Expression of PD-1 and PD-L1 in Endometrial Cancer: Molecular and Clinical Significance
Abstract
:1. Introduction
2. Materials and Methods
3. Molecular Classification of Endometrial Cancer
4. Immune Micro-Environment in Endometrial Cancer
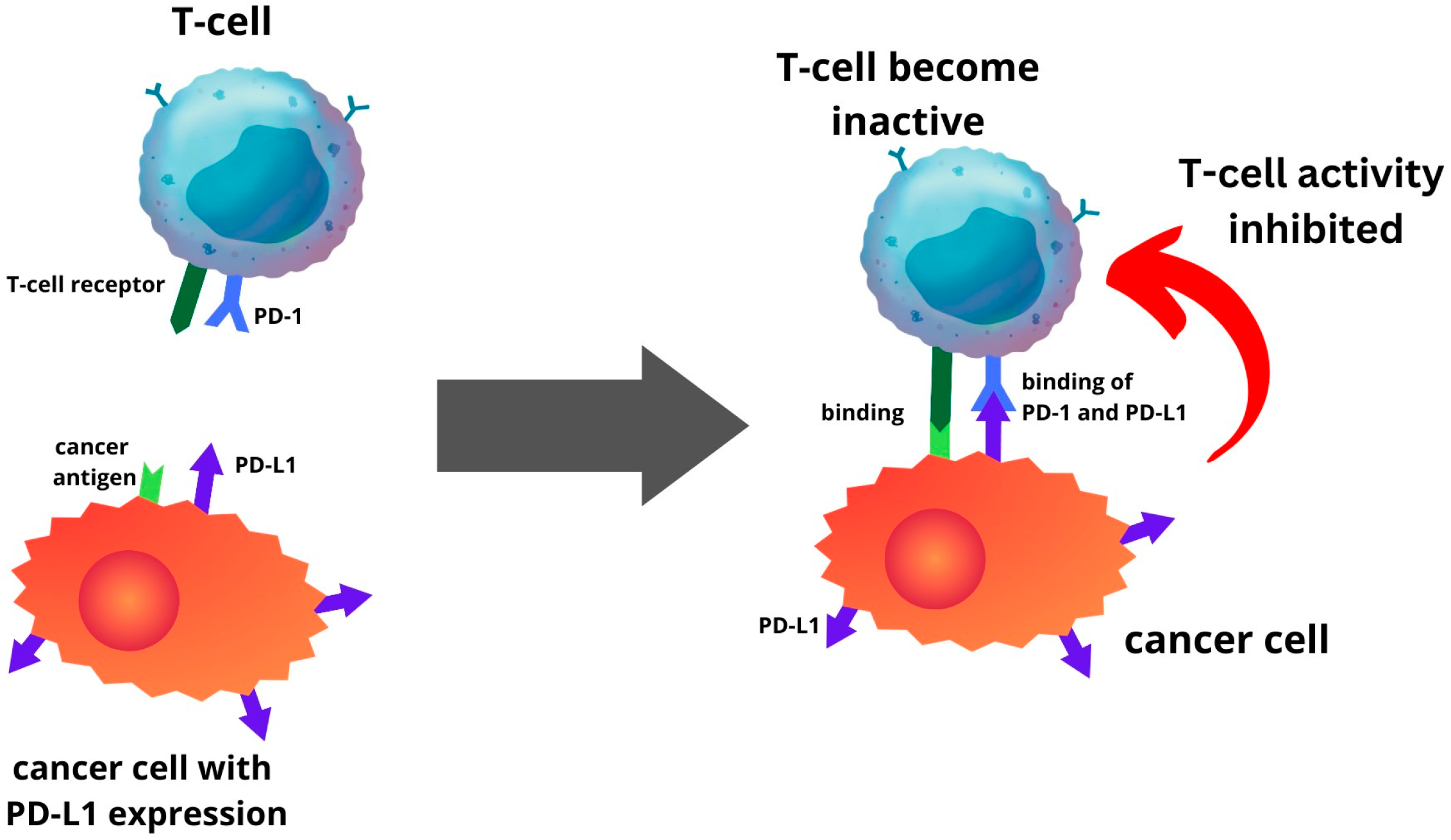
5. Expression of PD-1 and PD-L1 in Endometrial Cancer
6. Clinical Significance: Prognostic Role and Immune Checkpoint Inhibitors
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gentry-Maharaj, A.; Karpinskyj, C. Current and Future Approaches to Screening for Endometrial Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Hussein, Y.; Bandyopadhyay, S.; Cote, M.; Hassan, O.; Abdulfatah, E.; Alosh, B.; Guan, H.; Soslow, R.A.; Ali-Fehmi, R. Interobserver Variability in the Diagnosis of Uterine High-Grade Endometrioid Carcinoma. Arch. Pathol. Lab. Med. 2016, 140, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Gilks, C.B.; Oliva, E.; Soslow, R.A. Poor Interobserver Reproducibility in the Diagnosis of High-Grade Endometrial Carcinoma. Am. J. Surg. Pathol. 2013, 37, 874–881. [Google Scholar] [CrossRef]
- de Boer, S.M.; Wortman, B.G.; Bosse, T.; Powell, M.E.; Singh, N.; Hollema, H.; Wilson, G.; Chowdhury, M.N.; Mileshkin, L.; Pyman, J.; et al. Clinical Consequences of Upfront Pathology Review in the Randomised PORTEC-3 Trial for High-Risk Endometrial Cancer. Ann. Oncol. 2018, 29, 424–430. [Google Scholar] [CrossRef]
- Golia D’Augè, T.; Cuccu, I.; Santangelo, G.; Muzii, L.; Giannini, A.; Bogani, G.; Di Donato, V. Novel Insights into Molecular Mechanisms of Endometrial Diseases. Biomolecules 2023, 13, 499. [Google Scholar] [CrossRef]
- Getz, G.; Gabriel, S.B.; Cibulskis, K.; Lander, E.; Sivachenko, A.; Sougnez, C.; Lawrence, M.; Kandoth, C.; Dooling, D.; Fulton, R.; et al. Integrated Genomic Characterization of Endometrial Carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP Guidelines for the Management of Patients with Endometrial Carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Tomczak, K.; Czerwińska, P.; Wiznerowicz, M. The Cancer Genome Atlas (TCGA): An Immeasurable Source of Knowledge. Wspolczesna Onkol. 2015, 19, A68–A77. [Google Scholar] [CrossRef]
- Alexa, M.; Hasenburg, A.; Battista, M.J. The Tcga Molecular Classification of Endometrial Cancer and Its Possible Impact on Adjuvant Treatment Decisions. Cancers 2021, 13, 1478. [Google Scholar] [CrossRef]
- Abiko, K.; Matsumura, N.; Hamanishi, J.; Horikawa, N.; Murakami, R.; Yamaguchi, K.; Yoshioka, Y.; Baba, T.; Konishi, I.; Mandai, M. IFN-γ from Lymphocytes Induces PD-L1 Expression and Promotes Progression of Ovarian Cancer. Br. J. Cancer 2015, 112, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Church, D.N.; Stelloo, E.; Nout, R.A.; Valtcheva, N.; Depreeuw, J.; Haar, N.T.; Noske, A.; Amant, F.; Tomlinson, I.P.M.; Wild, P.J.; et al. Prognostic Significance of POLE Proofreading Mutations in Endometrial Cancer. J. Natl. Cancer Inst. 2015, 107, dju402. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Ma, X.; Fischer, J.V.; Sun, C.; Kong, B.; Zhang, Q. Immunotherapy in Endometrial Cancer: Rationale, Practice and Perspectives. Biomark. Res. 2021, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Jiang, S.; Cao, D.; Mao, M.; Xiang, Y. Immunologic Signatures across Molecular Subtypes and Potential Biomarkers for Sub-Stratification in Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 1791. [Google Scholar] [CrossRef] [PubMed]
- Leon-Castillo, A.; Horeweg, N.; Peters, E.E.M.; Rutten, T.; ter Haar, N.; Smit, V.T.H.B.M.; Kroon, C.D.; Boennelycke, M.; Hogdall, E.; Hogdall, C.; et al. Prognostic Relevance of the Molecular Classification in High-Grade Endometrial Cancer for Patients Staged by Lymphadenectomy and without Adjuvant Treatment. Gynecol. Oncol. 2022, 164, 577–586. [Google Scholar] [CrossRef]
- Bellone, S.; Roque, D.M.; Siegel, E.R.; Buza, N.; Hui, P.; Bonazzoli, E.; Guglielmi, A.; Zammataro, L.; Nagarkatti, N.; Zaidi, S.; et al. A Phase II Evaluation of Pembrolizumab in Recurrent Microsatellite Instability-High (MSI-H) Endometrial Cancer Patients with Lynch-like versus MLH-1 Methylated Characteristics (NCT02899793). Ann. Oncol. 2021, 32, 1045–1046. [Google Scholar] [CrossRef] [PubMed]
- Pasanen, A.; Ahvenainen, T.; Pellinen, T.; Vahteristo, P.; Loukovaara, M.; Bützow, R. PD-L1 Expression in Endometrial Carcinoma Cells and Intratumoral Immune Cells: Differences across Histologic and TCGA-Based Molecular Subgroups. Am. J. Surg. Pathol. 2020, 44, 174–181. [Google Scholar] [CrossRef]
- Momeni-Boroujeni, A.; Nguyen, B.; Vanderbilt, C.M.; Ladanyi, M.; Abu-Rustum, N.R.; Aghajanian, C.; Ellenson, L.H.; Weigelt, B.; Soslow, R.A. Genomic Landscape of Endometrial Carcinomas of No Specific Molecular Profile. Mod. Pathol. 2022, 35, 1269–1278. [Google Scholar] [CrossRef]
- Crumley, S.; Kurnit, K.; Hudgens, C.; Fellman, B.; Tetzlaff, M.T.; Broaddus, R. Identification of a Subset of Microsatellite-Stable Endometrial Carcinoma with High PD-L1 and CD8+ Lymphocytes. Mod. Pathol. 2019, 32, 396–404. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, W. High-Grade Endometrial Carcinomas: Morphologic Spectrum and Molecular Classification. Semin. Diagn. Pathol. 2022, 39, 176–186. [Google Scholar] [CrossRef]
- Hussein, Y.R.; Broaddus, R.; Weigelt, B.; Levine, D.A.; Soslow, R.A. The Genomic Heterogeneity of FIGO Grade 3 Endometrioid Carcinoma Impacts Diagnostic Accuracy and Reproducibility. Int. J. Gynecol. Pathol. 2016, 35, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; Jamieson, A.; Crosbie, E.J.; Taylor, A.; Chiu, D.; Leung, S.; Grube, M.; Kommoss, S.; Gilks, C.B.; McAlpine, J.N.; et al. Targeted Molecular Testing in Endometrial Carcinoma: Validation of a Clinically Driven Selective ProMisE Testing Protocol. Int. J. Gynecol. Pathol. 2023, 42, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Cuccu, I.; D’Oria, O.; Sgamba, L.; De Angelis, E.; Golia D’Augè, T.; Turetta, C.; Di Dio, C.; Scudo, M.; Bogani, G.; Di Donato, V.; et al. Role of Genomic and Molecular Biology in the Modulation of the Treatment of Endometrial Cancer: Narrative Review and Perspectives. Healthcare 2023, 11, 571. [Google Scholar] [CrossRef]
- Mamat @ Yusof, M.N.; Chew, K.T.; Hafizz, A.M.H.A.; Abd Azman, S.H.; Ab Razak, W.S.; Hamizan, M.R.; Kampan, N.C.; Shafiee, M.N. Efficacy and Safety of PD-1/PD-L1 Inhibitor as Single-Agent Immunotherapy in Endometrial Cancer: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 4032. [Google Scholar] [CrossRef]
- Vanderstraeten, A.; Tuyaerts, S.; Amant, F. The Immune System in the Normal Endometrium and Implications for Endometrial Cancer Development. J. Reprod. Immunol. 2015, 109, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Ochiel, D.O.; Ghosh, M.; Fahey, J.V.; Guyre, P.M.; Wira, C.R. Human Uterine Epithelial Cell Secretions Regulate Dendritic Cell Differentiation and Responses to TLR Ligands. J. Leukoc. Biol. 2010, 88, 435–444. [Google Scholar] [CrossRef]
- Agostinis, C.; Mangogna, A.; Bossi, F.; Ricci, G.; Kishore, U.; Bulla, R. Uterine Immunity and Microbiota: A Shifting Paradigm. Front. Immunol. 2019, 10, 2387. [Google Scholar] [CrossRef]
- Wira, C.R.; Fahey, J.V.; Rodriguez-Garcia, M.; Shen, Z.; Patel, M.V. Regulation of Mucosal Immunity in the Female Reproductive Tract: The Role of Sex Hormones in Immune Protection against Sexually Transmitted Pathogens. Am. J. Reprod. Immunol. 2014, 72, 236–258. [Google Scholar] [CrossRef]
- Wang, F.; Qualls, A.E.; Marques-Fernandez, L.; Colucci, F. Biology and Pathology of the Uterine Microenvironment and Its Natural Killer Cells. Cell. Mol. Immunol. 2021, 18, 2101–2113. [Google Scholar] [CrossRef]
- Aflatoonian, R.; Amjadi, F.; Mehdizadeh, M.; Salehi, E. Role of the Innate Immunity in Female Reproductive Tract. Adv. Biomed. Res. 2014, 3, 1. [Google Scholar] [CrossRef]
- Oertelt-Prigione, S. Immunology and the Menstrual Cycle. Autoimmun. Rev. 2012, 11, A486–A492. [Google Scholar] [CrossRef] [PubMed]
- Zwahlen, M.; Stute, P. Impact of Progesterone on the Immune System in Women: A Systematic Literature Review. Arch. Gynecol. Obstet. 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Mandal, M.; Goldsmith, L.T.; Kashani, B.N.; Ponzio, N.M. The Maternal Immune System during Pregnancy and Its Influence on Fetal Development. Res. Rep. Biol. 2015, 6, 171–189. [Google Scholar] [CrossRef]
- Chen, R.Y.; Zhu, Y.; Shen, Y.Y.; Xu, Q.Y.; Tang, H.Y.; Cui, N.X.; Jiang, L.; Dai, X.M.; Chen, W.Q.; Lin, Q.; et al. The Role of PD-1 Signaling in Health and Immune-Related Diseases. Front. Immunol. 2023, 14, 1163633. [Google Scholar] [CrossRef] [PubMed]
- Chikuma, S. Basics of PD-1 in Self-Tolerance, Infection, and Cancer Immunity. Int. J. Clin. Oncol. 2016, 21, 448–455. [Google Scholar] [CrossRef]
- Laba, S.; Mallett, G.; Amarnath, S. The Depths of PD-1 Function within the Tumor Microenvironment beyond CD8+ T Cells. Semin. Cancer Biol. 2022, 86, 1045–1055. [Google Scholar] [CrossRef]
- Jubel, J.M.; Barbati, Z.R.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. The Role of PD-1 in Acute and Chronic Infection. Front. Immunol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer Immunoediting: From Immunosurveillance to Tumor Escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Burnet, M. Cancer-A Biological Approach* Iii. Viruses Associated with Neoplastic Conditions. Br. Med. J. 1957, 1, 841. [Google Scholar] [CrossRef]
- Stutman, O. Tumor Development after 3-Methylcholanthrene in Immunologically Deficient Athymic-Nude Mice. Science 1974, 183, 534–536. [Google Scholar] [CrossRef]
- Ikeda, H.; Old, L.J.; Schreiber, R.D. The Roles of IFNγ in Protection against Tumor Development and Cancer Immunoediting. Cytokine Growth Factor Rev. 2002, 13, 95–109. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer Immunoediting and Resistance to T Cell-Based Immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; Balega, J.; Buckley, L.; Clamp, A.; Crosbie, E.; Drew, Y.; Durrant, L.; Forrest, J.; Fotopoulou, C.; Gajjar, K.; et al. British Gynaecological Cancer Society (BGCS) Uterine Cancer Guidelines: Recommendations for Practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 270, 50–89. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Steinman, R.M. Dendritic Cells: Specialized and Regulated Antigen Processing Machines. Cell 2001, 106, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Palucka, K.; Banchereau, J.; Mellman, I. Designing Vaccines Based on Biology of Human Dendritic Cell Subsets. Immunity 2010, 33, 464–478. [Google Scholar] [CrossRef]
- Chimal-Ramírez, G.K.; Espinoza-Sánchez, N.A.; Fuentes-Pananá, E.M. Protumor Activities of the Immune Response: Insights in the Mechanisms of Immunological Shift, Oncotraining, and Oncopromotion. J. Oncol. 2013, 2013, 835956. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Quezada, S.A.; Peggs, K.S.; Simpson, T.R.; Allison, J.P. Shifting the Equilibrium in Cancer Immunoediting: From Tumor Tolerance to Eradication. Immunol. Rev. 2011, 241, 104–118. [Google Scholar] [CrossRef]
- Aguirre-Ghiso, J.A. Models, Mechanisms and Clinical Evidence for Cancer Dormancy. Nat. Rev. Cancer 2007, 7, 834–846. [Google Scholar] [CrossRef]
- Koebel, C.M.; Vermi, W.; Swann, J.B.; Zerafa, N.; Rodig, S.J.; Old, L.J.; Smyth, M.J.; Schreiber, R.D. Adaptive Immunity Maintains Occult Cancer in an Equilibrium State. Nature 2007, 450, 903–907. [Google Scholar] [CrossRef]
- Gabrilovich, D.; Ishida, T.; Oyama, T.; Ran, S.; Kravtsov, V.; Nadaf, S.; Carbone, D.P. Vascular Endothelial Growth Factor Inhibits the Development of Dendritic Cells and Dramatically Affects the Differentiation of Multiple Hematopoietic Lineages in Vivo. Blood 1998, 92, 4150–4166. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Muto, G. TGF-β Function in Immune Suppression. In Negative Co-Receptors and Ligands; Springer: Berlin/Heidelberg, Germany, 2010; Volume 350, pp. 127–147. [Google Scholar] [CrossRef]
- Löb, S.; Königsrainer, A.; Rammensee, H.G.; Opelz, G.; Terness, P. Inhibitors of Indoleamine-2,3-Dioxygenase for Cancer Therapy: Can We See the Wood for the Trees? Nat. Rev. Cancer 2009, 9, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Garrido, F.; Ruiz-Cabello, F.; Cabrera, T.; Pérez-Villar, J.J.; López-Botet, M.; Duggan-Keen, M.; Stern, P.L. Implications for Immunosurveillance of Altered HLA Class I Phenotypes in Human Tumours. Immunol. Today 1997, 18, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Ventriglia, J.; Paciolla, I.; Pisano, C.; Cecere, S.C.; Di Napoli, M.; Tambaro, R.; Califano, D.; Losito, S.; Scognamiglio, G.; Setola, S.V.; et al. Immunotherapy in Ovarian, Endometrial and Cervical Cancer: State of the Art and Future Perspectives. Cancer Treat. Rev. 2017, 59, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, P.A. A Two-Step, Two-Signal Model for the Primary Activation of Precursor Helper T Cells. Proc. Natl. Acad. Sci. USA 1999, 96, 185–190. [Google Scholar] [CrossRef]
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The Future of Cancer Treatment: Immunomodulation, CARs and Combination Immunotherapy. Nat. Rev. Clin. Oncol. 2016, 13, 273–290. [Google Scholar] [CrossRef]
- Appleman, L.J.; Boussiotis, V.A. T Cell Anergy and Costimulation. Immunol. Rev. 2003, 192, 161–180. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The Future of Immune Checkpoint Therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Townsend, S.E.; Allison, J.P. Tumor Rejection after Direct Costimulation of CD8+ T Cells by B7-Transfected Melanoma Cells. Science 1993, 259, 368–370. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Linsley, P.S.; Greene, J.A.L.; Brady, W.; Bajorath, J.; Ledbetter, J.A.; Peach, R. Human B7-1 (CD80) and B7-2 (CD86) Bind with Similar Avidities but Distinct Kinetics to CD28 and CTLA-4 Receptors. Immunity 1994, 1, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Linsley, P.S.; Brady, W.; Urnes, M.; Grosmaire, L.S.; Damle, N.K.; Ledbetter, J.A. CTLA4 Is a Second Receptor for the b Cell Activation Antigen B7. J. Exp. Med. 1991, 174, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, T.; Takamatsu, M.; Kobayashi-Imanishi, W.; Hashimoto-Tane, A.; Azuma, M.; Saito, T. Programmed Cell Death 1 Forms Negative Costimulatory Microclusters That Directly Inhibit T Cell Receptor Signaling by Recruiting Phosphatase SHP2. J. Exp. Med. 2012, 209, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumor Antigen-Specific CD8 T Cells Infiltrating the Tumor Express High Levels of PD-1 and Are Functionally Impaired. Blood 2009, 114, 1537–1544. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Simon, S.; Labarriere, N. PD-1 Expression on Tumor-Specific T Cells: Friend or Foe for Immunotherapy? OncoImmunology 2018, 7, e1364828. [Google Scholar] [CrossRef]
- Staron, M.M.; Gray, S.M.; Marshall, H.D.; Parish, I.A.; Chen, J.H.; Perry, C.J.; Cui, G.; Li, M.O.; Kaech, S.M. The Transcription Factor FoxO1 Sustains Expression of the Inhibitory Receptor PD-1 and Survival of Antiviral CD8+ T Cells during Chronic Infection. Immunity 2014, 41, 802–814. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Xiao, J.; Jiao, S.; Teng, F.; Xue, S.; Zhang, C.; Sheng, C.; Leng, Q.; Rudd, C.E.; et al. ADAP and SKAP 55 Deficiency Suppresses PD-1 Expression in CD 8 + Cytotoxic T Lymphocytes for Enhanced Anti-tumor Immunotherapy. EMBO Mol. Med. 2015, 7, 754–769. [Google Scholar] [CrossRef]
- Salmaninejad, A.; Khoramshahi, V.; Azani, A.; Soltaninejad, E.; Aslani, S.; Zamani, M.R.; Zal, M.; Nesaei, A.; Hosseini, S.M. PD-1 and Cancer: Molecular Mechanisms and Polymorphisms. Immunogenetics 2018, 70, 73–86. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, L.; Li, J.; Li, Y.; Wang, Y.; Xu, Z.X. Recent Findings in the Regulation of Programmed Death Ligand 1 Expression. Front. Immunol. 2019, 10, 455837. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Wherry, E.J.; Ahmed, R.; Freeman, G.J. The Function of Programmed Cell Death 1 and Its Ligands in Regulating Autoimmunity and Infection. Nat. Immunol. 2007, 8, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Ohaegbulam, K.C.; Assal, A.; Lazar-Molnar, E.; Yao, Y.; Zang, X. Human Cancer Immunotherapy with Antibodies to the PD-1 and PD-L1 Pathway. Trends Mol. Med. 2015, 21, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Liu, Y.; Li, Q.; Li, X.D.; Zhao, W.Q.; Zhang, H.; Zhang, X.; Jiang, J.T.; Wu, C.P. PD-1/PD-L1 Pathway in Non-Small-Cell Lung Cancer and Its Relation with EGFR Mutation. J. Transl. Med. 2015, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, R.; Martin, A.; Bommarito, D.; Wang, K.; Hansen, S.H.; Freeman, G.J.; Ritz, J. Interferon-γ-Induced Activation of JAK1 and JAK2 Suppresses Tumor Cell Susceptibility to NK Cells through Upregulation of PD-L1 Expression. Oncoimmunology 2015, 4, e1008824. [Google Scholar] [CrossRef]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef]
- Dong, P.; Xiong, Y.; Yue, J.; Hanley, S.J.B.; Watari, H. Tumor-Intrinsic PD-L1 Signaling in Cancer Initiation, Development and Treatment: Beyond Immune Evasion. Front. Oncol. 2018, 8, 386. [Google Scholar] [CrossRef]
- Nunes-Xavier, C.E.; Angulo, J.C.; Pulido, R.; López, J.I. A Critical Insight into the Clinical Translation of PD-1/PD-L1 Blockade Therapy in Clear Cell Renal Cell Carcinoma. Curr. Urol. Rep. 2019, 20, 1. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Heinzerling, L.; Kirchberger, M.C.; Walter, L.; Schuler, G. Predicting the Response to Anti-PD1 Therapy in Metastatic Melanoma. Transl. Cancer Res. 2016, 5, S576–S579. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Z.; Zheng, X.; Tao, H.; Zhang, S.; Ma, J.; Liu, Z.; Wang, J.; Qian, Y.; Cui, P.; et al. Response Efficacy of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 562315. [Google Scholar] [CrossRef]
- Adkins, D.R.; Haddad, R.I. Clinical Trial Data of Anti–PD-1/PD-L1 Therapy for Recurrent or Metastatic Nasopharyngeal Carcinoma: A Review. Cancer Treat. Rev. 2022, 109, 102428. [Google Scholar] [CrossRef] [PubMed]
- Mamat @ Yusof, M.N.; Chew, K.T.; Kampan, N.; Nor, N.H.; Md Zin, R.R.; Tan, G.C.; Shafiee, M.N. PD-L1 Expression in Endometrial Cancer and Its Association with Clinicopathological Features: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Minaguchi, T.; Xu, C.; Qi, N.; Itagaki, H.; Shikama, A.; Tasaka, N.; Akiyama, A.; Sakurai, M.; Ochi, H.; et al. PD-L1 and CD4 Are Independent Prognostic Factors for Overall Survival in Endometrial Carcinomas. BMC Cancer 2020, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Sungu, N.; Yildirim, M.; Desdicioglu, R.; Aydoǧdu, Ö.B.; Kiliçarslan, A.; Doǧan, H.T.; Yazgan, A.K.; Akyol, M.; Erdoǧan, F. Expression of Immunomodulatory Molecules PD-1, PD-L1, and PD-L2, and Their Relationship with Clinicopathologic Characteristics in Endometrial Cancer. Int. J. Gynecol. Pathol. 2019, 38, 404–413. [Google Scholar] [CrossRef]
- Engerud, H.; Berg, H.F.; Myrvold, M.; Halle, M.K.; Bjorge, L.; Haldorsen, I.S.; Hoivik, E.A.; Trovik, J.; Krakstad, C. High Degree of Heterogeneity of PD-L1 and PD-1 from Primary to Metastatic Endometrial Cancer. Gynecol. Oncol. 2020, 157, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Chew, M.; Wong, Y.P.; Karim, N.; Mustangin, M.; Alfian, N.; Tan, G.C. Programmed Death Ligand 1: A Poor Prognostic Marker in Endometrial Carcinoma. Diagnostics 2020, 10, 394. [Google Scholar] [CrossRef]
- Mo, Z.; Liu, J.; Zhang, Q.; Chen, Z.; Mei, J.; Liu, L.; Yang, S.; Li, H.; Zhou, L.; You, Z. Expression of PD-1, PD-L1 and PD-L2 Is Associated with Differentiation Status and Histological Type of Endometrial Cancer. Oncol. Lett. 2016, 12, 944–950. [Google Scholar] [CrossRef]
- Howitt, B.E.; Shukla, S.A.; Sholl, L.M.; Ritterhouse, L.L.; Watkins, J.C.; Rodig, S.; Stover, E.; Strickland, K.C.; D’Andrea, A.D.; Wu, C.J.; et al. Association of Polymerase E-Mutated and Microsatellite-Instable Endometrial Cancers with Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015, 1, 1319–1323. [Google Scholar] [CrossRef]
- Luchini, C.; Bibeau, F.; Ligtenberg, M.J.L.; Singh, N.; Nottegar, A.; Bosse, T.; Miller, R.; Riaz, N.; Douillard, J.Y.; Andre, F.; et al. ESMO Recommendations on Microsatellite Instability Testing for Immunotherapy in Cancer, and Its Relationship with PD-1/PD-L1 Expression and Tumour Mutational Burden: A Systematic Review-Based Approach. Ann. Oncol. 2019, 30, 1232–1243. [Google Scholar] [CrossRef]
- Vanderwalde, A.; Spetzler, D.; Xiao, N.; Gatalica, Z.; Marshall, J. Microsatellite Instability Status Determined by Next-Generation Sequencing and Compared with PD-L1 and Tumor Mutational Burden in 11,348 Patients. Cancer Med. 2018, 7, 746–756. [Google Scholar] [CrossRef]
- Li, Z.; Joehlin-Price, A.S.; Rhoades, J.; Ayoola-Adeola, M.; Miller, K.; Parwani, A.V.; Backes, F.J.; Felix, A.S.; Suarez, A.A. Programmed Death Ligand 1 Expression among 700 Consecutive Endometrial Cancers: Strong Association with Mismatch Repair Protein Deficiency. Int. J. Gynecol. Cancer 2018, 28, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Sun, Z.; Mo, S.; Lu, Z.; Yu, S.; Xiang, Y.; Chen, J. PD-L1 Expression in Tumor Cells Is Associated with a Favorable Prognosis in Patients with High-Risk Endometrial Cancer. Gynecol. Oncol. 2021, 162, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Asaka, S.; Yen, T.T.; Wang, T.L.; Shih, I.M.; Gaillard, S. T Cell-Inflamed Phenotype and Increased Foxp3 Expression in Infiltrating T-Cells of Mismatch-Repair Deficient Endometrial Cancers. Mod. Pathol. 2019, 32, 576–584. [Google Scholar] [CrossRef]
- Talhouk, A.; Derocher, H.; Schmidt, P.; Leung, S.; Milne, K.; Blake Gilks, C.; Anglesio, M.S.; Nelson, B.H.; McAlpine, J.N. Molecular Subtype Not Immune Response Drives Outcomes in Endometrial Carcinoma. Clin. Cancer Res. 2019, 25, 2537–2548. [Google Scholar] [CrossRef]
- Bregar, A.; Deshpande, A.; Grange, C.; Zi, T.; Stall, J.; Hirsch, H.; Reeves, J.; Sathyanarayanan, S.; Growdon, W.B.; Rueda, B.R. Characterization of Immune Regulatory Molecules B7-H4 and PD-L1 in Low and High Grade Endometrial Tumors. Gynecol. Oncol. 2017, 145, 446–452. [Google Scholar] [CrossRef]
- Kucukgoz Gulec, U.; Kilic Bagir, E.; Paydas, S.; Guzel, A.B.; Gumurdulu, D.; Vardar, M.A. Programmed Death-1 (PD-1) and Programmed Death-Ligand 1 (PD-L1) Expressions in Type 2 Endometrial Cancer. Arch. Gynecol. Obstet. 2019, 300, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Nakayama, K.; Ishikawa, M.; Nakamura, K.; Ishibashi, T.; Sanuki, K.; Ono, R.; Sasamori, H.; Minamoto, T.; Iida, K.; et al. Microsatellite Instability Is a Biomarker for Immune Checkpoint Inhibitors in Endometrial Cancer. Oncotarget 2018, 9, 5652–5664. [Google Scholar] [CrossRef]
- Mitchard, J.; Hirschowitz, L. Concordance of FIGO Grade of Endometrial Adenocarcinomas in Biopsy and Hysterectomy Specimens. Histopathology 2003, 42, 372–378. [Google Scholar] [CrossRef]
- Nguyen, M.; Han, L.; Pua, T.; Mares, A.; Karsy, M.; LaFargue, C.; Ardacheva, O.; Pradhan, T.; Fallon, J.; Tedjarati, S. Comparison of FIGO Grade 3 Endometrioid Endometrial Carcinomas with Type 2 Uterine Cancers. Can Grade 3 Tumors Be Classified as Type 2 Cancers? A Clinicopathological and Immunohistochemical Analysis. Gynecol. Oncol. 2013, 130, e91–e92. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, X.; Liu, X.; Ma, K.; Meng, Y.T.; Yin, H.F.; Wen, J.; Yang, J.H.; Zhen, Z.; Feng, Z.H.; et al. Clinical Characteristics and Prognostic Characterization of Endometrial Carcinoma: A Comparative Analysis of Molecular Typing Protocols. BMC Cancer 2023, 23, 243. [Google Scholar] [CrossRef]
- Raedler, L.A. Opdivo (Nivolumab): Second PD-1 Inhibitor Receives FDA Approval for Unresectable or Metastatic Melanoma. Am. Heal. Drug Benefits 2015, 8, 180–183. [Google Scholar]
- Raedler, L.A. Keytruda (Pembrolizumab): First PD-1 Inhibitor Approved for Previously Treated Unresectable or Metastatic Melanoma. Am. Heal. Drug Benefits 2015, 8, 96–100. [Google Scholar]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-Year Survival Outcomes for Patients with Advanced Melanoma Treated with Pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanovszki, D.; Kiss, N.; Tóth, B.; Kerner, T.; Tóth, V.; Szakonyi, J.; Lőrincz, K.; Hársing, J.; Imrédi, E.; Pfund, A.; et al. Anti-PD-1 Monotherapy in Advanced Melanoma—Real-World Data from a 77-Month-Long Retrospective Observational Study. Biomedicines 2022, 10, 1737. [Google Scholar] [CrossRef] [PubMed]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab for Advanced Melanoma: Final Overall Survival Results of a Multicentre, Randomised, Open-Label Phase 3 Study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Schinzari, G.; Maiorano, B.A.; Indellicati, G.; Di Stefani, A.; Pagliara, M.M.; Fragomeni, S.M.; De Luca, E.V.; Sammarco, M.G.; Garganese, G.; et al. Efficacy of Immune Checkpoint Inhibitors in Different Types of Melanoma. Hum. Vaccines Immunother. 2020, 17, 4–13. [Google Scholar] [CrossRef]
- Aroldi, F.; Middleton, M.R. Long-Term Outcomes of Immune Checkpoint Inhibition in Metastatic Melanoma. Am. J. Clin. Dermatol. 2022, 23, 331–338. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; de Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1. [Google Scholar] [CrossRef]
- Hasegawa, K.; Tamura, K.; Katsumata, N.; Matsumoto, K.; Takahashi, S.; Mukai, H.; Nomura, H.; Minami, H. Efficacy and Safety of Nivolumab (Nivo) in Patients (Pts) with Advanced or Recurrent Uterine Cervical or Corpus Cancers. J. Clin. Oncol. 2018, 36 (Suppl. 15), 5594. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Luo, W.; Liu, J.F.; Gulhan, D.C.; Krasner, C.; Ishizuka, J.J.; Gockley, A.A.; Buss, M.; Growdon, W.B.; Crowe, H.; et al. Phase II Study of Avelumab in Patients with Mismatch Repair Deficient and Mismatch Repair Proficient Recurrent/Persistent Endometrial Cancer. J. Clin. Oncol. 2019, 37, 2786–2794. [Google Scholar] [CrossRef]
- Antill, Y.; Kok, P.S.; Stockler, M.R.; Robledo, K.; Yip, S.; Parry, M.; Smith, D.; Spurdle, A.; Barnes, E.; Friedlander, M.L.; et al. Updated Results of Activity of Durvalumab in Advanced Endometrial Cancer (AEC) According to Mismatch Repair (MMR) Status: The Phase II PHAEDRA Trial (ANZGOG1601). Ann. Oncol. 2019, 30, ix192. [Google Scholar] [CrossRef]
- Oaknin, A.; Tinker, A.V.; Gilbert, L.; Samouëlian, V.; Mathews, C.; Brown, J.; Barretina-Ginesta, M.P.; Moreno, V.; Gravina, A.; Abdeddaim, C.; et al. Clinical Activity and Safety of the Anti-Programmed Death 1 Monoclonal Antibody Dostarlimab for Patients with Recurrent or Advanced Mismatch Repair-Deficient Endometrial Cancer: A Nonrandomized Phase 1 Clinical Trial. JAMA Oncol. 2020, 6, 1766–1772. [Google Scholar] [CrossRef]
- Consortium, R.R. Refining Adjuvant Treatment in Endometrial Cancer Based on Molecular Features: The RAINBO Clinical Trial Program. Int. J. Gynecol. Cancer 2022, 33, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.; Bosse, T.; McAlpine, J.N. The Emerging Role of Molecular Pathology in Directing the Systemic Treatment of Endometrial Cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211035959. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Taylor, M.H.; Aghajanian, C.; Oaknin, A.; Mier, J.; Cohn, A.L.; Romeo, M.; Bratos, R.; Brose, M.S.; DiSimone, C.; et al. Lenvatinib plus Pembrolizumab in Patients with Advanced Endometrial Cancer. J. Clin. Oncol. 2020, 38, 2981–2992. [Google Scholar] [CrossRef]
- Makker, V.; Colombo, N.; Herráez, A.C.; Monk, B.J.; MacKay, H.; Santin, A.D.; Miller, D.S.; Moore, R.G.; Baron-Hay, S.; Ray-Coquard, I.; et al. Lenvatinib Plus Pembrolizumab in Previously Treated Advanced Endometrial Cancer: Updated Efficacy and Safety From the Randomized Phase III Study 309/KEYNOTE-775. J. Clin. Oncol. 2023, 41, 2904–2910. [Google Scholar] [CrossRef]
- Lheureux, S.; Matei, D.; Konstantinopoulos, P.A.; Block, M.S.; Jewell, A.; Gaillard, S.; McHale, M.S.; McCourt, C.K.; Temkin, S.; Girda, E.; et al. A Randomized Phase II Study of Cabozantinib and Nivolumab versus Nivolumab in Recurrent Endometrial Cancer. J. Clin. Oncol. 2020, 38 (Suppl. 15), 6010. [Google Scholar] [CrossRef]
- Eskander, R.N.; Sill, M.W.; Beffa, L.; Moore, R.G.; Hope, J.M.; Musa, F.B.; Mannel, R.; Shahin, M.S.; Cantuaria, G.H.; Girda, E.; et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [CrossRef]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; dePont Christensen, R.; Novák, Z.; Black, D.; Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.M.; et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef]
- Siraj, A.K.; Parvathareddy, S.K.; Annaiyappanaidu, P.; Siraj, N.; Al-Rasheed, M.; Al-Badawi, I.A.; Al-Dayel, F.; Al-Kuraya, K.S. PD-L1 Expression Is an Independent Marker for Lymph Node Metastasis in Middle Eastern Endometrial Cancer. Diagnostics 2021, 11, 394. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Han, X. Anti-PD-1/PD-L1 Therapy of Human Cancer: Past, Present, and Future. J. Clin. Investig. 2015, 125, 3384–3391. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Day, D.; Nicholls, S.J.; Segelov, E. Immune Checkpoint Inhibitor Therapy in Oncology: Current Uses and Future Directions: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2022, 4, 579–597. [Google Scholar] [CrossRef]
- Xu, D.; Dong, P.; Xiong, Y.; Chen, R.; Konno, Y.; Ihira, K.; Yue, J.; Watari, H. PD-L1 Is a Tumor Suppressor in Aggressive Endometrial Cancer Cells and Its Expression Is Regulated by MiR-216a and LncRNA MEG3. Front. Cell Dev. Biol. 2020, 8, 598205. [Google Scholar] [CrossRef]
- Lee, H.H.; Wang, Y.N.; Xia, W.; Chen, C.H.; Rau, K.M.; Ye, L.; Wei, Y.; Chou, C.K.; Wang, S.C.; Yan, M.; et al. Removal of N-Linked Glycosylation Enhances PD-L1 Detection and Predicts Anti-PD-1/PD-L1 Therapeutic Efficacy. Cancer Cell 2019, 36, 168–178.e4. [Google Scholar] [CrossRef]
- Ren, D.; Hua, Y.; Yu, B.; Ye, X.; He, Z.; Li, C.; Wang, J.; Mo, Y.; Wei, X.; Chen, Y.; et al. Predictive Biomarkers and Mechanisms Underlying Resistance to PD1/PD-L1 Blockade Cancer Immunotherapy. Mol. Cancer 2020, 19, 19. [Google Scholar] [CrossRef] [PubMed]

| Molecular Classes | Copy Number High (High-CN) | Copy Number Low (Low-CN) | Microsatellite Instability (MSI) | POLE-Mutant |
|---|---|---|---|---|
| Associated gene mutation | TP53 PIK3CA FBXW7 PPP2R1A PTEN | PTEN PIK3CA CTNNB1 ARID1A PIK3R1 | PTEN PIK3CA PIK3R1 RPL22 ARID1A | POLE DMD CSMD1 FAT4 PTEN |
| Mutations | Low | Low | High | Extremely high |
| Somatic copy number alteration | High | Low | Low | Very low |
| Associated histology | Serous type | Endometroid grade 1–2 | Endometroid grade 3 | Endometroid grade 3 |
| PD-L1 expression | Low | Low | High | High |
| Prognosis | Poor | Good/intermediate | Intermediate | Good |
| Benefit of PD-1/PD-L1 inhibitors | No | No | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamat @ Yusof, M.N.; Chew, K.T.; Kampan, N.C.; Shafiee, M.N. Expression of PD-1 and PD-L1 in Endometrial Cancer: Molecular and Clinical Significance. Int. J. Mol. Sci. 2023, 24, 15233. https://doi.org/10.3390/ijms242015233
Mamat @ Yusof MN, Chew KT, Kampan NC, Shafiee MN. Expression of PD-1 and PD-L1 in Endometrial Cancer: Molecular and Clinical Significance. International Journal of Molecular Sciences. 2023; 24(20):15233. https://doi.org/10.3390/ijms242015233
Chicago/Turabian StyleMamat @ Yusof, Mohd Nazzary, Kah Teik Chew, Nirmala Chandralega Kampan, and Mohamad Nasir Shafiee. 2023. "Expression of PD-1 and PD-L1 in Endometrial Cancer: Molecular and Clinical Significance" International Journal of Molecular Sciences 24, no. 20: 15233. https://doi.org/10.3390/ijms242015233
APA StyleMamat @ Yusof, M. N., Chew, K. T., Kampan, N. C., & Shafiee, M. N. (2023). Expression of PD-1 and PD-L1 in Endometrial Cancer: Molecular and Clinical Significance. International Journal of Molecular Sciences, 24(20), 15233. https://doi.org/10.3390/ijms242015233








