Identifying MicroRNAs Suitable for Detection of Breast Cancer: A Systematic Review of Discovery Phases Studies on MicroRNA Expression Profiles
Abstract
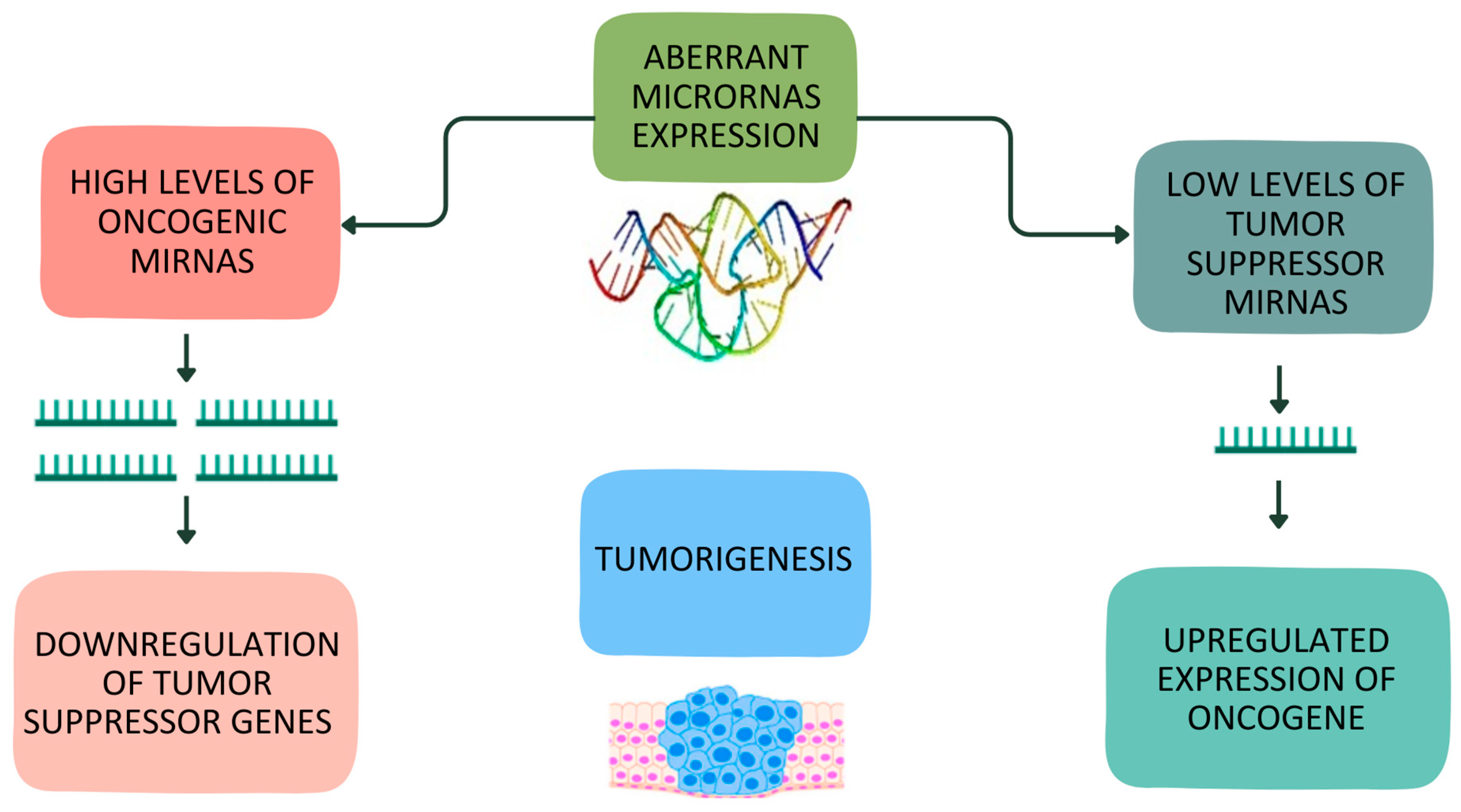
:1. Introduction
2. Materials and Methods
2.1. Publication Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
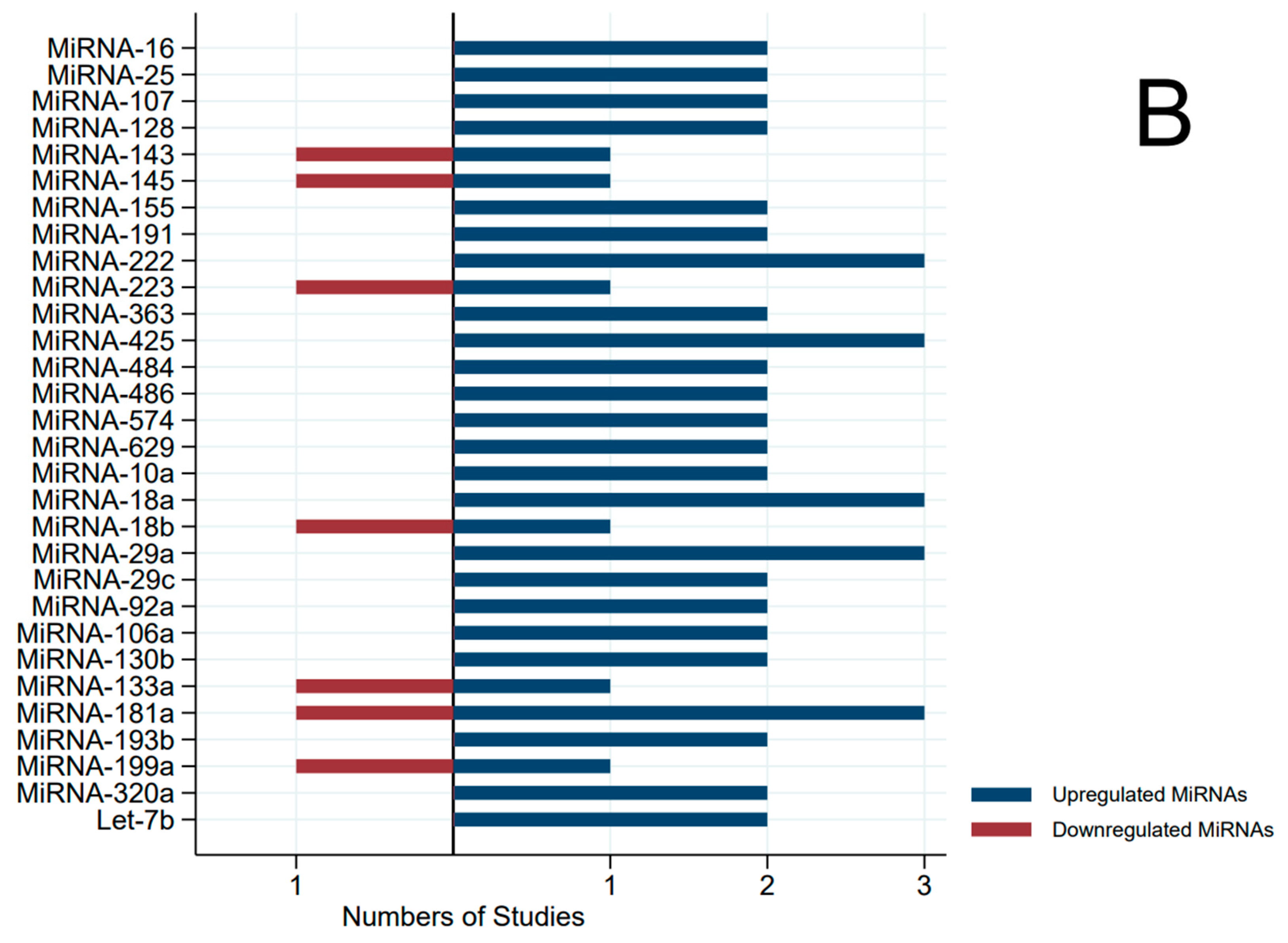
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loh, H.Y.; Norman, B.P.; Lai, K.S.; Rahman, N.M.A.N.A.; Alitheen, N.B.M.; Osman, M.A. The Regulatory Role of MicroRNAs in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 4940. [Google Scholar] [CrossRef] [PubMed]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in control of gene expression: An overview of nuclear functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef]
- Reddy, K.B. MicroRNA (miRNA) in cancer. Cancer Cell Int. 2015, 15, 38. [Google Scholar] [CrossRef]
- Melo, S.A.; Esteller, M. Dysregulation of microRNAs in cancer: Playing with fire. FEBS Lett. 2011, 585, 2087–2099, Erratum in FEBS Lett. 2021, 595, 2068. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Luo, Y.P. MicroRNAs in breast cancer: Oncogene and tumor suppressors with clinical potential. J. Zhejiang Univ. Sci. B 2015, 16, 18–31. [Google Scholar] [CrossRef]
- Strati, A.; Markou, A.; Kyriakopoulou, E.; Lianidou, E. Detection and Molecular Characterization of Circulating Tumour Cells: Challenges for the Clinical Setting. Cancers 2023, 15, 2185. [Google Scholar] [CrossRef] [PubMed]
- Cuk, K.; Obernosterer, G.; Leuschner, P.J.; Alenius, M.; Martinez, J. Post-transcriptional regulation of microRNA expression. RNA 2006, 12, 1161–1167. [Google Scholar]
- Hamam, R.; Hamam, D.; Alsaleh, K.A.; Kassem, M.; Zaher, W.; Alfayez, M.; Aldahmash, A.; Alajez, N.M. Circulating microRNAs in breast cancer; novel diagnostic and prognostic biomarkers. Cell Death Dis. 2017, 8, e3045. [Google Scholar] [CrossRef]
- Zheng, Y. Study Design Considerations for Cancer Biomarker Discoveries. J. Appl. Lab. Med. 2018, 3, 282–289. [Google Scholar] [CrossRef]
- Qin, L.-X.; Levine, D.A. Study design and data analysis considerations for the discovery of prognostic molecular biomarkers: A case study of progression free survival in advanced serous ovarian cancer. BMC Med. Genom. 2016, 9, 27. [Google Scholar] [CrossRef]
- Diamandis, E.P. Cancer biomarkers: Can we turn recent failures into success? J. Natl. Cancer Inst. 2010, 102, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Pepe, M.S.; Li, C.I.; Feng, Z. Improving the quality of biomarker discovery research: The right samples and enough of them. Cancer Epidemiol. Biomark. Prev. 2015, 24, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Padroni, L.; De Marco, L.; Dansero, L.; Fiano, V.; Milani, L.; Vasapolli, P.; Manfredi, L.; Caini, S.; Agnoli, C.; Ricceri, F.; et al. An Epidemiological Systematic Review with Meta-Analysis on Biomarker Role of Circulating MicroRNAs in Breast Cancer Incidence. Int. J. Mol. Sci. 2023, 24, 3910. [Google Scholar] [CrossRef]
- Bahramy, A.; Zafari, N.; Rajabi, F.; Aghakhani, A.; Jayedi, A.; Khaboushan, A.S.; Zolbin, M.M.; Yekaninejad, M.S. Prognostic and diagnostic values of non-coding RNAs as biomarkers for breast cancer: An umbrella review and pan-cancer analysis. Front. Mol. Biosci. 2023, 10, 1096524. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.N.; Nguyen, T.T.N.; Nguyen, T.T.M.; Nguyen, L.H.M.; Huynh, L.H.; Phan, H.N.; Nguyen, H.T. Panels of circulating microRNAs as potential diagnostic biomarkers for breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2022, 196, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sehovic, E.; Urru, S.; Chiorino, G.; Doebler, P. Meta-analysis of diagnostic cell-free circulating microRNAs for breast cancer detection. BMC Cancer 2022, 22, 634. [Google Scholar] [CrossRef] [PubMed]
- Dabi, Y.; Bendifallah, S.; Suisse, S.; Haury, J.; Touboul, C.; Puchar, A.; Favier, A.; Daraï, E. Overview of non-coding RNAs in breast cancers. Transl. Oncol. 2022, 25, 101512. [Google Scholar] [CrossRef] [PubMed]
- McGrath, T.A.; Alabousi, M.; Skidmore, B.; Korevaar, D.A.; Bossuyt, P.M.M.; Moher, D.; Thombs, B.; McInnes, M.D.F. Recommendations for reporting of systematic reviews and meta-analyses of diagnostic test accuracy; a systematic review. Syst. Rev. 2017, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Schrauder, M.G.; Strick, R.; Schulz-Wendtland, R.; Strissel, P.L.; Kahmann, L.; Loehberg, C.R.; Lux, M.P.; Jud, S.M.; Hartmann, A.; Hein, A.; et al. Circulating micro-RNAs as potential blood-based markers for early stage breast cancer detection. PLoS ONE 2012, 7, e29770. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, C.; Lu, Z.; Guo, L.; Ge, Q. Analysis of serum genome-wide microRNAs for breast cancer detection. Clin. Chim. Acta 2012, 413, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Liaw, C.S.; Ji, S.M.; Tan, H.H.; Wong, C.Y.; Thike, A.A.; Tan, P.H.; Ho, G.H.; Lee, A.S. Identification of circulating microRNA signatures for breast cancer detection. Clin. Cancer Res. 2013, 19, 4477–4487. [Google Scholar] [CrossRef]
- Cuk, K.; Zucknick, M.; Heil, J.; Madhavan, D.; Schott, S.; Turchinovich, A.; Arlt, D.; Rath, M.; Sohn, C.; Benner, A.; et al. Circulating microRNAs in plasma as early detection markers for breast cancer. Int. J. Cancer 2013, 132, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, A.C.; Xu, Z.; Weinberg, C.R.; Getts, R.C.; Wade, P.A.; DeRoo, L.A.; Sandler, D.P.; Taylor, J.A. Serum microRNA expression as an early marker for breast cancer risk in prospectively collected samples from the Sister Study cohort. Breast Cancer Res. 2013, 15, R42. [Google Scholar] [CrossRef]
- Ng, E.K.; Li, R.; Shin, V.Y.; Jin, H.C.; Leung, C.P.; Ma, E.S.; Pang, R.; Chua, D.; Chu, K.M.; Law, W.L.; et al. Circulating microRNAs as specific biomarkers for breast cancer detection. PLoS ONE 2013, 8, e53141. [Google Scholar] [CrossRef]
- Kodahl, A.R.; Lyng, M.B.; Binder, H.; Cold, S.; Gravgaard, K.; Knoop, A.S.; Ditzel, H.J. Novel circulating microRNA signature as a potential non-invasive multi-marker test in ER-positive early-stage breast cancer; a case control study. Mol. Oncol. 2014, 8, 874–883. [Google Scholar] [CrossRef]
- McDermott, A.M.; Miller, N.; Wall, D.; Martyn, L.M.; Ball, G.; Sweeney, K.J.; Kerin, M.J. Identification and validation of oncologic miRNA biomarkers for luminal A-like breast cancer. PLoS ONE 2014, 9, e87032. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Q.; Schrauder, M.; Yan, L.; Wang, D.; Medico, L.; Guo, Y.; Yao, S.; Zhu, Q.; Liu, B.; et al. Circulating miR-148b and miR-133a as biomarkers for breast cancer detection. Oncotarget 2014, 5, 5284–5294. [Google Scholar] [CrossRef]
- Zearo, S.; Kim, E.; Zhu, Y.; Zhao, J.T.; Sidhu, S.B.; Robinson, B.G.; Soon, P.S. MicroRNA-484 is more highly expressed in serum of early breast cancer patients compared to healthy volunteers. BMC Cancer 2014, 14, 200. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Y.; Jin, X.; Wang, Z.; Wu, Y.; Zhao, D.; Chen, G.; Li, D.; Wang, X.; Cao, H.; et al. A circulating miRNA signature as a diagnostic biomarker for non-invasive early detection of breast cancer. Breast Cancer Res. Treat. 2015, 154, 423–434. [Google Scholar] [CrossRef]
- Ferracin, M.; Lupini, L.; Salamon, I.; Saccenti, E.; Zanzi, M.V.; Rocchi, A.; Da Ros, L.; Zagatti, B.; Musa, G.; Bassi, C.; et al. Absolute quantification of cell-free microRNAs in cancer patients. Oncotarget 2015, 6, 14545–14555. [Google Scholar] [CrossRef] [PubMed]
- Shin, V.Y.; Siu, J.M.; Cheuk, I.; Ng, E.K.; Kwong, A. Circulating cell-free miRNAs as biomarker for triple-negative breast cancer. Br. J. Cancer 2015, 112, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Hamam, R.; Ali, A.M.; Alsaleh, K.A.; Kassem, M.; Alfayez, M.; Aldahmash, A.; Alajez, N.M. microRNA expression profiling on individual breast cancer patients identifies novel panel of circulating microRNA for early detection. Sci. Rep. 2016, 6, 25997. [Google Scholar] [CrossRef]
- Jusoh, A.R.; Mohan, S.V.; Ping, T.L.; Bin, T.A.; Din, T.; Haron, J.; Romli, R.C.; Jaafar, H.; Nafi, S.N.; Salwani, T.I.; et al. Plasma Circulating Mirnas Profiling for Identification of Potential Breast Cancer Early Detection Biomarkers. Asian Pac. J. Cancer Prev. 2021, 22, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Záveský, L.; Jandáková, E.; Weinberger, V.; Minář, L.; Hanzíková, V.; Dušková, D.; Faridová, A.; Turyna, R.; Slanař, O.; Hořínek, A.; et al. Small non-coding RNA profiling in breast cancer: Plasma U6 snRNA, miR-451a and miR-548b-5p as novel diagnostic and prognostic biomarkers. Mol. Biol. Rep. 2022, 49, 1955–1971. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhu, Y.; Wang, H.; Khadka, V.S.; Hu, L.; Ai, J.; Dou, Y.; Li, Y.; Dai, S.; Mason, C.E.; et al. Ratio-based method to identify true biomarkers by normalizing circulating ncRNA sequencing and quantitative PCR Data. Anal. Chem. 2019, 91, 6746–6753. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gallo, V.; Egger, M.; McCormack, V.; Farmer, P.B.; Ioannidis, J.P.; Kirsch-Volders, M.; Matullo, G.; Phillips, D.H.; Schoket, B.; Stromberg, U.; et al. STrengthening the Reporting of OBservational studies in Epidemiology—Molecular Epidemiology STROBE-ME: An extension of the STROBE statement. J. Clin. Epidemiol. 2011, 64, 1350–1363. [Google Scholar] [CrossRef]

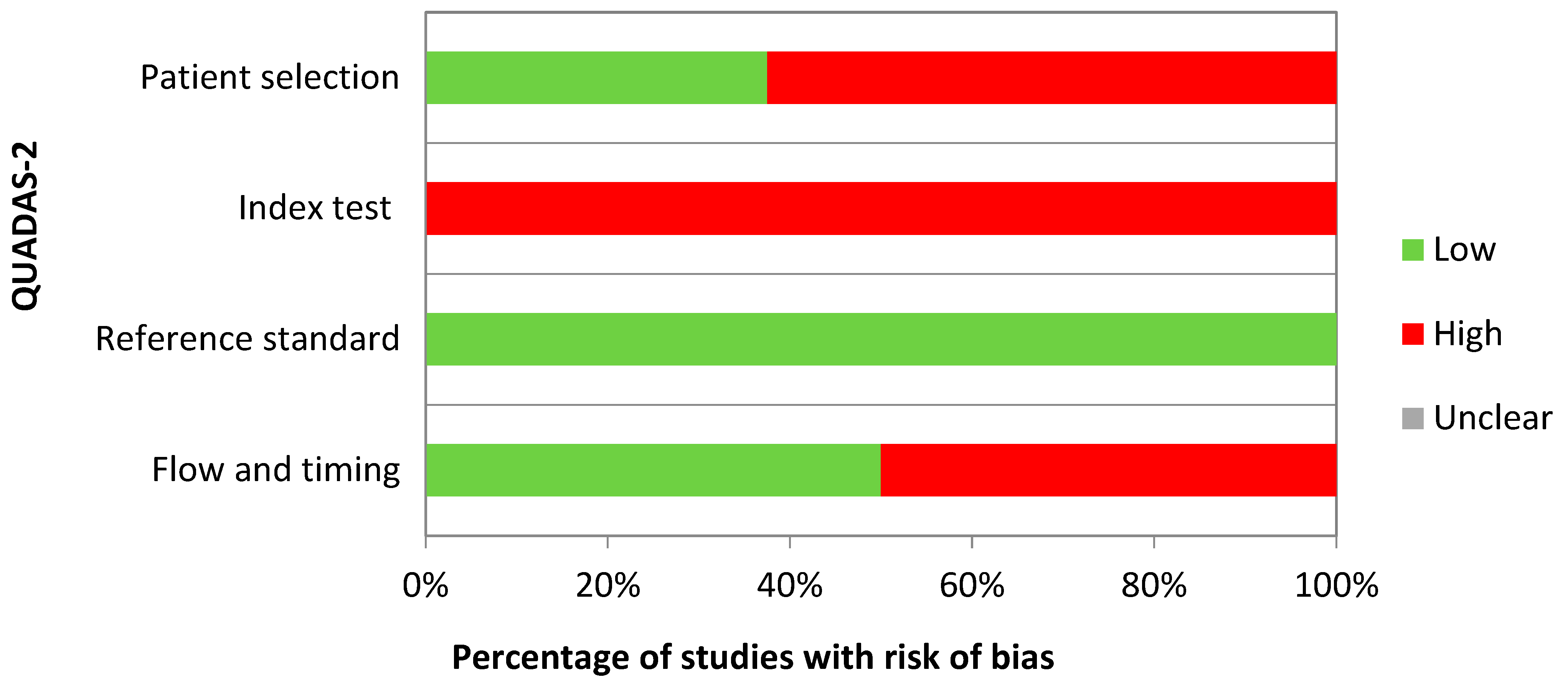
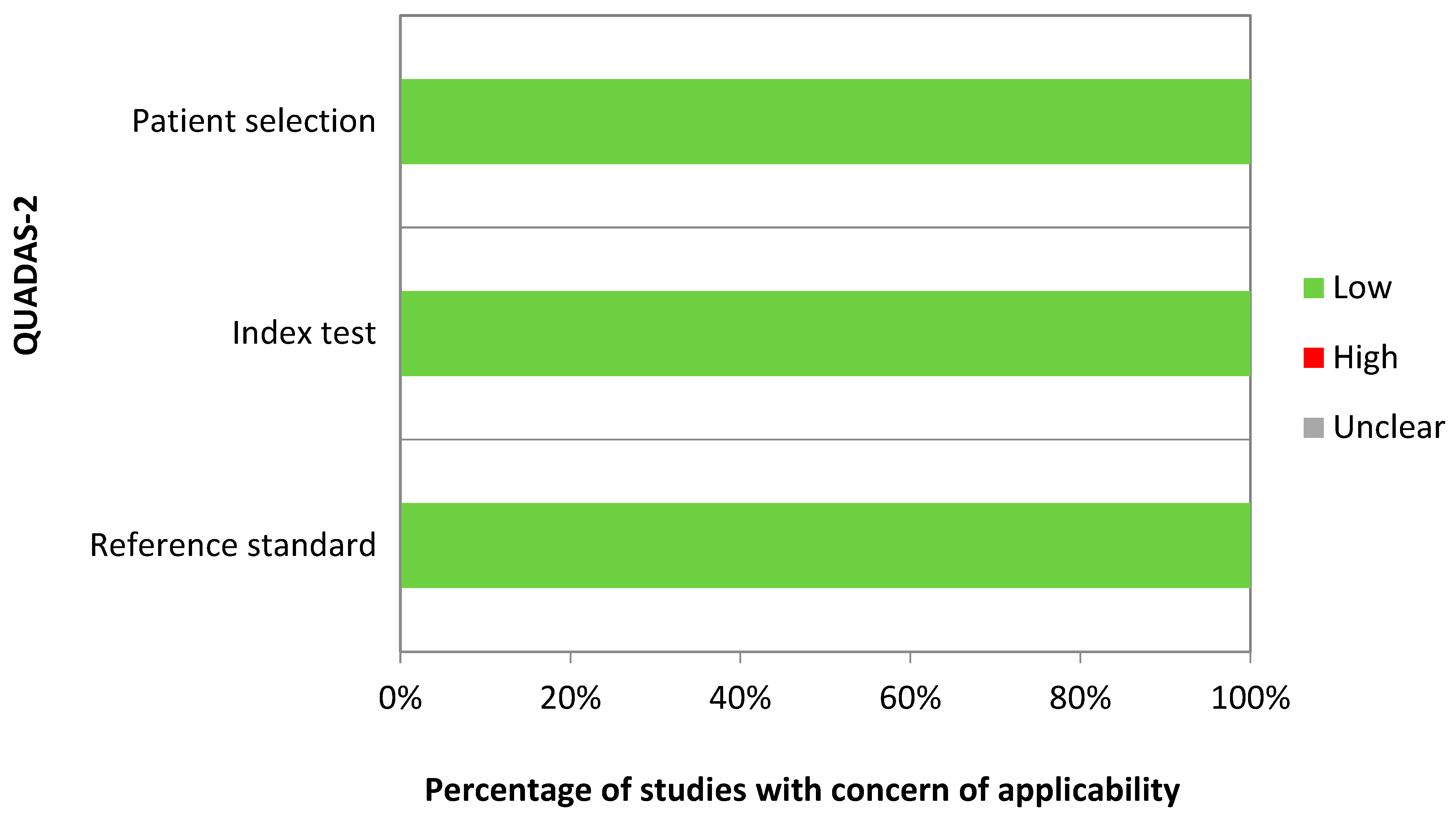

| First Author, Year | Country | Specimen Source | Lab Technique | Case–Control Size | QUADAS-2 Domains with Risk of Bias | Applied Multiple Testing Correction |
|---|---|---|---|---|---|---|
| Schrauder MG, 2012 [20] | Germany | Blood | Geniom Biochip miRNA homo sapiens | 48/57 | Index test | Benjamini–Hochberg |
| Wu Q, 2012 [21] | China | Serum | Life Technologies SOLiD™ sequencing base miRNA expression profiling | 13/10 | Patient selection, Index test | Not applied |
| Chan M, 2013 [22] | Singapore | Serum | Agilent Human miRNA microarray | 32/22 | Patient selection, Index test, Flow and timing | Benjamini–Hochberg |
| Cuk K, 2013 [23] | Germany | Plasma | TLDA human MicroRNA Cards A v2.1 and B v2.0 | 10/10 | Patient selection, Index test | Benjamini–Hochberg * |
| Ng E K, 2013 [25] | USA | Plasma | TLDA human MicroRNA Cards A v2.1 and B v2.0 | 5/5 | Patient selection, Index test | Not applied |
| Godfrey AC, 2013 [24] | USA | Serum | Affimetrix GeneChip miRNA 2.0 array | 205/205 | Index test | Not applied |
| Kodahl AR, 2014 [26] | Denmark | Serum | Exiqon microRNA panel (miRCURY) | 48/24 | Index test | Bonferroni * |
| McDermott AM, 2014 [27] | Ireland | Blood | TLDA human MicroRNA Cards A v2.1 and B v2.0 | 10/10 | Index test | Not applied |
| Shen J, 2014 [28] | USA | Plasma | Exiqon microRNA panel (miRCURY) | 52/35 | Index test, Flow and timing | Benjamini–Hochberg |
| Zearo S, 2014 [29] | Australia | Serum | TLDA human MicroRNA Cards A and B v3.0 | 39/10 | Patient selection, Index test, Flow and timing | Bonferroni |
| Ferracin M, 2015 [31] | Italy | Plasma | Agilent Human miRNA microarray | 18/18 | Patient selection, Index test, Flow and timing | Not applied |
| Shin VY, 2015 [32] | China | Plasma | Exiqon microRNA panel (miRCURY) | 5/5 | Patient selection, Index test, Flow and timing | Not applied |
| Zhang L, 2015 [30] | China | Serum | Serum-direct multiplex qRT-PCR (SdM-qRT-PCR) | 25/20 | Patient selection, Index test, Flow and timing | Bonferroni, Benjamini–Hochberg |
| Hamam R, 2016 [33] | Saudi Arabia | Blood | Agilent Human miRNA microarray | 23/9 | Patient selection, Index test, Flow and timing | Benjamini–Hochberg |
| Jusoh A, 2021 [34] | Malaysia | Plasma | Qiagen miScript miRNA PCR Array | 8/9 | Index test, Flow and timing | Not applied |
| Záveský L, 2022 [35] | Czech Republic | Plasma | TLDA human MicroRNA Cards A v2.1 and B v2.0 | 7/7 | Patient selection, Index test | Benjamini–Hochberg |
| MIR | First Author, Year | Specimen Source | Direction | AUC | p-Value |
|---|---|---|---|---|---|
| 1 | Chan M, 2013 [22] | Serum | up | <0.001 | |
| 7 | Chan M, 2013 [22] | Serum | up | <0.001 | |
| 16 | Chan M, 2013 [22] | Serum | up | <0.001 | |
| Ng E K, 2013 [25] | Plasma | up | |||
| Shin VY, 2015 [32] | Plasma | down | <0.05 | ||
| Zhang L, 2015 [30] | Serum | up | 0.001 | ||
| Záveský L, 2022 [35] | Plasma | down | 0.038 | ||
| 17 | Chan M, 2013 [22] | Serum | up | 0.001 | |
| Záveský L, 2022 [35] | Plasma | down | 0.017 | ||
| 21 | Ng E K, 2013 [25] | Plasma | up | ||
| Ferracin M, 2015 [31] | Plasma | up | |||
| Shin VY, 2015 [32] | Plasma | down | <0.05 | ||
| 22 | Shen J, 2014 [28] | Plasma | up | 0.85 | <0.001 |
| Jusoh A, 2021 [34] | Plasma | up | 0.83 | 0.020 | |
| 24 | Schrauder MG, 2012 [20] | Blood | down | 0.65 | 0.023 |
| Wu Q, 2012 [21] | Serum | up | |||
| 25 | Wu Q, 2012 [21] | Serum | up | ||
| Chan M, 2013 [22] | Serum | up | <0.001 | ||
| Ng E K, 2013 [25] | Plasma | up | |||
| 28 | Chan M, 2013 [22] | Serum | down | 0.005 | |
| Shen J, 2014 [28] | Plasma | up | 0.85 | <0.001 | |
| 93 | Chan M, 2013 [22] | Serum | up | <0.001 | |
| 95 | Chan M, 2013 [22] | Serum | up | 0.023 | |
| 96 | Chan M, 2013 [22] | Serum | up | 0.008 | |
| 100 | Zhang L, 2015 [30] | Serum | up | 0.79 | 0.003 |
| 101 | Zhang L, 2015 [30] | Serum | up | 0.024 | |
| 103 | Wu Q, 2012 [21] | Serum | up | ||
| 107 | Schrauder MG, 2012 [20] | Blood | down | 0.68 | 0.041 |
| Chan M, 2013 [22] | Serum | up | 0.013 | ||
| Kodahl AR, 2014 [26] | Serum | up | 0.006 | ||
| Shen J, 2014 [28] | Plasma | up | 0.87 | <0.001 | |
| 126 | Ng E K, 2013 [25] | Plasma | down | ||
| Shen J, 2014 [28] | Plasma | up | 0.77 | <0.001 | |
| Zearo S, 2014 [29] | Serum | up | <0.001 | ||
| 127 | Cuk K, 2013 [23] | Plasma | up | 0.459 | |
| Shen J, 2014 [28] | Plasma | up | 0.75 | <0.001 | |
| 128 | Chan M, 2013 [22] | Serum | up | 0.010 | |
| Zhang L, 2015 [30] | Serum | up | 0.039 | ||
| 134 | Chan M, 2013 [22] | Serum | up | 0.044 | |
| Hamam R, 2016 [33] | Blood | up | 0.042 | ||
| 136 | Shen J, 2014 [28] | Plasma | up | 0.87 | <0.001 |
| 139 | Cuk K, 2013 [23] | Plasma | down | 0.320 | |
| Kodahl AR, 2014 [26] | Serum | down | 0.623 | ||
| Shen J, 2014 [28] | Plasma | up | 0.79 | <0.001 | |
| 140 | Zearo S, 2014 [29] | Serum | up | <0.001 | |
| 141 | Zhang L, 2015 [30] | Serum | up | 0.89 | 0.027 |
| 142 | Chan M, 2013 [22] | Serum | down | 0.001 | |
| Shen J, 2014 [28] | Plasma | up | 0.82 | <0.001 | |
| 143 | Chan M, 2013 [22] | Serum | up | <0.001 | |
| Kodahl AR, 2014 [26] | Serum | down | 0.073 | ||
| Shin VY, 2015 [32] | Plasma | down | <0.05 | ||
| 144 | Chan M, 2013 [22] | Serum | up | <0.001 | |
| Shen J, 2014 [28] | Plasma | down | 0.94 | <0.001 | |
| 145 | Chan M, 2013 [22] | Serum | up | 0.036 | |
| Ng E K, 2013 [25] | Plasma | down | |||
| Kodahl AR, 2014 [26] | Serum | down | <0.001 | ||
| Jusoh A, 2021 [34] | Plasma | up | 0.82 | 0.040 | |
| 149 | Godfrey AC, 2013 [24] | Serum | up | 0.030 | |
| 150 | Ng E K, 2013 [25] | Plasma | up | ||
| Hamam R, 2016 [33] | Blood | up | 0.033 | ||
| 151 | Godfrey AC, 2013 [24] | Serum | up | 0.030 | |
| Shen J, 2014 [28] | Plasma | up | 0.88 | <0.001 | |
| 152 | Shen J, 2014 [28] | Plasma | up | 0.75 | 0.002 |
| 154 | Ng E K, 2013 [25] | Plasma | up | ||
| 155 | Zearo S, 2014 [29] | Serum | up | 0.008 | |
| Zhang L, 2015 [30] | Serum | up | 0.017 | ||
| 182 | Schrauder MG, 2012 [20] | Blood | down | 0.71 | 0.008 |
| Chan M, 2013 [22] | Serum | up | 0.009 | ||
| 183 | Zhang L, 2015 [30] | Serum | up | 0.79 | 0.003 |
| 184 | Cuk K, 2013 [23] | Plasma | up | 0.332 | |
| 185 | Chan M, 2013 [22] | Serum | up | <0.001 | |
| Shin VY, 2015 [32] | Plasma | down | <0.05 | ||
| 186 | Ng E K, 2013 [25] | Plasma | up | ||
| Zearo S, 2014 [29] | Serum | up | <0.001 | ||
| 188 | Hamam R, 2016 [33] | Blood | up | 0.004 | |
| 190 | Cuk K, 2013 [23] | Plasma | up | 0.459 | |
| 191 | Ng E K, 2013 [25] | Plasma | up | ||
| Zearo S, 2014 [29] | Serum | up | <0.001 | ||
| Zhang L, 2015 [30] | Serum | up | 0.018 | ||
| 192 | Wu Q, 2012 [21] | Serum | up | ||
| 194 | Wu Q, 2012 [21] | Serum | up | ||
| Shen J, 2014 [28] | Plasma | down | 0.81 | 0.002 | |
| 195 | Chan M, 2013 [22] | Serum | up | 0.007 | |
| 202 | Schrauder MG, 2012 [20] | Blood | up | 0.72 | 0.020 |
| Zhang L, 2015 [30] | Serum | down | 0.005 | ||
| 205 | Chan M, 2013 [22] | Serum | up | 0.011 | |
| 206 | Cuk K, 2013 [23] | Plasma | down | 0.320 | |
| 210 | Chan M, 2013 [22] | Serum | up | 0.044 | |
| Ng E K, 2013 [25] | Plasma | up | |||
| 214 | Chan M, 2013 [22] | Serum | up | <0.001 | |
| Záveský L, 2022 [35] | Plasma | up | 0.017 | ||
| 221 | Shen J, 2014 [28] | Plasma | up | 0.84 | <0.001 |
| Shin VY, 2015 [32] | Plasma | down | <0.05 | ||
| 222 | Wu Q, 2012 [21] | Serum | up | ||
| Godfrey AC, 2013 [24] | Serum | up | 0.020 | ||
| Zearo S, 2014 [29] | Serum | up | <0.001 | ||
| 223 | Wu Q, 2012 [21] | Serum | up | ||
| Chan M, 2013 [22] | Serum | down | <0.001 | ||
| 296 | Chan M, 2013 [22] | Serum | up | <0.001 | |
| 320 | Ng E K, 2013 [25] | Plasma | down | ||
| Zearo S, 2014 [29] | Serum | up | <0.001 | ||
| 324 | Ng E K, 2013 [25] | Plasma | down | ||
| Zhang L, 2015 [30] | Serum | up | 0.88 | <0.001 | |
| 326 | Shen J, 2014 [28] | Plasma | up | 0.88 | <0.001 |
| 328 | Ng E K, 2013 [25] | Plasma | up | ||
| Shen J, 2014 [28] | Plasma | up | 0.80 | <0.001 | |
| 330 | Záveský L, 2022 [35] | Plasma | up | 0.017 | |
| 331 | Shen J, 2014 [28] | Plasma | up | 0.71 | 0.006 |
| 335 | Schrauder MG, 2012 [20] | Blood | up | 0.74 | 0.040 |
| Chan M, 2013 [22] | Serum | up | 0.009 | ||
| Shen J, 2014 [28] | Plasma | up | 0.73 | 0.006 | |
| 338 | Chan M, 2013 [22] | Serum | down | <0.001 | |
| 339 | Chan M, 2013 [22] | Serum | down | 0.021 | |
| Shen J, 2014 [28] | Plasma | up | 0.76 | <0.001 | |
| 342 | Zearo S, 2014 [29] | Serum | up | <0.001 | |
| Shin VY, 2015 [32] | Plasma | up | <0.05 | ||
| 363 | Chan M, 2013 [22] | Serum | up | 0.003 | |
| Godfrey AC, 2013 [24] | Serum | up | 0.030 | ||
| Záveský L, 2022 [35] | Plasma | down | 0.011 | ||
| 365 | Kodahl AR, 2014 [26] | Serum | down | 0.006 | |
| 374 | Záveský L, 2022 [35] | Plasma | down | 0.022 | |
| 375 | Shen J, 2014 [28] | Plasma | down | 0.74 | 0.003 |
| 378 | Chan M, 2013 [22] | Serum | up | 0.013 | |
| 382 | Shen J, 2014 [28] | Plasma | up | 0.72 | <0.001 |
| 409 | Cuk K, 2013 [23] | Plasma | up | 0.332 | |
| Shen J, 2014 [28] | Plasma | up | 0.78 | <0.001 | |
| 421 | Chan M, 2013 [22] | Serum | up | 0.009 | |
| 423 | Chan M, 2013 [22] | Serum | up | <0.001 | |
| Shen J, 2014 [28] | Plasma | up | 0.82 | <0.001 | |
| 424 | Cuk K, 2013 [23] | Plasma | up | 0.322 | |
| Zhang L, 2015 [30] | Serum | up | 0.86 | 0.002 | |
| Hamam R, 2016 [33] | Blood | up | 0.044 | ||
| 425 | Chan M, 2013 [22] | Serum | up | 0.020 | |
| Kodahl AR, 2014 [26] | Serum | up | 0.119 | ||
| Zearo S, 2014 [29] | Serum | up | <0.001 | ||
| Ferracin M, 2015 [31] | Plasma | up | |||
| 429 | Wu Q, 2012 [21] | Serum | up | ||
| 451 | Chan M, 2013 [22] | Serum | up | 0.002 | |
| Ng E K, 2013 [25] | Plasma | up | |||
| 454 | Zearo S, 2014 [29] | Serum | up | <0.001 | |
| 483 | Zearo S, 2014 [29] | Serum | up | 0.016 | |
| Hamam R, 2016 [33] | Blood | up | 0.038 | ||
| Záveský L, 2022 [35] | Plasma | up | 0.004 | ||
| 484 | Chan M, 2013 [22] | Serum | up | 0.008 | |
| Shen J, 2014 [28] | Plasma | up | 0.84 | <0.001 | |
| Zearo S, 2014 [29] | Serum | up | <0.001 | ||
| 485 | Ng E K, 2013 [25] | Plasma | up | ||
| Shen J, 2014 [28] | Plasma | up | 0.87 | <0.001 | |
| 486 | Chan M, 2013 [22] | Serum | up | <0.001 | |
| Ng E K, 2013 [25] | Plasma | up | |||
| Zearo S, 2014 [29] | Serum | up | <0.001 | ||
| 494 | Ng E K, 2013 [25] | Plasma | down | ||
| 495 | Shen J, 2014 [28] | Plasma | up | 0.85 | <0.001 |
| 497 | Schrauder MG, 2012 [20] | Blood | up | 0.75 | 0.010 |
| 501 | Chan M, 2013 [22] | Serum | up | 0.023 | |
| 543 | Shen J, 2014 [28] | Plasma | up | 0.87 | <0.001 |
| 564 | Schrauder MG, 2012 [20] | Blood | down | 0.67 | 0.012 |
| 571 | Cuk K, 2013 [23] | Plasma | down | 0.100 | |
| 574 | Chan M, 2013 [22] | Serum | up | 0.027 | |
| Ng E K, 2013 [25] | Plasma | up | |||
| Zearo S, 2014 [29] | Serum | up | <0.001 | ||
| 576 | Chan M, 2013 [22] | Serum | up | <0.001 | |
| 584 | Chan M, 2013 [22] | Serum | up | 0.005 | |
| 598 | Chan M, 2013 [22] | Serum | up | 0.020 | |
| 605 | Godfrey AC, 2013 [24] | Serum | down | 0.050 | |
| 624 | Chan M, 2013 [22] | Serum | up | 0.027 | |
| 625 | Schrauder MG, 2012 [20] | Blood | down | 0.77 | 0.002 |
| 627 | Chan M, 2013 [22] | Serum | up | 0.030 | |
| 629 | Chan M, 2013 [22] | Serum | up | 0.009 | |
| Godfrey AC, 2013 [24] | Serum | up | 0.050 | ||
| 652 | Godfrey AC, 2013 [24] | Serum | up | 0.030 | |
| 660 | Chan M, 2013 [22] | Serum | up | 0.004 | |
| 664 | Chan M, 2013 [22] | Serum | down | 0.050 | |
| 671 | Godfrey AC, 2013 [24] | Serum | up | 0.010 | |
| Záveský L, 2022 [35] | Plasma | down | 0.029 | ||
| 718 | Schrauder MG, 2012 [20] | Blood | down | 0.77 | 0.004 |
| 744 | Godfrey AC, 2013 [24] | Serum | up | 0.020 | |
| 760 | Godfrey AC, 2013 [24] | Serum | down | 0.020 | |
| 762 | Hamam R, 2016 [33] | Blood | up | 0.042 | |
| 766 | Chan M, 2013 [22] | Serum | down | 0.011 | |
| Shen J, 2014 [28] | Plasma | up | 0.86 | <0.001 | |
| Ferracin M, 2015 [31] | Plasma | down | |||
| 801 | Cuk K, 2013 [23] | Plasma | up | 0.320 | |
| 874 | Schrauder MG, 2012 [20] | Blood | down | 0.74 | 0.001 |
| Ng E K, 2013 [25] | Plasma | down | |||
| 877 | Chan M, 2013 [22] | Serum | up | 0.043 | |
| 922 | Schrauder MG, 2012 [20] | Blood | up | 0.65 | 0.030 |
| 1202 | Hamam R, 2016 [33] | Blood | up | 0.006 | |
| 1207 | Hamam R, 2016 [33] | Blood | up | 0.020 | |
| 1225 | Hamam R, 2016 [33] | Blood | up | 0.004 | |
| 1234 | Godfrey AC, 2013 [24] | Serum | down | 0.030 | |
| 1290 | Hamam R, 2016 [33] | Blood | up | 0.022 | |
| 1323 | Schrauder MG, 2012 [20] | Blood | up | 0.69 | 0.040 |
| 1469 | Schrauder MG, 2012 [20] | Blood | down | 0.68 | 0.008 |
| 1471 | Schrauder MG, 2012 [20] | Blood | down | 0.70 | 0.012 |
| 1827 | Godfrey AC, 2013 [24] | Serum | up | 0.010 | |
| 1914 | Hamam R, 2016 [33] | Blood | up | 0.044 | |
| 1915 | Schrauder MG, 2012 [20] | Blood | down | 0.75 | 0.002 |
| 1974 | Shen J, 2014 [28] | Plasma | up | 0.85 | <0.001 |
| 2355 | Schrauder MG, 2012 [20] | Blood | down | 0.73 | 0.004 |
| 3130 | Schrauder MG, 2012 [20] | Blood | down | 0.73 | 0.004 |
| 3136 | Godfrey AC, 2013 [24] | Serum | up | 0.050 | |
| 3141 | Hamam R, 2016 [33] | Blood | up | 0.029 | |
| 3156 | Ferracin M, 2015 [31] | Plasma | down | ||
| 3186 | Schrauder MG, 2012 [20] | Blood | down | 0.75 | 0.002 |
| 3652 | Hamam R, 2016 [33] | Blood | up | 0.044 | |
| 4257 | Schrauder MG, 2012 [20] | Blood | up | 0.65 | 0.040 |
| 4270 | Hamam R, 2016 [33] | Blood | up | 0.001 | |
| 4281 | Hamam R, 2016 [33] | Blood | up | 0.019 | |
| 4298 | Hamam R, 2016 [33] | Blood | up | 0.035 | |
| 4306 | Schrauder MG, 2012 [20] | Blood | up | 0.71 | 0.020 |
| Godfrey AC, 2013 [24] | Serum | up | 0.030 | ||
| 106a | Chan M, 2013 [22] | Serum | up | <0.001 | |
| Ng E K, 2013 [25] | Plasma | down | |||
| Zhang L, 2015 [30] | Serum | up | 0.018 | ||
| Záveský L, 2022 [35] | Plasma | down | 0.038 | ||
| 106b | Schrauder MG, 2012 [20] | Blood | up | 0.72 | 0.010 |
| Záveský L, 2022 [35] | Plasma | down | 0.017 | ||
| 10a | Wu Q, 2012 [21] | Serum | up | ||
| Chan M, 2013 [22] | Serum | up | 0.029 | ||
| Ng E K, 2013 [25] | Plasma | up | |||
| 10b | Chan M, 2013 [22] | Serum | up | <0.001 | |
| 1255a | Godfrey AC, 2013 [24] | Serum | up | <0.01 | |
| 125a | Wu Q, 2012 [21] | Serum | up | ||
| Ferracin M, 2015 [31] | Plasma | up | |||
| 125b | Zhang L, 2015 [30] | Serum | up | 0.017 | |
| Záveský L, 2022 [35] | Plasma | down | 0.014 | ||
| 130a | Chan M, 2013 [22] | Serum | up | 0.020 | |
| Shen J, 2014 [28] | Plasma | up | 0.87 | <0.001 | |
| 130b | Chan M, 2013 [22] | Serum | up | 0.002 | |
| Godfrey AC, 2013 [24] | Serum | up | 0.030 | ||
| 133a | Chan M, 2013 [22] | Serum | up | <0.001 | |
| Kodahl AR, 2014 [26] | Serum | down | 0.479 | ||
| Shen J, 2014 [28] | Plasma | up | 0.80 | <0.001 | |
| 133b | Chan M, 2013 [22] | Serum | up | <0.001 | |
| 135b | Zhang L, 2015 [30] | Serum | up | 0.87 | <0.001 |
| 146b | Zearo S, 2014 [29] | Serum | up | <0.001 | |
| 148a | Ng E K, 2013 [25] | Plasma | up | ||
| 148b | Cuk K, 2013 [23] | Plasma | up | 0.320 | |
| Shen J, 2014 [28] | Plasma | up | 0.81 | <0.001 | |
| 15a | Kodahl AR, 2014 [26] | Serum | up | =1 | |
| 15b | Chan M, 2013 [22] | Serum | up | 0.003 | |
| 181a | Wu Q, 2012 [21] | Serum | up | ||
| Chan M, 2013 [22] | Serum | down | 0.023 | ||
| Godfrey AC, 2013 [24] | Serum | up | 0.050 | ||
| Ferracin M, 2015 [31] | Plasma | down | |||
| Zhang L, 2015 [30] | Serum | up | 0.86 | <0.001 | |
| 181b | Wu Q, 2012 [21] | Serum | up | ||
| 181c | Chan M, 2013 [22] | Serum | down | 0.038 | |
| 18a | Chan M, 2013 [22] | Serum | up | 0.004 | |
| Godfrey AC, 2013 [24] | Serum | up | 0.040 | ||
| Kodahl AR, 2014 [26] | Serum | up | 0.007 | ||
| 18b | Chan M, 2013 [22] | Serum | up | 0.002 | |
| Godfrey AC, 2013 [24] | Serum | down | 0.040 | ||
| 193a | Schrauder MG, 2012 [20] | Blood | down | 0.79 | <0.001 |
| Cuk K, 2013 [23] | Plasma | down | 0.320 | ||
| Ng E K, 2013 [25] | Plasma | down | |||
| 193b | Wu Q, 2012 [21] | Serum | up | ||
| Ng E K, 2013 [25] | Plasma | up | |||
| Zhang L, 2015 [30] | Serum | up | 0.80 | 0.002 | |
| Záveský L, 2022 [35] | Plasma | up | 0.017 | ||
| 196b | Záveský L, 2022 [35] | Plasma | down | 0.041 | |
| 199a | Chan M, 2013 [22] | Serum | down | 0.013 | |
| Shen J, 2014 [28] | Plasma | up | 0.84 | <0.001 | |
| Shin VY, 2015 [32] | Plasma | down | <0.05 | ||
| Zhang L, 2015 [30] | Serum | up | 0.84 | 0.001 | |
| 19a | Chan M, 2013 [22] | Serum | up | 0.016 | |
| Záveský L, 2022 [35] | Plasma | down | 0.038 | ||
| 200b | Wu Q, 2012 [21] | Serum | up | ||
| 200c | Wu Q, 2012 [21] | Serum | up | ||
| Ng E K, 2013 [25] | Plasma | up | |||
| 20a | Chan M, 2013 [22] | Serum | up | <0.001 | |
| Záveský L, 2022 [35] | Plasma | down | 0.017 | ||
| 20b | Chan M, 2013 [22] | Serum | up | 0.001 | |
| Záveský L, 2022 [35] | Plasma | down | 0.011 | ||
| 23a | Wu Q, 2012 [21] | Serum | up | ||
| 23b | Wu Q, 2012 [21] | Serum | up | ||
| Shen J, 2014 [28] | Plasma | up | 0.76 | 0.009 | |
| Shin VY, 2015 [32] | Plasma | up | <0.05 | ||
| 26a | Wu Q, 2012 [21] | Serum | up | ||
| 26b | Chan M, 2013 [22] | Serum | down | 0.005 | |
| Záveský L, 2022 [35] | Plasma | down | 0.011 | ||
| 27a | Wu Q, 2012 [21] | Serum | up | ||
| Ng E K, 2013 [25] | Plasma | up | |||
| 27b | Wu Q, 2012 [21] | Serum | up | ||
| Jusoh A, 2021 [34] | Plasma | up | 0.82 | 0.010 | |
| 29a | Wu Q, 2012 [21] | Serum | up | ||
| Zearo S, 2014 [29] | Serum | up | <0.001 | ||
| Zhang L, 2015 [30] | Serum | up | 0.029 | ||
| 29b | Wu Q, 2012 [21] | Serum | up | ||
| 29c | Wu Q, 2012 [21] | Serum | up | ||
| Zhang L, 2015 [30] | Serum | up | 0.81 | 0.001 | |
| 30a | Chan M, 2013 [22] | Serum | up | 0.029 | |
| 30b | Chan M, 2013 [22] | Serum | down | 0.027 | |
| Shen J, 2014 [28] | Plasma | up | 0.76 | <0.001 | |
| 30c | Shen J, 2014 [28] | Plasma | up | 0.77 | <0.001 |
| 30d | Chan M, 2013 [22] | Serum | up | 0.008 | |
| 30e | Wu Q, 2012 [21] | Serum | up | ||
| 320a | Wu Q, 2012 [21] | Serum | up | ||
| Chan M, 2013 [22] | Serum | up | <0.001 | ||
| Ferracin M, 2015 [31] | Plasma | up | |||
| 320b | Chan M, 2013 [22] | Serum | up | <0.001 | |
| 320d | Godfrey AC, 2013 [24] | Serum | up | 0.040 | |
| 33a | Shen J, 2014 [28] | Plasma | up | 0.79 | <0.001 |
| 34a | Hamam R, 2016 [33] | Blood | up | 0.044 | |
| 374a | Shen J, 2014 [28] | Plasma | up | 0.75 | 0.004 |
| 374b | Chan M, 2013 [22] | Serum | down | 0.007 | |
| 376a | Cuk K, 2013 [23] | Plasma | up | 0.386 | |
| 376c | Cuk K, 2013 [23] | Plasma | up | 0.224 | |
| 449b | Zhang L, 2015 [30] | Serum | up | 0.89 | <0.001 |
| 516b | Schrauder MG, 2012 [20] | Blood | up | 0.67 | 0.030 |
| Zhang L, 2015 [30] | Serum | up | 0.038 | ||
| 519a | Cuk K, 2013 [23] | Plasma | down | 0.407 | |
| 519c | Zhang L, 2015 [30] | Serum | up | 0.85 | 0.003 |
| 520c | Zhang L, 2015 [30] | Serum | up | 0.80 | 0.003 |
| 526a | Schrauder MG, 2012 [20] | Blood | down | 0.72 | 0.013 |
| 526b | Cuk K, 2013 [23] | Plasma | down | 0.386 | |
| 548b | Záveský L, 2022 [35] | Plasma | up | 0.001 | |
| 548c | Záveský L, 2022 [35] | Plasma | up | 0.035 | |
| 548d | Godfrey AC, 2013 [24] | Serum | down | 0.010 | |
| Záveský L, 2022 [35] | Plasma | up | 0.002 | ||
| 551a | Chan M, 2013 [22] | Serum | down | 0.002 | |
| 642b | Hamam R, 2016 [33] | Blood | up | 0.020 | |
| 92a | Wu Q, 2012 [21] | Serum | up | ||
| Chan M, 2013 [22] | Serum | up | <0.001 | ||
| Shin VY, 2015 [32] | Plasma | up | <0.05 | ||
| 92b | Chan M, 2013 [22] | Serum | up | 0.003 | |
| 99b | Shen J, 2014 [28] | Plasma | up | 0.81 | <0.001 |
| let7a | Schrauder MG, 2012 [20] | Blood | up | 0.65 | 0.030 |
| let-7a | Chan M, 2013 [22] | Serum | up | 0.005 | |
| let-7b | Chan M, 2013 [22] | Serum | up | <0.001 | |
| Zearo S, 2014 [29] | Serum | up | <0.001 | ||
| Záveský L, 2022 [35] | Plasma | down | 0.026 | ||
| let-7c | Chan M, 2013 [22] | Serum | up | 0.009 | |
| Záveský L, 2022 [35] | Plasma | down | 0.038 | ||
| let-7d | Shen J, 2014 [28] | Plasma | up | 0.89 | <0.001 |
| let-7f | Chan M, 2013 [22] | Serum | up | 0.016 | |
| Shen J, 2014 [28] | Plasma | up | 0.81 | <0.001 | |
| let-7g | Chan M, 2013 [22] | Serum | up | 0.002 | |
| Ng E K, 2013 [25] | Plasma | up | |||
| let-7i | Chan M, 2013 [22] | Serum | up | <0.001 | |
| U6 snRNA | Záveský L, 2022 [35] | Plasma | up | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padroni, L.; De Marco, L.; Fiano, V.; Milani, L.; Marmiroli, G.; Giraudo, M.T.; Macciotta, A.; Ricceri, F.; Sacerdote, C. Identifying MicroRNAs Suitable for Detection of Breast Cancer: A Systematic Review of Discovery Phases Studies on MicroRNA Expression Profiles. Int. J. Mol. Sci. 2023, 24, 15114. https://doi.org/10.3390/ijms242015114
Padroni L, De Marco L, Fiano V, Milani L, Marmiroli G, Giraudo MT, Macciotta A, Ricceri F, Sacerdote C. Identifying MicroRNAs Suitable for Detection of Breast Cancer: A Systematic Review of Discovery Phases Studies on MicroRNA Expression Profiles. International Journal of Molecular Sciences. 2023; 24(20):15114. https://doi.org/10.3390/ijms242015114
Chicago/Turabian StylePadroni, Lisa, Laura De Marco, Valentina Fiano, Lorenzo Milani, Giorgia Marmiroli, Maria Teresa Giraudo, Alessandra Macciotta, Fulvio Ricceri, and Carlotta Sacerdote. 2023. "Identifying MicroRNAs Suitable for Detection of Breast Cancer: A Systematic Review of Discovery Phases Studies on MicroRNA Expression Profiles" International Journal of Molecular Sciences 24, no. 20: 15114. https://doi.org/10.3390/ijms242015114
APA StylePadroni, L., De Marco, L., Fiano, V., Milani, L., Marmiroli, G., Giraudo, M. T., Macciotta, A., Ricceri, F., & Sacerdote, C. (2023). Identifying MicroRNAs Suitable for Detection of Breast Cancer: A Systematic Review of Discovery Phases Studies on MicroRNA Expression Profiles. International Journal of Molecular Sciences, 24(20), 15114. https://doi.org/10.3390/ijms242015114







