Antibody-Based and Cell Therapies for Advanced Mastocytosis: Established and Novel Concepts
Abstract
:1. Introduction
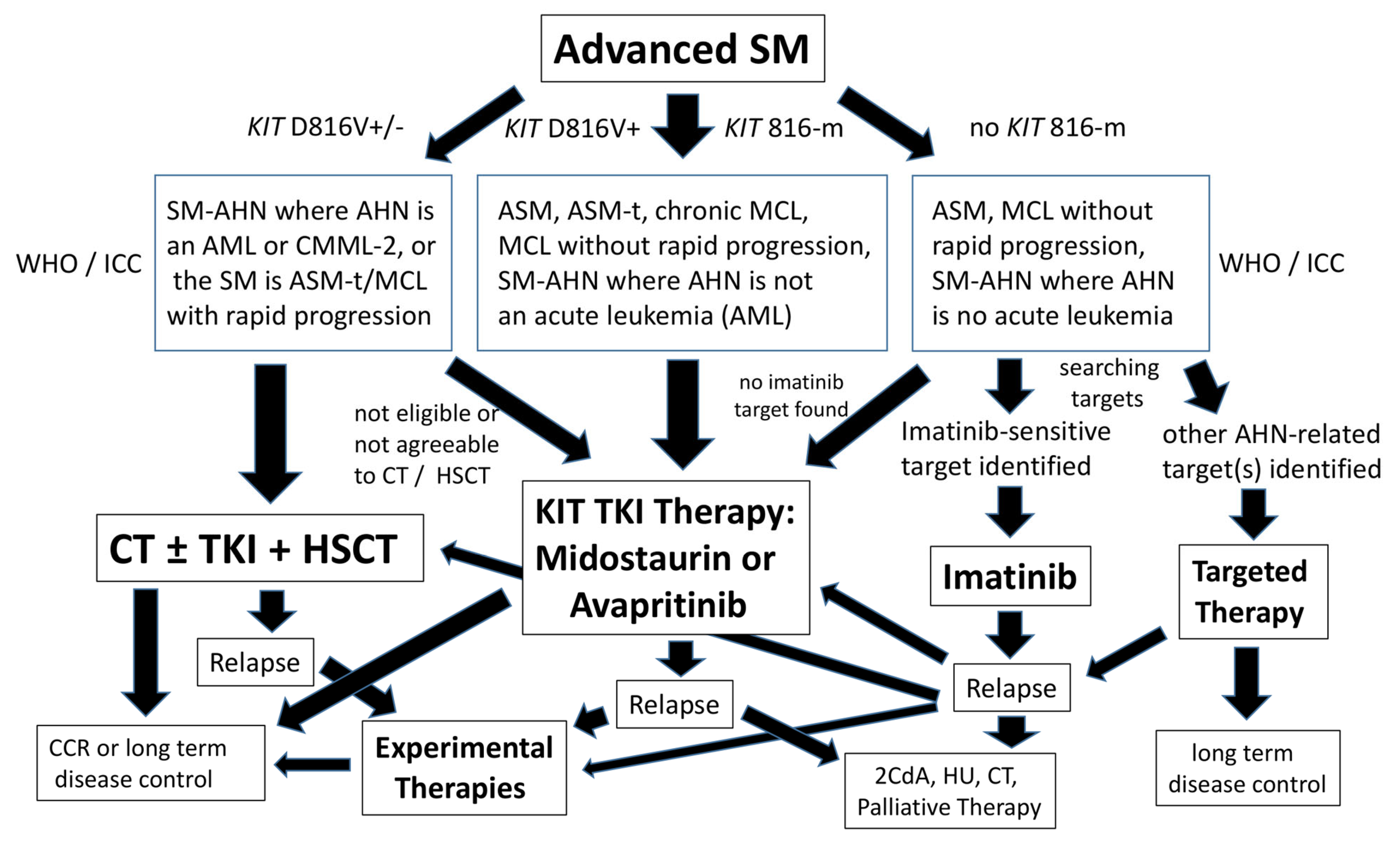
2. Standard Treatment Options for Patients with Advanced SM
3. Special Considerations for the Treatment of Patients with SM–AHN
4. Treatment Options for Patients with Drug-Resistant Advanced SM
5. Allogeneic HSCT
6. Antibody-Based Treatment of Drug-Resistant Advanced SM
7. Target Expression Profiles of NSC in Advanced SM
8. Novel Approaches to Target NSC in Advanced SM
9. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Valent, P.; Akin, C.; Metcalfe, D.D. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood 2017, 129, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Reiter, A.; George, T.I.; Gotlib, J.R. New developments in diagnosis, prognostication, and treatment of advanced systemic mastocytosis. Blood 2020, 135, 1365–1376. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Sperr, W.R.; Horny, H.-P.; Arock, M.; Metcalfe, D.D.; Galli, S.J. New Insights into the Pathogenesis of Mastocytosis: Emerging Concepts in Diagnosis and Therapy. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 361–386. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.; Escribano, L.; Grattan, C.; Brockow, K.; Carter, M.C.; Alvarez-Twose, I.; Matito, A.; Broesby-Olsen, S.; Siebenhaar, F.; Lange, M.; et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J. Allergy Clin. Immunol. 2016, 137, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Horny, H.-P.; Escribano, L.; Longley, B.; Li, C.Y.; Schwartz, L.B.; Marone, G.; Nuñez, R.; Akin, C.; Sotlar, K.; et al. Diagnostic criteria and classification of mastocytosis: A consensus proposal. Leuk. Res. 2001, 25, 603–625. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Escribano, L.; Födinger, M.; Hartmann, K.; Brockow, K.; Castells, M.; Sperr, W.R.; Kluin-Nelemans, H.C.; Hamdy, N.A.T.; et al. Standards and standardization in mastocytosis: Consensus Statements on Diagnostics, Treatment Recommendations and Response Criteria. Eur. J. Clin. Investig. 2007, 37, 435–453. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Arock, M.; Brockow, K.; Butterfield, J.H.; Carter, M.C.; Castells, M.; Escribano, L.; Hartmann, K.; Lieberman, P.; et al. Definitions, Criteria and Global Classification of Mast Cell Disorders with Special Reference to Mast Cell Activation Syndromes: A Consensus Proposal. Int. Arch. Allergy Immunol. 2012, 157, 215–225. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Hartmann, K.; Alvarez-Twose, I.; Brockow, K.; Hermine, O.; Niedoszytko, M.; Schwaab, J.; Lyons, J.J.; Carter, M.C.; et al. Updated Diagnostic Criteria and Classification of Mast Cell Disorders: A Consensus Proposal. Hemasphere 2021, 5, e646. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Sotlar, K.; Sperr, W.R.; Escribano, L.; Yavuz, S.; Reiter, A.; George, T.I.; Kluin-Nelemans, H.C.; Hermine, O.; Butterfield, J.H.; et al. Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): A consensus proposal. Ann. Oncol. 2014, 25, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Georgin-Lavialle, S.; Aguilar, C.; Guieze, R.; Lhermitte, L.; Bruneau, J.; Fraitag, S.; Canioni, D.; Chandesris, M.-O.; Suarez, F.; Grandpeix-Guyodo, C.; et al. Mast Cell Sarcoma: A Rare and Aggressive Entity—Report of Two Cases and Review of the Literature. J. Clin. Oncol. 2013, 31, e90–e97. [Google Scholar] [CrossRef] [PubMed]
- Monnier, J.; Georgin-Lavialle, S.; Canioni, D.; Lhermitte, L.; Soussan, M.; Arock, M.; Bruneau, J.; Dubreuil, P.; Bodemer, C.; Chandesris, M.-O.; et al. Mast cell sarcoma: New cases and literature review. Oncotarget 2016, 7, 66299–66309. [Google Scholar] [CrossRef]
- Matsumoto, N.P.; Yuan, J.; Wang, J.; Shen, Q.; Chen, X.; Kim, Y.; Zuppan, C.W.; Chang, C.-C.; Cui, W.; Chen, D.; et al. Mast cell sarcoma: Clinicopathologic and molecular analysis of 10 new cases and review of literature. Mod. Pathol. 2022, 35, 865–874. [Google Scholar] [CrossRef]
- Féger, F.; Dumas, A.R.; Leriche, L.; Valent, P.; Arock, M. Kit and c-kit Mutations in Mastocytosis: A Short Overview with Special Reference to Novel Molecular and Diagnostic Concepts. Int. Arch. Allergy Immunol. 2002, 127, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Arock, M.; Sotlar, K.; Akin, C.; Broesby-Olsen, S.; Hoermann, G.; Escribano, L.; Kristensen, T.K.; Kluin-Nelemans, H.C.; Hermine, O.; Dubreuil, P.; et al. KIT mutation analysis in mast cell neoplasms: Recommendations of the European Competence Network on Mastocytosis. Leukemia 2015, 29, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Hoermann, G.; Sotlar, K.; Jawhar, M.; Kristensen, T.; Bachelot, G.; Nedoszytko, B.; Carter, M.C.; Horny, H.-P.; Bonadonna, P.; Sperr, W.R.; et al. Standards of Genetic Testing in the Diagnosis and Prognostication of Systemic Mastocytosis in 2022: Recommendations of the EU-US Cooperative Group. J. Allergy Clin. Immunol. Pract. 2022, 10, 1953–1963. [Google Scholar] [CrossRef]
- Arock, M.; Hoermann, G.; Sotlar, K.; Hermine, O.; Sperr, W.R.; Hartmann, K.; Brockow, K.; Akin, C.; Triggiani, M.; Broesby-Olsen, S.; et al. Clinical impact and proposed application of molecular markers, genetic variants, and cytogenetic analysis in mast cell neoplasms: Status 2022. J. Allergy Clin. Immunol. 2022, 149, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.M.; Maric, I.; Simakova, O.; Bai, Y.; Chan, E.C.; Olivares, N.; Carter, M.; Maric, D.; Robyn, J.; Metcalfe, D.D. Clonal analysis of NRAS activating mutations in KIT-D816V systemic mastocytosis. Haematologica 2011, 96, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Schwaab, J.; Schnittger, S.; Sotlar, K.; Walz, C.; Fabarius, A.; Pfirrmann, M.; Kohlmann, A.; Grossmann, V.; Meggendorfer, M.; Horny, H.-P.; et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood 2013, 122, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Damaj, G.; Joris, M.; Chandesris, O.; Hanssens, K.; Soucie, E.; Canioni, D.; Kolb, B.; Durieu, I.; Gyan, E.; Livideanu, C.; et al. ASXL1 but Not TET2 Mutations Adversely Impact Overall Survival of Patients Suffering Systemic Mastocytosis with Associated Clonal Hematologic Non-Mast-Cell Diseases. PLoS ONE 2014, 9, e85362. [Google Scholar] [CrossRef]
- Jawhar, M.; Schwaab, J.; Schnittger, S.; Meggendorfer, M.; Pfirrmann, M.; Sotlar, K.; Horny, H.-P.; Metzgeroth, G.; Kluger, S.; Naumann, N.; et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V+ advanced systemic mastocytosis. Leukemia 2016, 30, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A.; Lasho, T.; Elala, Y.; Wassie, E.; Finke, C.; Reichard, K.K.; Chen, D.; Hanson, C.A.; Ketterling, R.P.; Tefferi, A. Next-generation sequencing in systemic mastocytosis: Derivation of a mutation-augmented clinical prognostic model for survival. Am. J. Hematol. 2016, 91, 888–893. [Google Scholar] [CrossRef]
- Nedoszytko, B.; Arock, M.; Lyons, J.J.; Bachelot, G.; Schwartz, L.B.; Reiter, A.; Jawhar, M.; Schwaab, J.; Lange, M.; Greiner, G.; et al. Clinical Impact of Inherited and Acquired Genetic Variants in Mastocytosis. Int. J. Mol. Sci. 2021, 22, 411. [Google Scholar] [CrossRef] [PubMed]
- Gleixner, K.V.; Mayerhofer, M.; Aichberger, K.J.; Derdak, S.; Sonneck, K.; Böhm, A.; Gruze, A.; Samorapoompichit, P.; Manley, P.W.; Fabbro, D.; et al. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of KIT: Comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood 2006, 107, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Ustun, C.; DeRemer, D.L.; Akin, C. Tyrosine kinase inhibitors in the treatment of systemic mastocytosis. Leuk. Res. 2011, 35, 1143–1152. [Google Scholar] [CrossRef]
- Gotlib, J.; Kluin-Nelemans, H.C.; George, T.I.; Akin, C.; Sotlar, K.; Hermine, O.; Awan, F.T.; Hexner, E.; Mauro, M.J.; Sternberg, D.W.; et al. Efficacy and Safety of Midostaurin in Advanced Systemic Mastocytosis. N. Engl. J. Med. 2016, 374, 2530–2541. [Google Scholar] [CrossRef]
- Chandesris, M.-O.; Damaj, G.; Canioni, D.; Brouzes, C.; Lhermitte, L.; Hanssens, K.; Frenzel, L.; Cherquaoui, Z.; Durieu, I.; Durupt, S.; et al. Midostaurin in Advanced Systemic Mastocytosis. N. Engl. J. Med. 2016, 374, 2605–2606. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Akin, C.; Hartmann, K.; George, T.I.; Sotlar, K.; Peter, B.; Gleixner, K.V.; Blatt, K.; Sperr, W.R.; Manley, P.W.; et al. Midostaurin: A magic bullet that blocks mast cell expansion and activation. Ann. Oncol. 2017, 28, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.H.; Gotlib, J. Clinical Validation of KIT Inhibition in Advanced Systemic Mastocytosis. Curr. Hematol. Malig. Rep. 2018, 13, 407–416. [Google Scholar] [CrossRef]
- Gotlib, J.; Reiter, A.; Radia, D.H.; Deininger, M.W.; George, T.I.; Panse, J.; Vannucchi, A.M.; Platzbecker, U.; Alvarez-Twose, I.; Mital, A.; et al. Efficacy and safety of avapritinib in advanced systemic mastocytosis: Interim analysis of the phase 2 PATHFINDER trial. Nat. Med. 2021, 27, 2192–2199. [Google Scholar] [CrossRef]
- DeAngelo, D.J.; Radia, D.H.; George, T.I.; Robinson, W.A.; Quiery, A.T.; Drummond, M.W.; Bose, P.; Hexner, E.O.; Winton, E.F.; Horny, H.-P.; et al. Safety and efficacy of avapritinib in advanced systemic mastocytosis: The phase 1 EXPLORER trial. Nat. Med. 2021, 27, 2183–2191. [Google Scholar] [CrossRef]
- Reiter, A.; Schwaab, J.; DeAngelo, D.J.; Gotlib, J.; Deininger, M.W.; Pettit, K.M.; Alvarez-Twose, I.; Vannucchi, A.M.; Panse, J.; Platzbecker, U.; et al. Efficacy and safety of avapritinib in previously treated patients with advanced systemic mastocytosis. Blood Adv. 2022, 6, 5750–5762. [Google Scholar] [CrossRef]
- Reiter, A.; Gotlib, J.; Álvarez-Twose, I.; Radia, D.H.; Lübke, J.; Bobbili, P.J.; Wang, A.; Norregaard, C.; Dimitrijevic, S.; Sullivan, E.; et al. Efficacy of avapritinib versus best available therapy in the treatment of advanced systemic mastocytosis. Leukemia 2022, 36, 2108–2120. [Google Scholar] [CrossRef] [PubMed]
- Kluin-Nelemans, H.C.; Oldhoff, J.M.; van Doormaal, J.J.; Van’t Wout, J.W.; Verhoef, G.; Gerrits, W.B.J.; van Dobbenburgh, O.A.; Pasmans, S.G.; Fijnheer, R. Cladribine therapy for systemic mastocytosis. Blood 2003, 102, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Barete, S.; Lortholary, O.; Damaj, G.; Hirsch, I.; Chandesris, M.O.; Elie, C.; Hamidou, M.; Durieu, I.; Suarez, F.; Grosbois, B.; et al. Long-term efficacy and safety of cladribine (2-CdA) in adult patients with mastocytosis. Blood 2015, 126, 1009–1016. [Google Scholar] [CrossRef]
- Tefferi, A.; Kittur, J.; Farrukh, F.; Begna, K.H.; Patnaik, M.M.; Al-Kali, A.; Elliott, M.A.; Reichard, K.K.; Gangat, N.; Pardanani, A. Cladribine therapy for advanced and indolent systemic mastocytosis: Mayo Clinic experience in 42 consecutive cases. Br. J. Haematol. 2021, 196, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Lübke, J.; Naumann, N.; Metzgeroth, G.; Kreil, S.; Brand, T.; Horny, H.-P.; Sotlar, K.; Cross, N.C.P.; Fabarius, A.; Valent, P.; et al. Response and resistance to cladribine in patients with advanced systemic mastocytosis: A registry-based analysis. Ann. Hematol. 2023, 102, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Lübke, J.; Schwaab, J.; Naumann, N.; Horny, H.-P.; Weiß, C.; Metzgeroth, G.; Kreil, S.; Cross, N.C.; Sotlar, K.; Fabarius, A.; et al. Superior Efficacy of Midostaurin over Cladribine in Advanced Systemic Mastocytosis: A Registry-Based Analysis. J. Clin. Oncol. 2022, 40, 1783–1794. [Google Scholar] [CrossRef]
- Akin, C.; Fumo, G.; Yavuz, A.S.; Lipsky, P.E.; Neckers, L.; Metcalfe, D.D. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood 2004, 103, 3222–3225. [Google Scholar] [CrossRef]
- Mital, A.; Piskorz, A.; Lewandowski, K.; Wasąg, B.; Limon, J.; Hellmann, A. A case of mast cell leukaemia with exon 9 KIT mutation and good response to imatinib. Eur. J. Haematol. 2011, 86, 531–535. [Google Scholar] [CrossRef]
- Campos, P.D.M.; Machado-Neto, J.A.; Scopim-Ribeiro, R.; Visconte, V.; Tabarroki, A.; Duarte, A.S.; Barra, F.F.; Vassalo, J.; Rogers, H.J.; Lorand-Metze, I.; et al. Familial systemic mastocytosis with germline KIT K509I mutation is sensitive to treatment with imatinib, dasatinib and PKC412. Leuk. Res. 2014, 38, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Twose, I.; Matito, A.; Morgado, J.M.; Sánchez-Muñoz, L.; Jara-Acevedo, M.; García-Montero, A.; Mayado, A.; Caldas, C.; Teodósio, C.; Muñoz-González, J.I.; et al. Imatinib in systemic mastocytosis: A phase IV clinical trial in patients lacking exon 17 KIT mutations and review of the literature. Oncotarget 2016, 8, 68950–68963. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Twose, I.; Jara-Acevedo, M.; Morgado, J.M.; García-Montero, A.; Sánchez-Muñoz, L.; Teodósio, C.; Matito, A.; Mayado, A.; Caldas, C.; Mollejo, M.; et al. Clinical, immunophenotypic, and molecular characteristics of well-differentiated systemic mastocytosis. J. Allergy Clin. Immunol. 2016, 137, 168–178.e1. [Google Scholar] [CrossRef] [PubMed]
- Ustun, C.; Reiter, A.; Scott, B.L.; Nakamura, R.; Damaj, G.; Kreil, S.; Shanley, R.; Hogan, W.J.; Perales, M.-A.; Shore, T.; et al. Hematopoietic Stem-Cell Transplantation for Advanced Systemic Mastocytosis. J. Clin. Oncol. 2014, 32, 3264–3274. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Sperr, W.R.; Akin, C. How I treat patients with advanced systemic mastocytosis. Blood 2010, 116, 5812–5817. [Google Scholar] [CrossRef] [PubMed]
- Ustun, C.; Gotlib, J.; Popat, U.; Artz, A.; Litzow, M.; Reiter, A.; Nakamura, R.; Kluin-Nelemans, H.C.; Verstovsek, S.; Gajewski, J.; et al. Consensus Opinion on Allogeneic Hematopoietic Cell Transplantation in Advanced Systemic Mastocytosis. Biol. Blood Marrow Transplant. 2016, 22, 1348–1356. [Google Scholar] [CrossRef]
- Valent, P.; Hartmann, K.; Schwaab, J.; Alvarez-Twose, I.; Brockow, K.; Bonadonna, P.; Hermine, O.; Niedoszytko, M.; Carter, M.C.; Hoermann, G.; et al. Personalized Management Strategies in Mast Cell Disorders: ECNM-AIM User’s Guide for Daily Clinical Practice. J. Allergy Clin. Immunol. Pract. 2022, 10, 1999–2012.e6. [Google Scholar] [CrossRef] [PubMed]
- Kubasch, A.S.; Franke, G.-N.; Aldaoud, A.; Weibl, K.; Jentzsch, M.; Sabri, O.; Horny, H.-P.; Fend, F.; Behre, G.; Platzbecker, U.; et al. Allogeneic Hematopoietic Stem Cell Transplantation in a Rare Case of Tonsillar Mast Cell Sarcoma. Front. Oncol. 2020, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Sotlar, K.; Marafioti, T.; Griesser, H.; Theil, J.; Aepinus, C.; Jaussi, R.; Stein, H.; Valent, P.; Horny, H.-P. Detection of c-kit mutation Asp 816 to Val in microdissected bone marrow infiltrates in a case of systemic mastocytosis associated with chronic myelomonocytic leukaemia. Mol. Pathol. 2000, 53, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Sotlar, K.; Colak, S.; Bache, A.; Berezowska, S.; Krokowski, M.; Bültmann, B.; Valent, P.; Horny, H.-P. Variable presence of KITD816V in clonal haematological non-mast cell lineage diseases associated with systemic mastocytosis (SM-AHNMD). J. Pathol. 2009, 220, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Fritsche-Polanz, R.; Fritz, M.; Huber, A.; Sotlar, K.; Sperr, W.R.; Mannhalter, C.; Födinger, M.; Valent, P. High frequency of concomitant mastocytosis in patients with acute myeloid leukemia exhibiting the transforming KIT mutation D816V. Mol. Oncol. 2010, 4, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Jawhar, M.; Döhner, K.; Kreil, S.; Schwaab, J.; Shoumariyeh, K.; Meggendorfer, M.; Span, L.L.F.; Fuhrmann, S.; Naumann, N.; Horny, H.-P.; et al. KIT D816 mutated/CBF-negative acute myeloid leukemia: A poor-risk subtype associated with systemic mastocytosis. Leukemia 2019, 33, 1124–1134. [Google Scholar] [CrossRef]
- Craig, J.W.; Hasserjian, R.P.; Kim, A.S.; Aster, J.C.; Pinkus, G.S.; Hornick, J.L.; Steensma, D.P.; Lindsley, R.C.; DeAngelo, D.J.; Morgan, E.A. Detection of the KIT mutation in myelodysplastic and/or myeloproliferative neoplasms and acute myeloid leukemia with myelodysplasia-related changes predicts concurrent systemic mastocytosis. Mod. Pathol. 2020, 33, 1135–1145. [Google Scholar] [CrossRef]
- Yamada, A.; Kinoshita, M.; Sawa, D.; Saito, Y.; Kamimura, S.; Miyachi, H.; Moritake, H. Long-term Remission of Acute Myeloid Leukemia Developed From Systemic Mastocytosis by Conventional Chemotherapy. J. Pediatr. Hematol. 2019, 41, e402–e404. [Google Scholar] [CrossRef] [PubMed]
- Pourhassan, H.; Kim, Y.; Pullarkat, V. Clearance of bone marrow mast cells after hypomethylating agent and venetoclax for systemic mastocytosis associated with myeloid neoplasia. Eur. J. Haematol. 2022, 110, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Hernández Alconchel, I.H.; González de Villambrosía, S.; Insunza Gaminde, A.; Montes Moreno, S. Systemic Mastocytosis with Associated Hematological Neoplasms. Diagnostic features and unique response pattern to tyrosine kinase inhibitors and allo-bone marrow transplantation therapy. Rev. Esp. Patol. 2023, 56, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Reiter, A.; Gotlib, J.; Álvarez-Twose, I.; Radia, D.; Lübke, J.; ScD, P.B.; Wang, A.; Norregaard, C.; Dimitrijević, S.; Sullivan, E.; et al. MPN-435 Overall Survival in Patients with Systemic Mastocytosis with Associated Hematologic Neoplasm Treated with Avapritinib versus Best Available Therapy. Clin. Lymphoma Myeloma Leuk. 2022, 22, S337. [Google Scholar] [CrossRef]
- Sriskandarajah, P.; McLornan, D.P.; Oni, C.; Wilson, A.; Woodley, C.; Ciesielska, M.; Raj, K.; Dillon, R.; Ethell, M.; Chacko, J.; et al. Advanced Systemic Mastocytosis with associated haematological neoplasm: Treatment with avapritinib can facilitate successful bridge to allogeneic haematopoietic cell transplant. Curr. Res. Transl. Med. 2023, 71, 103398. [Google Scholar] [CrossRef]
- Navarro-Navarro, P.; Álvarez-Twose, I.; Pérez-Pons, A.; Henriques, A.; Mayado, A.; García-Montero, A.C.; Sánchez-Muñoz, L.; González-López, O.; Matito, A.; Caldas, C.; et al. KITD816V mutation in blood for the diagnostic screening of systemic mastocytosis and mast cell activation syndromes. Allergy 2023, 78, 1347–1359. [Google Scholar] [CrossRef]
- Fazio, M.; Vetro, C.; Markovic, U.; Duminuco, A.; Parisi, M.; Maugeri, C.; Mauro, E.; Parrinello, N.L.; Stagno, F.; Villari, L.; et al. A case of high-risk AML in a patient with advanced systemic mastocytosis. Clin. Case Rep. 2023, 11, e7134. [Google Scholar] [CrossRef]
- Prats-Martín, C.; Jiménez-Guerrero, P.; Morales-Camacho, R.M.; Caballero-Velázquez, T.; Vargas, M.T.; Pérez, O.; Montero, I.; Falantes, J.; Burillo-Sanz, S.; Carrillo, E.; et al. KIT D816V− chronic myelomonocytic leukemia progressing to KIT D816V+ associated to mast cell leukemia responding to allogeneic hematopoietic cell transplantation. Ann. Hematol. 2018, 97, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Hägglund, H.; Yavuz, A.S.; Dreimane, A.; Malm, C.; Sundin, A.; Sander, B.; Nilsson, G. Graft-versus-mastocytosis effect after donor lymphocyte infusion: Proof of principle. Eur. J. Haematol. 2021, 106, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Spyridonidis, A.; Thomas, A.K.; Bertz, H.; Zeiser, R.; Schmitt-Gräff, A.; Lindemann, A.; Waller, C.F.; Finke, J. Evidence for a graft-versus-mast-cell effect after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004, 34, 515–519. [Google Scholar] [CrossRef]
- Martynova, A.; Nael, A.; O’neill, C.; Ramsingh, G.; Merchant, A.; Yaghmour, B.; Yaghmour, G. Aggressive systemic mastocytosis: Midostaurin is safe, feasible and associated with durable response post-haploidentical allogeneic stem cell transplant. Br. J. Haematol. 2019, 186, e139–e141. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Zheng, F.-M.; Wang, Z.-D.; Zhang, Y.-Y.; Cheng, Y.-F.; Fu, H.-X.; Lv, M.; Chen, H.; Xu, L.-P.; Zhang, X.-H.; et al. Avapritinib is effective for treatment of minimal residual disease in acute myeloid leukemia with t (8;21) and kit mutation failing to immunotherapy after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2023, 58, 777–783. [Google Scholar] [CrossRef]
- Escribano, L.; Orfao, A.; Díaz-Agustin, B.; Villarrubia, J.; Cerveró, C.; López, A.; Marcos, M.A.G.; Bellas, C.; Fernández-Cañadas, S.; Cuevas, M.; et al. Indolent Systemic Mast Cell Disease in Adults: Immunophenotypic Characterization of Bone Marrow Mast Cells and Its Diagnostic Implications. Blood 1998, 91, 2731–2736. [Google Scholar] [CrossRef]
- Krauth, M.-T.; Böhm, A.; Agis, H.; Sonneck, K.; Samorapoompichit, P.; Florian, S.; Sotlar, K.; Valent, P. Effects of the CD33-targeted drug gemtuzumab ozogamicin (Mylotarg) on growth and mediator secretion in human mast cells and blood basophils. Exp. Hematol. 2007, 35, 108–116. [Google Scholar] [CrossRef]
- Eisenwort, G.; Sadovnik, I.; Schwaab, J.; Jawhar, M.; Keller, A.; Stefanzl, G.; Berger, D.; Blatt, K.; Hoermann, G.; Bilban, M.; et al. Identification of a leukemia-initiating stem cell in human mast cell leukemia. Leukemia 2019, 33, 2673–2684. [Google Scholar] [CrossRef]
- Dasilva-Freire, N.; Mayado, A.; Teodosio, C.; Jara-Acevedo, M.; Álvarez-Twose, I.; Matito, A.; Sánchez-Muñoz, L.; Caldas, C.; Henriques, A.; Muñoz-González, J.I.; et al. Bone Marrow Mast Cell Antibody-Targetable Cell Surface Protein Expression Profiles in Systemic Mastocytosis. Int. J. Mol. Sci. 2019, 20, 552. [Google Scholar] [CrossRef]
- Alvarez-Twose, I.; Martínez-Barranco, P.; Gotlib, J.; García-Montero, A.; Morgado, J.M.; Jara-Acevedo, M.; Merker, J.D.; Peñalver, F.J.; Matito, A.; Hou, Y.; et al. Complete response to gemtuzumab ozogamicin in a patient with refractory mast cell leukemia. Leukemia 2016, 30, 1753–1756. [Google Scholar] [CrossRef]
- Escribano, L.; Díaz-Agustín, B.; Bellas, C.; Navalón, R.; Nuñez, R.; Sperr, W.R.; Schernthaner, G.-H.; Valent, P.; Orfao, A. Utility of flow cytometric analysis of mast cells in the diagnosis and classification of adult mastocytosis. Leuk. Res. 2001, 25, 563–570. [Google Scholar] [CrossRef]
- Hatch, E.W.; Geeze, M.B.; Martin, C.; Salama, M.E.; Hartmann, K.; Eisenwort, G.; Blatt, K.; Valent, P.; Gotlib, J.; Lee, J.-H.; et al. Variability of PD-L1 expression in mastocytosis. Blood Adv. 2018, 2, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Hoermann, G.; Blatt, K.; Greiner, G.; Putz, E.M.; Berger, A.; Herrmann, H.; Gleixner, K.V.; Walz, C.; Hoetzenecker, K.; Müllauer, L.; et al. CD52 is a molecular target in advanced systemic mastocytosis. FASEB J. 2014, 28, 3540–3551. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A.; Lasho, T.; Chen, D.; Kimlinger, T.K.; Finke, C.; Zblewski, D.; Patnaik, M.M.; Reichard, K.K.; Rowinsky, E.; Hanson, C.A.; et al. Aberrant expression of CD123 (interleukin-3 receptor-α) on neoplastic mast cells. Leukemia 2015, 29, 1605–1608. [Google Scholar] [CrossRef]
- Mueller, N.; Wicklein, D.; Eisenwort, G.; Jawhar, M.; Berger, D.; Stefanzl, G.; Greiner, G.; Boehm, A.; Kornauth, C.; Muellauer, L.; et al. CD44 is a RAS/STAT5-regulated invasion receptor that triggers disease expansion in advanced mastocytosis. Blood 2018, 132, 1936–1950. [Google Scholar] [CrossRef] [PubMed]
- Morgado, J.M.; Perbellini, O.; Johnson, R.C.; Teodósio, C.; Matito, A.; Álvarez-Twose, I.; Bonadonna, P.; Zamò, A.; Jara-Acevedo, M.; Mayado, A.; et al. CD30 expression by bone marrow mast cells from different diagnostic variants of systemic mastocytosis. Histopathology 2013, 63, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Blatt, K.; Cerny-Reiterer, S.; Schwaab, J.; Sotlar, K.; Eisenwort, G.; Stefanzl, G.; Hoermann, G.; Mayerhofer, M.; Schneeweiss, M.; Knapp, S.; et al. Identification of the Ki-1 antigen (CD30) as a novel therapeutic target in systemic mastocytosis. Blood 2015, 126, 2832–2841. [Google Scholar] [CrossRef]
- Smiljkovic, D.; Herrmann, H.; Sadovnik, I.; Gamperl, S.; Berger, D.; Stefanzl, G.; Eisenwort, G.; Hoermann, G.; Kopanja, S.; Dorofeeva, Y.; et al. Expression and regulation of Siglec-6 (CD327) on human mast cells and basophils. J. Allergy Clin. Immunol. 2023, 151, 202–211. [Google Scholar] [CrossRef]
- Laszlo, G.S.; Estey, E.H.; Walter, R.B. The past and future of CD33 as therapeutic target in acute myeloid leukemia. Blood Rev. 2014, 28, 143–153. [Google Scholar] [CrossRef]
- Kalmanti, L.; Saussele, S.; Lauseker, M.; Müller, M.C.; Dietz, C.T.; Heinrich, L.; Hanfstein, B.; Proetel, U.; Fabarius, A.; Krause, S.W.; et al. Safety and efficacy of imatinib in CML over a period of 10 years: Data from the randomized CML-study IV. Leukemia 2015, 29, 1123–1132. [Google Scholar] [CrossRef]
- Yun, H.D.; Felices, M.; Vallera, D.A.; Hinderlie, P.; Cooley, S.; Arock, M.; Gotlib, J.; Ustun, C.; Miller, J.S. Trispecific killer engager CD16xIL15xCD33 potently induces NK cell activation and cytotoxicity against neoplastic mast cells. Blood Adv. 2018, 2, 1580–1584. [Google Scholar] [CrossRef] [PubMed]

| Disease Variant | Prevalence of KIT D816V | Other KIT Mutations | Other Gene Variants * |
|---|---|---|---|
| SSM | >90% | <10% | <10% |
| SM–AHN | >90% | <10% | >70% |
| ASM | 80–90% | 10–20% ** | 10–20% |
| AdvSMWD | 20% | 50–80% ** | <10% |
| MCL | 70–80% | 20–30% ** | 10% |
| MCS *** | <10% *** | <10% | 10–30% *** |
| Treatment | Indications |
|---|---|
| Interferon-alpha (IFN-A) * | Treatment-refractory osteoporosis (low dose IFN-A). ASM with liver involvement and ascites (patient not eligible for TKI therapy or 2CdA). IFN-A responsive AHN. |
| Cladribine (2CdA) | Patient not eligible for KIT TKI therapy or patient resistant against KIT TKI or no KIT TKI available. |
| Imatinib | KIT D816V-negative advanced SM, including ASM with KIT K509I. |
| Masitinib | KIT D816V-negative advanced SM. |
| Midostaurin | ASM, ASM-t, ASM–AHN, MCL, MCL–AHN. ISM–AHN where the AHN is a KIT-driven (KIT D816V+) aggressive neoplasm. |
| Avapritinib | ASM, ASM-t, ASM–AHN, MCL, MCL–AHN. ISM–AHN where the AHN is a KIT-driven (KIT D816V+) aggressive neoplasm. |
| Hydroxyurea (HU) | Multi-drug-resistant advanced SM, including ASM, MCL and SM–AHN. HU is a standard palliative drug. |
| Local radiation | Mast cell sarcoma (MCS) or MCS-like progression of ASM. Huge splenomegaly: as debulking prior to CT. Skeletal disease (huge osteolysis with local tumor mass). |
| Poly-chemotherapy | Drug-resistant advanced SM. Debulking as preparation for HSCT. SM–CMML with progressing CMML or SM–AML in patients not eligible for HSCT. |
| Mono-chemotherapy: demethylating agents and other drugs, such as venetoclax | Patients with advanced, KIT TKI-resistant SM not eligible for poly-chemotherapy or HSCT (and/or resistant against 2CdA). SM–AHN patients not eligible for HSCT in whom the AHN may be responsive: (example: azacitidine in MDS or AML). |
| Allogeneic HSCT | Drug resistant advanced SM, ASM-t or MCL. ASM with rapid progression. SM–CMML, SM–AML. |
| CD | Antigen, Target | Cell Surface Expression Detected by Flow Cytometry * on | |||||
|---|---|---|---|---|---|---|---|
| Neoplastic Mast Cells | CD34+/CD38− Stem Cells | ||||||
| ASM | SM–AHN | MCL | ASM | SM–AHN | MCL | ||
| CD2 | LFA-2 | + | + | +/− | − | − | − |
| CD13 | A-Pept-N | + | + | + | + | + | + |
| CD25 | IL-2RA | + | + | + | +/− | +/- | +/- |
| CD30 | Ki-1 | +/− | +/− | +/− | −/+ | −/+ | −/+ |
| CD33 | Siglec-3 | + | + | + | + | + | + |
| CD44 | Hermes | + | + | + | + | + | + |
| CD47 | IAP | + | + | + | + | + | + |
| CD52 | Campath-1 | + | + | + | −/+ | −/+ | −/+ |
| CD117 | KIT | + | + | + | + | + | + |
| CD123 | IL-3RA | +/− | +/− | +/− | + | + | + |
| CD184 | CXCR4 | + | + | + | + | + | + |
| CD274 | PD-L1 | + | + | + | n.k. | n.k. | n.k. |
| CD327 | Siglec-6 | + | + | + | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valent, P.; Akin, C.; Arock, M.; Gleixner, K.V.; Greinix, H.; Hermine, O.; Horny, H.-P.; Ivanov, D.; Orfao, A.; Rabitsch, W.; et al. Antibody-Based and Cell Therapies for Advanced Mastocytosis: Established and Novel Concepts. Int. J. Mol. Sci. 2023, 24, 15125. https://doi.org/10.3390/ijms242015125
Valent P, Akin C, Arock M, Gleixner KV, Greinix H, Hermine O, Horny H-P, Ivanov D, Orfao A, Rabitsch W, et al. Antibody-Based and Cell Therapies for Advanced Mastocytosis: Established and Novel Concepts. International Journal of Molecular Sciences. 2023; 24(20):15125. https://doi.org/10.3390/ijms242015125
Chicago/Turabian StyleValent, Peter, Cem Akin, Michel Arock, Karoline V. Gleixner, Hildegard Greinix, Olivier Hermine, Hans-Peter Horny, Daniel Ivanov, Alberto Orfao, Werner Rabitsch, and et al. 2023. "Antibody-Based and Cell Therapies for Advanced Mastocytosis: Established and Novel Concepts" International Journal of Molecular Sciences 24, no. 20: 15125. https://doi.org/10.3390/ijms242015125
APA StyleValent, P., Akin, C., Arock, M., Gleixner, K. V., Greinix, H., Hermine, O., Horny, H.-P., Ivanov, D., Orfao, A., Rabitsch, W., Reiter, A., Schulenburg, A., Sotlar, K., Sperr, W. R., & Ustun, C. (2023). Antibody-Based and Cell Therapies for Advanced Mastocytosis: Established and Novel Concepts. International Journal of Molecular Sciences, 24(20), 15125. https://doi.org/10.3390/ijms242015125






