Fully Automated Production of [68Ga]GaFAPI-46 with Gallium-68 from Cyclotron Using Liquid Targets
Abstract
:1. Introduction
2. Results
2.1. [68Ga]GaFAPI-46 Synthesis
2.2. Validation of Analytical Methods
HPLC, TLC, and GC Methods Validation
2.3. Quality Control
2.4. [68Ga]GaFAPI-46 Stability
3. Discussion
4. Materials and Methods
4.1. Irradiation and Purification of [68Ga]GaCl3
4.2. Synthesis of [68Ga]GaFAPI-46 Using a Synthera® Extension Synthesizer
- The C18 plus short cartridge is preconditioned with ethanol (10 mL) followed by water (10 mL) prior to use.
- Next, 50 µg of FAPI-46 precursor, dissolved in 1 mL of 0.5 M HEPES, is added to the reaction vial.
- Purified gallium-68 (10 mL) is loaded into the SCX bound elute cartridge using a peristaltic pump to prevent cross-contamination of the tubing system.
- The loaded SCX cartridge is eluted with a 5 M NaCl (in 0.05 M HCl) solution in the reactor vial.
- Radiolabeling reaction takes 5 min at a 90 °C temperature.
- Reaction mixture is cooled down with water (5 mL) and passed through the C18 plus short cartridge, at 5 mL/min flow, to the waste container.
- C18 cartridge is then rinsed with water (10 mL) at a 5 mL/min flow.
- Finally, [68Ga]GaFAPI-46 is eluted from the C18 column with a solution of 2 mL water/EtOH (1:1) and filtered into the final product vial, which is infused with 500 mg of sodium ascorbate.
- After purification and synthesis, [68Ga]GaFAPI-46 is transferred to the Quality Control (QC) laboratory and all the components are measured, after which the decay-corrected RY is determined.
4.3. Radionuclidic Identity and Purity
4.3.1. HPGe Analysis
4.3.2. Half-Life Measurements
4.4. Radiochemical Purity and Identity
4.4.1. HPLC Analysis
4.4.2. TLC Analysis
4.5. Residual Solvents
4.5.1. Ethanol
4.5.2. HEPES
4.6. Stability of [68Ga]GaFAPI-46
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindner, T.; Loktev, A.; Giesel, F.; Kratochwil, C.; Altmann, A.; Haberkorn, U. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm. Chem. 2019, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Gao, J.; Zheng, Z.; Chen, Y.; Lv, S.; Zhao, Z.; Yu, C.; Yang, X.; Zhang, R. Fibroblast Activation Protein-α as a Target in the Bench-to-Bedside Diagnosis and Treatment of Tumors: A Narrative Review. Front. Oncol. 2021, 11, 648187. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marmé, F.; Jäger, D.; Mier, W.; et al. Development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef]
- Cankaya, A.; Balzer, M.; Amthauer, H.; Brenner, W.; Spreckelmeyer, S. Optimization of 177Lu-labelling of DOTA-TOC, PSMA-I&T and FAPI-46 for clinical application. EJNMMI Radiopharm. Chem. 2023, 8, 10. [Google Scholar] [PubMed]
- Eryilmaz, K.; Kilbas, B. Fully-automated synthesis of 177Lu labelled FAPI derivatives on the module modular lab-Eazy. EJNMMI Radiopharm. Chem. 2021, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Alfteimi, A.; Lützen, U.; Helm, A.; Jüptner, M.; Marx, M.; Zhao, Y.; Zuhayra, M. Automated synthesis of [68Ga]Ga-FAPI-46 without pre-purification of the generator eluate on three common synthesis modules and two generator types. EJNMMI Radiopharm. Chem. 2022, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Spreckelmeyer, S.; Balzer, M.; Poetzsch, S.; Brenner, W. Fully-automated production of [68Ga]Ga-FAPI-46 for clinical application. EJNMMI Radiopharm. Chem. 2020, 5, 31. [Google Scholar] [CrossRef]
- Notni, J.; Wester, H.J. Re-thinking the role of radiometal isotopes: Towards a future concept for theranostic radiopharmaceuticals. J. Label. Compd. Radiopharm. 2018, 61, 141–153. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency. Gallium-68 Cyclotron Production; IAEA-TECDOS-1863; IAEA: Vienna, Austria, 2019. [Google Scholar]
- Alves, F.; Alves, V.H.; Carmo, S.J.C.; Neves, A.C.B.; Silva, M.; Abrunhosa, A.J. Production of copper-64 and gallium-68 with a medical cyclotron using liquid targets. Mod. Phys. Lett. A 2017, 32, 1740013. [Google Scholar] [CrossRef]
- Council of Europe. Approximate PH of Solutions. In European Pharmacopoeia 10.0.; European Council: Brussels, Belgium, 2019. [Google Scholar]
- Gillings, N.; Todde, S.; Behe, M.; Decristoforo, C.; Elsinga, P.; Ferrari, V.; Hjelstuen, O.; Peitl, P.K.; Koziorowski, J.; Laverman, P.; et al. EANM guideline on the validation of analytical methods for radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2020, 5, 7. [Google Scholar] [CrossRef]
- Tremblay, S.; Beaudoin, J.-F.; Benesty, O.B.; Ait-Mohand, S.; Dumulon-Perreault, V.; Rousseau, É.; Turcotte, E.E.; Guerin, B. Ga-DOTATATE Prepared from Cyclotron Produced Gallium-68:An Integrated Solution from Cyclotron Vault to Safety Assessment and Diagnostic Efficacy in Neuroendocrine Cancer Patients. J. Nucl. Med. 2022, 64, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.H.; do Carmo, S.J.C.; Alves, F.; Abrunhosa, A.J. Automated Purification of Radiometals Produced by Liquid Targets. Instruments 2018, 2, 17. [Google Scholar] [CrossRef]
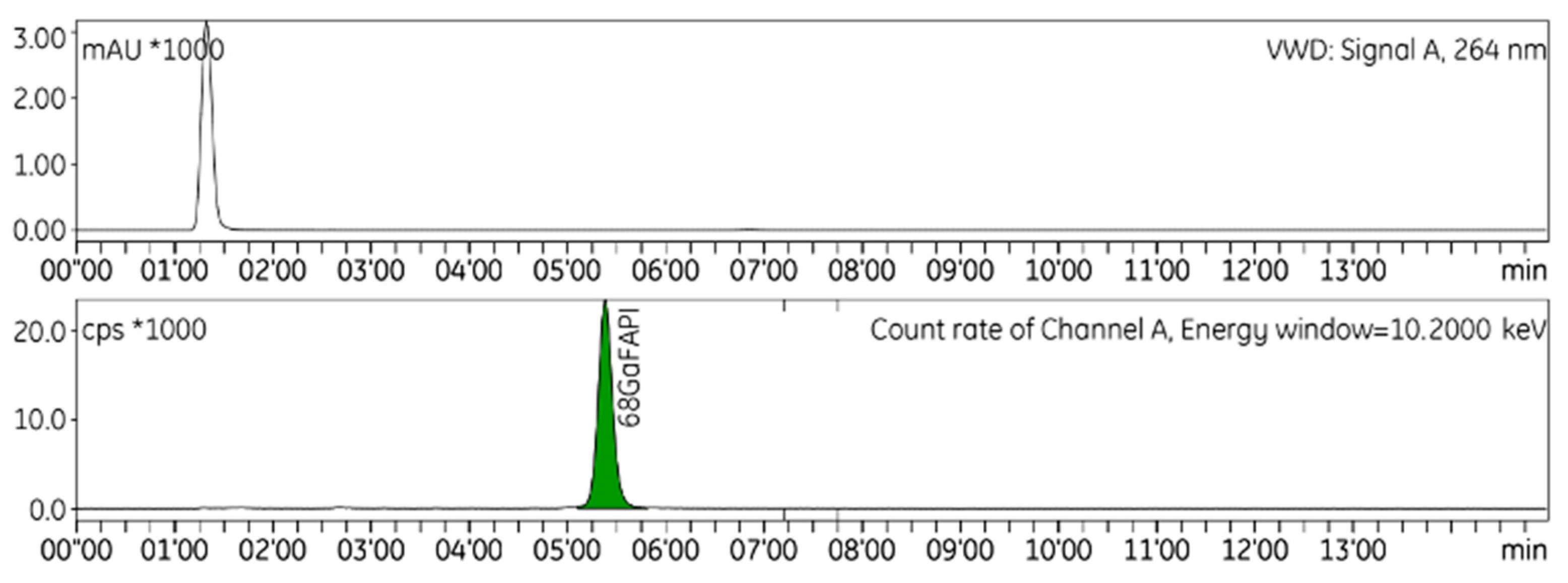



| Synthesis No | Starting Activity (GBq) | Amount of Peptide (µg/GBq) | Decay Corrected RCY (%) | Purity HPLC (%) | Purity TLC (%) |
|---|---|---|---|---|---|
| 1 | 4.68 | 10.67 | 90.20 | 99.61 | 99.86 |
| 2 | 4.27 | 11.70 | 91.00 | 98.18 | 99.94 |
| 3 | 3.96 | 12.62 | 90.04 | 99.04 | 99.13 |
| Mean ± SD | 4.31 ± 0.36 | 11.67 ± 0.97 | 90.53 ± 0.42 | 98.94 ± 0.72 | 99.64 ± 0.45 |
| Spreckelmeyer et al. [7], on MLPT | Spreckelmeyer et al. [7], on ML Eazy | Alfeimi et al. [6], on Trasis EasyOne | Alfeimi et al. [6], on Synthra | Alfeimi et al. [6], on Scintomics | |
|---|---|---|---|---|---|
| Reaction Temp. | 95 °C | 98 °C | 90 °C | 90 °C | 90 °C |
| Reaction time | 10 min | 10 min | 4 min | 4 min | 4 min |
| RCY (%) | 95.20 ± 1.40 | 89.70 ± 6.70 | 92.45 | 92.32 | 92.86 |
| RCP (%) HPLC | 99.70 | 99.40 | 99.70 | 99.80 | |
| RCP (%) TLC | 99.90 | - | - | - | |
| Validation Results Summary of the HPLC Method | ||||
|---|---|---|---|---|
| Test Parameter | Acceptance Criteria | Results | ||
| Repeatability | 6 repetitions of [68Ga]GaFAPI-46 | RSD ≤ 5% | 4.89 | Table S1a |
| 6 repetitions of [natGa]GaFAPI-46 | RSD ≤ 5% | 3.40 | Table S1b | |
| Specificity/Selectivity | Resolution between peaks: | |||
| [68Ga]GaFAPI-46 | 5.0 ≤ RT ≤ 6.0 | 5.37 | Figure S1c | |
| [natGa]GaFAPI-46 | 5 ≤ RT ≤ 6 | 5.28 | Figure S1b | |
| [68Ga]GaCl3 | 2 ≤ RT ≤ 2 | 1.33 | Figure S1a | |
| RRT ([natGa]GaFAPI-46/[68Ga]GaFAPI-46) | 0.9 ≤ RRT ≤ 1.1 | 0.98 | ||
| LOQ | S/N ratio ≥ 10 | ≤0.5 MBq/mL | 10.70 | Figure S3a |
| Linearity | MBq/mL (5 concentrations) | R2 ≥ 0.99 | 1.00 | Figure S4a |
| Range | Reported Value | 0.13–99.77 MBq/mL | ||
| Validation Results Summary of the TLC method | ||||
| Test Parameter | Acceptance criteria | Results | ||
| Repeatability | 6 repetitions of [natGa]GaFAPI-46 | RSD ≤ 0.2% | 0.12 | Table S2 |
| Specificity/Selectivity | Resolution between peaks: | |||
| [68Ga]GaFAPI-46 | R/F > 0.55 | 0.63 | Figure S2b | |
| [68Ga]GaCl3 | R/F < 0.15 | 0.12 | Figure S2a | |
| LOQ | S/N ratio ≥ 10 | (0.17 MBq/mL) | 23.1 | Figure S3b |
| Linearity | MBq/mL (5 concentrations) | R2 ≥ 0.99 | 1.00 | Figure S4b |
| Range | Reported Value | 0.15–47.00 MBq/mL | ||
| Validation Results Summary of the GC method | ||||
| Test Parameter | Acceptance criteria | Results | ||
| Repeatability | 6 repetitions of [68Ga]GaFAPI-46 | RSD ≤ 5% | 1.90 | Table S3 |
| LOQ | S/N ratio ≥ 10 | (50 mg/10 mL) | 247.70 | Figure S3c |
| Linearity | 50–2500 mg/10 mL (6 concentrations) | R2 ≥ 0.99 | 1.00 | Figure S4c |
| Range | Reported Value | 50–2500 mg/10 mL | ||
| Precision | 6 repetitions of EtOH 2500 mg/10 mL | RSD ≤ 5% | 3.88 | |
| Accuracy | Spiked conc. 1500 mg/10 mL | ≤10% | 6.83 | |
| Tests | Method | Specifications | Results (n = 3) |
|---|---|---|---|
| Appearance | Visual Inspection | Clear, colorless, or slightly yellow solution | Comply |
| pH | Potentiometric or strips | 4 to 8 | 6.5 |
| Identification | |||
| Radionuclidic Identification—Energy photons γ | Gamma-ray spectrometry | The principal gamma photons have energies of 0.511 MeV and 1.077 MeV, and a sum peak of 1.022 MeV may be observed; peaks due to gamma photons with energy of 1.883 MeV may be observed. | Comply |
| Half-life | Ionization Chamber | 61 min to 75 min | 67.6 |
| Chemical Purity | |||
| HEPES | TLC | ≤0.5 mg/10 mL | Comply |
| Radiochemical Purity | |||
| [68Ga]GaFAPI-46 | Radio-HPLC | ≥95% | 97.8 |
| Peak area of gallium-68 species RF < 0.2 | TLC (Radioactivity detector) | ≤3% | 1.3 |
| Radionuclidic Purity | |||
| Gallium-68 | Gamma-ray spectrometry | ≥98% | 99.8 |
| Gallium-66 and Gallium-67 1,2 | Gamma-ray spectrometry | ≤2% | 0.2 |
| Other gamma-ray-emitting impurities 1,3 | Gamma-ray spectrometry | ≤0.1% | 0.0 |
| Residual Solvents | |||
| Ethanol 4 | GC-FID | ≤2500 mg/10 mL | 732.1 |
| Biological Tests | |||
| Endotoxin analysis | Direct inoculation | No evidence of growth should be found | Comply |
| Column: | Avantor/ACE, ACE 3 C18, 3 µm, 150 × 3 mm S/N: A210625054 | ||
| Detector: | VWD 1260 Infinity II G7114A | ||
| Data acquisition software: | Software Gina X | ||
| Wavelength: | 264 nm | ||
| Scintillation: | Allow LOQ ≤ 0.05 MBq/mL | ||
| Column temperature: | Room temperature (not controlled) | ||
| Flow: | 0.6 mL/min | ||
| Injection volume: | 20 µL | ||
| Run time: | 15 min | ||
| Program: | Time (min) | % Mobile phase A | % Mobile phase B |
| 0.0 | 87 | 13 | |
| 15.0 | 87 | 13 | |
| Mobile Phase A: | Water/TFA = 1000/1 (v/v) | ||
| Mobile Phase B: | Acetonitrile/TFA = 1000/1 (v/v) | ||
| Diluent: | Water for injection | ||
| Detector: | Scintillation | |
| Data acquisition software: | TLC Control software, version 2.30 | |
| Detector | Scintillation: | Allow LOQ ≤ 0.5 MBq/mL |
| Others | Chromatographic paper: | Agilent iTLC-SG |
| Application volume: | 5 µL | |
| Elution length: | 80 mm (origin: 1.0 cm from the bottom end; elution front: 2.0 cm from the top end) | |
| Mobile Phase A: | Ammonium acetate 1.0 M/methanol = 1/1 (v/v) | |
| Diluent: | Water for injection | |
| Injector | |
| Mode | Split |
| Temperature | 250 °C |
| Split ratio | 15:1 |
| Gas | Helium |
| Liner | Cone liner with glass wool, 4.0 mm ID, PN#5183-4647. |
| Oven | |
| Equilibrium time | 0.00 min |
| Run time | 15.0 min |
| Detector | |
| Temperature | 260 °C |
| Mode | Constant Makeup |
| Makeup flow | 30 mL/min (He) |
| Hydrogen flow | 30 mL/min |
| Air flow | 300 mL/min |
| Column (HP-Fast Residual Solvent; PN#1095V-420E or equivalent) | |
| Mode | Constant flow |
| Flow | 3.0 mL/min |
| Length | 30 m |
| Internal diameter | 530 µm |
| Film Thickness | 1.0 µm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, A.I.; Alves, V.H.; Hrynchak, I.; Alves, F.; Abrunhosa, A.J. Fully Automated Production of [68Ga]GaFAPI-46 with Gallium-68 from Cyclotron Using Liquid Targets. Int. J. Mol. Sci. 2023, 24, 15101. https://doi.org/10.3390/ijms242015101
Fonseca AI, Alves VH, Hrynchak I, Alves F, Abrunhosa AJ. Fully Automated Production of [68Ga]GaFAPI-46 with Gallium-68 from Cyclotron Using Liquid Targets. International Journal of Molecular Sciences. 2023; 24(20):15101. https://doi.org/10.3390/ijms242015101
Chicago/Turabian StyleFonseca, Alexandra I., Vítor H. Alves, Ivanna Hrynchak, Francisco Alves, and Antero J. Abrunhosa. 2023. "Fully Automated Production of [68Ga]GaFAPI-46 with Gallium-68 from Cyclotron Using Liquid Targets" International Journal of Molecular Sciences 24, no. 20: 15101. https://doi.org/10.3390/ijms242015101
APA StyleFonseca, A. I., Alves, V. H., Hrynchak, I., Alves, F., & Abrunhosa, A. J. (2023). Fully Automated Production of [68Ga]GaFAPI-46 with Gallium-68 from Cyclotron Using Liquid Targets. International Journal of Molecular Sciences, 24(20), 15101. https://doi.org/10.3390/ijms242015101










