Insensitive Effects of Inflammatory Cytokines on the Reference Genes of Synovial Fluid Resident-Mesenchymal Stem Cells Derived from Rheumatoid Arthritis Patients
Abstract
:1. Introduction
2. Results
2.1. Characterization of H-SF-MSCs and RA-SF-MSCs
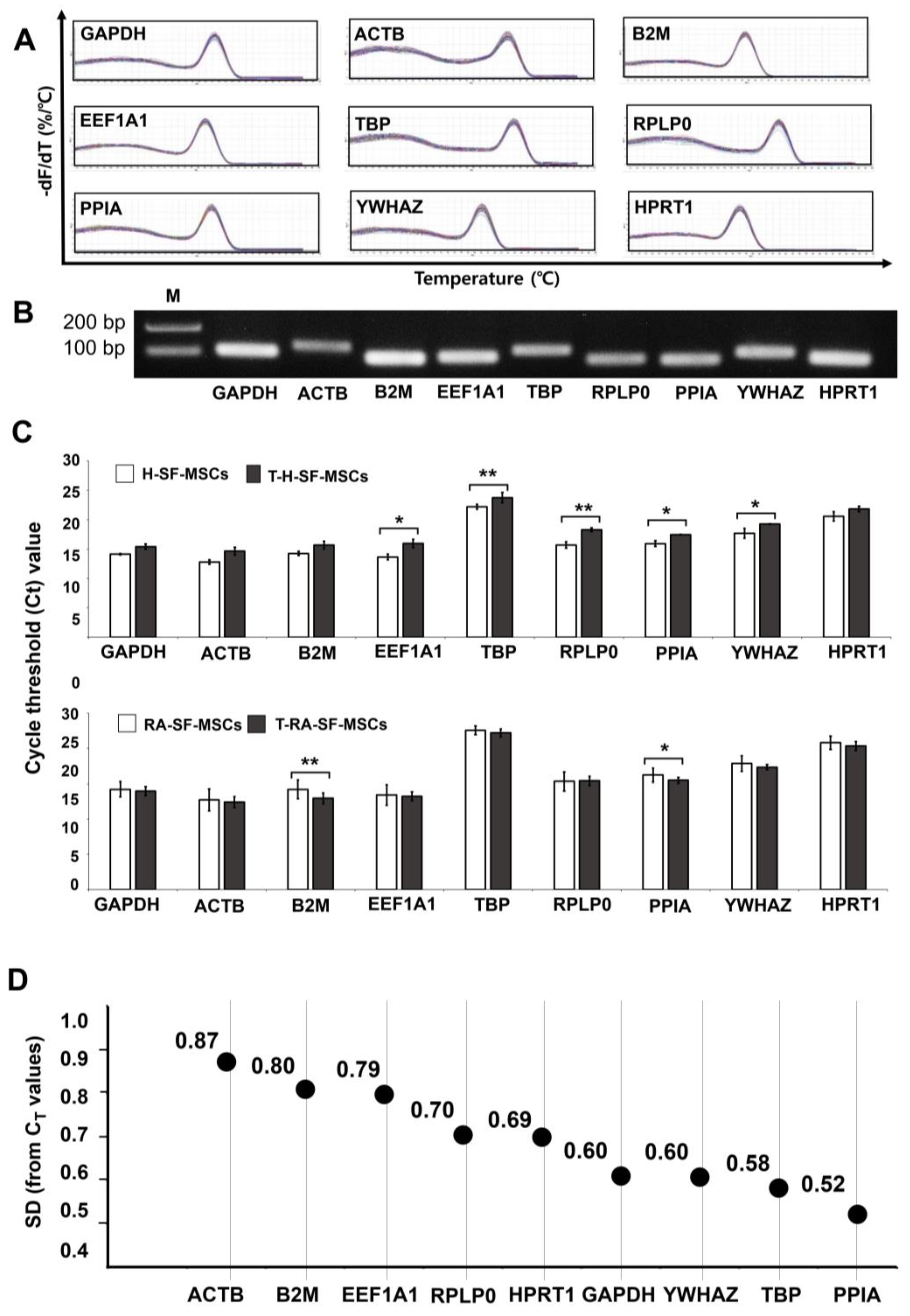
2.2. CT Values of the RGs in the SF-MSCs upon Inflammatory Cytokine Induction
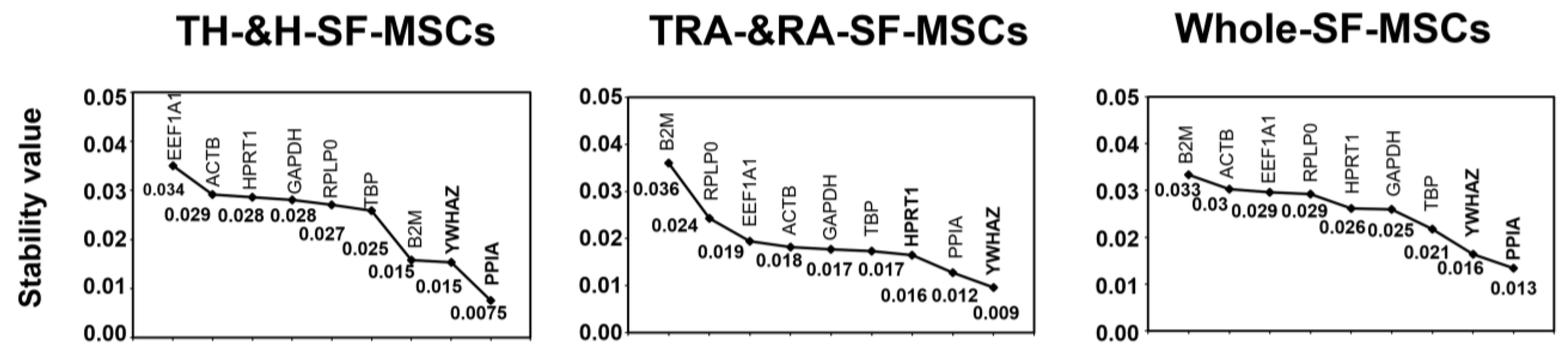
2.3. Analysis of the Most Stable Reference Gene Using geNorm
2.4. Analysis of the Most Stable Reference Gene Using NormFinder
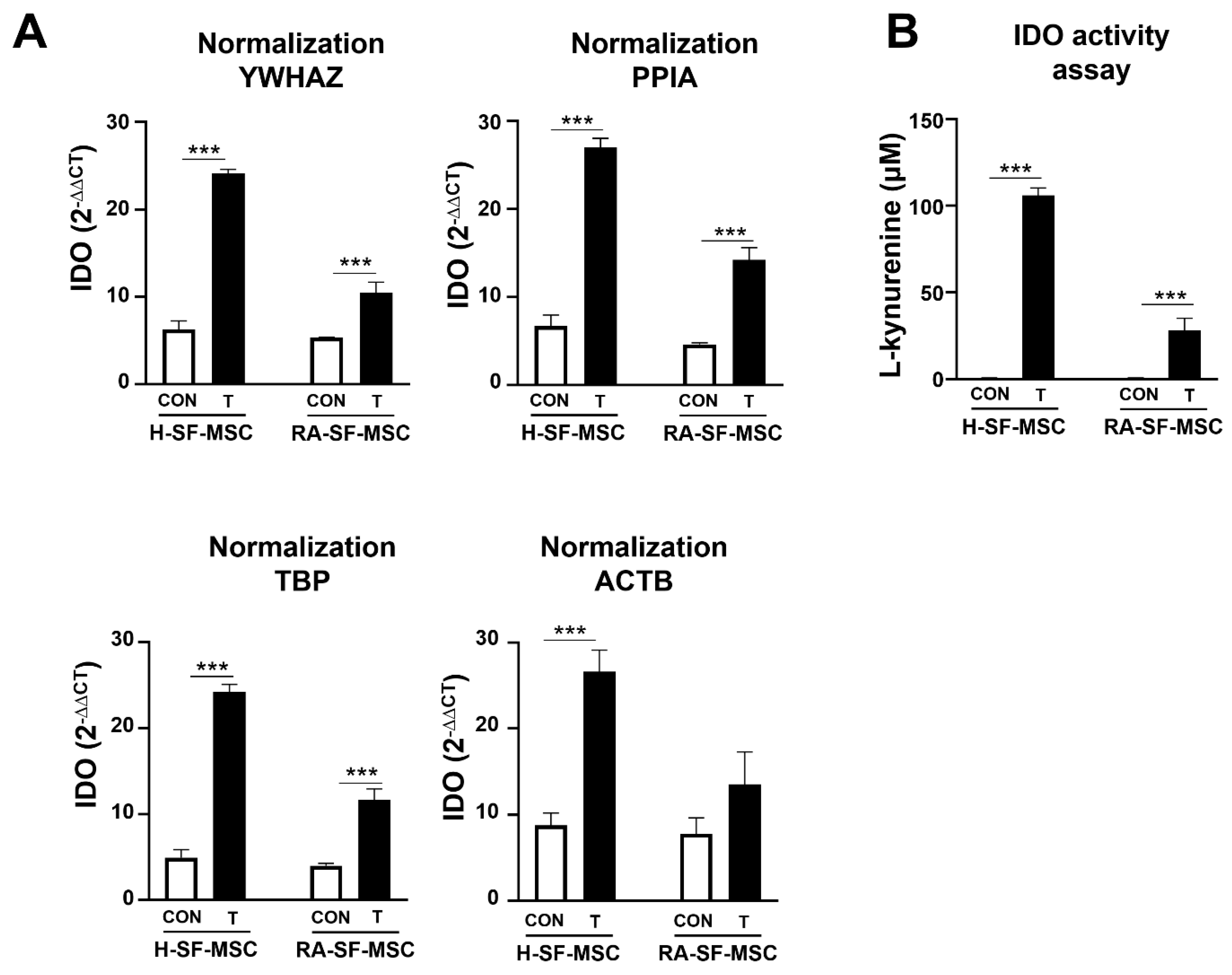
2.5. The Impact of Using an Unstable Reference Gene in the Normalization of the GOI
3. Discussion
4. Materials and Methods
4.1. Chemicals and Ethics Used in the Experiments
4.2. Characterization of SF-MSCs from Healthy and RA Patients
4.3. Treatment of H-SF-MSCs and RA-SF-MSCs with Pro-Inflammatory Cytokines
4.4. Candidate Reference Genes and Primer Sequences
4.5. RNA Extraction, cDNA Synthesis, and RT-qPCR
4.6. Analysis of Stable Reference Gene Expression
4.7. Application of Different RGs to Normalize the Gene of Interest (GOI)
4.8. IDO Activity Measurements
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grskovic, M.; Javaherian, A.; Strulovici, B.; Daley, G.Q. Induced pluripotent stem cells—Opportunities for disease modeling and drug discovery. Nat. Rev. Drug Discov. 2011, 10, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, W.J.; Hwang, S.C.; Choe, Y.; Kim, S.; Bok, E.; Lee, S.; Kim, S.J.; Kim, H.O.; Ock, S.A.; et al. Chronic inflammation-induced senescence impairs immunomodulatory properties of synovial fluid mesenchymal stem cells in rheumatoid arthritis. Stem Cell Res. Ther. 2021, 12, 502. [Google Scholar] [CrossRef] [PubMed]
- Studeny, M.; Marini, F.C.; Champlin, R.E.; Zompetta, C.; Fidler, I.J.; Andreeff, M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumors. Cancer Res. 2002, 62, 3603–3608. [Google Scholar]
- McInnes, I.B.; Schett, G. Citocinas en la patogenia de la artritis reumatoide. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Djouad, F.; Fritz, V.; Apparailly, F.; Louis-Plence, P.; Bony, C.; Sany, J.; Jorgensen, C.; Noël, D. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor α in collagen-induced arthritis. Arthritis Rheum. 2005, 52, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Hu, J.; Liao, L.; Sun, Z.; Han, Q.; Song, Z.; Zhao, R. Flk-1+ mesenchymal stem cells aggravate collagen-induced arthritis by up-regulating interleukin-6. Clin. Exp. Immunol. 2010, 159, 292–302. [Google Scholar] [CrossRef]
- Schurgers, E.; Kelchtermans, H.; Mitera, T.; Geboes, L.; Matthys, P. Discrepancy between the in vitro and in vivoeffects of murine mesenchymal stem cells on T-cell proliferation and collagen-induced arthritis. Arthritis Res. Ther. 2010, 12, R31. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Hah, Y.S.; Ock, S.A.; Lee, J.H.; Jeon, R.H.; Park, J.S.; Lee, S.I.; Rho, N.Y.; Rho, G.J.; Lee, S.L. Cell source-dependent in vivo immunosuppressive properties of mesenchymal stem cells derived from the bone marrow and synovial fluid of minipigs. Exp. Cell Res. 2015, 333, 273–288. [Google Scholar] [CrossRef]
- Chen, X.I.; Armstrong, M.A.; Li, G. Mesenchymal stem cells in immunoregulation. Immunol. Cell Biol. 2006, 84, 413–421. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef]
- Zwingenberger, S.; Yao, Z.; Jacobi, A.; Vater, C.; Valladares, R.D.; Li, C.; Nich, C.; Rao, A.J.; Christman, J.E.; Antonios, J.K.; et al. Enhancement of BMP-2 induced bone regeneration by SDF-1α mediated stem cell recruitment. Tissue Eng. Part A 2014, 20, 810–818. [Google Scholar] [CrossRef]
- Kay, J.; Calabrese, L. The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rheumatology 2004, 43 (Suppl. S3), iii2–iii9. [Google Scholar] [CrossRef]
- van der Pouw Kraan, T.C.; van Gaalen, F.A.; Kasperkovitz, P.V.; Verbeet, N.L.; Smeets, T.J.; Kraan, M.C.; Fero, M.; Tak, P.P.; Huizinga, T.W.; Pieterman, E.; et al. Rheumatoid arthritis is a heterogeneous disease: Evidence for differences in the activation of the STAT-1 pathway between rheumatoid tissues. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2003, 48, 2132–2145. [Google Scholar] [CrossRef]
- El-Jawhari, J.J.; El-Sherbiny, Y.M.; Jones, E.A.; McGonagle, D. Mesenchymal stem cells, autoimmunity and rheumatoid arthritis. QJM Int. J. Med. 2014, 107, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Tratwal, J.; Follin, B.; Ekblond, A.; Kastrup, J.; Haack-Sørensen, M. Identification of a common reference gene pair for qPCR in human mesenchymal stromal cells from different tissue sources treated with VEGF. BMC Mol. Biol. 2014, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, M.; Jäger, A.; Konermann, A. The stability of different housekeeping genes in human periodontal ligament cells under inflammatory conditions. Ann. Anat.-Anat. Anz. 2019, 224, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Thellin, O.; Zorzi, W.; Lakaye, B.; De Borman, B.; Coumans, B.; Hennen, G.; Grisar, T.; Igout, A.; Heinen, E. Housekeeping genes as internal standards: Use and limits. J. Biotechnol. 1999, 75, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, I.; Ojima, M.; Suzuki, S.; Yamaga, M.; Horie, M.; Koga, H.; Tsuji, K.; Miyaguchi, K.; Ogishima, S.; Tanaka, H.; et al. Human mesenchymal stem cells in synovial fluid increase in the knee with degenerated cartilage and osteoarthritis. J. Orthop. Res. 2012, 30, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Morito, T.; Muneta, T.; Hara, K.; Ju, Y.J.; Mochizuki, T.; Makino, H.; Umezawa, A.; Sekiya, I. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology 2008, 47, 1137–1143. [Google Scholar] [CrossRef]
- Jones, E.; Churchman, S.M.; English, A.; Buch, M.H.; Horner, E.A.; Burgoyne, C.H.; Reece, R.; Kinsey, S.; Emery, P.; McGonagle, D.; et al. Mesenchymal stem cells in rheumatoid synovium: Enumeration and functional assessment in relation to synovial inflammation level. Ann. Rheum. Dis. 2010, 69, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Gao, J.; Mi, L.; Zhang, G.; Zhang, L.; Zhang, N.; Huo, R.; Hu, J.; Xu, K. Synovial membrane mesenchymal stem cells: Past life, current situation, and application in bone and joint diseases. Stem Cell Res. Ther. 2020, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, Z.; Yan, S.; Mao, A.; Wang, B.; Ren, H.; Chi, Y.; Han, Z. Evaluation of suitable reference gene for real-time PCR in human umbilical cord mesenchymal stem cells with long-term in vitro expansion. Vitr. Cell. Dev. Biol.-Anim. 2010, 46, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, F.G.; Posada, O.M.; Gallego-Perez, D.; Higuita-Castro, N.; Sarassa, C.; Hansford, D.J.; Agudelo-Florez, P.; López, L.E. Housekeeping gene stability influences the quantification of osteogenic markers during stem cell differentiation to the osteogenic lineage. Cytotechnology 2010, 62, 109–120. [Google Scholar] [CrossRef]
- Jacobi, A.; Rauh, J.; Bernstein, P.; Liebers, C.; Zou, X.; Stiehler, M. Comparative analysis of reference gene stability in human mesenchymal stromal cells during osteogenic differentiation. Biotechnol. Prog. 2013, 29, 1034–1042. [Google Scholar] [CrossRef]
- Jacob, F.; Guertler, R.; Naim, S.; Nixdorf, S.; Fedier, A.; Hacker, N.F.; Heinzelmann-Schwarz, V. Careful selection of reference genes is required for reliable performance of RT-qPCR in human normal and cancer cell lines. PLoS ONE 2013, 8, e59180. [Google Scholar] [CrossRef]
- Schildberg, T.; Rauh, J.; Bretschneider, H.; Stiehler, M. Identification of suitable reference genes in bone marrow stromal cells from osteoarthritic donors. Stem Cell Res. 2013, 11, 1288–1298. [Google Scholar] [CrossRef]
- Pombo-Suarez, M.; Calaza, M.; Gomez-Reino, J.J.; Gonzalez, A. Reference genes for normalization of gene expression studies in human osteoarthritic articular cartilage. BMC Mol. Biol. 2008, 9, 17. [Google Scholar] [CrossRef]
- Wang, X.N.; Yang, Q.W.; Du, Z.W.; Yu, T.; Qin, Y.G.; Song, Y.; Xu, M.; Wang, J.C. Evaluation of the stability of reference genes in bone mesenchymal stem cells from patients with avascular necrosis of the femoral head. Genet. Mol. Res. 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Watanabe, H.; Ishii, H.; Takahashi, K.; Takai, S.; Ozawa, H. Suitable reference gene selection for gene expression studies in knee osteoarthritis synovium using quantitative PCR analysis. Connect. Tissue Res. 2018, 59, 356–368. [Google Scholar] [CrossRef]
- Tanaka, A.; To, J.; O’Brien, B.; Donnelly, S.; Lund, M. Selection of reliable reference genes for the normalisation of gene expression levels following time course LPS stimulation of murine bone marrow derived macrophages. BMC Immunol. 2017, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Montero-Melendez, T.; Perretti, M. Gapdh gene expression is modulated by inflammatory arthritis and is not suitable for qPCR normalization. Inflammation 2014, 37, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Qureshi MA, A.; Ahmed, A.S.; Li, J.; Stark, A.; Eriksson, P.; Ahmed, M. Validation of reference genes for mRNA quantification in adjuvant arthritis. Open J. Rheumatol. Autoimmune Dis. 2012, 2, 64–72. [Google Scholar] [CrossRef]
- Jiang, C.; Meng, L.; Zhu, W.; Shahzad, M.; Yang, X.; Lu, S. Housekeeping gene stability in pristane-induced arthritis and antigen-induced pulmonary inflammation of rats. Inflamm. Res. 2009, 58, 601–609. [Google Scholar] [CrossRef]
- Jeon, R.H.; Lee, W.J.; Son, Y.B.; Bharti, D.; Shivakumar, S.B.; Lee, S.L.; Rho, G.J. PPIA, HPRT1, and YWHAZ genes are suitable for normalization of mRNA expression in long-term expanded human mesenchymal stem cells. BioMed Res. Int. 2019, 2019, 3093545. [Google Scholar] [CrossRef]
- Krampera, M.; Cosmi, L.; Angeli, R.; Pasini, A.; Liotta, F.; Andreini, A.; Santarlasci, V.; Mazzinghi, B.; Pizzolo, G.; Vinante, F.; et al. Role for interferon-γ in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 2006, 24, 386–398. [Google Scholar] [CrossRef]
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in human mesenchymal stem cells: Functional changes and implications in stem cell-based therapy. Int. J. Mol. Sci. 2016, 17, 1164. [Google Scholar] [CrossRef]
- Lee, W.J.; Jang, S.J.; Lee, S.C.; Park, J.S.; Jeon, R.H.; Subbarao, R.B.; Bharti, D.; Shin, J.; Park, B.; Rho, G.J. Selection of reference genes for quantitative real-time polymerase chain reaction in porcine embryos. Reprod. Fertil. Dev. 2017, 29, 357–367. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, Research0034. [Google Scholar] [CrossRef]


| Primer Information | Standard Curve Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Gene Name (Symbol) | Sequence | Base Pair | Accession | R2 | M | B | E | |
| Actin beta (ACTB) | F: | TCCACGAAAC TACCTTCAACTC | 131 | NM_001101.5 | 0.996 | −3.514 | 34.524 | 0.95 |
| R: | GATCTCCTTCT CATCCTGTCG | |||||||
| Beta-2-microglobulin (B2M) | F: | CAGCTACTCCA AAGATTCAGG | 116 | AF_072097.1 | 0.993 | −3.356 | 39.045 | 1.04 |
| R: | GGATGAAACCC AGACACATAGC | |||||||
| Eukaryotic translation elongation factor 1 alpha 1 (EEF1A1) | F: | ACTATCATGAT GCCCCAGGAC | 121 | NM_001402.5 | 0.997 | −3.356 | 32.288 | 0.96 |
| R: | ACACCAGCAG CAACAATCAG | |||||||
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | F: | GTCAACGGATTTGGTCGTATTGG | 108 | NM_002046.7 | 0.995 | −3.499 | 33.652 | 1.03 |
| R: | CATGTAGTTGAG GTCAATGAAGGG | |||||||
| Hypoxanthine phosphoribosyltransferase 1 (HPRT1) | F: | CTGGCGTCGTGA TTAGTGAT | 90 | M_31642.1 | 0.996 | −3.485 | 32.587 | 1.01 |
| R: | ACACCCTTTCCA AATCCTCA | |||||||
| Peptidylprolyl isomerase A (PPIA) | F: | TGCTGGACCCA ACACAAATG | 89 | NM_001002.3 | 0.995 | −3.468 | 32.145 | 0.97 |
| R: | AACACCACATG CTTGCCATC | |||||||
| Ribosomal protein, large, P0 (RPLP0) | F: | TGGGCAAGAAC ACCATGATG | 98 | NM_001402.5 | 0.997 | −3.358 | 38.541 | 1.03 |
| R: | TTTGTGGGACAGCATGGATG | |||||||
| Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta (YWHAZ) | F: | CGAAGCTGAAG CAGGAGAAG | 111 | NM_021130.3 | 0.995 | −3.477 | 35.568 | 1.01 |
| R: | TTTGTGGGACAG CATGGATG | |||||||
| Characteristics | H-SF-MSCs (n = 4) | RA-SF-MSCs (n = 10) | p Value * |
|---|---|---|---|
| Age (years) | 30.5 (3.69) | 58.8 (2.5) | 0.0002 |
| Women | 4 (100%) | 10 (100%) | - |
| Positive for rheumatoid factor | - | 8 (80%) | - |
| Positive for anti-CCP antibody | - | 8 (80%) | - |
| DAS28-ESR | - | 4.75 (0.3) | - |
| Disease duration (years) | - | 7.9 (2.9) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bok, E.-Y.; Kim, S.-B.; Thakur, G.; Choe, Y.-H.; Oh, S.-J.; Hwang, S.-C.; Ock, S.-A.; Rho, G.-J.; Lee, S.-I.; Lee, W.-J.; et al. Insensitive Effects of Inflammatory Cytokines on the Reference Genes of Synovial Fluid Resident-Mesenchymal Stem Cells Derived from Rheumatoid Arthritis Patients. Int. J. Mol. Sci. 2023, 24, 15159. https://doi.org/10.3390/ijms242015159
Bok E-Y, Kim S-B, Thakur G, Choe Y-H, Oh S-J, Hwang S-C, Ock S-A, Rho G-J, Lee S-I, Lee W-J, et al. Insensitive Effects of Inflammatory Cytokines on the Reference Genes of Synovial Fluid Resident-Mesenchymal Stem Cells Derived from Rheumatoid Arthritis Patients. International Journal of Molecular Sciences. 2023; 24(20):15159. https://doi.org/10.3390/ijms242015159
Chicago/Turabian StyleBok, Eun-Yeong, Saet-Byul Kim, Gitika Thakur, Yong-Ho Choe, Seong-Ju Oh, Sun-Chul Hwang, Sun-A. Ock, Gyu-Jin Rho, Sang-Il Lee, Won-Jae Lee, and et al. 2023. "Insensitive Effects of Inflammatory Cytokines on the Reference Genes of Synovial Fluid Resident-Mesenchymal Stem Cells Derived from Rheumatoid Arthritis Patients" International Journal of Molecular Sciences 24, no. 20: 15159. https://doi.org/10.3390/ijms242015159






