Prevalence of Congenital Color Vision Deficiency in Southern Taiwan and Detection of Female Carriers by Visual Pigment Gene Analysis
Abstract
:1. Introduction
2. Results
2.1. Part I: Survey of Color Vision of Students
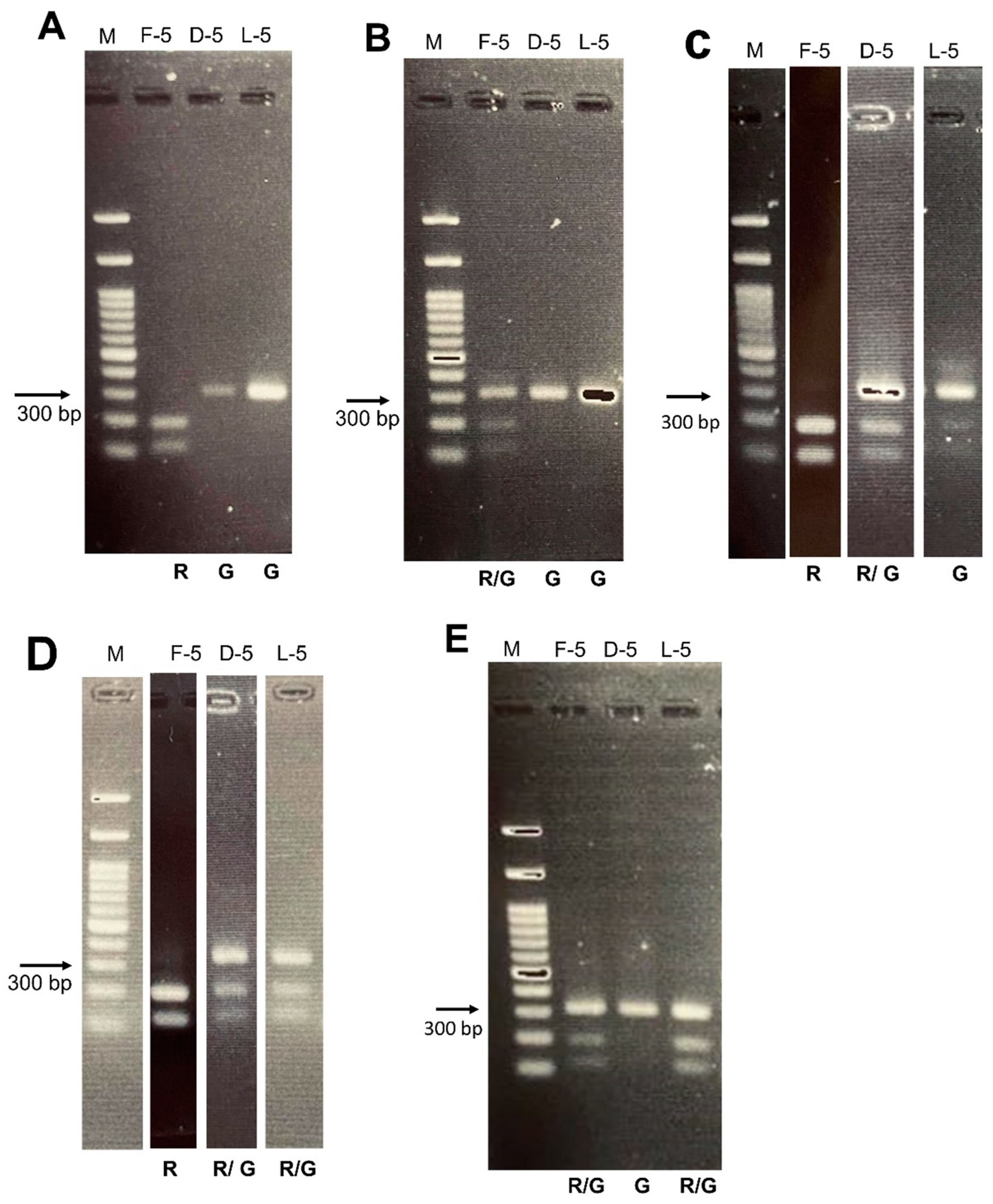
2.2. Part II: Detection of Female Carriers
3. Discussion
4. Materials and Methods
4.1. Part I: Survey of the Color Vision of Students
4.2. Part II: Detection of Female Carriers
4.2.1. Color Vision Screening Tests
4.2.2. DNA Extraction
4.2.3. Long-Range and Secondary PCR (Two-Step)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics approval and consent to participate
References
- Simunovic, M.P. Colour vision deficiency. Eye 2010, 24, 747–755. [Google Scholar] [CrossRef]
- Neitz, J.; Neitz, M. The genetics of normal and defective color vision. Vision Res. 2011, 51, 633–651. [Google Scholar] [CrossRef]
- Birch, J. Worldwide prevalence of red-green color deficiency. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2012, 29, 313–320. [Google Scholar] [CrossRef]
- Harrington, S.; Davison, P.A.; O’Dwyer, V. Prevalence of colour vision deficiency in the Republic of Ireland schoolchildren and associated socio-demographic factors. Clin. Exp. Optom. 2021, 104, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.Z.; Tarczy-Hornoch, K.; Lin, J.; Cotter, S.A.; Torres, M.; Varma, R.; Multi-Ethnic Pediatric Eye Disease Study Group. Color vision deficiency in preschool children: The multi-ethnic pediatric eye disease study. Ophthalmology 2014, 121, 1469–1474. [Google Scholar] [CrossRef] [PubMed]
- Nathans, J.; Piantanida, T.P.; Eddy, R.L.; Shows, T.B.; Hogness, D.S. Molecular genetics of inherited variation in human color vision. Science 1986, 232, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Nathans, J.; Thomas, D.; Hogness, D.S. Molecular genetics of human color vision: The genes encoding blue, green, and red pigments. Science 1986, 232, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; Ueyama, H.; Tanabe, S.; Tanaka, Y.; Yamade, S.; Kani, K. Detection of female carriers of congenital color-vision deficiencies by visual pigment gene analysis. Curr. Eye Res. 2000, 21, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; Ueyama, H.; Nishida, Y.; Tanabe, S.; Yamade, S. Analysis of L-cone/M-cone visual pigment gene arrays in females by long-range PCR. Vision Res. 2003, 43, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Kao, L.Y.; See, L.C.; Lin, S.M.; Liang, Y.S. The Survey of Color Vision in an Aboriginal Village-Fu Hsing. Trans. Soc. Ophthalmol. 1999, 38, 57–62. [Google Scholar]
- Chia, A.; Gazzard, G.; Tong, L.; Zhang, X.; Sim, E.L.; Fong, A.; Mei Saw, S. Red-green colour blindness in Singaporean children. Clin. Exp. Ophthalmol. 2008, 36, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.S.; Rangavittal, S.; Chandrasekar, A.; Narayanan, A. Prevalence of color vision deficiency among school-going boys in South India. Indian. J. Ophthalmol. 2021, 69, 2021–2025. [Google Scholar] [PubMed]
- Mashige, K.P.; van Staden, D.B. Prevalence of congenital colour vision deficiency among Black school children in Durban, South Africa. BMC. Res. Notes. 2019, 12, 324. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, N.A.; Togoo, R.A.; Alqahtani, M.M.; Suliman, N.S.; Alasmari, F.A.; Alqahtani, F.M.; Alshahrani, F.T. Frequency of Color Vision Deficiency among Saudi Dental Students: A Cross-Sectional Study. Eur. J. Dent. 2021, 15, 27–32. [Google Scholar] [CrossRef]
- Shah, A.; Hussain, R.; Fareed, M.; Afzal, M. Prevalence of Red-Green Color Vision Defects among Muslim Males and Females of Manipur, India. Iran. J. Public Health 2013, 42, 16–24. [Google Scholar]
- Barry, J.A.; Mollan, S.; Burdon, M.A.; Jenkins, M.; Denniston, A.K. Development and validation of a questionnaire assessing the quality of life impact of Colour Blindness (CBQoL). BMC Ophthalmol. 2017, 17, 179. [Google Scholar] [CrossRef]
- Kainz, P.M.; Neitz, M.; Neitz, J. Molecular genetic detection of female carriers of protan defects. Vision Res. 1998, 38, 3365–3369. [Google Scholar] [CrossRef]
- Deeb, S.S.; Lindsey, D.T.; Hibiya, Y.; Sanocki, E.; Winderickx, J.; Teller, D.Y.; Motulsky, A.G. Genotype-phenotype relationships in human red/green color-vision defects: Molecular and psychophysical studies. Am. J. Hum. Genet. 1992, 51, 687–700. [Google Scholar]
- Neitz, M.; Neitz, J. Numbers and ratios of visual pigment genes for normal red-green color vision. Science 1995, 267, 1013–1016. [Google Scholar] [CrossRef]
- Sjoberg, S.A.; Neitz, M.; Balding, S.D.; Neitz, J. L-cone pigment genes expressed in normal colour vision. Vision Res. 1998, 38, 3213–3219. [Google Scholar] [CrossRef]
- Hayashi, S.; Ueyama, H.; Tanabe, S.; Yamade, S.; Kani, K. Number and variations of the red and green visual pigment genes in Japanese men with normal color vision. Jpn. J. Ophthalmol. 2001, 45, 60–67. [Google Scholar] [CrossRef]
- Ueyama, H.; Kuwayama, S.; Imai, H.; Oda, S.; Nishida, Y.; Tanabe, S.; Shichida, Y.; Yamade, S. Analysis of L-cone/M-cone visual pigment gene arrays in Japanese males with protan color-vision deficiency. Vision Res. 2004, 44, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, H.; Kuwayama, S.; Imai, H.; Tanabe, S.; Oda, S.; Nishida, Y.; Wada, A.; Shichida, Y.; Yamade, S. Novel missense mutations in red/green opsin genes in congenital color-vision deficiencies. Biochem. Biophys. Res. Commun. 2002, 294, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, H.; Li, Y.H.; Fu, G.L.; Lertrit, P.; Atchaneeyasakul, L.O.; Oda, S.; Tanabe, S.; Nishida, Y.; Yamade, S.; Ohkubo, I. An A-71C substitution in a green gene at the second position in the red/green visual-pigment gene array is associated with deutan color-vision deficiency. Proc. Natl. Acad. Sci. USA 2003, 100, 3357–3362. [Google Scholar] [CrossRef] [PubMed]
- Scimone, C.; Bramanti, P.; Ruggeri, A.; Donato, L.; Alafaci, C.; Crisafulli, C.; Mucciardi, M.; Rinaldi, C.; Sidoti, A.; D’Angelo, R. CCM3/SERPINI1 bidirectional promoter variants in patients with cerebral cavernous malformations: A molecular and functional study. BMC. Med. Genet. 2016, 17, 74. [Google Scholar] [CrossRef]
- Scimone, C.; Bramanti, P.; Ruggeri, A.; Donato, L.; Alafaci, C.; Crisafulli, C.; Mucciardi, M.; Rinaldi, C.; Sidoti, A.; D’Angelo, R. Detection of Novel Mutation in Ccm3 Causes Familial Cerebral Cavernous Malformations. J. Mol. Neurosci. 2015, 57, 400–403. [Google Scholar] [CrossRef]
- Scimone, C.; Donato, L.; Alafaci, C.; Granata, F.; Rinaldi, C.; Longo, M.; D’Angelo, R.; Sidoti, A. High-Throughput Sequencing to Detect Novel Likely Gene-Disrupting Variants in Pathogenesis of Sporadic Brain Arteriovenous Malformations. Front. Genet. 2020, 11, 146. [Google Scholar] [CrossRef]
- Birch, J. Identification of red-green colour deficiency: Sensitivity of the Ishihara and American Optical Company (Hard, Rand and Rittler) pseudo-isochromatic plates to identify slight anomalous trichromatism. Ophthalmic Physiol. Opt. 2010, 30, 667–671. [Google Scholar] [CrossRef]
| 2019 | Age | Students No. of Total | % Of CVD in Male | % Of CVD in Female | % Of CVD in Total |
|---|---|---|---|---|---|
| Grade 1 | 7 | 22,681 | 3.07% | 0.12% | 1.63% |
| Grade 4 | 11 | 18,494 | 3.41% | 0.09% | 1.80% |
| Grade 7 | 13 | 21,055 | 3.88% | 0.09% | 2.07% |
| 2020 | |||||
| Grade 1 | 7 | 21,218 | 3.25% | 0.21% | 1.78% |
| Grade 4 | 11 | 19,374 | 3.05% | 0.14% | 1.65% |
| Grade 7 | 13 | 20,956 | 3.41% | 0.15% | 1.85% |
| 2021 | |||||
| Grade 1 | 7 | 22,511 | 3.91% | 0.25% | 2.16% |
| Grade 4 | 11 | 23,231 | 3.29% | 0.13% | 1.77% |
| Grade 7 | 13 | 20,063 | 3.88% | 0.12% | 2.05% |
| 2019–2021 | |||||
| Total students | 189,583 | 3.46% | 0.14% | 1.86% | |
| Sex | Age | Ishihara Color Test | Gel | |
|---|---|---|---|---|
| OC1 | F | 61 | miss 1 plate | R/G-G-R/G |
| OC2 | F | 40 | normal | R/G-G-X |
| OC3 | F | 40 | normal | R/G-G-G |
| OC4 | F | 55 | miss 1 plate | R/G-G-G |
| RC1 | F | 34 | normal | R/G-G-G |
| RC2 | F | 37 | normal | R-R/G-R/G |
| RC3 | F | 34 | normal | R-G-R/G |
| RC4 | F | 30 | normal | R-R/G-G |
| RC5 | F | 31 | normal | R-G-R/G |
| RC6 | F | 51 | normal | R-G-R/G |
| RC7 | F | 38 | normal | R/G-G-G |
| N (n = 73) | F | 37.48 (20~48) | 4 miss 1 plate, 1 miss 2 plate | R-G-G |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, H.-K.; Tsao, S.-T.; Wu, P.-C. Prevalence of Congenital Color Vision Deficiency in Southern Taiwan and Detection of Female Carriers by Visual Pigment Gene Analysis. Int. J. Mol. Sci. 2023, 24, 15247. https://doi.org/10.3390/ijms242015247
Kuo H-K, Tsao S-T, Wu P-C. Prevalence of Congenital Color Vision Deficiency in Southern Taiwan and Detection of Female Carriers by Visual Pigment Gene Analysis. International Journal of Molecular Sciences. 2023; 24(20):15247. https://doi.org/10.3390/ijms242015247
Chicago/Turabian StyleKuo, Hsi-Kung, Shih-Ting Tsao, and Pei-Chang Wu. 2023. "Prevalence of Congenital Color Vision Deficiency in Southern Taiwan and Detection of Female Carriers by Visual Pigment Gene Analysis" International Journal of Molecular Sciences 24, no. 20: 15247. https://doi.org/10.3390/ijms242015247
APA StyleKuo, H.-K., Tsao, S.-T., & Wu, P.-C. (2023). Prevalence of Congenital Color Vision Deficiency in Southern Taiwan and Detection of Female Carriers by Visual Pigment Gene Analysis. International Journal of Molecular Sciences, 24(20), 15247. https://doi.org/10.3390/ijms242015247







