Scholar] [CrossRef]
- Uzun, S.; Kozumplik, O.; Jakovljević, M.; Sedić, B. Side Effects of Treatment with Benzodiazepines. Psychiatr. Danub. 2010, 22, 90–93. [Google Scholar]
- Nabavi, S.M.; Daglia, M.; Braidy, N.; Nabavi, S.F. Natural Products, Micronutrients, and Nutraceuticals for the Treatment of Depression: A Short Review. Nutr. Neurosci. 2017, 20, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Hira, S.; Saleem, U.; Anwar, F.; Ahmad, B. Antioxidants Attenuate Isolation- and L-DOPA-Induced Aggression in Mice. Front. Pharmacol. 2018, 8, 945. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Jope, R.S. Inflammation and Lithium: Clues to Mechanisms Contributing to Suicide-Linked Traits. Transl. Psychiatry 2014, 4, e488. [Google Scholar] [CrossRef] [PubMed]
- Mccleery, J.; Abraham, R.P.; Denton, D.A.; Rutjes, A.W.S.; Chong, L.Y.; Al-Assaf, A.S.; Griffith, D.J.; Rafeeq, S.; Yaman, H.; Malik, M.A.; et al. Vitamin and Mineral Supplementation for Preventing Dementia or Delaying Cognitive Decline in People with Mild Cognitive Impairment. Cochrane Database Syst. Rev. 2018, 11, CD011905. [Google Scholar] [CrossRef] [PubMed]
- de la Ortí, J.E.R.; Cuerda-Ballester, M.; Drehmer, E.; Carrera-Juliá, S.; Motos-Muñoz, M.; Cunha-Pérez, C.; Benlloch, M.; López-Rodríguez, M.M. Vitamin B1 Intake in Multiple Sclerosis Patients and Its Impact on Depression Presence: A Pilot Study. Nutrients 2020, 12, 2655. [Google Scholar] [CrossRef]
- Borges-Vieira, J.G.; Cardoso, C.K.S. Efficacy of B-Vitamins and Vitamin D Therapy in Improving Depressive and Anxiety Disorders: A Systematic Review of Randomized Controlled Trials. Nutr. Neurosci. 2022, 1–21. [Google Scholar] [CrossRef]
- Petermann, A.B.; Reyna-Jeldes, M.; Ortega, L.; Coddou, C.; Yévenes, G.E. Roles of the Unsaturated Fatty Acid Docosahexaenoic Acid in the Central Nervous System: Molecular and Cellular Insights. Int. J. Mol. Sci. 2022, 23, 5390. [Google Scholar] [CrossRef]
- Kidd, P.M. Omega-3 DHA and EPA for Cognition, Behavior, and Mood: Clinical Findings and Structural-Functional Synergies with Cell Membrane Phospholipids. Altern. Med. Rev. 2007, 12, 207–227. [Google Scholar]
- DeMar, J.C.; Ma, K.; Bell, J.M.; Igarashi, M.; Greenstein, D.; Rapoport, S.I. One Generation of N-3 Polyunsaturated Fatty Acid Deprivation Increases Depression and Aggression Test Scores in Rats. J. Lipid Res. 2006, 47, 172–180. [Google Scholar] [CrossRef]
- O’Donnell, K.C.; Gould, T.D. The Behavioral Actions of Lithium in Rodent Models: Leads to Develop Novel Therapeutics. Neurosci. Biobehav. Rev. 2007, 31, 932–962. [Google Scholar] [CrossRef]
- Kovacsics, C.E.; Gould, T.D. Shock-Induced Aggression in Mice Is Modified by Lithium. Pharmacol. Biochem. Behav. 2010, 94, 380–386. [Google Scholar] [CrossRef]
- Markova, N.; Bazhenova, N.; Anthony, D.C.; Vignisse, J.; Svistunov, A.; Lesch, K.P.; Bettendorff, L.; Strekalova, T. Thiamine and Benfotiamine Improve Cognition and Ameliorate GSK-3β-Associated Stress-Induced Behaviours in Mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 75, 148–156. [Google Scholar] [CrossRef]
- Costa-Nunes, J.; Cline, B.H.; Araújo-Correia, M.; Valença, A.; Markova, N.; Dolgov, O.; Kubatiev, A.; Yeritsyan, N.; Steinbusch, H.W.M.; Strekalova, T. Animal Models of Depression and Drug Delivery with Food as an Effective Dosing Method: Evidences from Studies with Celecoxib and Dicholine Succinate. BioMed Res. Int. 2015, 2015, 596126. [Google Scholar] [CrossRef]
- Strekalova, T.; Costa-Nunes, J.P.; Veniaminova, E.; Kubatiev, A.; Lesch, K.-P.; Chekhonin, V.P.; Evans, M.C.; Steinbusch, H.W.M. Insulin Receptor Sensitizer, Dicholine Succinate, Prevents Both Toll-like Receptor 4 (TLR4) Upregulation and Affective Changes Induced by a High-Cholesterol Diet in Mice. J. Affect. Disord. 2016, 196, 109–116. [Google Scholar] [CrossRef]
- Guo, M.; Li, C.; Lei, Y.; Xu, S.; Zhao, D.; Lu, X.-Y. Role of the Adipose PPARγ-Adiponectin Axis in Susceptibility to Stress and Depression/Anxiety-Related Behaviors. Mol. Psychiatry 2017, 22, 1056–1068. [Google Scholar] [CrossRef]
- Banerjee, N.; Kim, H.; Talcott, S.T.; Turner, N.D.; Byrne, D.H.; Mertens-Talcott, S.U. Plum polyphenols inhibit colorectal aberrant crypt foci formation in rats: Potential role of the miR-143/protein kinase B/mammalian target of rapamycin axis. Nutr. Res. 2016, 36, 1105–1113. [Google Scholar] [CrossRef]
- Georgiev, V.G.; Weber, J.; Kneschke, E.M.; Denev, P.N.; Bley, T.; Pavlov, A.I. Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot Beta vulgaris cv. Detroit dark red. Plant Foods Hum. Nutr. 2010, 65, 105–111. [Google Scholar] [CrossRef]
- Violle, N.; Rozan, P.; Demais, H.; Nyvall Collen, P.; Bisson, J.F. Evaluation of the Antidepressant- and Anxiolytic-like Effects of a Hydrophilic Extract from the Green Seaweed Ulva Sp. in Rats. Nutr. Neurosci. 2018, 21, 248–256. [Google Scholar] [CrossRef]
- Schapovalova, O.; Gorlova, A.; de Munter, J.; Sheveleva, E.; Eropkin, M.; Gorbunov, N.; Sicker, M.; Umriukhin, A.; Lyubchyk, S.; Lesch, K.-P.; et al. Immunomodulatory Effects of New Phytotherapy on Human Macrophages and TLR4- and TLR7/8-Mediated Viral-like Inflammation in Mice. Front. Med. 2022, 9, 952977. [Google Scholar] [CrossRef]
- Ghaleiha, A.; Davari, H.; Jahangard, L.; Haghighi, M.; Ahmadpanah, M.; Seifrabie, M.A.; Bajoghli, H.; Holsboer-Trachsler, E.; Brand, S. Adjuvant Thiamine Improved Standard Treatment in Patients with Major Depressive Disorder: Results from a Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 695–702. [Google Scholar] [CrossRef]
- Onodera, K. Abnormal Behavior Induced by Thiamine Deficiency in Rats: Muricide and Its Behavioral and Pharmacological Characteristics. Folia Pharmacol. Jpn. 1992, 100, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bâ, A. Perinatal Thiamine Deficiency-Induced Spontaneous Abortion and Pup-Killing Responses in Rat Dams. Nutr. Neurosci. 2013, 16, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Rasgon, N.L.; Kenna, H.A.; Williams, K.E.; Powers, B.; Wroolie, T.; Schatzberg, A.F. Rosiglitazone Add-on in Treatment of Depressed Patients with Insulin Resistance: A Pilot Study. ScientificWorldJournal 2010, 10, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Kemp, D.E.; Ismail-Beigi, F.; Ganocy, S.J.; Conroy, C.; Gao, K.; Obral, S.; Fein, E.; Findling, R.L.; Calabrese, J.R. Use of Insulin Sensitizers for the Treatment of Major Depressive Disorder: A Pilot Study of Pioglitazone for Major Depression Accompanied by Abdominal Obesity. J. Affect. Disord. 2012, 136, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Adzick, N.S.; de Leon, D.D.; States, L.J.; Lord, K.; Bhatti, T.R.; Becker, S.A.; Stanley, C.A. Surgical Treatment of Congenital Hyperinsulinism: Results from 500 Pancreatectomies in Neonates and Children. J. Pediatr. Surg. 2019, 54, 27–32. [Google Scholar] [CrossRef]
- Tufano, M.; Pinna, G. Is There a Future for PPARs in the Treatment of Neuropsychiatric Disorders? Molecules 2020, 25, 1062. [Google Scholar] [CrossRef]
- Igarashi, M.; Hirata, A.; Yamaguchi, H.; Jimbu, Y.; Tominaga, M. Pioglitazone Reduces Atherogenic Outcomes in Type 2 Diabetic Patients. J. Atheroscler. Thromb. 2008, 15, 34–40. [Google Scholar] [CrossRef]
- Saubermann, L.J.; Nakajima, A.; Wada, K.; Zhao, S.; Terauchi, Y.; Kadowaki, T.; Aburatani, H.; Matsuhashi, N.; Nagai, R.; Blumberg, R.S. Peroxisome Proliferator-Activated Receptor Gamma Agonist Ligands Stimulate a Th2 Cytokine Response and Prevent Acute Colitis. Inflamm. Bowel Dis. 2002, 8, 330–339. [Google Scholar] [CrossRef]
- Storozheva, Z.I.; Proshin, A.T.; Sherstnev, V.V.; Storozhevykh, T.P.; Senilova, Y.E.; Persiyantseva, N.A.; Pinelis, V.G.; Semenova, N.A.; Zakharova, E.I.; Pomytkin, I.A. Dicholine salt of succinic acid, a neuronal insulin sensitizer, ameliorates cognitive deficits in rodent models of normal aging, chronic cerebral hypoperfusion, and beta-amyloid peptide-(25-35)-induced amnesia. BMC Pharmacol. 2008, 8, 1. [Google Scholar] [CrossRef]
- Storozhevykh, T.P.; Senilova, Y.E.; Persiyantseva, N.A.; Pinelis, V.G.; Pomytkin, I.A. Mitochondrial respiratory chain is involved in insulin-stimulated hydrogen peroxide production and plays an integral role in insulin receptor autophosphorylation in neurons. BMC Neurosci. 2007, 8, 84. [Google Scholar] [CrossRef]
- de La Rossa, A.; Laporte, M.H.; Astori, S.; Marissal, T.; Montessuit, S.; Sheshadri, P.; Ramos-Fernández, E.; Mendez, P.; Khani, A.; Quairiaux, C.; et al. Paradoxical Neuronal Hyperexcitability in a Mouse Model of Mitochondrial Pyruvate Import Deficiency. eLife 2022, 11, e72595. [Google Scholar] [CrossRef]
- Oyebode, O.; Kandala, N.-B.; Chilton, P.J.; Lilford, R.J. Use of Traditional Medicine in Middle-Income Countries: A WHO-SAGE Study. Health Policy Plan 2016, 31, 984–991. [Google Scholar] [CrossRef]
- Bystritsky, A.; Hovav, S.; Sherbourne, C.; Stein, M.B.; Rose, R.D.; Campbell-Sills, L.; Golinelli, D.; Sullivan, G.; Craske, M.G.; Roy-Byrne, P.P. Use of Complementary and Alternative Medicine in a Large Sample of Anxiety Patients. Psychosomatics 2012, 53, 266–272. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Umukoro, S.; Adebesin, A.; Agu, G.; Omorogbe, O.; Asehinde, S.B. Antidepressant-like Activity of Methyl Jasmonate Involves Modulation of Monoaminergic Pathways in Mice. Adv. Med. Sci. 2018, 63, 36–42. [Google Scholar] [CrossRef]
- Schiavone, S.; Colaianna, M.; Curtis, L. Impact of Early Life Stress on the Pathogenesis of Mental Disorders: Relation to Brain Oxidative Stress. Curr. Pharm. Des. 2015, 21, 1404–1412. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Dicko, A.; Younos, C.; Soulimani, R. Chlorogenic Acid, a Polyphenol from Prunus Domestica (Mirabelle), with Coupled Anxiolytic and Antioxidant Effects. J. Neurol. Sci. 2007, 262, 77–84. [Google Scholar] [CrossRef]
- Sulakhiya, K.; Patel, V.; Saxena, R.; Dashore, J.; Srivastava, A.; Rathore, M. Effect of Beta Vulgaris Linn. Leaves Extract on Anxiety- and Depressive-like Behavior and Oxidative Stress in Mice after Acute Restraint Stress. Pharmacogn. Res. 2016, 8, 1–7. [Google Scholar] [CrossRef]
- McIntyre, E.; Saliba, A.J.; Moran, C.C. Herbal Medicine Use in Adults Who Experience Anxiety: A Qualitative Exploration. Int. J. Qual. Stud. Health Well-Being 2015, 10, 29275. [Google Scholar] [CrossRef]
- Montivero, A.J.; Ghersi, M.S.; Silvero, C.M.J.; Artur de la Villarmois, E.; Catalan-Figueroa, J.; Herrera, M.; Becerra, M.C.; Hereñú, C.B.; Pérez, M.F. Early IGF-1 Gene Therapy Prevented Oxidative Stress and Cognitive Deficits Induced by Traumatic Brain Injury. Front. Pharmacol. 2021, 12, 672392. [Google Scholar] [CrossRef]
- Yong, H.Y.F.; Rawji, K.S.; Ghorbani, S.; Xue, M.; Yong, V.W. The Benefits of Neuroinflammation for the Repair of the Injured Central Nervous System. Cell Mol. Immunol. 2019, 16, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Kıray, H.; Lindsay, S.L.; Hosseinzadeh, S.; Barnett, S.C. The Multifaceted Role of Astrocytes in Regulating Myelination. Exp. Neurol. 2016, 283, 541–549. [Google Scholar] [CrossRef] [PubMed]
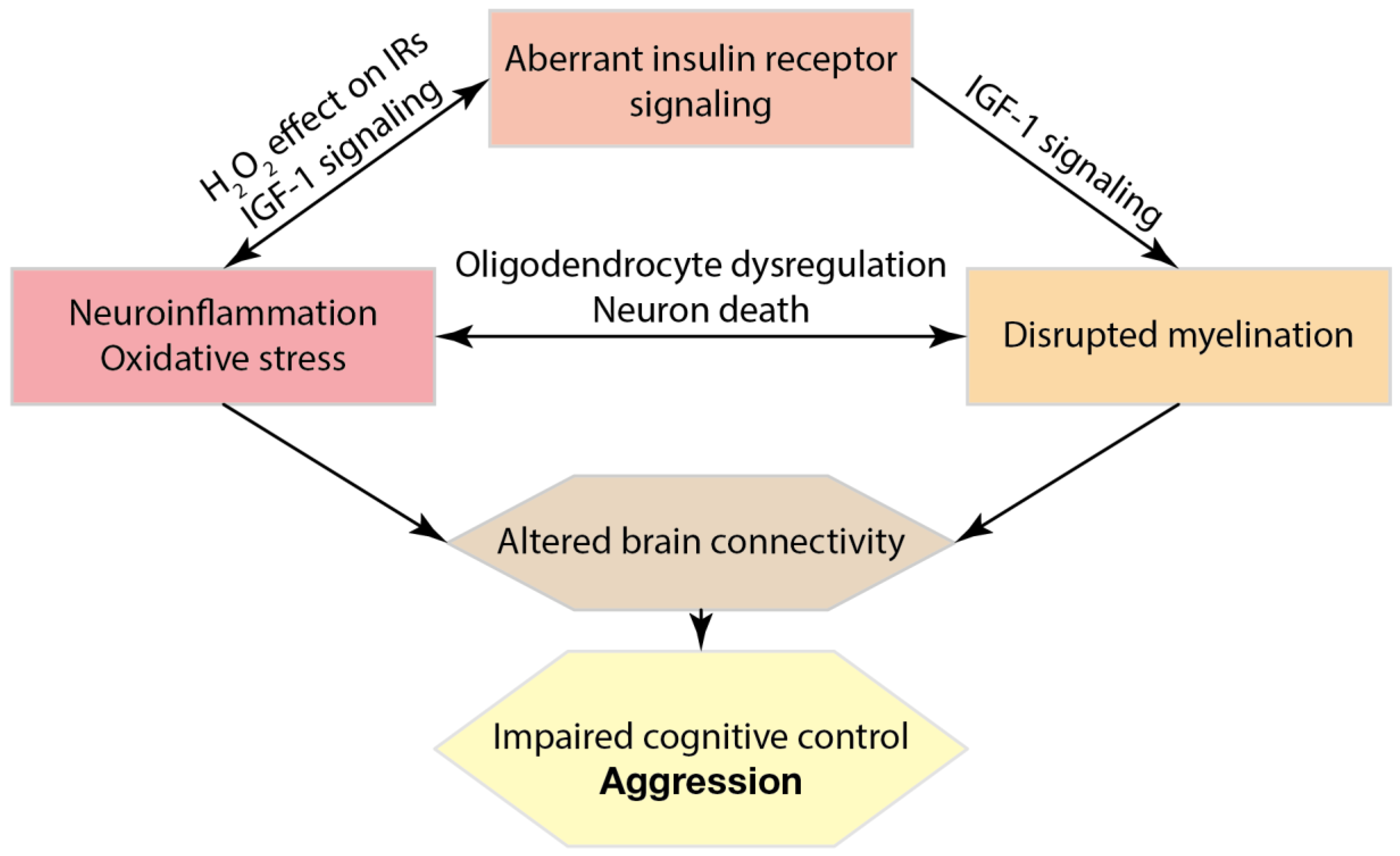
| Drug Class | Drug Examples | Strains | Core Targets | References |
|---|---|---|---|---|
| Benzodiazepine | Adinazolam Diazepam | Sprague-Dawley rats | Facilitating GABAergic transmission, decreasing neuronal excitability | [223,224] |
| TCA | Amitriptyline Desipramine Imipramine | Sprague-Dawley rats, Wistar rats, C57BL/6J mice | Blocking of serotonin transporter SERT and norepinephrine transporter NET, inhibition of sodium channels, reversed lipid peroxidation | [225,226,227,228] |
| SSRI | Citalopram Escitalopram Fluoxetine | Sprague-Dawley rats, Wistar rats | Inhibition of serotonin reuptake, increased norepinephrine transmission, upregulation of BDNF, anti-inflammatory effects | [229,230] |
| MAOI | Moclobemide | Sprague-Dawley rats | Inhibition of monoamine oxidase activity, deamination of serotonin and norepinephrine | [231] |
| NMDA antagonist | Ketamine MK-801 | Wistar rats | Inhibition of ionotropic NMDA receptors, anti-inflammatory effects | [232,233,234] |
| SNRI | Duloxetine | Sprague-Dawley rats | Inhibition of serotonin and norepinephrine reuptake, antioxidant activity | [235] |
| Typical antipsychotic | Haloperidol | Wistar rats | Blocking of dopamine receptor type 2 | [236,237] |
| Essential vitamins and their synthetic derivates | Thiamine Benfotiamine Dibenzoylthiamine Vitamin E | BALB/C, CD1, C57BL/6J mice | Antioxidant activity, increased neuroplasticity, overexpression of BDNF, anti-inflammatory effects, downregulation of GSK3-β, increased glutathione content | [56,62,66,238,239,240] |
| Insulin receptor sensitizers | Rosiglitazone Pioglitazone | BALB/C, CD1, C57BL/6J mice | Decreased neuronal damage, anti-inflammatory effects, increased mitochondrial biogenesis | [187,241,242] |
| Treatment | Model | Effects on Aggression | Other Effects | References |
|---|---|---|---|---|
| Ascorbic acid Beta carotene Vitamin E N-acetyl cysteine | Isolation, or L-DOPA, male Swiss albino mice | ↓ | Increased levels of GSH, SOD, CAT in brain | [261] |
| Lithium chloride | Shock-induced, Sprague-Dawley rats; shock induced plus d-AMP or scopolamine, Walter Reed rats; isolation, AB mice; resident-intruder, TO mice | ↓ | — | [269] |
| Shock-induced, CD-1, C57BL/6J and FVB/N mice | ↓ | Increased brain norepinephrine turnover | [270] | |
| Thiamine Benfothiamine Dibenzoylthiamine | Ultrasound-induced, BALB/c mice | ↓ | Anxiolytic, anti-depressant, reduced inflammation and oxidative stress | [56,66,238,240,271] |
| Dicholine succinate | C57BL/6N mice; Western diet, C57BL/6 mice; chronic stress, CD-1 mice; defeat stress, C57BL/6J mice | ↓ (pilot data) | Anxiolytic, anti-depressant, prevention of Tlr4 upregulation in brain | [188,272,273] |
| Rosiglitazone | Chronic social defeat, C57BL/6J mice | Not assessed | Anxiolytic, anti-depressant | [274] |
| Chlorogenic acid extract from Prunus domestica or Beta vulgaris | Swiss albino mice, alone or with restraint stress | Not assessed | Anxiolytic, anti-depressant, reduced ROS production by immune cells in vitro, increased GSH level and decreased MDA in brain tissue | [275,276] |
| Ulva sp. extract | Wistar rats | Not assessed | Anti-depressant | [277] |
| Extract of blackberry chamomile, garlic, cloves, and elderberry | Resiquimod- or LPS-induced inflammation, CD-1 mice | Not assessed | Anxiolytic, anti-depressant. Reduced expression of SAA2, ACE2, CXCL1, CXCL10, Il-1β, Il-6 in spleen and liver. Normalized counts of neutrophiles, monocytes, eosinophiles. | [278] |
| Standardized herbal cocktail (see [63] for composition) | Ultrasound stress, BALB/c and C57BL/6 mice | ↓ (pilot data) | Anti-depressant. Decreased brain MDA and protein-carbonyl, decreased brain expression of IL-1β and IL-6 | [63] |
| Standardized herbal cocktail (see [62] for composition) | Ultrasound stress, BALB/c mice | ↓ | Anti-depressant. Decreased brain expression of Il-1β, Il-6, TNF, GSK-3β. Increased expression of Ki67, decreased brain MDA | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorlova, A.; Svirin, E.; Pavlov, D.; Cespuglio, R.; Proshin, A.; Schroeter, C.A.; Lesch, K.-P.; Strekalova, T. Understanding the Role of Oxidative Stress, Neuroinflammation and Abnormal Myelination in Excessive Aggression Associated with Depression: Recent Input from Mechanistic Studies. Int. J. Mol. Sci. 2023, 24, 915. https://doi.org/10.3390/ijms24020915
Gorlova A, Svirin E, Pavlov D, Cespuglio R, Proshin A, Schroeter CA, Lesch K-P, Strekalova T. Understanding the Role of Oxidative Stress, Neuroinflammation and Abnormal Myelination in Excessive Aggression Associated with Depression: Recent Input from Mechanistic Studies. International Journal of Molecular Sciences. 2023; 24(2):915. https://doi.org/10.3390/ijms24020915
Chicago/Turabian StyleGorlova, Anna, Evgeniy Svirin, Dmitrii Pavlov, Raymond Cespuglio, Andrey Proshin, Careen A. Schroeter, Klaus-Peter Lesch, and Tatyana Strekalova. 2023. "Understanding the Role of Oxidative Stress, Neuroinflammation and Abnormal Myelination in Excessive Aggression Associated with Depression: Recent Input from Mechanistic Studies" International Journal of Molecular Sciences 24, no. 2: 915. https://doi.org/10.3390/ijms24020915
APA StyleGorlova, A., Svirin, E., Pavlov, D., Cespuglio, R., Proshin, A., Schroeter, C. A., Lesch, K.-P., & Strekalova, T. (2023). Understanding the Role of Oxidative Stress, Neuroinflammation and Abnormal Myelination in Excessive Aggression Associated with Depression: Recent Input from Mechanistic Studies. International Journal of Molecular Sciences, 24(2), 915. https://doi.org/10.3390/ijms24020915






