An Enzymatic Strategy for the Selective Methylation of High-Value-Added Tetrahydroprotoberberine Alkaloids
Abstract
:1. Introduction
2. Results and Discussion
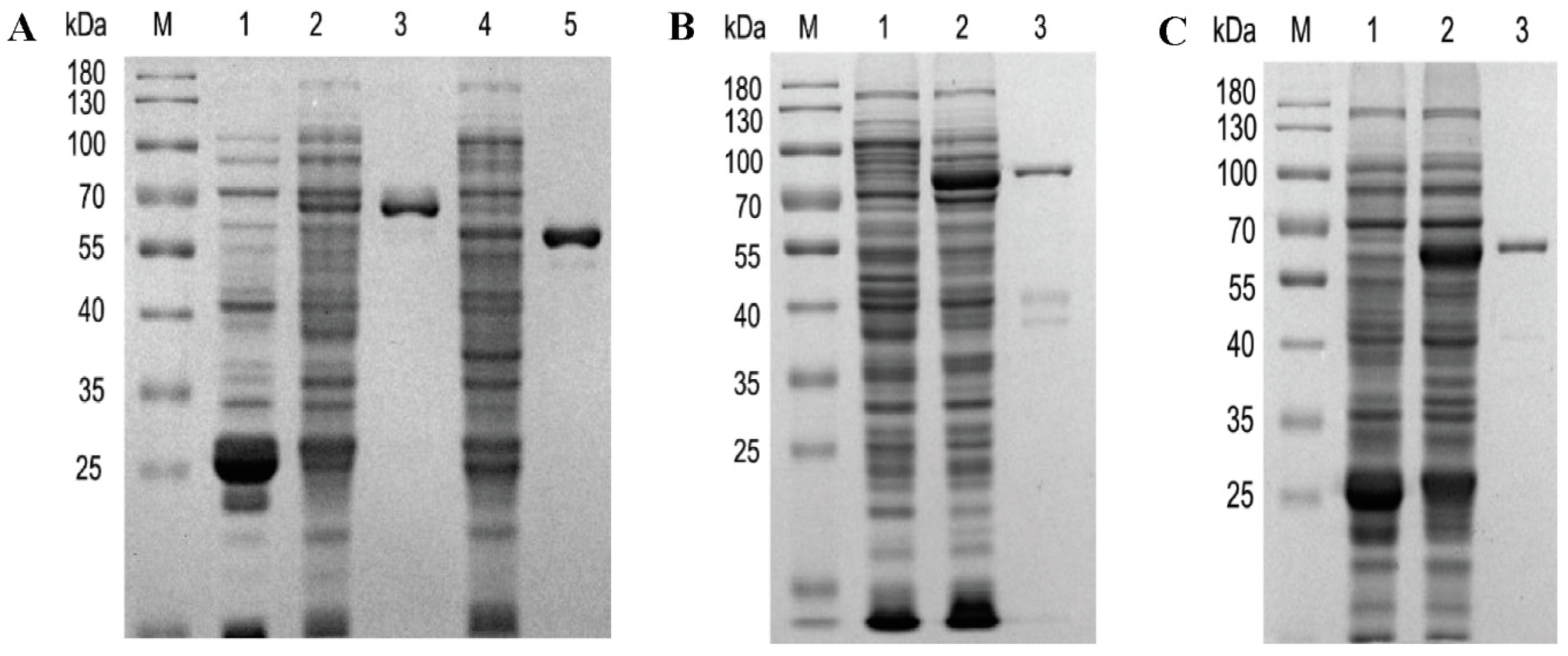
2.1. Heterologous Expression of Recombinant Proteins and Enzyme Assays
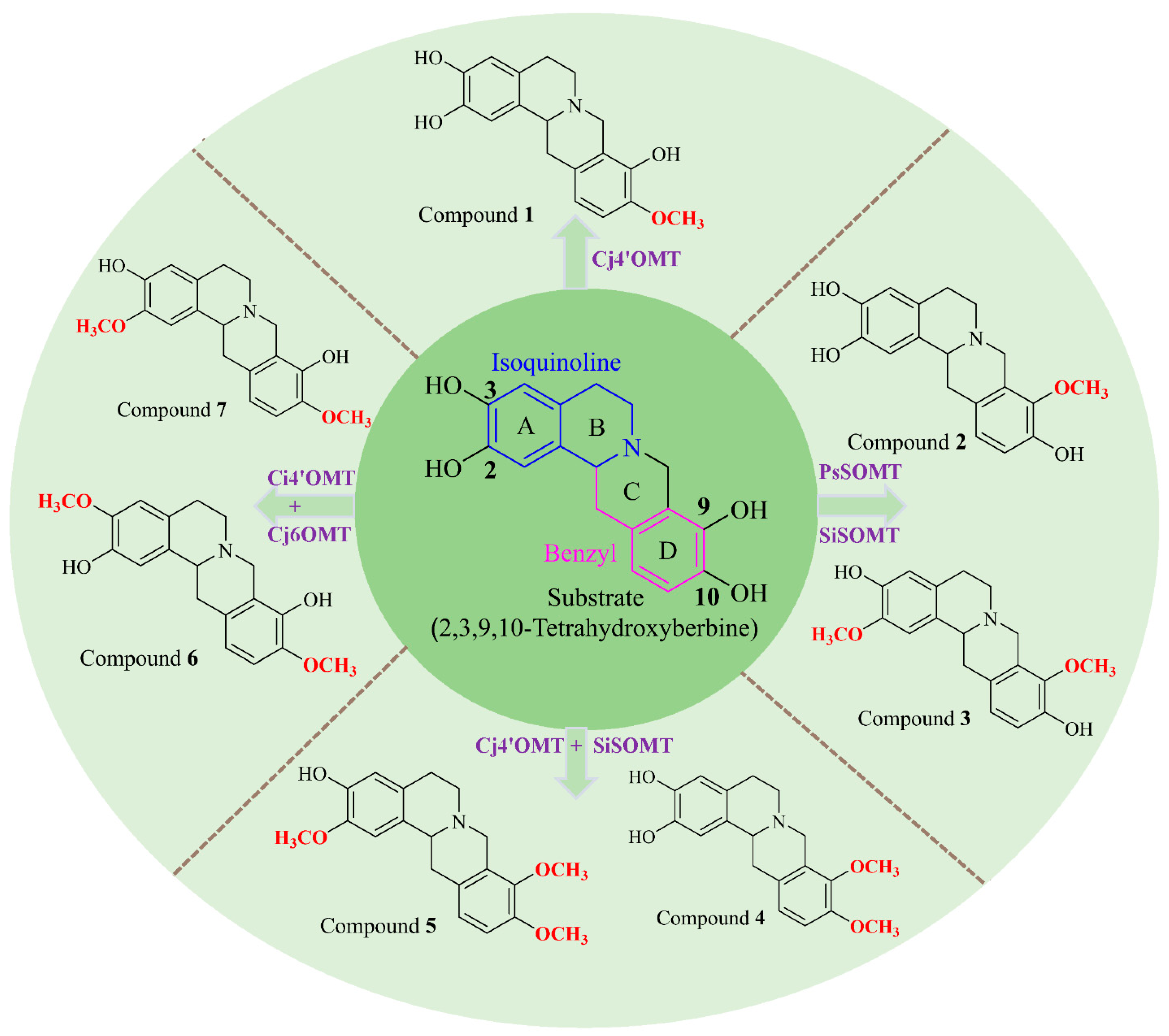
2.2. Structural Elucidation of Compounds
2.3. Molecular Docking of SOMT to the Substrate
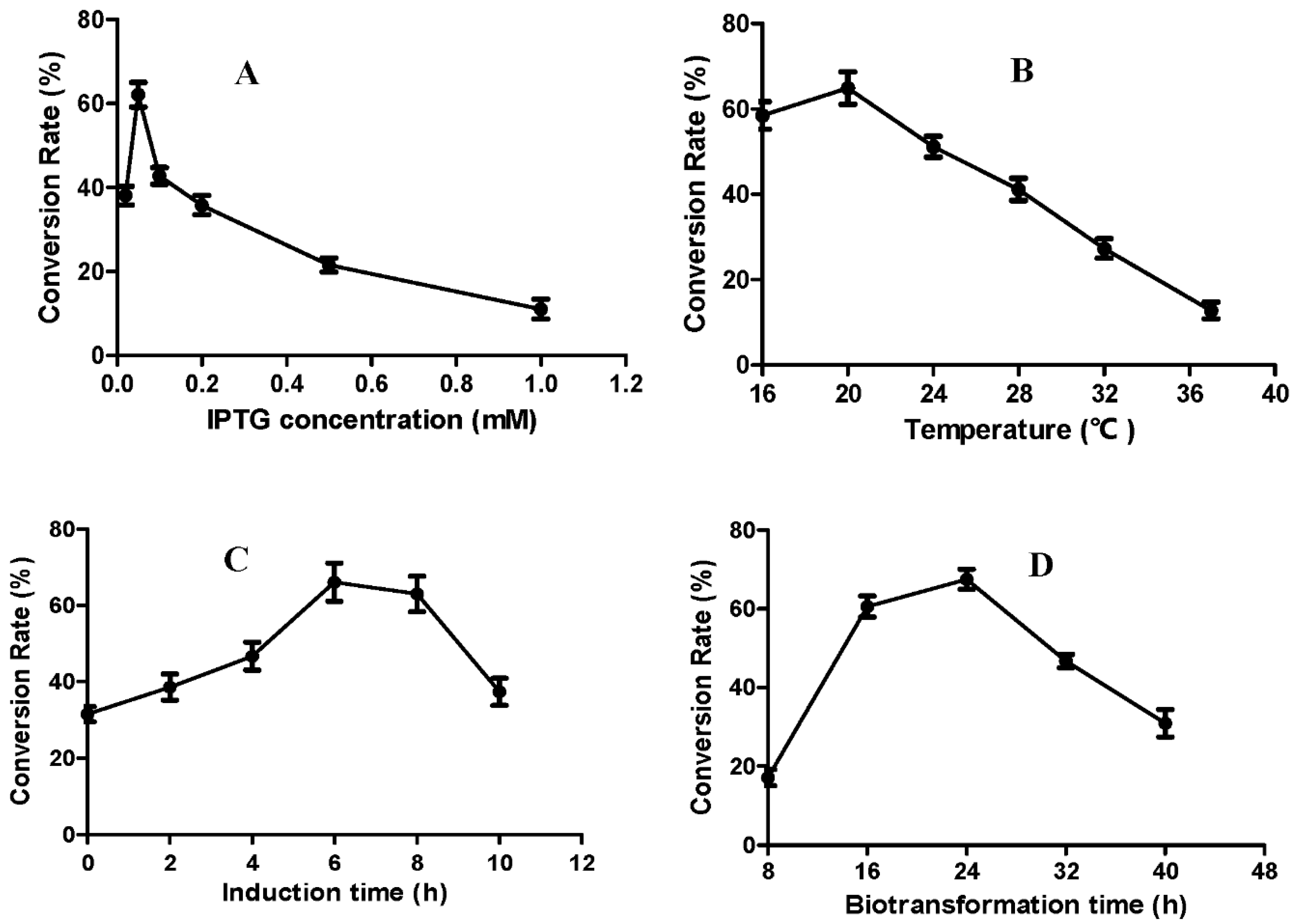
2.4. Optimization of the Fermentation Conditions for Producing Compound 2
3. Materials and Methods
3.1. Materials, Reagents, and Instruments
3.2. Plasmid Construction
3.3. Heterologous Expression of Recombinant Proteins in E. coli
3.4. In Vitro Screening of Selective Methylation by the Methyltransferases
3.5. Whole-Cell Biotransformation for Compound Production
3.6. Product Analysis
3.7. Product Separation and Structural Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zein, A.L.; Dawe, L.N.; Georghiou, P.E. Enantioselective total synthesis and X-ray structures of the tetrahydroprotoberberine alkaloids (-)-(S)-tetrahydropalmatrubine and (-)-(S)-corytenchine. J. Nat. Prod. 2010, 73, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Mantsch, J.R.; Li, S.-J.; Risinger, R.; Awad, S.; Katz, E.; Baker, D.A.; Yang, Z. Levo-tetrahydropalmatine attenuates cocaine self-administration and cocaine-induced reinstatement in rats. Psychopharmacology 2007, 192, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Zhang, Y.; Yang, Z.; Qiang, K.; Chen, C.; Sun, L.; Chen, M.; Zhang, J. Chemical synthesis, microbial transformation and biological evaluation of tetrahydroprotoberberines as dopamine D1/D2 receptor ligands. Bioorganic Med. Chem. 2019, 27, 2100–2111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sha, R.; Wang, K.; Li, H.; Yan, B.; Zhou, N. Protective effects of tetrahydropalmatine against ketamine-induced learning and memory injury via antioxidative, anti-inflammatory and anti-apoptotic mechanisms in mice. Mol. Med. Rep. 2018, 17, 6873–6880. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-T.; Peng, J.-G.; Zhang, J.-Q.; Wang, Z.-X.; Zhang, Y.; Zhou, X.-R.; Miao, J.; Tang, L. Novel berberine-based derivatives with potent hypoglycemic activity. Bioorganic Med. Chem. Lett. 2019, 29, 126709. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.; Srivastava, J.S.; Jha, R.N.; Pandey, V.B.; Singh, U.P. Efficacy of alkaloid (-)-corypalmine against spore germination of some fungi. Folia Microbiol. 2002, 47, 287–290. [Google Scholar] [CrossRef]
- Ge, H.; Zhang, W.; Yuan, K.; Xue, H.; Cheng, H.; Chen, W.; Xie, Y.; Zhang, J.; Xu, X.; Yang, P. Design, synthesis, and biological evaluation of novel tetrahydroprotoberberine derivatives to reduce SREBPs expression for the treatment of hyperlipidemia. Eur. J. Med. Chem. 2021, 221, 113522. [Google Scholar] [CrossRef]
- Almeida, J.R.; de Lima, J.T.; de Oliveira, H.R.; de Oliveira, M.R.; Meira, P.R.; Lucio, A.S.; Barbosa Filho, J.M.; Quintans, L.J., Jr. Antinociceptive activity of discretamine isolated from Duguetia moricandiana. Nat. Prod. Res. 2011, 25, 1908–1915. [Google Scholar] [CrossRef]
- Chaichompoo, W.; Rojsitthisak, P.; Pabuprapap, W.; Siriwattanasathien, Y.; Yotmanee, P.; Haritakun, W.; Suksamrarn, A. Stephapierrines A-H, new tetrahydroprotoberberine and aporphine alkaloids from the tubers of Stephania pierrei Diels and their anti-cholinesterase activities. RSC Adv. 2021, 11, 21153–21169. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Bi, J.L.; Sun, Q.Y.; Yang, F.M.; Wang, Y.H.; Tang, G.H.; Zhao, F.W.; Wang, H.; Xu, J.J.; Kennelly, E.J.; et al. Bioactive isoquinoline alkaloids from Corydalis saxicola. Planta Medica 2012, 78, 65–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Wang, L.; Parks, G.S.; Zhang, X.; Guo, Z.; Ke, Y.; Li, K.W.; Kim, M.K.; Vo, B.; et al. A novel analgesic isolated from a traditional Chinese medicine. Curr. Biol. 2014, 24, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Nestl, B.M.; Hammer, S.C.; Nebel, B.A.; Hauer, B. New generation of biocatalysts for organic synthesis. Angew. Chem. Int. Ed. Engl. 2014, 53, 3070–3095. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.J.; Yeon, Y.J.; Seo, J.H.; Lee, J.H.; Boñgol, J.P.; Oh, Y.; Park, J.M.; Lim, S.M.; Lee, C.G.; Park, J.B. Enzyme/whole-cell biotransformation of plant oils, yeast derived oils, and microalgae fatty acid methyl esters into n-nonanoic acid, 9-hydroxynonanoic acid, and 1,9-nonanedioic acid. Bioresour. Technol. 2018, 251, 288–294. [Google Scholar] [CrossRef]
- Schmitz, E.; Leontakianakou, S.; Norlander, S.; Nordberg Karlsson, E.; Adlercreutz, P. Lignocellulose degradation for the bioeconomy: The potential of enzyme synergies between xylanases, ferulic acid esterase and laccase for the production of arabinoxylo-oligosaccharides. Bioresour. Technol. 2022, 343, 126114. [Google Scholar] [CrossRef]
- Won, Y.; Jeon, H.; Pagar, A.D.; Patil, M.D.; Nadarajan, S.P.; Flood, D.T.; Dawson, P.E.; Yun, H. In vivo biosynthesis of tyrosine analogs and their concurrent incorporation into a residue-specific manner for enzyme engineering. Chem. Commun. 2019, 55, 15133–15136. [Google Scholar] [CrossRef] [PubMed]
- Roddan, R.; Subrizi, F.; Broomfield, J.; Ward, J.M.; Keep, N.H.; Hailes, H.C. Chemoenzymatic Cascades toward Methylated Tetrahydroprotoberberine and Protoberberine Alkaloids. Org. Lett. 2021, 23, 6342–6347. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, H.; Zhu, H.; Wen, L.; Yu, J.; Li, L.; Chen, L.; Geng, Y. A novel method for highly efficient biotransformation and separation of isoflavone aglycones from soybean with high-speed counter-current chromatography. Ind. Crops Prod. 2019, 129, 224–230. [Google Scholar] [CrossRef]
- Xu, W.; Lou, Q.; Hao, L.; Hu, K.; Cao, M.; Liu, Y.; Han, R.; He, C.; Song, J. O-methyltransferases catalyze the last step of geniposide biosynthesis in Gardenia jasminoides. Ind. Crops Prod. 2022, 177, 114438. [Google Scholar] [CrossRef]
- Wu, X.; Yuwen, M.; Pu, Z.; Zhao, Z.; Yu, H.; Zha, J. Engineering of flavonoid 3′-O-methyltransferase for improved biosynthesis of chrysoeriol in Escherichia coli. Appl. Microbiol. Biotechnol. 2023, 107, 1663–1672. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, M.; Shen, C.; Liu, K.; Liu, H.; Ou, C.; Dai, W.; Liu, X.; Liu, J. Biosynthesis of plant-specific alkaloids tetrahydroprotoberberines in engineered Escherichia coli. Green Chem. 2021, 23, 5944–5955. [Google Scholar] [CrossRef]
- Jamil, O.K.; Cravens, A.; Payne, J.T.; Kim, C.Y.; Smolke, C.D. Biosynthesis of tetrahydropapaverine and semisynthesis of papaverine in yeast. Proc. Natl. Acad. Sci. USA 2022, 119, e2205848119. [Google Scholar] [CrossRef] [PubMed]
- Inui, T.; Kawano, N.; Shitan, N.; Yazaki, K.; Kiuchi, F.; Kawahara, N.; Sato, F.; Yoshimatsu, K. Improvement of benzylisoquinoline alkaloid productivity by overexpression of 3′-hydroxy-N-methylcoclaurine 4′-O-methyltransferase in transgenic Coptis japonica plants. Biol. Pharm. Bull. 2012, 35, 650–659. [Google Scholar] [CrossRef] [PubMed]
- He, S.M.; Liang, Y.L.; Cong, K.; Chen, G.; Zhao, X.; Zhao, Q.M.; Zhang, J.J.; Wang, X.; Dong, Y.; Yang, J.L.; et al. Identification and Characterization of Genes Involved in Benzylisoquinoline Alkaloid Biosynthesis in Coptis Species. Front. Plant Sci. 2018, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- Malla, S.; Sommer, M.O.A. A sustainable route to produce the scytonemin precursor using Escherichia coli(Article). Green Chem. 2014, 16, 3255–3265. [Google Scholar] [CrossRef]
- Du, J.; Shao, Z.; Zhao, H. Engineering microbial factories for synthesis of value-added products. J. Ind. Microbiol. Biotechnol. 2011, 38, 873–890. [Google Scholar] [CrossRef]
- Nakagawa, A.; Minami, H.; Kim, J.S.; Koyanagi, T.; Katayama, T.; Sato, F.; Kumagai, H. A bacterial platform for fermentative production of plant alkaloids. Nat. Commun. 2011, 2, 326. [Google Scholar] [CrossRef]
- Matsumura, E.; Nakagawa, A.; Tomabechi, Y.; Ikushiro, S.; Sakaki, T.; Katayama, T.; Yamamoto, K.; Kumagai, H.; Sato, F.; Minami, H. Microbial production of novel sulphated alkaloids for drug discovery. Sci. Rep. 2018, 8, 7980. [Google Scholar] [CrossRef]
- Pontrelli, S.; Chiu, T.Y.; Lan, E.I.; Chen, F.Y.; Chang, P.; Liao, J.C. Escherichia coli as a host for metabolic engineering. Metab. Eng. 2018, 50, 16–46. [Google Scholar] [CrossRef]
- Nakagawa, A.; Matsuzaki, C.; Matsumura, E.; Koyanagi, T.; Katayama, T.; Yamamoto, K.; Sato, F.; Kumagai, H.; Minami, H. (R,S)-tetrahydropapaveroline production by stepwise fermentation using engineered Escherichia coli. Sci. Rep. 2014, 4, 6695. [Google Scholar] [CrossRef]
- Trenchard, I.J.; Siddiqui, M.S.; Thodey, K.; Smolke, C.D. De novo production of the key branch point benzylisoquinoline alkaloid reticuline in yeast. Metab. Eng. 2015, 31, 74–83. [Google Scholar] [CrossRef]
- Nakagawa, A.; Matsumura, E.; Koyanagi, T.; Katayama, T.; Kawano, N.; Yoshimatsu, K.; Yamamoto, K.; Kumagai, H.; Sato, F.; Minami, H. Total biosynthesis of opiates by stepwise fermentation using engineered Escherichia coli. Nat. Commun. 2016, 7, 10390. [Google Scholar] [CrossRef] [PubMed]
- DeLoache, W.C.; Russ, Z.N.; Narcross, L.; Gonzales, A.M.; Martin, V.J.; Dueber, J.E. An enzyme-coupled biosensor enables (S)-reticuline production in yeast from glucose. Nat. Chem. Biol. 2015, 11, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Shen, C.; Zhu, J.; Ou, C.; Liu, M.; Dai, W.; Liu, X.; Liu, J. Identification and characterization of methyltransferases involved in benzylisoquinoline alkaloids biosynthesis from Stephania intermedia. Biotechnol. Lett. 2020, 42, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Facchini, P.J. Characterization of three O-methyltransferases involved in noscapine biosynthesis in opium poppy. Plant Physiol. 2012, 159, 618–631. [Google Scholar] [CrossRef]
- Costa, E.V.; Sampaio, M.F.C.; Salvador, M.J.; Nepel, A.; Barison, A. Chemical constituents from the stem bark of Annona pickelii (Annonaceae). Química Nova 2015, 38, 769–776. [Google Scholar]
- Akdemir, H.; Silva, A.; Zha, J.; Zagorevski, D.V.; Koffas, M.A.G. Production of pyranoanthocyanins using Escherichia coli co-cultures. Metab. Eng. 2019, 55, 290–298. [Google Scholar] [CrossRef]
- Jones, J.A.; Vernacchio, V.R.; Sinkoe, A.L.; Collins, S.M.; Ibrahim, M.H.A.; Lachance, D.M.; Hahn, J.; Koffas, M.A.G. Experimental and computational optimization of an Escherichia coli co-culture for the efficient production of flavonoids. Metab. Eng. 2016, 35, 55–63. [Google Scholar] [CrossRef]
- Fang, Z.; Jones, J.A.; Zhou, J.; Koffas, M.A.G. Engineering Escherichia coli Co-Cultures for Production of Curcuminoids from Glucose. Biotechnol. J. 2018, 13, e1700576. [Google Scholar] [CrossRef]
- Lemos, D.E.C.V.; Cavalcante-Silva, L.H.A.; de Almeida Lima, É.; Alves, A.F.; Lúcio, A.S.S.C.; Barbosa-Filho, J.M.; Mascarenhas, S.R. Anti-inflammatory Effect of Discretamine, a Protoberberine Alkaloid Isolated from Duguetia moricandiana. Nat. Prod. Commun. 2017, 12, 1595–1597. [Google Scholar] [CrossRef]
- Habartova, K.; Havelek, R.; Seifrtova, M.; Kralovec, K.; Cahlikova, L.; Chlebek, J.; Cermakova, E.; Mazankova, N.; Marikova, J.; Kunes, J.; et al. Scoulerine affects microtubule structure, inhibits proliferation, arrests cell cycle and thus culminates in the apoptotic death of cancer cells. Sci. Rep. 2018, 8, 4829. [Google Scholar] [CrossRef]
- Mo, J.; Guo, Y.; Yang, Y.S.; Shen, J.S.; Jin, G.Z.; Zhen, X. Recent developments in studies of l-stepholidine and its analogs: Chemistry, pharmacology and clinical implications. Curr. Med. Chem. 2007, 14, 2996–3002. [Google Scholar] [CrossRef] [PubMed]
- Nesbit, M.O.; Phillips, A.G. Tetrahydroprotoberberines: A Novel Source of Pharmacotherapies for Substance Use Disorders? Trends Pharmacol. Sci. 2020, 41, 147–161. [Google Scholar] [CrossRef] [PubMed]
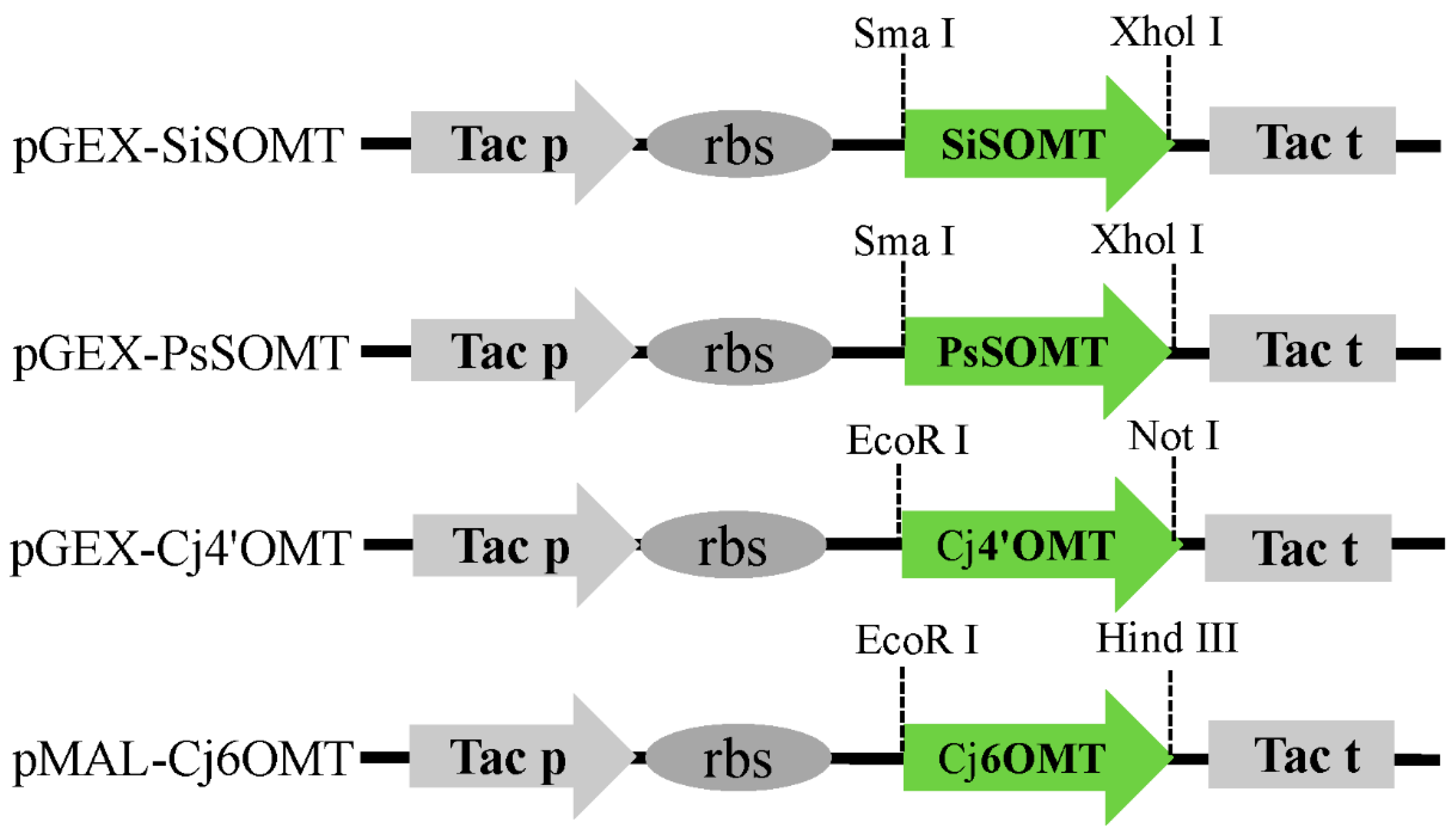

| Plasmids/Strains | Detailed Information | Source/Usge |
|---|---|---|
| pGEX-PsSOMT | pGEX-6p-1-Tac Prom-PsSOMT-Tac Term, Sma I/Xhol I | This work |
| pGEX-SiSOMT | pGEX-6p-1-Tac Prom-SiSOMT-Tac Term, Sma I/Xhol I | This work |
| pGEX-Cj4’OMT | pGEX-6p-1-Tac Prom-Cj4’OMT-Tac Term, EcoR I/Not I | This work |
| pMAL-Cj6OMT | pMAL-c4x, Tac Prom-Cj6OMT-Tac Term, EcoR I/Hind III | This work |
| E. coli DH5α | F-φ80 lacZΔM15Δ (lacZYA-argF) U169 end A1 recA1 hsdR17 (rk-, mk-) supE44λ- thi-1 gyrA96 relA1 phoA | Plasmid amplification |
| E. coli BL21(DE3) | F-ompT hsdS (rB-mB-) gal dcm (DE3) | Protein expression |
| BL21-pGEX-PsSOMT | E. coli BL21(DE3) with pGEX-PsSOMT | Producing alkaliods |
| BL21-pGEX-SiSOMT | E. coli BL21(DE3) with pGEX-SiSOMT | Producing alkaliods |
| BL21-pGEX-Cj4’OMT | E. coli BL21(DE3) with pGEX-Cj4’OMT | Producing alkaliods |
| BL21-pMAL-Cj6OMT | E. coli BL21(DE3) with pMAL-Cj6OMT | Producing alkaliods |
| Enzyme | Substrate | Km (μΜ) | Vmax nmol min−1 mg−1 Protein | kcat (S−1) | kcat/Km (M−1 S−1) |
|---|---|---|---|---|---|
| PsSOMT | 2,3,9,10-tetrahydroxyberbine | 53.77 ± 12.03 | 522 ± 27 | 0.52 ± 0.03 | 9671 |
| SiSOMT | 2,3,9,10-tetrahydroxyberbine | 44.09 ± 10.91 | 1682 ± 92 | 1.68 ± 0.09 | 38,149 |
| Cj4’OMT | 2,3,9,10-tetrahydroxyberbine | 83.50 ± 19.72 | 1088 ± 79 | 1.09 ± 0.08 | 13,018 |
| Cj6OMT | compound 1 | 59.46 ± 15.98 | 505 ± 36 | 0.13 ± 0.01 | 2186 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Liu, M.; Liu, K.; Liu, H.; Liu, X.; Liu, J. An Enzymatic Strategy for the Selective Methylation of High-Value-Added Tetrahydroprotoberberine Alkaloids. Int. J. Mol. Sci. 2023, 24, 15214. https://doi.org/10.3390/ijms242015214
Zhao W, Liu M, Liu K, Liu H, Liu X, Liu J. An Enzymatic Strategy for the Selective Methylation of High-Value-Added Tetrahydroprotoberberine Alkaloids. International Journal of Molecular Sciences. 2023; 24(20):15214. https://doi.org/10.3390/ijms242015214
Chicago/Turabian StyleZhao, Wanli, Manyu Liu, Kemeng Liu, Hanqing Liu, Xiufeng Liu, and Jihua Liu. 2023. "An Enzymatic Strategy for the Selective Methylation of High-Value-Added Tetrahydroprotoberberine Alkaloids" International Journal of Molecular Sciences 24, no. 20: 15214. https://doi.org/10.3390/ijms242015214
APA StyleZhao, W., Liu, M., Liu, K., Liu, H., Liu, X., & Liu, J. (2023). An Enzymatic Strategy for the Selective Methylation of High-Value-Added Tetrahydroprotoberberine Alkaloids. International Journal of Molecular Sciences, 24(20), 15214. https://doi.org/10.3390/ijms242015214






