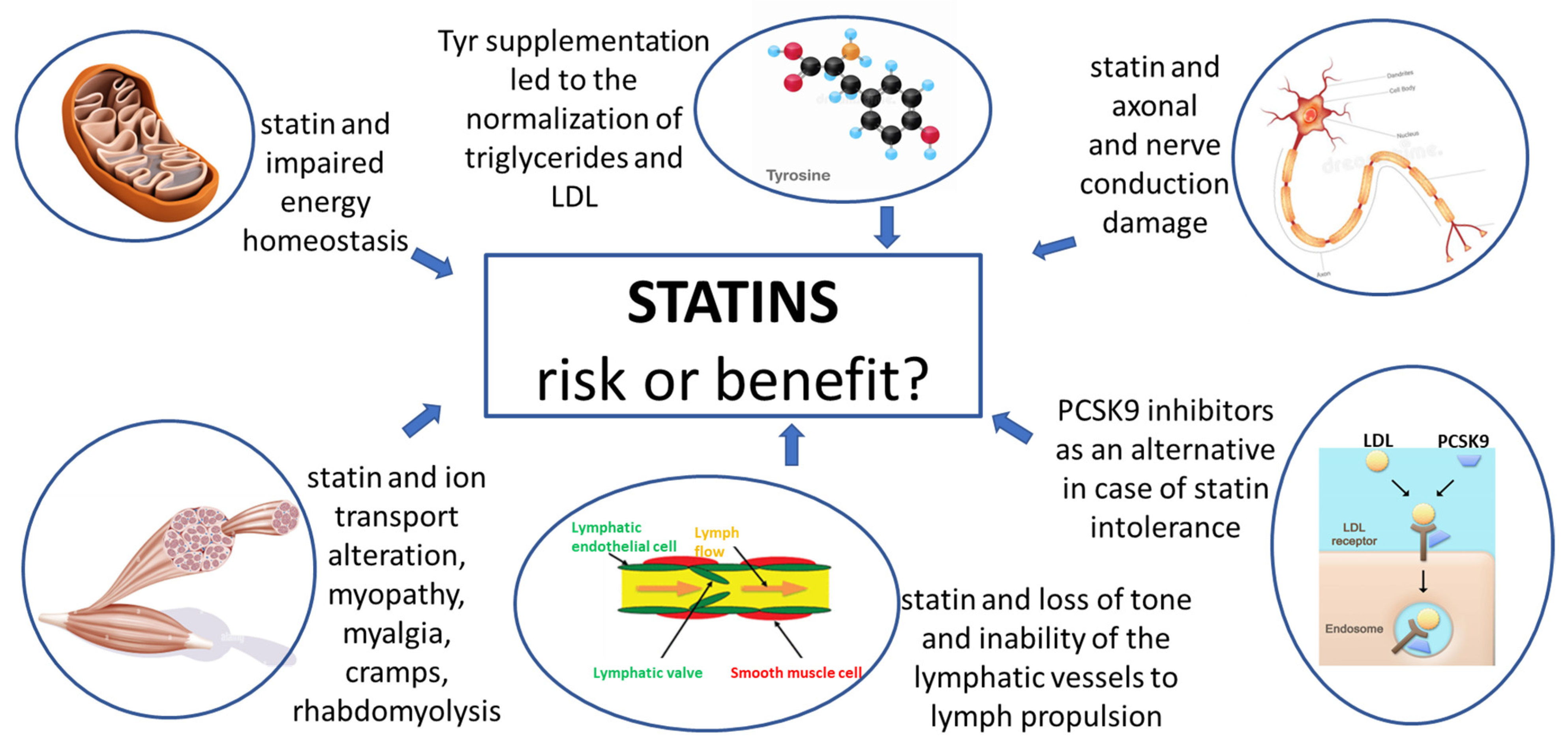
Pharmacotherapy of the Lipid-Lowering Drugs: Update on Efficacy and Risk
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity. Circ Res. 2019, 124, 328–350. [Google Scholar] [CrossRef]
- Staffa, J.A.; Chang, J.; Green, L. Cerivastatin and reports of fatal rhabdomyolysis. New Engl. J. Med. 2002, 346, 539–540. [Google Scholar] [CrossRef]
- Camerino, G.M.; Tarantino, N.; Canfora, I.; De Bellis, M.; Musumeci, O.; Pierno, S. Statin-Induced Myopathy: Translational Studies from Preclinical to Clinical Evidence. Int. J. Mol. Sci. 2021, 22, 2070. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Pierno, S.; Camerino, D.C. Electrical properties of diaphragm and EDL muscles during the life of dystrophic mice. Am. J. Physiol. Cell Physiol. 1997, 272, C333–C340. [Google Scholar] [CrossRef] [PubMed]
- Capogrosso, R.F.; Mantuano, P.; Uaesoontrachoon, K.; Cozzoli, A.; Giustino, A.; Dow, T.; Srinivassane, S.; Filipovic, M.; Bell, C.; Vandermeulen, J.; et al. Ryanodine channel complex stabilizer compound S48168/ARM210 as a disease modifier in dystrophin-deficient mdx mice: Proof-of-concept study and independent validation of efficacy. FASEB J. 2018, 32, 1025–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cozzoli, A.; Liantonio, A.; Conte, E.; Cannone, M.; Massari, A.M.; Giustino, A.; Scaramuzzi, A.; Pierno, S.; Mantuano, P.; Capogrosso, R.F.; et al. Angiotensin II modulates mouse skeletal muscle resting conductance to chloride and potassium ions and calcium homeostasis via the AT1 receptor and NADPH oxidase. Am. J. Physiol. Cell Physiol. 2014, 307, C634–C647. [Google Scholar] [CrossRef] [Green Version]
- Camerino, G.M.; Musumeci, O.; Conte, E.; Musaraj, K.; Fonzino, A.; Barca, E.; Marino, M.; Rodolico, C.; Tricarico, D.; Camerino, C.; et al. Risk of Myopathy in Patients in Therapy with Statins: Identification of Biological Markers in a Pilot Study. Front. Pharmacol. 2017, 8, 500. [Google Scholar] [CrossRef] [Green Version]
- Camerino, G.M.; De Bellis, M.; Conte, E.; Liantonio, A.; Musaraj, K.; Cannone, M.; Fonzino, A.; Giustino, A.; De Luca, A.; Romano, R.; et al. Statin-induced myotoxicity is exacerbated by aging: A biophysical and molecular biology study in rats treated with atorvastatin. Toxicol. Appl. Pharmacol. 2016, 306, 36–46. [Google Scholar] [CrossRef]
- Stroes, E.S.; Thompson, P.D.; Corsini, A.; Vladutiu, G.D.; Raal, F.J.; Ray, K.K.; Roden, M.; Stein, E.; Tokgözoglu, L.; Nordestgaard, B.G.; et al. Statin-associated muscle symptoms: Impact on statin therapy—European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 2015, 36, 1012–1022. [Google Scholar] [CrossRef] [Green Version]
- Nikolic, D.; Banach, M.; Chianetta, R.; Luzzu, L.M.; Stoian, A.P.; Diaconu, C.C.; Citarrella, R.; Montalto, G.; Rizzo, M. An overview of statin-induced myopathy and perspectives for the future. Expert Opin. Drug Saf. 2020, 19, 601–615. [Google Scholar] [CrossRef]
- Norata, G.D.; Tibolla, G.; Catapano, A.L. Targeting PCSK9 for hypercholesterolemia. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.; Sheth, S.; Jacoby, D. A Clinical Guide to Combination Lipid-Lowering Therapy. Curr. Atheroscler. Rep. 2018, 20, 19. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Baker, S.; Banach, M.; Borow, K.M.; Braun, L.T.; Bruckert, E.; Brunham, L.R.; Catapano, A.L.; Elam, M.B.; Mancini, G.B.J.; et al. Optimizing Cholesterol Treatment in Patients With Muscle Complaints. J. Am. Coll. Cardiol. 2017, 70, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Banach, M.; Sahebkar, A.; Corsini, A.; Sirtori, C.R. ETC-1002 (Bempedoic acid) for the management of hyper-lipidemia: From preclinical studies to phase 3 trials. Expert Opin. Pharmacother. 2019, 20, 791–803. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.; Morris, P.; Ballantyne, C.M.; Birtcher, K.K.; Covington, A.M.; DePalma, S.M.; Minissian, M.B.; Orringer, C.E.; Smith, S.C.; Waring, A.A.; et al. ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk. J. Am. Coll. Cardiol. 2022, 80, 1366–1418. [Google Scholar] [CrossRef]
- Basiak, M.; Kosowski, M.; Cyrnek, M.; Bułdak, Ł.; Maligłówka, M.; Machnik, G.; Okopień, B. Pleiotropic Effects of PCSK-9 Inhibitors. Int. J. Mol. Sci. 2021, 22, 3144. [Google Scholar] [CrossRef] [PubMed]
- Attardo, S.; Musumeci, O.; Velardo, D.; Toscano, A. Statins Neuromuscular Adverse Effects. Int. J. Mol. Sci. 2022, 23, 8364. [Google Scholar] [CrossRef]
- Brunham, L.R.; Baker, S.; Mammen, A.; Mancini, G.; Rosenson, R.S. Role of genetics in the prediction of statin-associated muscle symptoms and optimization of statin use and adherence. Cardiovasc. Res. 2018, 114, 1073–1081. [Google Scholar] [CrossRef]
- Mohassel, P.; Mammen, A.L. Anti-HMGCR Myopathy. J. Neuromuscul. Dis. 2018, 5, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Macchi, C.; Bonalume, V.; Greco, M.F.; Mozzo, M.; Melfi, V.; Sirtori, C.R.; Magnaghi, V.; Corsini, A.; Ruscica, M. Impact of Atorvastatin on Skeletal Muscle Mitochondrial Activity, Locomotion and Axonal Excitability—Evidence from ApoE-/- Mice. Int. J. Mol. Sci. 2022, 23, 5415. [Google Scholar] [CrossRef]
- Ruscica, M.; Ferri, N.; Banach, M.; Sirtori, C.R.; Corsini, A. Side effects of statins: From pathophysiology and epidemiology to diagnostic and therapeutic implications. Cardiovasc. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.; Roizes, S.; von der Weid, P.-Y. Off-Target Effect of Lovastatin Disrupts Dietary Lipid Uptake and Dissemination through Pro-Drug Inhibition of the Mesenteric Lymphatic Smooth Muscle Cell Contractile Apparatus. Int. J. Mol. Sci. 2021, 22, 11756. [Google Scholar] [CrossRef] [PubMed]
- Shipelin, V.A.; Trusov, N.V.; Apryatin, S.A.; Shumakova, A.A.; Balakina, A.S.; Riger, N.A.; Gmoshinski, I.V.; Nikityuk, D.B. Effects of Tyrosine and Tryptophan in Rats with Diet-Induced Obesity. Int. J. Mol. Sci. 2021, 22, 2429. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierno, S.; Musumeci, O. Pharmacotherapy of the Lipid-Lowering Drugs: Update on Efficacy and Risk. Int. J. Mol. Sci. 2023, 24, 996. https://doi.org/10.3390/ijms24020996
Pierno S, Musumeci O. Pharmacotherapy of the Lipid-Lowering Drugs: Update on Efficacy and Risk. International Journal of Molecular Sciences. 2023; 24(2):996. https://doi.org/10.3390/ijms24020996
Chicago/Turabian StylePierno, Sabata, and Olimpia Musumeci. 2023. "Pharmacotherapy of the Lipid-Lowering Drugs: Update on Efficacy and Risk" International Journal of Molecular Sciences 24, no. 2: 996. https://doi.org/10.3390/ijms24020996




