Proteome Analysis of Alpine Merino Sheep Skin Reveals New Insights into the Mechanisms Involved in Regulating Wool Fiber Diameter
Abstract
:1. Introduction
2. Results
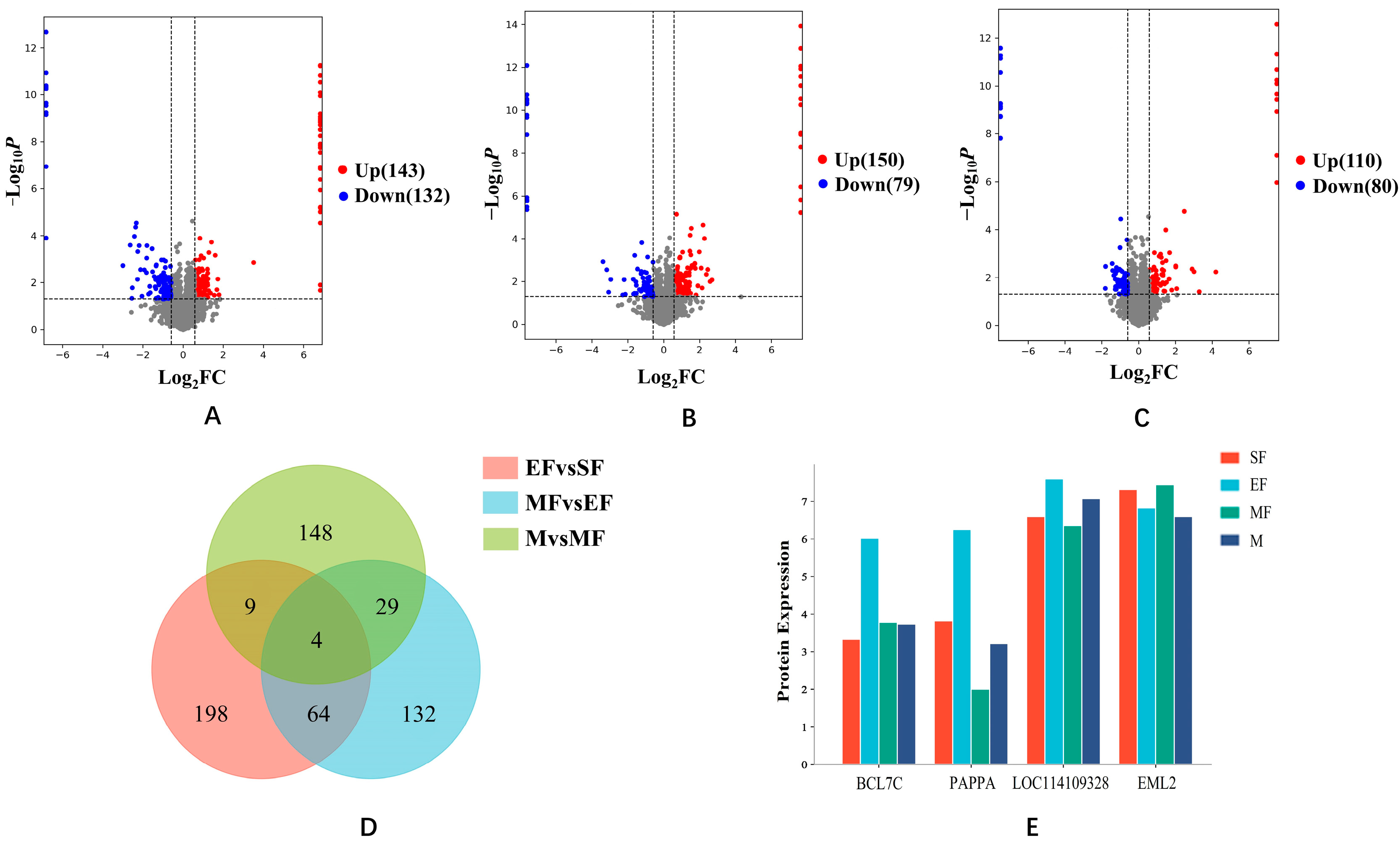
2.1. Differential Protein Analysis
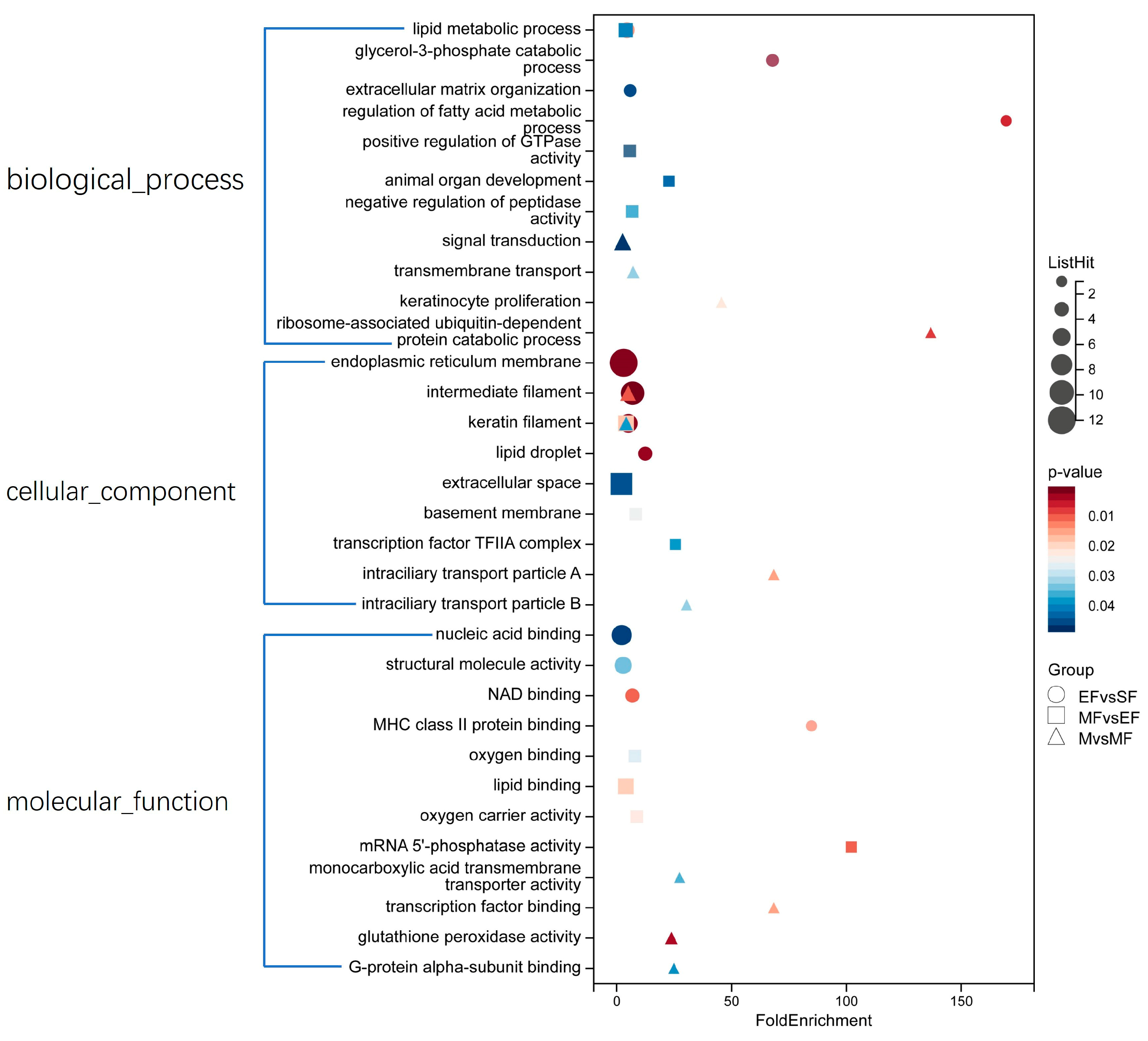
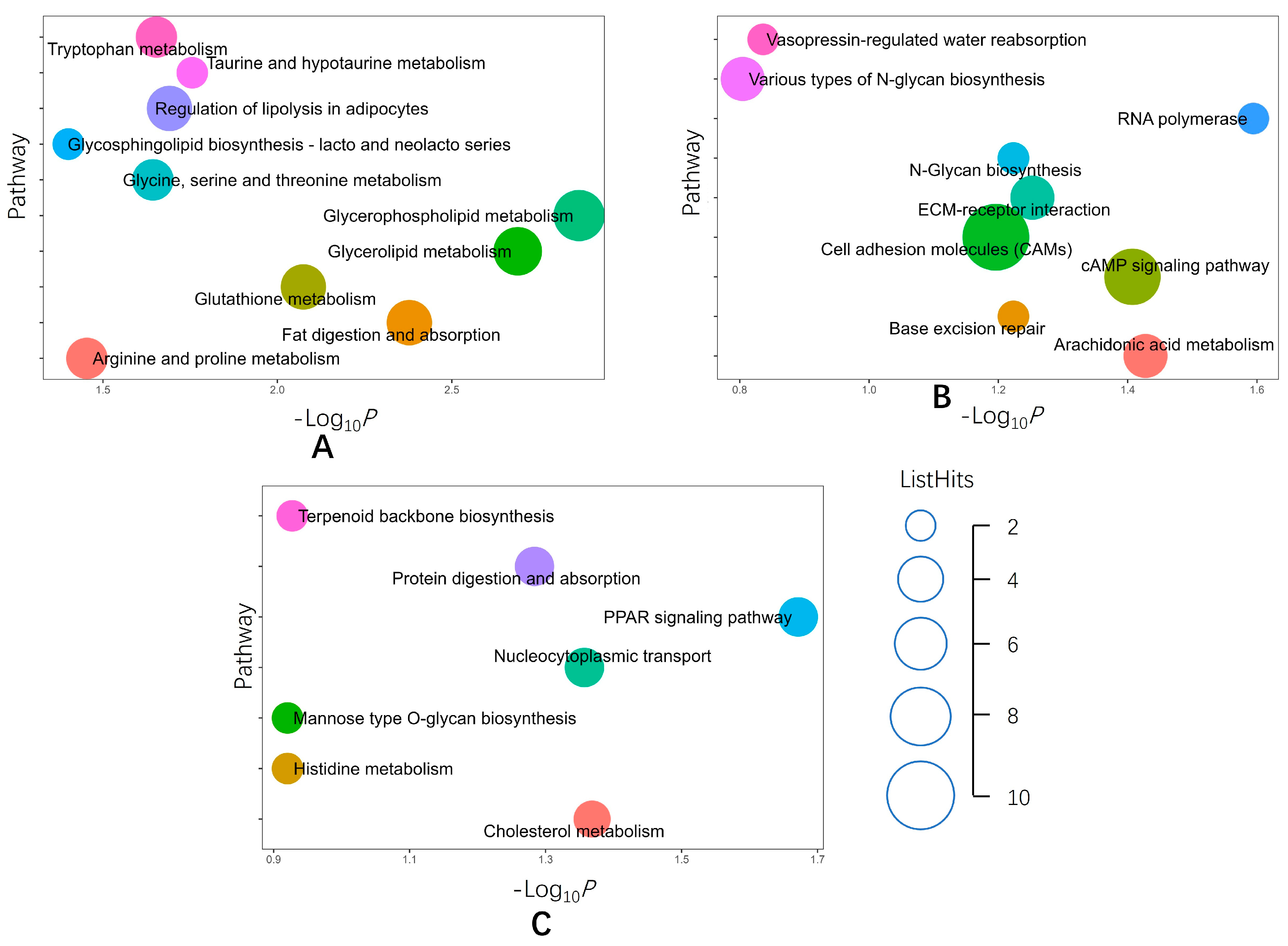
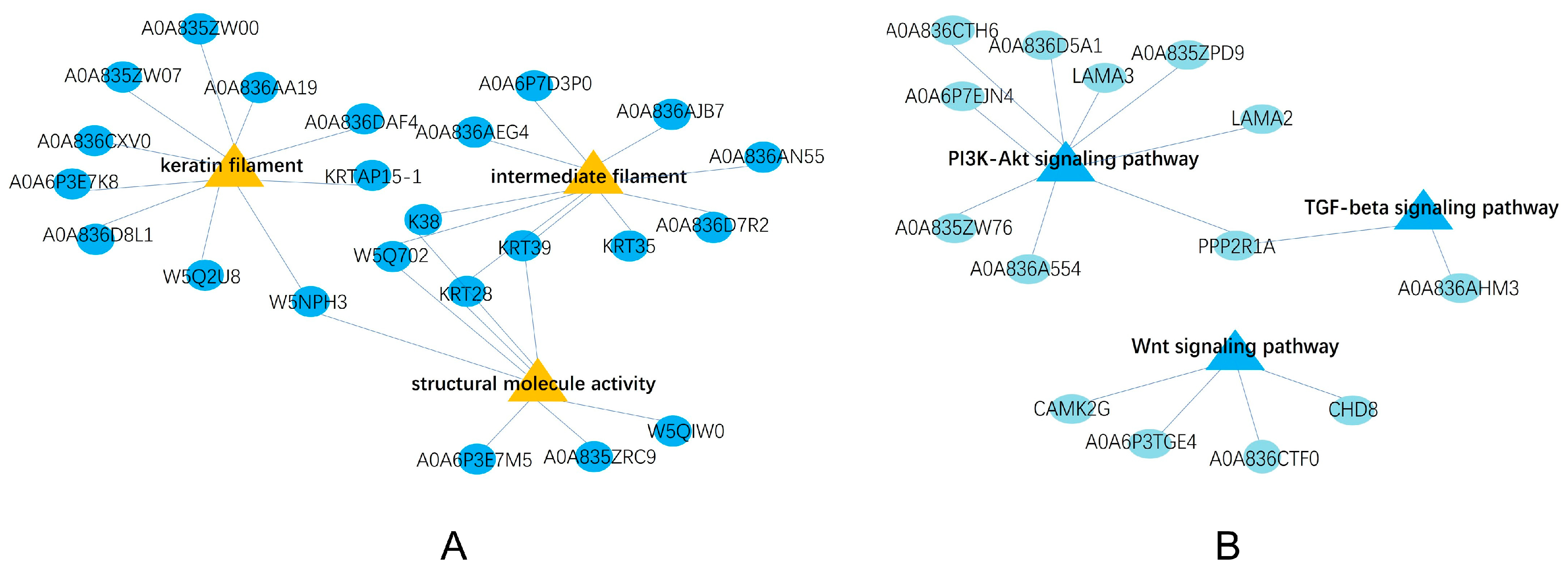
2.2. Functional Analyses of Identified DEPs
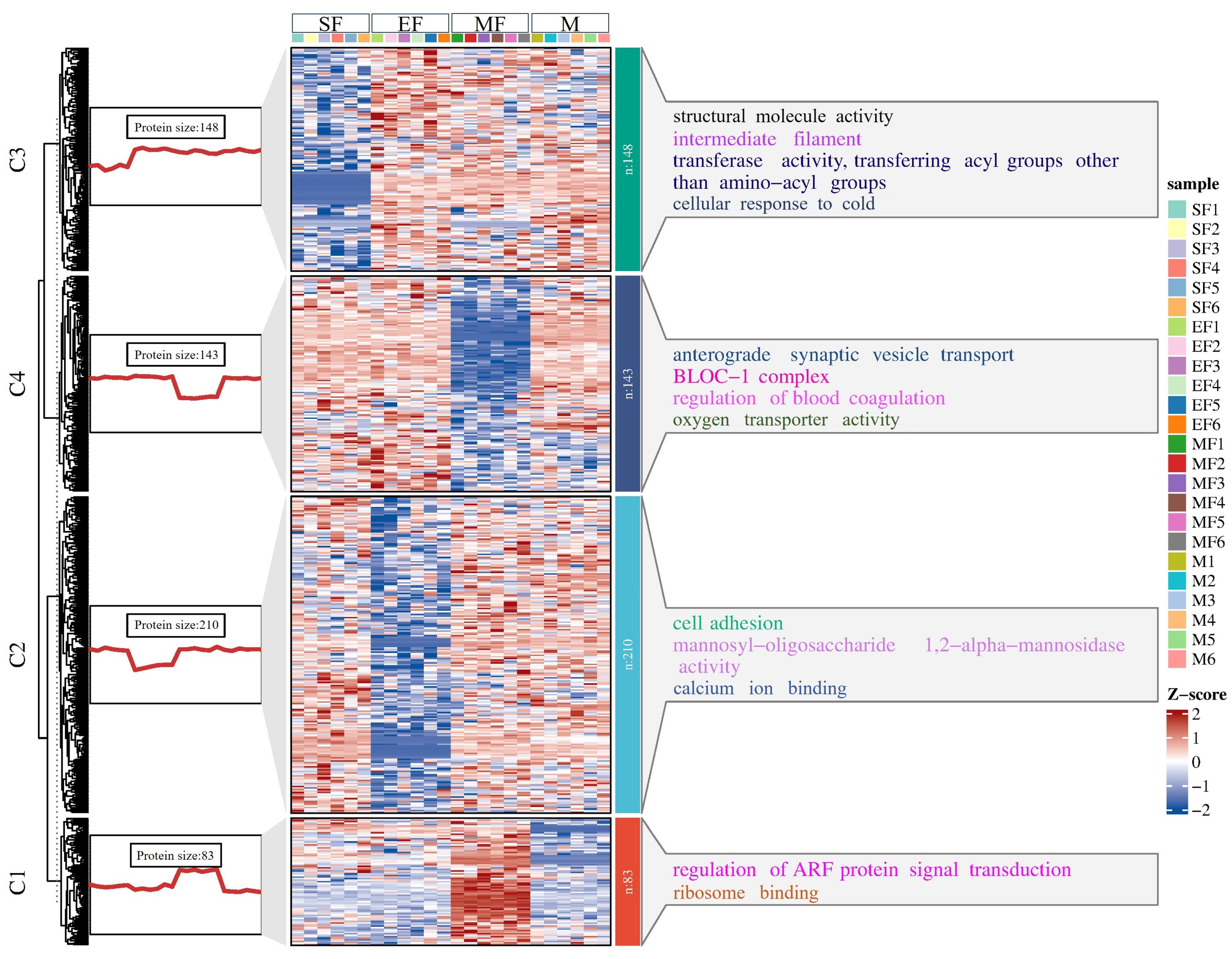
2.3. K-Means Analysis of DEPs
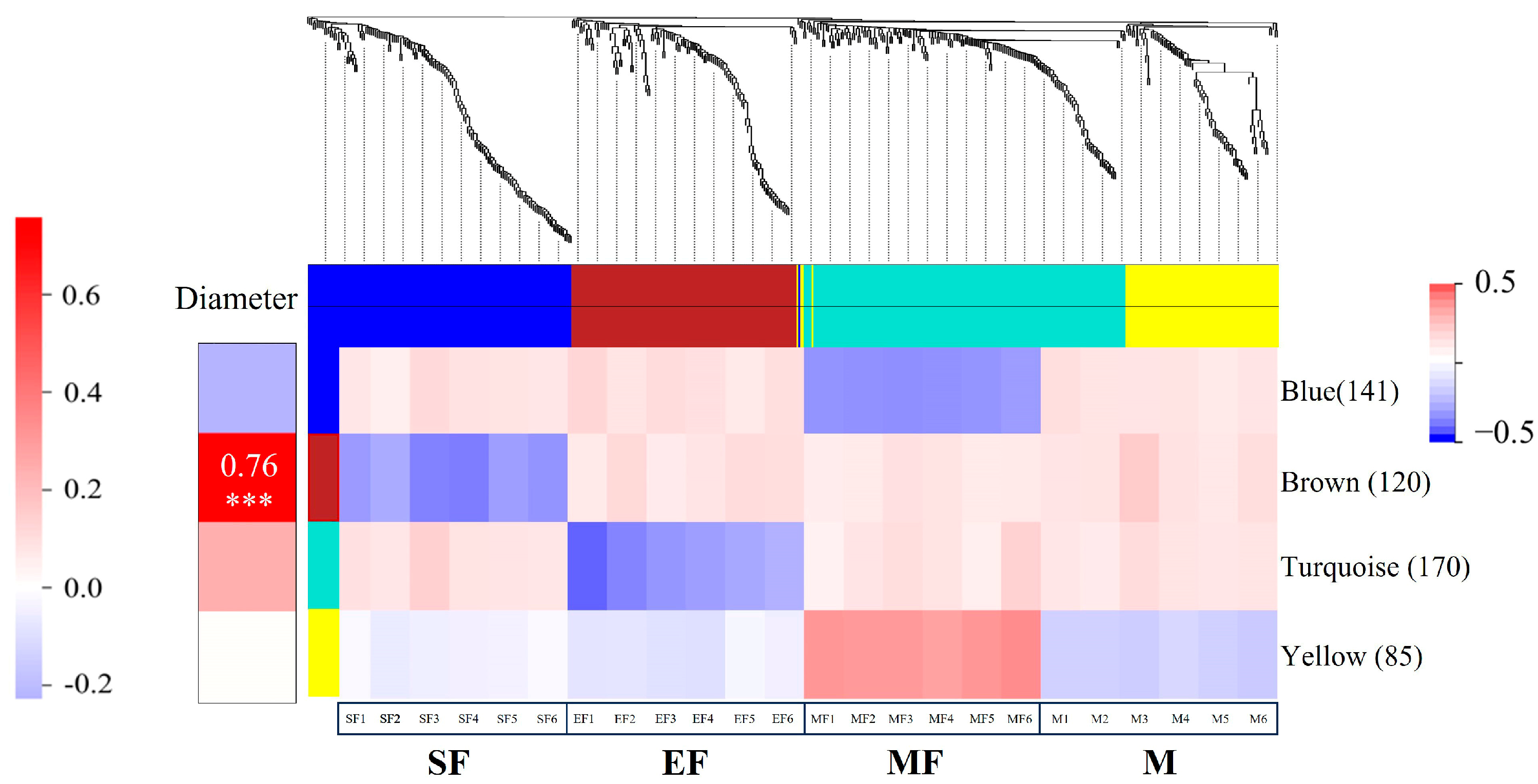
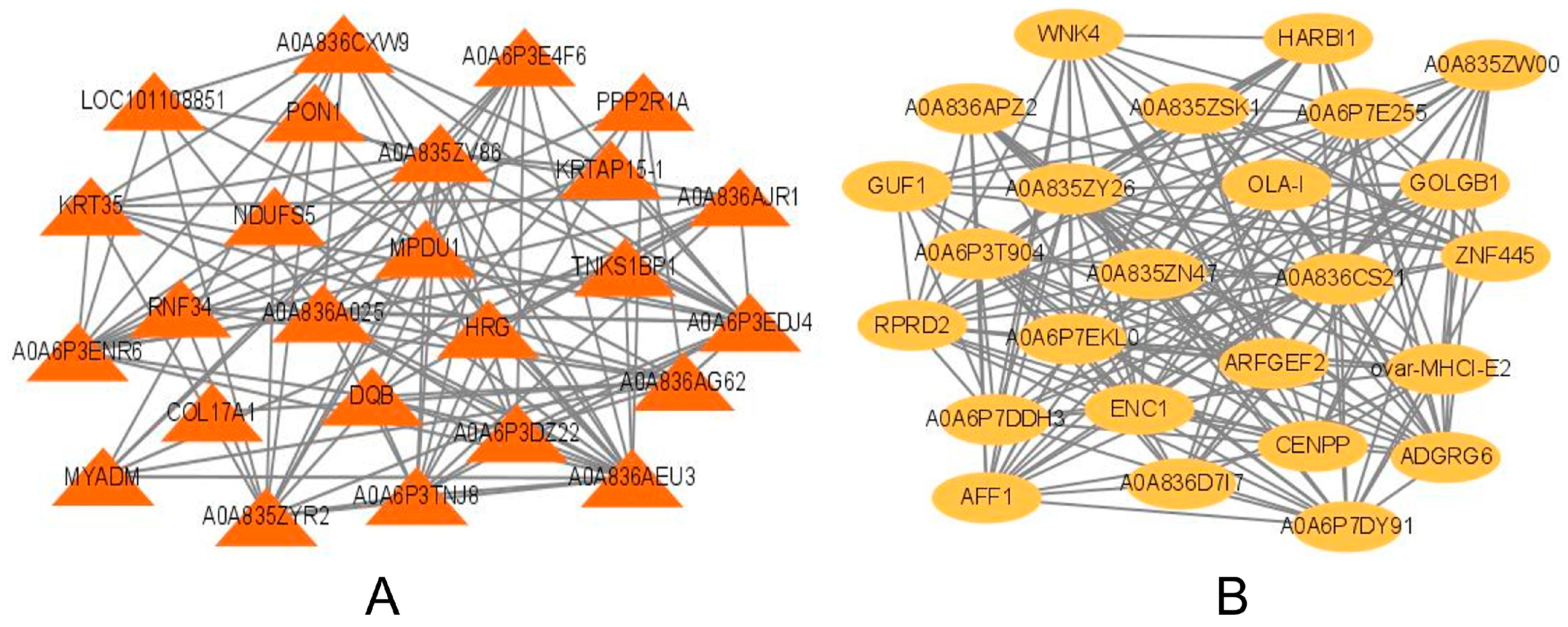
2.4. Weighted Gene Co-Expression Network Analysis
3. Discussion
4. Materials and Methods
4.1. Weighted Gene Co-Expression Network Analysis
4.2. Skin Tissue Protein Extraction and Digestion
4.3. High-Performance Liquid Chromatography
4.4. Mass Spectrometry Analysis
4.5. Proteome Data Processing Analysis
4.6. K-Means Analysis and WGCNA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hai, F.; Wang, X.J. Research on the Development Strategy of China’s Wool Industry. J. Wuhan Text. Univ. 2016, 29, 14–18. [Google Scholar]
- Wang, J.; Zhou, H.; Hickford, J.G.H.; Luo, Y.; Gong, H.; Hu, J.; Liu, X.; Li, S.; Song, Y.; Ke, N.; et al. Identification of the Ovine Keratin-Associated Protein 2-1 Gene and Its Sequence Variation in Four Chinese Sheep Breeds. Genes 2020, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Luo, Y.; Zhou, H.; Liu, X.; Li, S.; Hao, Z.; Shen, J.; Zhen, H.; Li, L.; Wang, J.; et al. Variation in caprine KRTAP1-3 and its association with cashmere fibre diameter. Gene 2022, 823, 146341–146347. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, H.; Luo, Y.; Zhao, M.; Gong, H.; Hao, Z.; Hu, J.; Hickford, J.G.H. Variation in the Caprine KAP24-1 Gene Affects Cashmere Fibre Diameter. Animals 2019, 9, 15. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, H.; Luo, Y.; Wang, J.; Hu, J.; Liu, X.; Li, S.; Hao, Z.; Jin, X.; Song, Y.; et al. Variation in the Caprine Keratin-Associated Protein 27-1 Gene is Associated with Cashmere Fiber Diameter. Genes 2020, 11, 934. [Google Scholar] [CrossRef]
- Zhang, M.Y.H.; Wei, Q.L.; He, G.M.; Shen, M. The Effect of EEF1D Gene Expression on Wool Fiber Diameters and Hair Follicle Development. J. Domest. Anim. Ecol. 2023, 44, 27–31. [Google Scholar]
- Fu, X.F.; Huang, X.X.; Liu, C.J.; Xu, X.M.; Zhang, Y.H.; Wu, W.W.; Yu, L.J.; Shi, G.; Tian, K.C. Study on Clone and Differential Expression of MYL6 Gene in Fine-Wool Sheep with Different Fiber Diameter. Chin. J. Anim. Sci. 2016, 52, 5–11. [Google Scholar]
- Wang, S.; Li, F.; Liu, J.; Zhang, Y.; Zheng, Y.; Ge, W.; Qu, L.; Wang, X. Integrative Analysis of Methylome and Transcriptome Reveals the Regulatory Mechanisms of Hair Follicle Morphogenesis in Cashmere Goat. Cells 2020, 9, 969. [Google Scholar] [CrossRef]
- Zhao, B.; Luo, H.; He, J.; Huang, X.; Chen, S.; Fu, X.; Zeng, W.; Tian, Y.; Liu, S.; Li, C.J.; et al. Comprehensive transcriptome and methylome analysis delineates the biological basis of hair follicle development and wool-related traits in Merino sheep. BMC Biol. 2021, 19, 197–215. [Google Scholar] [CrossRef]
- He, J.; Zhao, B.; Huang, X.; Fu, X.; Liu, G.; Tian, Y.; Wu, C.; Mao, J.; Liu, J.; Gun, S.; et al. Gene network analysis reveals candidate genes related with the hair follicle development in sheep. BMC Genom. 2022, 23, 428–444. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, C.; Sammad, A.; Ma, Z.; Suo, L.; Wu, Y.; Fu, X. The fiber diameter traits of Tibetan cashmere goats are governed by the inherent differences in stress, hypoxic, and metabolic adaptations: An integrative study of proteome and transcriptome. BMC Genom. 2022, 23, 191–203. [Google Scholar] [CrossRef]
- Fu, X.; Zhao, B.; Tian, K.; Wu, Y.; Suo, L.; Ba, G.; Ciren, D.; De, J.; Awang, C.; Gun, S.; et al. Integrated analysis of lncRNA and mRNA reveals novel insights into cashmere fineness in Tibetan cashmere goats. PeerJ 2020, 8, e10217. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.W.; O’Regan, L.; Roth, D.; Montgomery, J.M.; Straube, A.; Fry, A.M.; Bayliss, R. Microtubule association of EML proteins and the EML4-ALK variant 3 oncoprotein require an N-terminal trimerization domain. Biochem. J. 2015, 467, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.M.; He, Y.Q.; Xue, W.Q.; Zhang, J.B.; Xia, Y.F.; Deng, C.M.; Zhang, W.L.; Xiao, R.W.; Liao, Y.; Yang, D.W.; et al. Whole-Exome Sequencing Study of Familial Nasopharyngeal Carcinoma and Its Implication for Identifying High-Risk Individuals. J. Natl. Cancer Inst. 2022, 114, 1689–1697. [Google Scholar] [CrossRef]
- Abak, A.; Shoorei, H.; Taheri, M.; Ghafouri-Fard, S. In vivo Engineering of Chromosome 19 q-arm by Employing the CRISPR/AsCpf1 and ddAsCpf1 Systems in Human Malignant Gliomas (Hypothesis). J. Mol. Neurosci. 2021, 71, 1648–1663. [Google Scholar] [CrossRef]
- Huang, C.; Hao, Q.; Shi, G.; Zhou, X.; Zhang, Y. BCL7C suppresses ovarian cancer growth by inactivating mutant p53. J. Mol. Cell Biol. 2021, 13, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Bao, Y.; Guo, D.; Yang, W. Pregnancy-associated plasma protein a in cancer: Expression, oncogenic functions and regulation. Am. J. Cancer Res. 2018, 8, 955–963. [Google Scholar]
- Laursen, L.S.; Overgaard, M.T.; Søe, R.; Boldt, H.B.; Sottrup-Jensen, L.; Giudice, L.C.; Conover, C.A.; Oxvig, C. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: Implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett. 2001, 504, 36–40. [Google Scholar] [CrossRef]
- Steinert, P.M. Keratins: Dynamic, flexible structural proteins of epithelial cells. Curr. Probl. Dermatol. 2001, 13, 65–68. [Google Scholar] [CrossRef]
- Planko, L.; BHse, K.; HHfeld, J.R.; Betz, R.C.; Hanneken, S.; Eigelshoven, S.; Kruse, R.; NThen, M.M.; Magin, T.M. Identification of a keratin-associated protein with a putative role in vesicle transport. Eur. J. Cell Biol. 2007, 86, 827–839. [Google Scholar] [CrossRef]
- Bragulla, H.H.; Homberger, D.G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 2009, 214, 516–559. [Google Scholar] [CrossRef] [PubMed]
- Plowman, J.E.; Harland, D.P.; Ganeshan, S.; Woods, J.L.; van Shaijik, B.; Deb-Choudhury, S.; Thomas, A.; Clerens, S.; Scobie, D.R. The proteomics of wool fibre morphogenesis. J. Struct. Biol. 2015, 191, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Plowman, J.E.; Harland, D.P.; Campos, A.M.O.; Rocha, E.S.S.; Thomas, A.; Vernon, J.A.; van Koten, C.; Hefer, C.; Clerens, S.; de Almeida, A.M. The wool proteome and fibre characteristics of three distinct genetic ovine breeds from Portugal. J. Proteom. 2020, 225, 103853. [Google Scholar] [CrossRef] [PubMed]
- Gniadecki, R. Regulation of keratinocyte proliferation. Gen. Pharmacol. 1998, 30, 619–622. [Google Scholar] [CrossRef]
- Miettinen, P.J.; Berger, J.E.; Meneses, J.; Phung, Y.; Pedersen, R.A.; Werb, Z.; Derynck, R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature 1995, 376, 337–341. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, R.; Li, F.; Yue, X. Five SNPs Within the FGF5 Gene Significantly Affect Both Wool Traits and Growth Performance in Fine-Wool Sheep (Ovis aries). Front. Genet. 2021, 12, 732097. [Google Scholar] [CrossRef]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef]
- Sawyer, G.; Fox, D.R.; Narayan, E. Pre- and post-partum variation in wool cortisol and wool micron in Australian Merino ewe sheep (Ovis aries). PeerJ 2021, 9, e11288. [Google Scholar] [CrossRef]
- Thiboutot, D. Regulation of human sebaceous glands. J. Investig. Dermatol. 2004, 123, 1–12. [Google Scholar] [CrossRef]
- Smith, K.R.; Thiboutot, D.M. Thematic review series: Skin lipids. Sebaceous gland lipids: Friend or foe? J. Lipid Res. 2008, 49, 271–281. [Google Scholar] [CrossRef]
- Plikus, M.V.; Baker, R.E.; Chen, C.C.; Fare, C.; De La Cruz, D.; Andl, T.; Maini, P.K.; Millar, S.E.; Widelitz, R.; Chuong, C.M. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science 2011, 332, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Botchkarev, V.A.; Yaar, M.; Peters, E.M.; Raychaudhuri, S.P.; Botchkareva, N.V.; Marconi, A.; Raychaudhuri, S.K.; Paus, R.; Pincelli, C. Neurotrophins in skin biology and pathology. J. Investig. Dermatol. 2006, 126, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Kamberov, Y.G.; Karlsson, E.K.; Kamberova, G.L.; Lieberman, D.E.; Sabeti, P.C.; Morgan, B.A.; Tabin, C.J. A genetic basis of variation in eccrine sweat gland and hair follicle density. Proc. Natl. Acad. Sci. USA 2015, 112, 9932–9937. [Google Scholar] [CrossRef] [PubMed]
- Hanley, K.; Jiang, Y.; Crumrine, D.; Bass, N.M.; Appel, R.; Elias, P.M.; Williams, M.L.; Feingold, K.R. Activators of the nuclear hormone receptors PPARalpha and FXR accelerate the development of the fetal epidermal permeability barrier. J. Clin. Investig. 1997, 100, 705–712. [Google Scholar] [CrossRef]
- Hanley, K.; Kömüves, L.G.; Bass, N.M.; He, S.S.; Jiang, Y.; Crumrine, D.; Appel, R.; Friedman, M.; Bettencourt, J.; Min, K.; et al. Fetal epidermal differentiation and barrier development In vivo is accelerated by nuclear hormone receptor activators. J. Investig. Dermatol. 1999, 113, 788–795. [Google Scholar] [CrossRef]
- Zeller, E.; Hammer, K.; Kirschnick, M.; Braeuning, A. Mechanisms of RAS/β-catenin interactions. Arch. Toxicol. 2013, 87, 611–632. [Google Scholar] [CrossRef]
- Thatcher, J.D. The Ras-MAPK signal transduction pathway. Sci. Signal 2010, 3, tr1. [Google Scholar] [CrossRef]
- Kolodziejczyk, S.M.; Hall, B.K. Signal transduction and TGF-beta superfamily receptors. Biochem. Cell Biol. 1996, 74, 299–314. [Google Scholar] [CrossRef]
- Colicelli, J. Signal transduction: RABGEF1 fingers RAS for ubiquitination. Curr. Biol. 2010, 20, R630–R632. [Google Scholar] [CrossRef]
- Andl, T.; Reddy, S.T.; Gaddapara, T.; Millar, S.E. WNT signals are required for the initiation of hair follicle development. Dev. Cell 2002, 2, 643–653. [Google Scholar] [CrossRef]
- Headon, D.J.; Overbeek, P.A. Involvement of a novel Tnf receptor homologue in hair follicle induction. Nat. Genet. 1999, 22, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Foitzik, K.; Paus, R.; Doetschman, T.; Dotto, G.P. The TGF-beta2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Dev. Biol. 1999, 212, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L.; Corey, R.B.; Branson, H.R. The structure of proteins; two hydrogen-bonded helical configurations of the polypeptide chain. Proc. Natl. Acad. Sci. USA 1951, 37, 205–211. [Google Scholar] [CrossRef]
- Huggins, M.L. The structure of alpha-keratin. Macromolecules 1977, 10, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef] [PubMed]
- Li, P. Cidea, brown fat and obesity. Mech. Ageing Dev. 2004, 125, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Kinney, C.J.; Bloch, R.J. µ-Crystallin: A thyroid hormone binding protein. Endocr. Regul. 2021, 55, 89–102. [Google Scholar] [CrossRef]
- Nagarajan, S.R.; Livingstone, E.J.; Monfeuga, T.; Lewis, L.C.; Ali, S.H.L.; Chandran, A.; Dearlove, D.J.; Neville, M.J.; Chen, L.; Maroteau, C.; et al. MLX plays a key role in lipid and glucose metabolism in humans: Evidence from in vitro and in vivo studies. Metabolism 2023, 144, 155563. [Google Scholar] [CrossRef] [PubMed]
- Klüssendorf, M.; Song, I.; Schau, L.; Morellini, F.; Dityatev, A.; Koliwer, J.; Kreienkamp, H.J. The Golgi-Associated PDZ Domain Protein Gopc/PIST Is Required for Synaptic Targeting of mGluR5. Mol. Neurobiol. 2021, 58, 5618–5634. [Google Scholar] [CrossRef]
- Miyauchi, M.; Sasaki, K.; Kagoya, Y.; Taoka, K.; Masamoto, Y.; Yamazaki, S.; Arai, S.; Mizuno, H.; Kurokawa, M. CAMK2G is identified as a novel therapeutic target for myelofibrosis. Blood Adv. 2022, 6, 1585–1597. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Xue, H.; Kong, L.; Wang, Y. Network-based methods for identifying critical pathways of complex diseases: A survey. Mol. Biosyst. 2016, 12, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, L.; Lu, Z.; Guo, T.; Liu, J.; Yang, B.; Yuan, C. Proteome Analysis of Alpine Merino Sheep Skin Reveals New Insights into the Mechanisms Involved in Regulating Wool Fiber Diameter. Int. J. Mol. Sci. 2023, 24, 15227. https://doi.org/10.3390/ijms242015227
Yue L, Lu Z, Guo T, Liu J, Yang B, Yuan C. Proteome Analysis of Alpine Merino Sheep Skin Reveals New Insights into the Mechanisms Involved in Regulating Wool Fiber Diameter. International Journal of Molecular Sciences. 2023; 24(20):15227. https://doi.org/10.3390/ijms242015227
Chicago/Turabian StyleYue, Lin, Zengkui Lu, Tingting Guo, Jianbin Liu, Bohui Yang, and Chao Yuan. 2023. "Proteome Analysis of Alpine Merino Sheep Skin Reveals New Insights into the Mechanisms Involved in Regulating Wool Fiber Diameter" International Journal of Molecular Sciences 24, no. 20: 15227. https://doi.org/10.3390/ijms242015227





