Current and Novel Therapies for Eosinophilic Gastrointestinal Diseases
Abstract
:1. Introduction
2. Eosinophils’ Distribution and Activity in the Gastrointestinal Tract
3. Eosinophilic Esophagitis (EOE)
3.1. Pathophysiology
3.2. Clinical Manifestations
3.3. Diagnostic Criteria
3.4. Current Standard Therapies
3.5. Emerging Therapies

3.6. Therapeutic Targets and Current Evidence
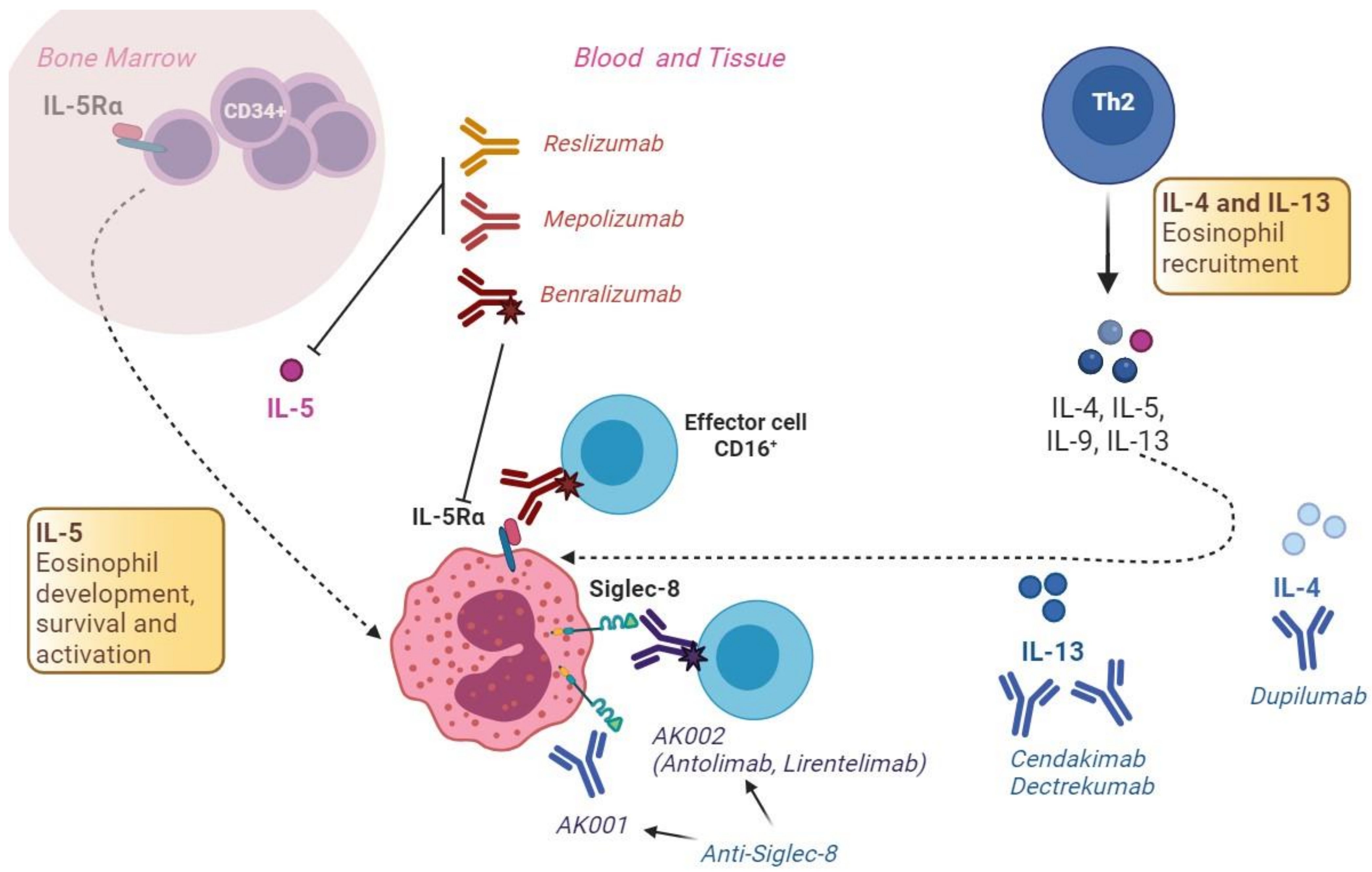
3.6.1. The IL-5 Pathway
3.6.2. The IL-13 Pathway
3.6.3. The IL-4 Pathway
3.6.4. IgE
3.6.5. Integrins
3.6.6. CRTH2
3.6.7. The IL-15 Pathway
3.6.8. The TNF-α Pathway
3.6.9. The JAK/STAT Pathway
3.6.10. TGF-β
3.6.11. TSLP
3.6.12. Sphingosine 1-Phosphate [S1P] Receptor
3.6.13. The KIT Pathway
4. Eosinophilic Gastritis and Enteritis
4.1. Pathophysiology
4.2. Clinical Manifestations
4.3. Diagnostic Criteria
4.4. Current Treatment
4.5. Emerging Therapies
4.5.1. The IL-5 Pathway
4.5.2. Siglec-8
5. Eosinophilic Colitis (EOC)
5.1. Epidemiology
5.2. Pathophysiology
5.3. Clinical Manifestations
5.4. Diagnostic Criteria
5.5. Current Treatment
5.6. Emerging Therapies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hahn, J.W.; Lee, K.; Shin, J.I.; Cho, S.H.; Turner, S.; Shin, J.U.; Yeniova, A.; Koyanagi, A.; Jacob, L.; Smith, L.; et al. Global Incidence and Prevalence of Eosinophilic Esophagitis, 1976–2022: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2023, in press. [Google Scholar] [CrossRef]
- Dellon, E.S.; Gonsalves, N.; Abonia, J.P.; Alexander, J.A.; Arva, N.C.; Atkins, D.; Attwood, S.E.; Auth, M.K.H.; Bailey, D.D.; Biederman, L.; et al. International Consensus Recommendations for Eosinophilic Gastrointestinal Disease Nomenclature. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2022, 20, 2474–2484.e3. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, J.R.; Plotnick, L.M.; Fende, J.M.; Collins, M.H.; Putnam, P.E.; Rothenberg, M.E. Eosinophil-associated gastrointestinal disorders: A world-wide-web based registry. J. Pediatr. 2002, 141, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Díaz del Arco, C.; Taxonera, C.; Olivares, D.; Fernández Aceñero, M.J. Eosinophilic colitis: Case series and literature review. Pathol. Res. Pract. 2018, 214, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Visaggi, P.; Savarino, E.; Del Corso, G.; Hunter, H.; Svizzero, F.B.; Till, S.J.; Wong, T.; de Bortoli, N.; Zeki, S. Six-Food Elimination Diet is Less Effective During Pollen Season in Adults with Eosinophilic Esophagitis Sensitized to Pollens. Am. J. Gastroenterol. 2023. [Google Scholar] [CrossRef]
- Sciumè, G.D.; Visaggi, P.; Sostilio, A.; Tarducci, L.; Pugno, C.; Frazzoni, M.; Ricchiuti, A.; Bellini, M.; Giannini, E.G.; Marchi, S.; et al. Eosinophilic esophagitis: Novel concepts regarding pathogenesis and clinical manifestations. Minerva Gastroenterol. 2022, 68, 23–39. [Google Scholar] [CrossRef]
- Allen-Brady, K.; Colletier, K.J.; Woller, S.; Eliason, K.; Uchida, A.M.; Ro, G.; Newman, M.; Peterson, K.A. Eosinophilic Gastritis and Enteritis Are Increased in Families With Eosinophilic Esophagitis. Am. J. Gastroenterol. 2023, 118, 263–268. [Google Scholar] [CrossRef]
- Visaggi, P.; Savarino, E.; Sciume, G.; Chio, T.D.; Bronzini, F.; Tolone, S.; Frazzoni, M.; Pugno, C.; Ghisa, M.; Bertani, L.; et al. Eosinophilic esophagitis: Clinical, endoscopic, histologic and therapeutic differences and similarities between children and adults. Ther. Adv. Gastroenterol. 2021, 14, 1756284820980860. [Google Scholar] [CrossRef]
- Talley, N.J.; Shorter, R.G.; Phillips, S.F.; Zinsmeister, A.R. Eosinophilic gastroenteritis: A clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut 1990, 31, 54–58. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Storr, M.A.; Shaffer, E.A. Eosinophilic colitis: Epidemiology, clinical features, and current management. Ther. Adv. Gastroenterol. 2011, 4, 301–309. [Google Scholar] [CrossRef]
- Uppal, V.; Kreiger, P.; Kutsch, E. Eosinophilic gastroenteritis and colitis: A comprehensive review. Clin. Rev. Allergy Immunol. 2016, 50, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Rothenberg, M.E. Roles and Regulation of Gastrointestinal Eosinophils in Immunity and Disease. J. Immunol. 2014, 193, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.E.; Chetty, R. Eosinophilic gastroenteritis: A review. J. Gastroenterol. 2008, 43, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Khan, S. Eosinophilic gastroenteritis. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 177–198. [Google Scholar] [CrossRef]
- Lowichik, A.; Weinberg, A.G. A quantitative evaluation of mucosal eosinophils in the pediatric gastrointestinal tract. Mod. Pathol. 1996, 9, 110–114. [Google Scholar]
- Zhang, M.; Li, Y. Eosinophilic gastroenteritis: A state-of-the-art review. J. Gastroenterol. Hepatol. 2017, 32, 64–72. [Google Scholar] [CrossRef]
- Visaggi, P.; Ghisa, M.; Barberio, B.; Maniero, D.; Greco, E.; Savarino, V.; Black, C.J.; Ford, A.C.; de Bortoli, N.; Savarino, E. Treatment Trends for Eosinophilic Esophagitis and the Other Eosinophilic Gastrointestinal Diseases: Systematic Review of Clinical Trials. Dig. Liver Dis. 2023, 55, 208–222. [Google Scholar] [CrossRef]
- Matsushita, T.; Maruyama, R.; Ishikawa, N.; Harada, Y.; Araki, A.; Chen, D.; Tauchi-Nishi, P.; Yuki, T.; Kinoshita, Y. The Number and Distribution of Eosinophils in the Adult Human Gastrointestinal Tract: A Study and Comparison of Racial and Environmental Factors. Am. J. Surg. Pathol. 2015, 39, 521–527. [Google Scholar] [CrossRef]
- Turner, K.O.; Sinkre, R.A.; Neumann, W.L.; Genta, R.M. Primary Colonic Eosinophilia and Eosinophilic Colitis in Adults. Am. J. Surg. Pathol. 2017, 41, 225–233. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Molina-Infante, J.; Arias, Á.; Von Arnim, U.; Bredenoord, A.J.; Bussmann, C.; Dias, J.A.; Bove, M.; González-Cervera, J.; Larsson, H.; et al. Guidelines on eosinophilic esophagitis: Evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur. Gastroenterol. J. 2017, 5, 335–358. [Google Scholar] [CrossRef]
- Hirano, I.; Chan, E.S.; Rank, M.A.; Sharaf, R.N.; Stollman, N.H.; Stukus, D.R.; Wang, K.; Greenhawt, M.; Falck-Ytter, Y.T.; Chachu, K.A.; et al. AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters Clinical Guidelines for the Management of Eosinophilic Esophagitis. Gastroenterology 2020, 158, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- de Bortoli, N.; Penagini, R.; Savarino, E.; Marchi, S. Eosinophilic esophagitis: Update in diagnosis and management. Position paper by the Italian Society of Gastroenterology and Gastrointestinal Endoscopy (SIGE). Dig. Liver Dis. 2017, 49, 254–260. [Google Scholar] [CrossRef]
- Furuta, G.T.; Liacouras, C.A.; Collins, M.H.; Gupta, S.K.; Justinich, C.; Putnam, P.E.; Bonis, P.; Hassall, E.; Straumann, A.; Rothenberg, M.E. Eosinophilic Esophagitis in Children and Adults: A Systematic Review and Consensus Recommendations for Diagnosis and Treatment: Sponsored by the American Gastroenterological Association (AGA) Institute and North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition. Gastroenterology 2007, 133, 1342–1363. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.M.; Potter, M.; Talley, N.J. Eosinophilic gastroenteritis and other eosinophilic gut diseases distal to the oesophagus. Lancet Gastroenterol. Hepatol. 2018, 3, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Lwin, T.; Melton, S.D.; Genta, R.M. Eosinophilic gastritis: Histopathological characterization and quantification of the normal gastric eosinophil content. Mod. Pathol. 2011, 24, 556–563. [Google Scholar] [CrossRef]
- Dellon, E.S.; Gonsalves, N.; Rothenberg, M.E.; Hirano, I.; Chehade, M.; Peterson, K.A.; Falk, G.W.; Murray, J.A.; Gehman, L.T.; Chang, A.T.; et al. Determination of Biopsy Yield That Optimally Detects Eosinophilic Gastritis and/or Duodenitis in a Randomized Trial of Lirentelimab. Clin. Gastroenterol. Hepatol. 2022, 20, 535–545.e15. [Google Scholar] [CrossRef]
- Collins, M.H. Histopathology Associated with Eosinophilic Gastrointestinal Diseases. Immunol. Allergy Clin. N. Am. 2009, 29, 109–117. [Google Scholar] [CrossRef]
- Yan, B.M.; Shaffer, E.A. Primary eosinophilic disorders of the gastrointestinal tract. Gut 2008, 58, 721–732. [Google Scholar] [CrossRef]
- Impellizzeri, G.; Marasco, G.; Eusebi, L.H.; Salfi, N.; Bazzoli, F.; Zagari, R.M. Eosinophilic colitis: A clinical review. Dig. Liver Dis. 2019, 51, 769–773. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Storr, M.A.; Shaffer, E.A. Eosinophilic colitis: An update on pathophysiology and treatment. Br. Med. Bull. 2011, 100, 59–72. [Google Scholar] [CrossRef]
- Azouz, N.P.; Ynga-Durand, M.A.; Caldwell, J.M.; Jain, A.; Rochman, M.; Fischesser, D.M.; Ray, L.M.; Bedard, M.C.; Mingler, M.K.; Forney, C.; et al. The antiprotease SPINK7 serves as an inhibitory checkpoint for esophageal epithelial inflammatory responses. Sci. Transl. Med. 2018, 10, eaap9736. [Google Scholar] [CrossRef] [PubMed]
- Sherrill, J.D.; Kc, K.; Wu, D.; Djukic, Z.; Caldwell, J.M.; Stucke, E.M.; Kemme, K.A.; Costello, M.S.; Mingler, M.K.; Blanchard, C.; et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol. 2013, 7, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Visaggi, P.; Ghisa, M.; Marabotto, E.; Venturini, A.; Donati, D.S.; Bellini, M.; Savarino, V.; de Bortoli, N.; Savarino, E. Esophageal dysmotility in patients with eosinophilic esophagitis: Pathogenesis, assessment tools, manometric characteristics, and clinical implications. Esophagus 2022, 20, 29–38. [Google Scholar] [CrossRef]
- Dellon, E.S.; Jensen, E.T.; Martin, C.F.; Shaheen, N.J.; Kappelman, M.D. Prevalence of eosinophilic esophagitis in the United States. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2014, 12, 589–596.e1. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, J.; Talley, N.J.; Aro, P.; Storskrubb, T.; Johansson, S.-E.; Lind, T.; Bolling-Sternevald, E.; Vieth, M.; Stolte, M.; Walker, M.M.; et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: The population-based Ka-lixanda study. Gut 2007, 56, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Straumann, A.; Simon, H.-U. Eosinophilic esophagitis: Escalating epidemiology? J. Allergy Clin. Immunol. 2005, 115, 418–419. [Google Scholar] [CrossRef]
- Navarro, P.; Arias, Á.; Arias-González, L.; Laserna-Mendieta, E.J.; Ruiz-Ponce, M.; Lucendo, A.J. Systematic review with meta-analysis: The growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment. Pharmacol. Ther. 2019, 49, 1116–1125. [Google Scholar] [CrossRef]
- Dellon, E.S.; Hirano, I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology 2018, 154, 319–332.e3. [Google Scholar] [CrossRef]
- Noel, R.J.; Putnam, P.E.; Rothenberg, M.E. Eosinophilic Esophagitis. N. Engl. J. Med. 2004, 351, 940–941. [Google Scholar] [CrossRef]
- Vitellas, K.M.; Bennett, W.F.; Bova, J.G.; Johnston, J.C.; Caldwell, J.H.; Mayle, J.E. Idiopathic eosinophilic esophagitis. Radiology 1993, 186, 789–793. [Google Scholar] [CrossRef]
- Dellon, E.S.; Liacouras, C.A. Advances in Clinical Management of Eosinophilic Esophagitis. Gastroenterology 2014, 147, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Visaggi, P.; Ghisa, M.; Barberio, B.; Marabotto, E.; de Bortoli, N.; Savarino, E. Systematic Review: Esophageal motility patterns in patients with eosinophilic esophagitis. Dig. Liver Dis. 2022, 54, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Haboubi, H.N.; Attwood, S.E.; Auth, M.K.H.; Dunn, J.M.; Sweis, R.; Morris, D.; Epstein, J.; Novelli, M.R.; Hunter, H.; et al. British Society of Gastroenterology (BSG) and British Society of Paediatric Gastroenterology, Hepatology and Nutrition (BSPGHAN) joint consensus guidelines on the diagnosis and management of eosinophilic oesophagitis in children and adults. Gut 2022, 71, 1459–1487. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Liacouras, C.A.; Molina-Infante, J.; Furuta, G.T.; Spergel, J.M.; Zevit, N.; Spechler, S.J.; Attwood, S.E.; Straumann, A.; Aceves, S.S.; et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018, 155, 1022–1033.e10. [Google Scholar] [CrossRef]
- Hirano, I.; Moy, N.; Heckman, M.G.; Thomas, C.S.; Gonsalves, N.; Achem, S.R. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: Validation of a novel classification and grading system. Gut 2012, 62, 489–495. [Google Scholar] [CrossRef]
- Protheroe, C.; Woodruff, S.A.; de Petris, G.; Mukkada, V.; Ochkur, S.I.; Janarthanan, S.; Lewis, J.C.; Pasha, S.; Lunsford, T.; Harris, L.; et al. A Novel Histologic Scoring System to Evaluate Mucosal Biopsies From Patients With Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2009, 7, 749–755.e11. [Google Scholar] [CrossRef]
- Kirsch, R.; Bokhary, R.; Marcon, M.A.; Cutz, E. Activated Mucosal Mast Cells Differentiate Eosinophilic (Allergic) Esophagitis From Gastroesophageal Reflux Disease. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 20–26. [Google Scholar] [CrossRef]
- Vicario, M.; Blanchard, C.; Stringer, K.F.; Collins, M.H.; Mingler, M.K.; Ahrens, A.; Putnam, P.E.; Abonia, J.P.; Santos, J.; Rothenberg, M.E. Local B cells and IgE production in the oesophageal mucosa in eosinophilic oesophagitis. Gut 2010, 59, 12–20. [Google Scholar] [CrossRef]
- Arias, A.; González-Cervera, J.; Tenias, J.M.; Lucendo, A.J. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: A systematic review and meta-analysis. Gastroenterology 2014, 146, 1639–1648. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Lucendo, A.J. Eosinophilic esophagitis: A practical approach to diagnosis and management. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 925–934. [Google Scholar] [CrossRef]
- Dellon, E.S.; Gupta, S.K. A Conceptual Approach to Understanding Treatment Response in Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2019, 17, 2149–2160. [Google Scholar] [CrossRef]
- Liacouras, C.A.; Furuta, G.T.; Hirano, I.; Atkins, D.; Attwood, S.E.; Bonis, P.A.; Burks, A.W.; Chehade, M.; Collins, M.H.; Dellon, E.S.; et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J. Allergy Clin. Immunol. 2011, 128, 3–20.e6. [Google Scholar] [CrossRef]
- Visaggi, P.; Mariani, L.; Pardi, V.; Rosi, E.M.; Pugno, C.; Bellini, M.; Zingone, F.; Ghisa, M.; Marabotto, E.; Giannini, E.G.; et al. Dietary Management of Eosinophilic Esophagitis: Tailoring the Approach. Nutrients 2021, 13, 1630. [Google Scholar] [CrossRef] [PubMed]
- Visaggi, P.; Baiano Svizzero, F.; Savarino, E. Food elimination diets in eosinophilic esophagitis: Practical tips in current management and future directions. Best Pract. Res. Clin. Gastroenterol. 2023, 62–63, 101825. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, J.P.; Hommel, K.A.; DeBrosse, C.W.; Greenberg, A.B.; Greenler, A.J.; Abonia, J.P.; Rothenberg, M.E.; Varni, J.W. Quality of life in paediatric eosinophilic oesophagitis: What is important to patients?: Paediatric eosinophilic oesophagitis QOL. Child. Care Health Dev. 2012, 38, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Laserna-Mendieta, E.J.; Casabona, S.; Guagnozzi, D.; Savarino, E.; Perelló, A.; Guardiola-Arévalo, A.; Barrio, J.; Pérez-Martínez, I.; Lund Krarup, A.; Alcedo, J.; et al. Efficacy of proton pump inhibitor therapy for eosinophilic oesophagitis in 630 patients: Results from the EoE connect registry. Aliment. Pharmacol. Ther. 2020, 52, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Lucendo, A.J.; Arias, Á.; Molina-Infante, J. Efficacy of Proton Pump Inhibitor Drugs for Inducing Clinical and Histologic Remission in Patients With Symptomatic Esophageal Eosinophilia: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 13–22.e1. [Google Scholar] [CrossRef]
- Remedios, M.; Campbell, C.; Jones, D.M.; Kerlin, P. Eosinophilic esophagitis in adults: Clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest. Endosc. 2006, 63, 3–12. [Google Scholar] [CrossRef]
- Alexander, J.A.; Jung, K.W.; Arora, A.S.; Enders, F.; Katzka, D.A.; Kephardt, G.M.; Kita, H.; Kryzer, L.A.; Romero, Y.; Smyrk, T.C.; et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2012, 10, 742–749.e1. [Google Scholar] [CrossRef] [PubMed]
- Konikoff, M.R.; Noel, R.J.; Blanchard, C.; Kirby, C.; Jameson, S.C.; Buckmeier, B.K.; Akers, R.; Cohen, M.B.; Collins, M.H.; Assa’ad, A.H.; et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology 2006, 131, 1381–1391. [Google Scholar] [CrossRef]
- Cotton, C.C.; Eluri, S.; Wolf, W.A.; Dellon, E.S. Six-Food Elimination Diet and Topical Steroids are Effective for Eosinophilic Esophagitis: A Meta-Regression. Dig. Dis. Sci. 2017, 62, 2408–2420. [Google Scholar] [CrossRef]
- Miehlke, S.; Schlag, C.; Lucendo, A.J.; Biedermann, L.; Vaquero, C.S.; Schmoecker, C.; Hayat, J.; Hruz, P.; Ciriza de Los Rios, C.; Bredenoord, A.J.; et al. Budesonide orodispersible tablets for induction of remission in patients with active eosinophilic oesophagitis: A 6-week open-label trial of the EOS-2 Programme. United Eur. Gastroenterol. J. 2022, 10, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Straumann, A.; Lucendo, A.J.; Miehlke, S.; Vieth, M.; Schlag, C.; Biedermann, L.; Vaquero, C.S.; Ciriza de Los Rios, C.; Schmoecker, C.; Madisch, A.; et al. Budesonide Orodispersible Tablets Maintain Remission in a Randomized, Placebo-Controlled Trial of Patients With Eosinophilic Esophagitis. Gastroenterology 2020, 159, 1672–1685.e5. [Google Scholar] [CrossRef] [PubMed]
- Visaggi, P.; Baiano Svizzero, F.; Del Corso, G.; Bellini, M.; Savarino, E.; de Bortoli, N. Efficacy of a Second PPI Course After Steroid-Induced Remission in Eosinophilic Esophagitis Refractory to Initial PPI Therapy. Am. J. Gastroenterol. 2022, 117, 1702–1705. [Google Scholar] [CrossRef] [PubMed]
- Schoepfer, A.M.; Gonsalves, N.; Bussmann, C.; Conus, S.; Simon, H.-U.; Straumann, A.; Hirano, I. Esophageal dilation in eosinophilic esophagitis: Effectiveness, safety, and impact on the underlying inflammation. Am. J. Gastroenterol. 2010, 105, 1062–1070. [Google Scholar] [CrossRef]
- Robles-Medranda, C.; Villard, F.; le Gall, C.; Lukashok, H.; Rivet, C.; Bouvier, R.; Dumortier, J.; Lachaux, A. Severe dysphagia in children with eosinophilic esophagitis and esophageal stricture: An indication for balloon dilation? J. Pediatr. Gastroenterol. Nutr. 2010, 50, 516–520. [Google Scholar] [CrossRef]
- Lucendo, A.J. Cellular and molecular immunological mechanisms in eosinophilic esophagitis: An updated overview of their clinical implications. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 669–685. [Google Scholar] [CrossRef]
- Hirano, I.; Aceves, S.S. Clinical implications and pathogenesis of esophageal remodeling in eosinophilic esophagitis. Gastroenterol. Clin. N. Am. 2014, 43, 297–316. [Google Scholar] [CrossRef]
- Farne, H.A.; Wilson, A.; Powell, C.; Bax, L.; Milan, S.J. Anti-IL5 therapies for asthma. Cochrane Database Syst. Rev. 2017, 9, CD010834. [Google Scholar] [CrossRef]
- Castro, M.; Zangrilli, J.; Wechsler, M.E.; Bateman, E.D.; Brusselle, G.G.; Bardin, P.; Murphy, K.; Maspero, J.F.; O’Brien, C.; Korn, S. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: Results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir. Med. 2015, 3, 355–366. [Google Scholar] [CrossRef]
- Spergel, J.M.; Rothenberg, M.E.; Collins, M.H.; Furuta, G.T.; Markowitz, J.E.; Fuchs, G., III; O’Gorman, M.A.; Abonia, J.P.; Young, J.; Henkel, T.; et al. Reslizumab in children and adolescents with eosinophilic esophagitis: Results of a double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2012, 129, 456–463.e3. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.E.; Klion, A.D.; Roufosse, F.E.; Kahn, J.E.; Weller, P.F.; Simon, H.-U.; Schwartz, L.B.; Rosenwasser, L.J.; Ring, J.; Griffin, E.F.; et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N. Engl. J. Med. 2008, 358, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Straumann, A.; Conus, S.; Grzonka, P.; Kita, H.; Kephart, G.; Bussmann, C.; Beglinger, C.; Smith, D.A.; Patel, J.; Byrne, M.; et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: A randomised, placebo-controlled, double-blind trial. Gut 2010, 59, 21–30. [Google Scholar] [CrossRef]
- Assa’ad, A.H.; Gupta, S.K.; Collins, M.H.; Thomson, M.; Heath, A.T.; Smith, D.A.; Perschy, T.L.; Jurgensen, C.H.; Ortega, H.G.; Aceves, S.S. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology 2011, 141, 1593–1604. [Google Scholar] [CrossRef]
- Dellon, E.S.; Peterson, K.A.; Mitlyng, B.L.; Iuga, A.; Bookhout, C.E.; Cortright, L.M.; Walker, K.B.; Gee, T.S.; McGee, S.J.; Cameron, B.A.; et al. Mepolizumab for treatment of adolescents and adults with eosinophilic oesophagitis: A multicentre, randomised, double-blind, placebo-controlled clinical trial. Gut 2023, 72, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Hirano, I.; Collins, M.H.; Assouline-Dayan, Y.; Evans, L.; Gupta, S.; Schoepfer, A.M.; Straumann, A.; Safroneeva, E.; Grimm, M.; Smith, H.; et al. RPC4046, a Monoclonal Antibody Against IL13, Reduces Histologic and Endoscopic Activity in Patients With Eosinophilic Esophagitis. Gastroenterology 2019, 156, 592–603.e10. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Collins, M.H.; Rothenberg, M.E.; Assouline-Dayan, Y.; Evans, L.; Gupta, S.; Schoepfer, A.; Straumann, A.; Safroneeva, E.; Rodriguez, C.; et al. Long-term Efficacy and Tolerability of RPC4046 in an Open-Label Extension Trial of Patients With Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2021, 19, 473–483.e17. [Google Scholar] [CrossRef]
- Rothenberg, M.E.; Wen, T.; Greenberg, A.; Alpan, O.; Enav, B.; Hirano, I.; Nadeau, K.; Kaiser, S.; Peters, T.; Perez, A.; et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J. Allergy Clin. Immunol. 2015, 135, 500–507. [Google Scholar] [CrossRef]
- Hirano, I.; Dellon, E.S.; Hamilton, J.D.; Collins, M.H.; Peterson, K.; Chehade, M.; Schoepfer, A.M.; Safroneeva, E.; Rothenberg, M.E.; Falk, G.W.; et al. Efficacy of Dupilumab in a Phase 2 Randomized Trial of Adults With Active Eosinophilic Esophagitis. Gastroenterology 2020, 158, 111–122.e10. [Google Scholar] [CrossRef]
- Dellon, E.S.; Rothenberg, M.E.; Collins, M.H.; Hirano, I.; Chehade, M.; Bredenoord, A.J.; Lucendo, A.J.; Spergel, J.M.; Aceves, S.; Sun, X.; et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. New Engl. J. Med. 2022, 387, 2317–2330. [Google Scholar] [CrossRef]
- Clayton, F.; Fang, J.C.; Gleich, G.J.; Lucendo, A.J.; Olalla, J.M.; Vinson, L.A.; Lowichik, A.; Chen, X.; Emerson, L.; Cox, K.; et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology 2014, 147, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Cadden, P.; Hunter, M.; Pearce Collins, L.; Perkins, M.; Pettipher, R.; Townsend, E.; Vinall, S.; O’Connor, B. Inhibition of the asthmatic allergen challenge response by the CRTH2 antagonist OC000459. Eur. Respir. J. 2013, 41, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Straumann, A.; Hoesli, S.; Bussmann, C.; Stuck, M.; Perkins, M.; Collins, L.P.; Payton, M.; Pettipher, R.; Hunter, M.; Steiner, J.; et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy 2013, 68, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Straumann, A.; Bussmann, C.; Conus, S.; Beglinger, C.; Simon, H.-U. Anti-TNF-alpha (infliximab) therapy for severe adult eosinophilic esophagitis. J. Allergy Clin. Immunol. 2008, 122, 425–427. [Google Scholar] [CrossRef]
- Banerjee, S.; Biehl, A.; Gadina, M.; Hasni, S.; Schwartz, D.M. JAK-STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs 2017, 77, 521–546. [Google Scholar] [CrossRef]
- Mendoza Alvarez, L.B.; Liu, X.; Glover, S. Treatment-resistant eosinophilic oesophagitis successfully managed with tofacitinib. BMJ Case Rep. 2019, 12, e232558. [Google Scholar] [CrossRef]
- Arias, Á.; Lucendo, A.J. Molecular basis and cellular mechanisms of eosinophilic esophagitis for the clinical practice. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 99–117. [Google Scholar] [CrossRef]
- Rothenberg, M.E.; Spergel, J.M.; Sherrill, J.D.; Annaiah, K.; Martin, L.J.; Cianferoni, A.; Gober, L.; Kim, C.; Glessner, J.; Frackelton, E.; et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat. Genet. 2010, 42, 289–291. [Google Scholar] [CrossRef]
- Abonia, J.P.; Wen, T.; Stucke, E.M.; Grotjan, T.; Griffith, M.S.; Kemme, K.A.; Collins, M.H.; Putnam, P.E.; Franciosi, J.P.; von Tiehl, K.F.; et al. High prevalence of eosinophilic esophagitis in patients with inherited connective tissue disorders. J. Allergy Clin. Immunol. 2013, 132, 378–386. [Google Scholar] [CrossRef]
- Blaho, V.A.; Hla, T. An update on the biology of sphingosine 1-phosphate receptors. J. Lipid Res. 2014, 55, 1596–1608. [Google Scholar] [CrossRef]
- Sherrill, J.D.; KC, K.; Blanchard, C.; Stucke, E.M.; Kemme, K.A.; Collins, M.H.; Abonia, J.P.; Putnam, P.E.; Mukkada, V.A.; Kaul, A.; et al. Analysis and expansion of the eosinophilic esophagitis transcriptome by RNA sequencing. Genes Immun. 2014, 15, 361–369. [Google Scholar] [CrossRef]
- Mansoor, E.; Saleh, M.A.; Cooper, G.S. Prevalence of Eosinophilic Gastroenteritis and Colitis in a Population-Based Study, From 2012 to 2017. Clin. Gastroenterol. Hepatol. 2017, 15, 1733–1741. [Google Scholar] [CrossRef]
- Jensen, E.T.; Martin, C.F.; Kappelman, M.D.; Dellon, E.S. Prevalence of eosinophilic gastritis, gastroenteritis, and colitis: Estimates from a national administrative database. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 36–42. [Google Scholar] [CrossRef]
- Hui, C.K.; Hui, N.K. A Prospective Study on the Prevalence, Extent of Disease and Outcome of Eosinophilic Gastroenteritis in Patients Presenting with Lower Abdominal Symptoms. Gut Liver 2018, 12, 288–296. [Google Scholar] [CrossRef]
- Sunkara, T.; Rawla, P.; Yarlagadda, K.S.; Gaduputi, V. Eosinophilic gastroenteritis: Diagnosis and clinical perspectives. Clin. Exp. Gastroenterol. 2019, 12, 239–253. [Google Scholar] [CrossRef]
- Klein, N.C.; Hargrove, R.L.; Sleisenger, M.H.; Jeffries, G.H. Eosinophilic gastroenteritis. Medicine (Baltimore) 1970, 49, 299–319. [Google Scholar] [CrossRef]
- Pineton de Chambrun, G.; Gonzalez, F.; Canva, J.; Gonzalez, S.; Houssin, L.; Desreumaux, P.; Cortot, A.; Colombel, J. Natural History of Eosinophilic Gastroenteritis. Clin. Gastroenterol. Hepatol. 2011, 9, 950–956.e1. [Google Scholar] [CrossRef]
- Cello, J.P. Eosinophilic gastroenteritis--a complex disease entity. Am. J. Med. 1979, 67, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, J.M.; Genta, R.M.; Melton, S.D. Histopathologic Diagnosis of Eosinophilic Conditions in the Gastrointestinal Tract. Adv. Anat. Pathol. 2011, 18, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Ulmer, F.A. Eosinophils and allergic diseases of the gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2008, 22, 455–479. [Google Scholar] [CrossRef] [PubMed]
- Egritas Gurkan, O.; Ozturk, H.; Karagol, H.I.E.; Ceylan, K.; Duztas, D.T.; Ekinci, O.; Sari, S.; Dalgic, B.; Bakirtas, A. Primary Eosinophilic Gastrointestinal Diseases Beyond Eosinophilic Esophagitis in Children. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.; Woosley, J.T.; Dellon, E.S. Clinical characteristics, treatment outcomes, and resource utilization in children and adults with eosinophilic gastroenteritis. Dig. Liver Dis. 2015, 47, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Manatsathit, W.; Sermsathanasawadi, R.; Pongpaiboon, A.; Pongprasobchai, S. Mucosal-type eosinophilic gastroenteritis in Thailand: 12-year retrospective study. J. Med. Assoc. Thai 2013, 96 (Suppl. 2), S194–S202. [Google Scholar] [PubMed]
- Ko, H.M.; Morotti, R.A.; Yershov, O.; Chehade, M. Eosinophilic gastritis in children: Clinicopathological correlation, disease course, and response to therapy. Am. J. Gastroenterol. 2014, 109, 1277–1285. [Google Scholar] [CrossRef]
- Yalon, M.; Tahboub Amawi, A.D.; Kelm, Z.S.; Wells, M.L.; Teo, L.L.S.; Heiken, J.P.; Sheedy, S.P.; Torbenson, M.S.; Fidler, J.L.; Venkatesh, S.K. Eosinophilic Disorders of the Gastrointestinal Tract and Associated Abdominal Viscera: Imaging Findings and Diagnosis. Radiographics 2022, 42, 1081–1102. [Google Scholar] [CrossRef]
- Marco-Doménech, S.F.; Gil-Sánchez, S.; Jornet-Fayos, J.; Ambit-Capdevila, S.; Gonzalez-Añón, M. Eosinophilic gastroenteritis: Percutaneous biopsy under ultrasound guidance. Abdom. Imaging 1998, 23, 286–288. [Google Scholar] [CrossRef]
- Zheng, X.; Cheng, J.; Pan, K.; Yang, K.; Wang, H.; Wu, E. Eosinophilic enteritis: CT features. Abdom. Imaging 2008, 33, 191–195. [Google Scholar] [CrossRef]
- Anuradha, C.; Mittal, R.; Yacob, M.; Manipadam, M.T.; Kurian, S.; Eapen, A. Eosinophilic disorders of the gastrointestinal tract: Imaging features. Diagn. Interv. Radiol. 2012, 18, 183–188. [Google Scholar] [CrossRef]
- Huang, X.; Liao, X.; Xiao, Z.; Huang, Z. Halo sign and araneid limb-like sign in eosinophilic enteritis. Lancet Gastroenterol. Hepatol. 2020, 5, 954. [Google Scholar] [CrossRef]
- Amruthesh, T.M.; Kini, D.; Yachha, S.K.; Rao, P.; Shetty, S.S.; Kumar, V. Eosinophilic gastroenteritis: Clinical characteristics and management. Indian. J. Gastroenterol. 2021, 40, 338–343. [Google Scholar] [CrossRef]
- Gonsalves, N. Eosinophilic Gastrointestinal Disorders. Clinic Rev. Allerg. Immunol. 2019, 57, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Lopes Vendrami, C.; Kelahan, L.; Escobar, D.J.; Goodhartz, L.; Hammond, N.; Nikolaidis, P.; Yang, G.-Y.; Hirano, I.; Miller, F.H. Imaging Findings of Eosinophilic Gastrointestinal Diseases in Adults. Curr. Probl. Diagn. Radiol. 2023, 52, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Ridolo, E.; Melli, V.; De’ Angelis, G.; Martignago, I. Eosinophilic disorders of the gastro-intestinal tract: An update. Clin. Mol. Allergy 2016, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.-W.; Lim, K.H.; Wan, W.K.; Low, S.C.; Kong, S.C. Eosinophilic gastroenteritis: Clinical profiles and treatment outcomes, a retrospective study of 18 adult patients in a Singapore Tertiary Hospital. Med. J. Malays. 2015, 70, 232–237. [Google Scholar]
- Abou Rached, A.; El Hajj, W. Eosinophilic gastroenteritis: Approach to diagnosis and management. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 513–523. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Serrano-Montalbán, B.; Arias, Á.; Redondo, O.; Tenias, J.M. Efficacy of Dietary Treatment for Inducing Disease Remission in Eosinophilic Gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 56–64. [Google Scholar] [CrossRef]
- Friesen, C.A.; Kearns, G.L.; Andre, L.; Neustrom, M.; Roberts, C.C.; Abdel-Rahman, S.M. Clinical efficacy and pharmacokinetics of montelukast in dyspeptic children with duodenal eosinophilia. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 343–351. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Pardi, D.S.; Murray, J.A. Use of montelukast as steroid-sparing agent for recurrent eosinophilic gastroenteritis. Dig. Dis. Sci. 2001, 46, 1787–1790. [Google Scholar] [CrossRef]
- El-Alali, E.A.; Abukhiran, I.M.; Alhmoud, T.Z. Successful use of montelukast in eosinophilic gastroenteritis: A case report and a literature review. BMC Gastroenterol. 2021, 21, 279. [Google Scholar] [CrossRef]
- Suzuki, J.; Kawasaki, Y.; Nozawa, R.; Isome, M.; Suzuki, S.; Takahashi, A.; Suzuki, H. Oral disodium cromoglycate and ketotifen for a patient with eosinophilic gastroenteritis, food allergy and protein-losing enteropathy. Asian Pac. J. Allergy Immunol. 2003, 21, 193–197. [Google Scholar]
- Pérez-Millán, A.; Martín-Lorente, J.L.; López-Morante, A.; Yuguero, L.; Sáez-Royuela, F. Subserosal eosinophilic gastroenteritis treated efficaciously with sodium cromoglycate. Dig. Dis. Sci. 1997, 42, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Van Dellen, R.G.; Lewis, J.C. Oral administration of cromolyn in a patient with protein-losing enteropathy, food allergy, and eosinophilic gastroenteritis. Mayo Clin. Proc. 1994, 69, 441–444. [Google Scholar] [CrossRef]
- Sheikh, R.A.; Prindiville, T.P.; Pecha, R.E.; Ruebner, B.H. Unusual presentations of eosinophilic gastroenteritis: Case series and review of literature. World J. Gastroenterol. 2009, 15, 2156–2161. [Google Scholar] [CrossRef] [PubMed]
- Beishuizen, A.; van Bodegraven, A.A.; Bronsveld, W.; Sindram, J.W. Eosinophilic gastroenteritis--a disease with a wide clinical spectrum. Neth. J. Med. 1993, 42, 212–217. [Google Scholar] [PubMed]
- Yamada, Y.; Toki, F.; Yamamoto, H.; Nishi, A.; Kato, M. Proton pump inhibitor treatment decreased duodenal and esophageal eosinophilia in a case of eosinophilic gastroenteritis. Allergol. Int. 2015, 64, S83–S85. [Google Scholar] [CrossRef]
- Kuang, F.L.; De Melo, M.S.; Makiya, M.; Kumar, S.; Brown, T.; Wetzler, L.; Ware, J.M.; Khoury, P.; Collins, M.H.; Quezado, M.; et al. Benralizumab Completely Depletes Gastrointestinal Tissue Eosinophils and Improves Symptoms in Eosinophilic Gastrointestinal Disease. J. Allergy Clin. Immunol. Pract. 2022, 10, 1598–1605.e2. [Google Scholar] [CrossRef]
- Kliewer, K.L.; Murray-Petzold, C.; Collins, M.H.; Abonia, J.P.; Bolton, S.M.; DiTommaso, L.A.; Martin, L.J.; Zhang, X.; Mukkada, V.A.; Putnam, P.E.; et al. Benralizumab for eosinophilic gastritis: A single-site, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol. Hepatol. 2023, 8, 803–815. [Google Scholar] [CrossRef]
- Youngblood, B.A.; Brock, E.C.; Leung, J.; Falahati, R.; Bryce, P.J.; Bright, J.; Williams, J.; Shultz, L.D.; Greiner, D.L.; Brehm, M.A.; et al. AK002, a Humanized Sialic Acid-Binding Immunoglobulin-Like Lectin-8 Antibody that Induces Antibody-Dependent Cell-Mediated Cytotoxicity against Human Eosinophils and Inhibits Mast Cell-Mediated Anaphylaxis in Mice. Int. Arch. Allergy Immunol. 2019, 180, 91–102. [Google Scholar] [CrossRef]
- Dellon, E.S.; Peterson, K.A.; Murray, J.A.; Falk, G.W.; Gonsalves, N.; Chehade, M.; Genta, R.M.; Leung, J.; Khoury, P.; Klion, A.D.; et al. Anti-Siglec-8 Antibody for Eosinophilic Gastritis and Duodenitis. N. Engl. J. Med. 2020, 383, 1624–1634. [Google Scholar] [CrossRef]
- Celgene A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Induction and Maintenance Study to Evaluate the Efficacy and Safety of CC-93538 in Adult and Adolescent Japanese Subjects With Eosinophilic Gastroenteritis. 2022. Available online: Clinicaltrials.gov (accessed on 23 April 2023).
- Children’s Hospital Medical Center, Cincinnati A Randomized, Double-blind, Placebo-controlled Clinical Trial to Evaluate the Efficacy of Dupilumab (Anti-IL4a) in Subjects With Eosinophilic Gastritis. 2022. Available online: Clinicaltrials.gov (accessed on 28 April 2023).
- Kim, H.P.; Reed, C.C.; Herfarth, H.H.; Dellon, E.S. Vedolizumab Treatment May Reduce Steroid Burden and Improve Histology in Patients With Eosinophilic Gastroenteritis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2018, 16, 1992–1994. [Google Scholar] [CrossRef]
- Mark, J.; Fernando, S.D.; Masterson, J.C.; Pan, Z.; Capocelli, K.E.; Furuta, G.T.; De Zoeten, E.F. Clinical Implications of Pediatric Colonic Eosinophilia. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Durieu, I.; Nove-Josserand, R.; Cathebras, P.; Durand, D.V.; Rousset, H.; Levrat, R. Ascites à éosinophiles. À propos de deux nouvelles observations. Rev. De Med. Interne 1992, 13, 446–448. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Shaffer, E.A.; Urbanski, S.J.; Storr, M.A. Eosinophilic colitis is a sporadic self-limited disease of middle-aged people: A population-based study. Colorectal Dis. 2014, 16, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.M.; Milla, P.J. Colitis caused by food allergy in infants. Arch. Dis. Child. 1990, 65, 132–133. [Google Scholar] [CrossRef]
- Troncone, R.; Discepolo, V. Colon in food allergy. J. Pediatr. Gastroenterol. Nutr. 2009, 48 (Suppl. 2), S89–S91. [Google Scholar] [CrossRef]
- Box, J.C.; Tucker, J.; Watne, A.L.; Lucas, G. Eosinophilic colitis presenting as a left-sided colocolonic intussusception with secondary large bowel obstruction: An uncommon entity with a rare presentation. Am. Surg. 1997, 63, 741–743. [Google Scholar]
- Velchuru, V.R.; Khan, M.A.B.; Hellquist, H.B.; Studley, J.G.N. Eosinophilic colitis. J. Gastrointest. Surg. 2007, 11, 1373–1375. [Google Scholar] [CrossRef]
- Carmona-Sánchez, R.; Carrera-Álvarez, M.A.; Peña-Zepeda, C. Prevalencia de colitis eosinofílica primaria en pacientes con diarrea crónica y síndrome de intestino irritable con predominio de diarrea. Rev. De Gastroenterol. De México 2022, 87, 135–141. [Google Scholar] [CrossRef]
- Van Sickle, G.J.; Powell, G.K.; McDonald, P.J.; Goldblum, R.M. Milk- and soy protein-induced enterocolitis: Evidence for lymphocyte sensitization to specific food proteins. Gastroenterology 1985, 88, 1915–1921. [Google Scholar] [CrossRef]
- Schoonbroodt, D.; Horsmans, Y.; Laka, A.; Geubel, A.P.; Hoang, P. Eosinophilic gastroenteritis presenting with colitis and cholangitis. Dig. Dis. Sci. 1995, 40, 308–314. [Google Scholar] [CrossRef]
- Brandon, J.L.; Schroeder, S.; Furuta, G.T.; Capocelli, K.; Masterson, J.C.; Fenton, L.Z. CT imaging features of eosinophilic colitis in children. Pediatr. Radiol. 2013, 43, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Han, J.; Ridley, W.E.; Ridley, L.J. Araneid limb-like sign: Eosinophilic enteritis. J. Med. Imaging Radiat. Oncol. 2018, 62, 53. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Kruimel, J.W.; Naber, T.H. Eosinophilic gastroenteritis treated with non-enteric-coated budesonide tablets. Eur. J. Gastroenterol. Hepatol. 2001, 13, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Russel, M.G.; Zeijen, R.N.; Brummer, R.J.; de Bruine, A.P.; van Kroonenburgh, M.J.; Stockbrügger, R.W. Eosinophilic enterocolitis diagnosed by means of technetium-99m albumin scintigraphy and treated with budesonide (CIR). Gut 1994, 35, 1490–1492. [Google Scholar] [CrossRef]
- Rothenberg, M.E. Eosinophilic gastrointestinal disorders (EGID). J. Allergy Clin. Immunol. 2004, 113, 11–28. [Google Scholar] [CrossRef]
- Lee, Y.K.; Mazmanian, S.K. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 2010, 330, 1768–1773. [Google Scholar] [CrossRef]
- Dai, Y.-X.; Shi, C.-B.; Cui, B.-T.; Wang, M.; Ji, G.-Z.; Zhang, F.-M. Fecal microbiota transplantation and prednisone for severe eosinophilic gastroenteritis. World J. Gastroenterol. 2014, 20, 16368–16371. [Google Scholar] [CrossRef]
- Song, D.J.; Cho, J.Y.; Miller, M.; Strangman, W.; Zhang, M.; Varki, A.; Broide, D.H. Anti-Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clin. Immunol. 2009, 131, 157–169. [Google Scholar] [CrossRef]
- Song, D.J.; Shim, M.H.; Lee, N.; Yoo, Y.; Choung, J.T. CCR3 monoclonal antibody inhibits eosinophilic inflammation and mucosal injury in a mouse model of eosinophilic gastroenteritis. Allergy Asthma Immunol. Res. 2017, 9, 360–367. [Google Scholar] [CrossRef]
- Sanofi A Phase 2, Multi-center, Randomized, Double-blind, Placebo-controlled Parallel-group Study to Evaluate the Efficacy and Safety of Dupilumab Therapy in Patients With Moderately to Severely Active Ulcerative Colitis With an Eosinophilic Phenotype. 2023. Available online: Clinicaltrials.gov (accessed on 15 July 2023).
- Kim, H.S.; Noh, G. Treatment of primary eosinophilic colitis using immunoglobulin/histamine complex. Clin. Case Rep. 2023, 11, e6885. [Google Scholar] [CrossRef]
| Disease | Number of Eosinophils |
|---|---|
| Eosinophilic esophagitis | ≥15 eosinophils /HPF [20,21,22,23] |
| Eosinophilic gastritis | ≥30 eosinophils/HPF in ≥5/HPF [24,25] |
| Eosinophilic duodenitis | ≥30 eosinophils/HPF in ≥3/HPF [26] |
| Eosinophilic gastroenteritis | >52 eosinophils/HPF [24,27] |
| Eosinophilic colitis | Right colon >50 eosinophils/HPF or >100 eosinophils /HPF Transverse colon >35 eosinophils /HPF; >eosinophils 84/HPF Left colon >25 eosinophils /HPF; >eosinophils 65/HPF [19] |
| Gastroesophageal reflux disease |
| Achalasia |
| Allergic gastroenteritis/colitis/proctitis |
| Drug reactions (e.g., clopidogrel, aspirin and ticlopidine, non-steroidal antiflammatory drugs (NSAIDs. e.g. ibuprofen and naproxen), estroprogestinic agents, rifampicin, carbamazepine, gold, and tracrolimus) |
| Parasitic, fungal, and viral infections (including spirochaetosis and strongyloides) |
| Inflammatory bowel disease |
| Microscopic colitis |
| Rheumatoid arthritis |
| Vasculitis |
| Collagen vascular diseases (e.g., Eosinophilic granulomatosis with polyangiitis (EGPA)) |
| Granulomatosis with polyangiitis (Wegener’s granulomatosis) |
| Churg–Strauss syndrome |
| Hypereosinophilic syndrome |
| Systemic lupus erythematosus |
| Lymphomas |
| EoE | EoG | EoN | EoC | |
|---|---|---|---|---|
| Benralizumab (anti-IL5Rα) | The study did not meet one of the two dual primary endpoints. Given the lack of clear benefit in this patient population, the study has been terminated (NCT04543409; phase III RCT). | Reduction of intraepithelial gastric eosinophil counts, but no other histologic modifications nor significant clinical improvement [127]. NCT05251909 has been interrupted. | Not evaluated. | Not evaluated. |
| Reslizumab (anti-IL5) | Reduction of intraepithelial esophageal eosinophil counts (Phase II and III RCTs) [71]. | Not evaluated. | Not evaluated. | Not evaluated. |
| Mepolizumab (anti-IL5) | Reduction of intraepithelial esophageal eosinophil counts (RCT) [73,74]. | Not evaluated. | Not evaluated. | Not evaluated. |
| Cendakimab (anti-IL13) | Endoscopic, histologic, and clinical improvements (phase II RCT) [76,77]. Phase III RCT is ongoing (NCT04753697). | Currently being tested (phase III RCT) [130]. | Currently being tested (phase III RCT). | Not evaluated. |
| Dupilumab (anti-IL4R) | Endoscopic, histologic, and clinical improvements and/or remission [80]. Currently the only monoclonal antibody licensed for EoE (phase III RCT). | Currently being tested (phase II RCT) [131]. | Currently being tested (phase II RCT) [131]. | Currently recruiting patients with ulcerative colitis and eosinophilic phenotype (phase II RCT) [152]. |
| Omalizumab (anti-IgE) | Failure in histologic and clinical improvements (phase II RCT) [81]. | Not evaluated. | Not evaluated. | Not evaluated. |
| Infliximab (anti-TNF) | Failure in histologic and clinical improvements (case series) [84]. | Not evaluated. | Not evaluated. | Not evaluated. |
| Tezepelumab (anti-TSLP) | Currently recruiting (NCT05583227; phase III RCT). | Not evaluated. | Not evaluated. | Not evaluated. |
| Etrasimod (S1P receptor modulation) | Currently being tested (NCT04682639; phase II RCT). | Not evaluated. | Not evaluated. | Not evaluated. |
| Barzolvolimab (anti-KIT pathway) | Currently being tested (NCT05774184; phase II RCT). | Not evaluated. | Not evaluated. | Not evaluated. |
| Lirentelimab (anti-Siglec8) | Not evaluated. | Reduction of gastrointestinal eosinophils and symptoms (phase II RCT) [129]. Histologic improvement but no significant clinical improvement (NCT04322604 and NCT04856891; phase III RCT). | Not evaluated. | Not evaluated. |
| Vedolizumab (anti-α4β7 integrin) | Not evaluated. | Histologic and clinical improvements (retrospective cohort study) [132]. | Not evaluated. | Not evaluated. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marasco, G.; Visaggi, P.; Vassallo, M.; Fiocca, M.; Cremon, C.; Barbaro, M.R.; De Bortoli, N.; Bellini, M.; Stanghellini, V.; Savarino, E.V.; et al. Current and Novel Therapies for Eosinophilic Gastrointestinal Diseases. Int. J. Mol. Sci. 2023, 24, 15165. https://doi.org/10.3390/ijms242015165
Marasco G, Visaggi P, Vassallo M, Fiocca M, Cremon C, Barbaro MR, De Bortoli N, Bellini M, Stanghellini V, Savarino EV, et al. Current and Novel Therapies for Eosinophilic Gastrointestinal Diseases. International Journal of Molecular Sciences. 2023; 24(20):15165. https://doi.org/10.3390/ijms242015165
Chicago/Turabian StyleMarasco, Giovanni, Pierfrancesco Visaggi, Mariagiulia Vassallo, Miriam Fiocca, Cesare Cremon, Maria Raffaella Barbaro, Nicola De Bortoli, Massimo Bellini, Vincenzo Stanghellini, Edoardo Vincenzo Savarino, and et al. 2023. "Current and Novel Therapies for Eosinophilic Gastrointestinal Diseases" International Journal of Molecular Sciences 24, no. 20: 15165. https://doi.org/10.3390/ijms242015165
APA StyleMarasco, G., Visaggi, P., Vassallo, M., Fiocca, M., Cremon, C., Barbaro, M. R., De Bortoli, N., Bellini, M., Stanghellini, V., Savarino, E. V., & Barbara, G. (2023). Current and Novel Therapies for Eosinophilic Gastrointestinal Diseases. International Journal of Molecular Sciences, 24(20), 15165. https://doi.org/10.3390/ijms242015165










