The PKG Inhibitor CN238 Affords Functional Protection of Photoreceptors and Ganglion Cells against Retinal Degeneration
Abstract
1. Introduction
2. Results
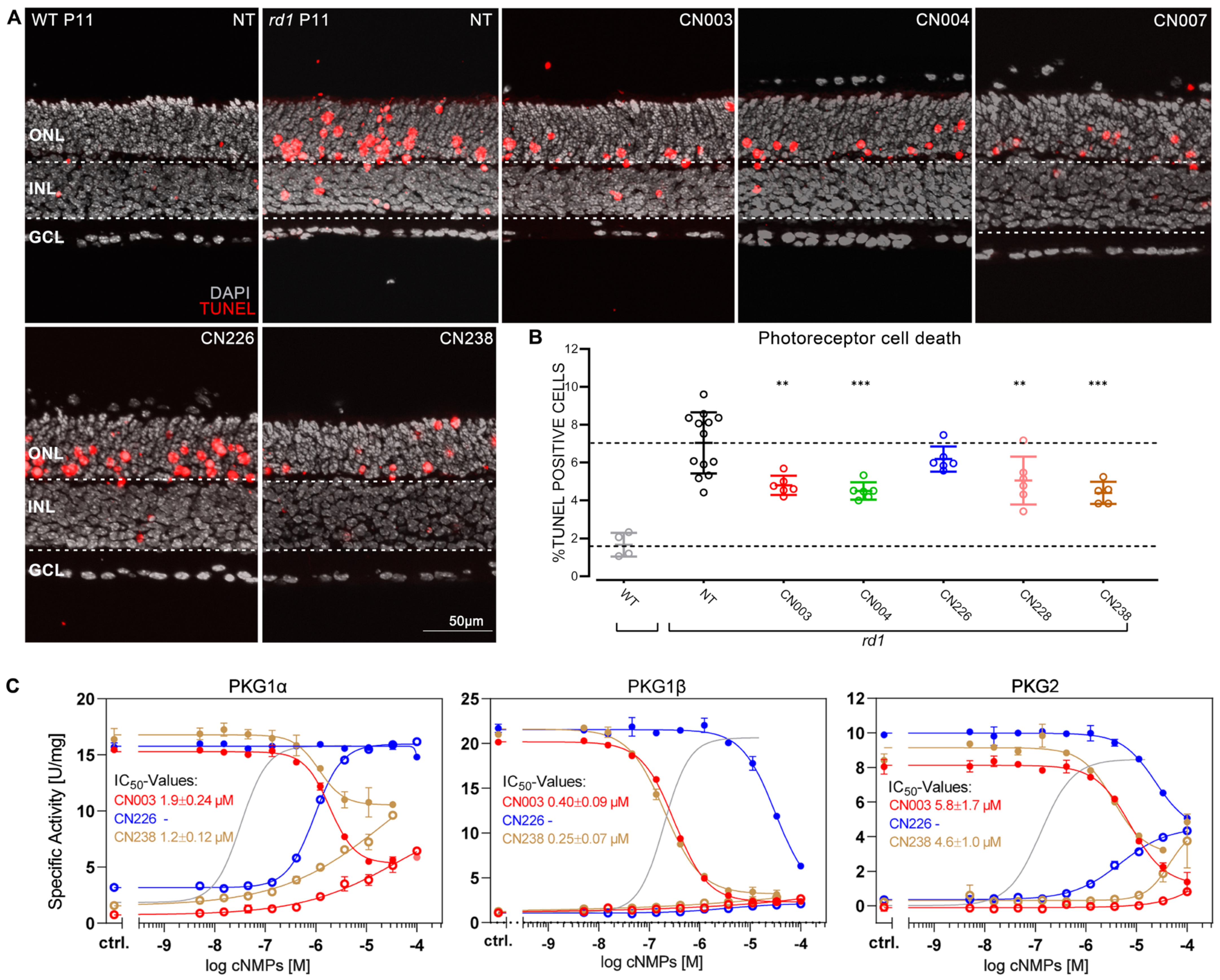
2.1. Novel PKG Inhibitors Protect rd1 Photoreceptors
2.2. PKG Inhibition Preserves Viability and Function of Photoreceptors in rd10 Retinal Explants
2.3. CN238 Prevents Axotomy-Induced Degeneration of Retinal Ganglion Cells
2.4. Effects of cGMP-Mediated Inhibition of PKG in Retinal Ganglion Cells
2.5. Down-Stream Effectors of CN238 Include Kv1 Channels
2.6. CN238 Inhibits Kv1-Mediated Ca2+ Extrusion
3. Discussion
3.1. cGMP Analogues as PKG Inhibitors
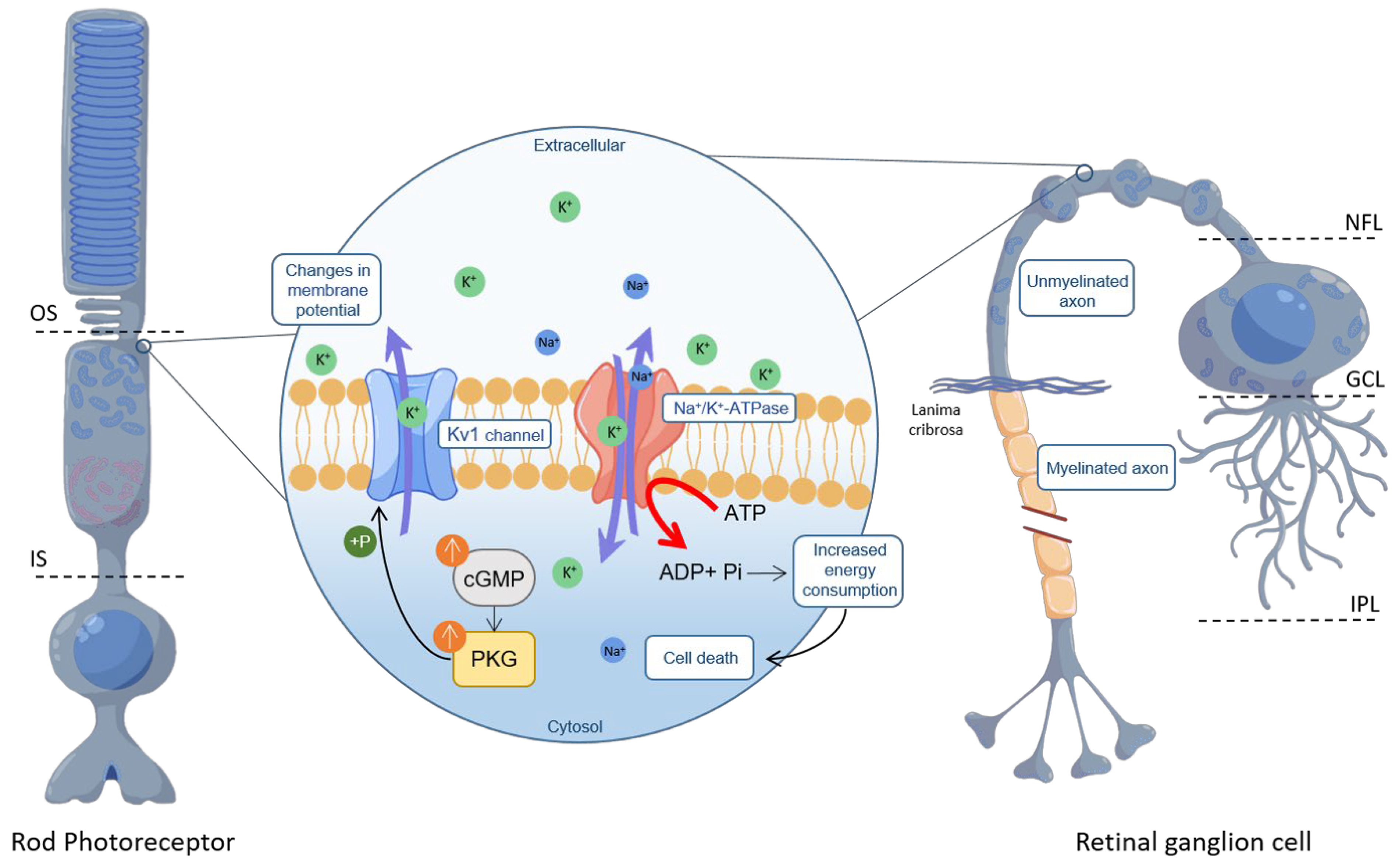
3.2. PKG Inhibition Affords Multilevel Neuronal Protection
3.3. PKG Inhibition and Neuronal Cell Survival: Significance of Kv1 and CNG Channels
4. Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berger, W.; Kloeckener-Gruissem, B.; Neidhardt, J. The molecular basis of human retinal and vitreoretinal diseases. Prog. Retin. Eye Res. 2010, 29, 335–375. [Google Scholar] [CrossRef] [PubMed]
- Sahel, J.A.; Marazova, K.; Audo, I. Clinical characteristics and current therapies for inherited retinal degenerations. Cold Spring Harb. Perspect. Med. 2014, 5, a017111. [Google Scholar] [CrossRef]
- Arango-Gonzalez, B.; Trifunović, D.; Sahaboglu, A.; Kranz, K.; Michalakis, S.; Farinelli, P.; Koch, S.; Koch, F.; Cottet, S.; Janssen-Bienhold, U.; et al. Identification of a common non-apoptotic cell death mechanism in hereditary retinal degeneration. PLoS ONE 2014, 9, e112142. [Google Scholar] [CrossRef] [PubMed]
- Power, M.; Das, S.; Schütze, K.; Marigo, V.; Ekström, P.; Paquet-Durand, F. Cellular mechanisms of hereditary photoreceptor degeneration–Focus on cGMP. Prog. Retin. Eye Res. 2020, 74, 100772. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Dinculescu, A.; Dai, X.; Du, W.; Smith, W.C.; Pang, J. Review: The history and role of naturally occurring mouse models with Pde6b mutations. Mol. Vis. 2013, 19, 2579–2589. [Google Scholar] [PubMed]
- Farber, D.B.; Lolley, R.N. Cyclic guanosine monophosphate: Elevation in degenerating photoreceptor cells of the C3H mouse retina. Science 1974, 186, 449–451. [Google Scholar] [CrossRef]
- Paquet-Durand, F.; Hauck, S.M.; van Veen, T.; Ueffing, M.; Ekström, P. PKG activity causes photoreceptor cell death in two retinitis pigmentosa models. J. Neurochem. 2009, 108, 796–810. [Google Scholar] [CrossRef]
- Kim, J.J.; Lorenz, R.; Arold, S.T.; Reger, A.S.; Sankaran, B.; Casteel, D.E.; Herberg, F.W.; Kim, C. Crystal Structure of PKG I:cGMP Complex Reveals a cGMP-Mediated Dimeric Interface that Facilitates cGMP-Induced Activation. Structure 2016, 24, 710–720. [Google Scholar] [CrossRef]
- Browning, D.D. Protein kinase G as a therapeutic target for the treatment of metastatic colorectal cancer. Expert Opin. Ther. Targets 2008, 12, 367–376. [Google Scholar] [CrossRef]
- Canals, S.; Casarejos, M.J.; de Bernardo, S.; Rodríguez-Martín, E.; Mena, M.A. Nitric oxide triggers the toxicity due to glutathione depletion in midbrain cultures through 12-lipoxygenase. J. Biol. Chem. 2003, 278, 21542–21549. [Google Scholar] [CrossRef]
- Canzoniero, L.M.; Adornetto, A.; Secondo, A.; Magi, S.; Dell’aversano, C.; Scorziello, A.; Amoroso, S.; Di Renzo, G. Involvement of the nitric oxide/protein kinase G pathway in polychlorinated biphenyl-induced cell death in SH-SY 5Y neuroblastoma cells. J. Neurosci. Res. 2006, 84, 692–697. [Google Scholar] [CrossRef]
- Fallahian, F.; Karami-Tehrani, F.; Salami, S.; Aghaei, M. Cyclic GMP induced apoptosis via protein kinase G in oestrogen receptor-positive and -negative breast cancer cell lines. FEBS J. 2011, 278, 3360–3369. [Google Scholar] [CrossRef]
- Leung, E.L.; Wong, J.C.; Johlfs, M.G.; Tsang, B.K.; Fiscus, R.R. Protein kinase G type Ialpha activity in human ovarian cancer cells significantly contributes to enhanced Src activation and DNA synthesis/cell proliferation. Mol. Cancer Res. 2010, 8, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Lolley, R.N.; Farber, D.B.; Rayborn, M.E.; Hollyfield, J.G. Cyclic GMP accumulation causes degeneration of photoreceptor cells: Simulation of an inherited disease. Science 1977, 196, 664–666. [Google Scholar] [CrossRef]
- Butt, E.; Eigenthaler, M.; Genieser, H.G. (Rp)-8-pCPT-cGMPS, a novel cGMP-dependent protein kinase inhibitor. Eur. J. Pharmacol. Mol. Pharmacol. 1994, 269, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Vighi, E.; Trifunović, D.; Veiga-Crespo, P.; Rentsch, A.; Hoffmann, D.; Sahaboglu, A.; Strasser, T.; Kulkarni, M.; Bertolotti, E.; van den Heuvel, A.; et al. Combination of cGMP analogue and drug delivery system provides functional protection in hereditary retinal degeneration. Proc. Natl. Acad. Sci. USA 2018, 115, E2997–E3006. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Trewhella, J.; Corbin, J.; Francis, S.; Mitchell, R.; Brushia, R.; Walsh, D. Progressive cyclic nucleotide-induced conformational changes in the cGMP-dependent protein kinase studied by small angle X-ray scattering in solution. J. Biol. Chem. 1997, 272, 31929–31936. [Google Scholar] [CrossRef]
- Haq, W.; Zrenner, E.; Ueffing, M.; Paquet-Durand, F. Using Micro-Electrode-Array Recordings and Retinal Disease Models to Elucidate Visual Functions: Simultaneous Recording of Local Electroretinograms and Ganglion Cell Action Potentials Reveals the Origin of Retinal Oscillatory Potentials. Bioengineering 2023, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Alarautalahti, V.; Ragauskas, S.; Hakkarainen, J.J.; Uusitalo-Järvinen, H.; Uusitalo, H.; Hyttinen, J.; Kalesnykas, G.; Nymark, S. Viability of Mouse Retinal Explant Cultures Assessed by Preservation of Functionality and Morphology. Investig. Opthalmol. Vis. Sci. 2019, 60, 1914–1927. [Google Scholar] [CrossRef]
- Osborne, A.; Hopes, M.; Wright, P.; Broadway, D.C.; Sanderson, J. Human organotypic retinal cultures (HORCs) as a chronic experimental model for investigation of retinal ganglion cell degeneration. Exp. Eye Res. 2016, 143, 28–38. [Google Scholar] [CrossRef]
- Rodriguez, A.R.; de Sevilla Müller, L.P.; Brecha, N.C. The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J. Comp. Neurol. 2014, 522, 1411–1443. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Tolone, A.; Hilhorst, R.; Groten, J.; Tomar, T.; Paquet-Durand, F. Kinase activity profiling identifies putative downstream targets of cGMP/PKG signaling in inherited retinal neurodegeneration. Cell Death Discov. 2022, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, P.D.; Wang, Y.; Schlichter, L.C. Kv1.1 and Kv1.3 channels contribute to the degeneration of retinal ganglion cells after optic nerve transection in vivo. Cell Death Differ. 2010, 17, 134–144. [Google Scholar] [CrossRef]
- Garcia-Calvo, M.; Leonard, R.J.; Novick, J.; Stevens, S.P.; Schmalhofer, W.; Kaczorowski, G.J.; Garcia, M.L. Purification, characterization, and biosynthesis of margatoxin, a component of Centruroides margaritatus venom that selectively inhibits voltage-dependent potassium channels. J. Biol. Chem. 1993, 268, 18866–18874. [Google Scholar] [CrossRef]
- Poppe, H.; Rybalkin, S.D.; Rehmann, H.; Hinds, T.R.; Tang, X.B.; Christensen, A.E.; Schwede, F.; Genieser, H.G.; Bos, J.L.; Doskeland, S.O.; et al. Cyclic nucleotide analogs as probes of signaling pathways. Nat. Methods 2008, 5, 277–278. [Google Scholar] [CrossRef]
- Paquet-Durand, F.; Beck, S.; Michalakis, S.; Goldmann, T.; Huber, G.; Mühlfriedel, R.; Trifunović, D.; Fischer, M.D.; Fahl, E.; Duetsch, G.; et al. A key role for cyclic nucleotide gated (CNG) channels in cGMP-related retinitis pigmentosa. Hum. Mol. Genet. 2011, 20, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Wucherpfennig, S.; Haq, W.; Popp, V.; Kesh, S.; Das, S.; Melle, C.; Rentsch, A.; Schwede, F.; Paquet-Durand, F.; Nache, V. Using cGMP analogues to modulate photoreceptor light sensitivity: Perspectives for the treatment of retinal degeneration. bioRxiv 2022. [Google Scholar] [CrossRef]
- Butt, E.; Pöhler, D.; Genieser, H.G.; Huggins, J.P.; Bucher, B. Inhibition of cyclic GMP-dependent protein kinase-mediated effects by (Rp)-8-bromo-PET-cyclic GMPS. Br. J. Pharmacol. 1995, 116, 3110–3116. [Google Scholar] [CrossRef]
- Atkinson, E.L.; Iegre, J.; Brear, P.D.; Zhabina, E.A.; Hyvönen, M.; Spring, D.R. Downfalls of Chemical Probes Acting at the Kinase ATP-Site: CK2 as a Case Study. Molecules 2021, 26, 1977. [Google Scholar] [CrossRef]
- Wei, J.Y.; Cohen, E.D.; Yan, Y.Y.; Genieser, H.G.; Barnstable, C.J. Identification of competitive antagonists of the rod photoreceptor cGMP-gated cation channel: Beta-phenyl-1,N2-etheno-substituted cGMP analogues as probes of the cGMP-binding site. Biochemistry 1996, 35, 16815–16823. [Google Scholar] [CrossRef]
- Fox, D.A.; Poblenz, A.T.; He, L. Calcium overload triggers rod photoreceptor apoptotic cell death in chemical-induced and inherited retinal degenerations. Ann. N. Y. Acad. Sci. 1999, 893, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Popp, V.; Power, M.; Groeneveld, K.; Yan, J.; Melle, C.; Rogerson, L.; Achury, M.; Schwede, F.; Strasser, T.; et al. Redefining the role of Ca(2+)-permeable channels in photoreceptor degeneration using diltiazem. Cell Death Dis. 2022, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Stett, A.; Egert, U.; Guenther, E.; Hofmann, F.; Meyer, T.; Nisch, W.; Haemmerle, H. Biological application of microelectrode arrays in drug discovery and basic research. Anal. Bioanal. Chem. 2003, 377, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Berkelaar, M.; Clarke, D.B.; Wang, Y.C.; Bray, G.M.; Aguayo, A.J. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J. Neurosci. 1994, 14, 4368–4374. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Liu, J.; Huang, X.; Liu, R.; Xia, H.; Brecha, N.C.; Pu, M.; Gao, J. Protective Effect of ALA in Crushed Optic Nerve Cat Retinal Ganglion Cells Using a New Marker RBPMS. PLoS ONE 2016, 11, e0160309. [Google Scholar] [CrossRef]
- Beykin, G.; Norcia, A.M.; Srinivasan, V.J.; Dubra, A.; Goldberg, J.L. Discovery and clinical translation of novel glaucoma biomarkers. Prog. Retin. Eye Res. 2021, 80, 100875. [Google Scholar] [CrossRef]
- Lynch, S.K.; Abràmoff, M.D. Diabetic retinopathy is a neurodegenerative disorder. Vis. Res. 2017, 139, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, N.E.; Curcio, C.A. Preservation of ganglion cell layer neurons in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2001, 42, 795–803. [Google Scholar]
- Yenice, E.; Şengün, A.; Soyugelen Demirok, G.; Turaçlı, E. Ganglion cell complex thickness in nonexudative age-related macular degeneration. Eye 2015, 29, 1076–1080. [Google Scholar] [CrossRef]
- Mueller-Buehl, A.M.; Tsai, T.; Hurst, J.; Theiss, C.; Peters, L.; Hofmann, L.; Herms, F.; Kuehn, S.; Schnichels, S.; Joachim, S.C. Reduced Retinal Degeneration in an Oxidative Stress Organ Culture Model through an iNOS-Inhibitor. Biology 2021, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, A.H.; Sawada, A.; Becker, B. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma. Proc. Natl. Acad. Sci. USA 1999, 96, 9944–9948. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Abdul, Y.; Singh, S.; Ahmad, A.; Husain, M. Regulation of nitric oxide production by δ-opioid receptors during glaucomatous injury. PLoS ONE 2014, 9, e110397. [Google Scholar] [CrossRef]
- Bian, K.; Murad, F. What is next in nitric oxide research? From cardiovascular system to cancer biology. Nitric Oxide 2014, 43, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Howell, G.R.; Soto, I.; Libby, R.T.; John, S.W. Intrinsic axonal degeneration pathways are critical for glaucomatous damage. Exp. Neurol. 2013, 246, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Vrabec, J.P.; Levin, L.A. The neurobiology of cell death in glaucoma. Eye 2007, 21 (Suppl. S1), S11–S14. [Google Scholar] [CrossRef]
- Rainer, P.P.; Kass, D.A. Old dog, new tricks: Novel cardiac targets and stress regulation by protein kinase G. Cardiovasc. Res. 2016, 111, 154–162. [Google Scholar] [CrossRef]
- Calamaras, T.D.; Pande, S.; Baumgartner, R.A.; Kim, S.K.; McCarthy, J.C.; Martin, G.L.; Tam, K.; McLaughlin, A.L.; Wang, G.-r.; Aronovitz, M.J. MLK3 mediates impact of PKG1α on cardiac function and controls blood pressure through separate mechanisms. JCI Insight 2021, 6, e149075. [Google Scholar] [CrossRef] [PubMed]
- Diem, R.; Meyer, R.; Weishaupt, J.H.; Bahr, M. Reduction of potassium currents and phosphatidylinositol 3-kinase-dependent AKT phosphorylation by tumor necrosis factor-(alpha) rescues axotomized retinal ganglion cells from retrograde cell death in vivo. J. Neurosci. 2001, 21, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, P.D.; Schlichter, L.C. Targeting K(V) channels rescues retinal ganglion cells in vivo directly and by reducing inflammation. Channels 2010, 4, 337–346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Höltje, M.; Brunk, I.; Grosse, J.; Beyer, E.; Veh, R.W.; Bergmann, M.; Grosse, G.; Ahnert-Hilger, G. Differential distribution of voltage-gated potassium channels Kv 1.1-Kv1.6 in the rat retina during development. J. Neurosci. Res. 2007, 85, 19–33. [Google Scholar] [CrossRef]
- Roy, A.; Groten, J.; Marigo, V.; Tomar, T.; Hilhorst, R. Identification of Novel Substrates for cGMP Dependent Protein Kinase (PKG) through Kinase Activity Profiling to Understand Its Putative Role in Inherited Retinal Degeneration. Int. J. Mol. Sci. 2021, 22, 1180. [Google Scholar] [CrossRef]
- Pinto, L.H.; Klumpp, D.J. Localization of potassium channels in the retina. Prog. Retin. Eye Res. 1998, 17, 207–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Prokosch, V. Energy Metabolism in the Inner Retina in Health and Glaucoma. Int. J. Mol. Sci. 2021, 22, 3689. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dong, J.; Cull, G.; Fortune, B.; Cioffi, G.A. Varicosities of intraretinal ganglion cell axons in human and nonhuman primates. Investig. Opthalmol. Vis. Sci. 2003, 44, 2–9. [Google Scholar] [CrossRef]
- Pivovarov, A.S.; Calahorro, F.; Walker, R.J. Na(+)/K(+)-pump and neurotransmitter membrane receptors. Invertebr. Neurosci. 2018, 19, 1. [Google Scholar] [CrossRef]
- Cook, P.F.; Neville, M.E., Jr.; Vrana, K.E.; Hartl, F.T.; Roskoski, R., Jr. Adenosine cyclic 3’,5’-monophosphate dependent protein kinase: Kinetic mechanism for the bovine skeletal muscle catalytic subunit. Biochemistry 1982, 21, 5794–5799. [Google Scholar] [CrossRef]
- Liman, E.R.; Tytgat, J.; Hess, P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 1992, 9, 861–871. [Google Scholar] [CrossRef]
- Peng, C.; Rich, E.D.; Varnum, M.D. Subunit configuration of heteromeric cone cyclic nucleotide-gated channels. Neuron 2004, 42, 401–410. [Google Scholar] [CrossRef]
- Shammat, I.M.; Gordon, S.E. Stoichiometry and arrangement of subunits in rod cyclic nucleotide-gated channels. Neuron 1999, 23, 809–819. [Google Scholar] [CrossRef]
- Zheng, J.; Trudeau, M.C.; Zagotta, W.N. Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron 2002, 36, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Scholle, A.; Koopmann, R.; Leicher, T.; Ludwig, J.; Pongs, O.; Benndorf, K. Structural elements determining activation kinetics in Kv2.1. Recept. Channels 2000, 7, 65–75. [Google Scholar]
- Belhadj, S.; Tolone, A.; Christensen, G.; Das, S.; Chen, Y.; Paquet-Durand, F. Long-Term, Serum-Free Cultivation of Organotypic Mouse Retina Explants with Intact Retinal Pigment Epithelium. J. Vis. Exp. 2020, 165, e61868. [Google Scholar] [CrossRef]
- Loo, D.T. In situ detection of apoptosis by the TUNEL assay: An overview of techniques. Methods Mol. Biol. 2011, 682, 3–13. [Google Scholar] [CrossRef]
- Haq, W.; Arango-Gonzalez, B.; Zrenner, E.; Euler, T.; Schubert, T. Synaptic remodeling generates synchronous oscillations in the degenerated outer mouse retina. Front. Neural Circuits 2014, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Briggman, K.L.; Euler, T. Bulk electroporation and population calcium imaging in the adult mammalian retina. J. Neurophysiol. 2011, 105, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Chirumamilla, C.S.; Fazil, M.; Perez-Novo, C.; Rangarajan, S.; de Wijn, R.; Ramireddy, P.; Verma, N.K.; Vanden Berghe, W. Profiling Activity of Cellular Kinases in Migrating T-Cells. Methods Mol. Biol. 2019, 1930, 99–113. [Google Scholar] [CrossRef]
- Hilhorst, R.; Houkes, L.; Mommersteeg, M.; Musch, J.; van den Berg, A.; Ruijtenbeek, R. Peptide microarrays for profiling of serine/threonine kinase activity of recombinant kinases and lysates of cells and tissue samples. Methods Mol. Biol. 2013, 977, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M.; Aartsma-Rus, A.; Alves, S.; Borgos, S.E.; Buijsen, R.A.M.; Collin, R.W.J.; Covello, G.; Denti, M.A.; Desviat, L.R.; Echevarría, L.; et al. Delivery of oligonucleotide-based therapeutics: Challenges and opportunities. EMBO Mol. Med. 2021, 13, e13243. [Google Scholar] [CrossRef] [PubMed]
- Maguire, A.M.; Bennett, J.; Aleman, E.M.; Leroy, B.P.; Aleman, T.S. Clinical Perspective: Treating RPE65-Associated Retinal Dystrophy. Mol. Ther. 2021, 29, 442–463. [Google Scholar] [CrossRef]
- Kitiratschky, V.B.; Stingl, K.; Wilhelm, B.; Peters, T.; Besch, D.; Sachs, H.; Gekeler, F.; Bartz-Schmidt, K.U.; Zrenner, E. Safety evaluation of “retina implant alpha IMS”--a prospective clinical trial. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 381–387. [Google Scholar] [CrossRef]
- Yang, J.; Luo, L.; Oh, Y.; Meng, T.; Chai, G.; Xia, S.; Emmert, D.; Wang, B.; Eberhart, C.G.; Lee, S.; et al. Sunitinib malate-loaded biodegradable microspheres for the prevention of corneal neovascularization in rats. J. Control. Release 2020, 327, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.; Barut, L.; Urimi, D.; Schipper, N.; Paquet-Durand, F. Investigating Ex Vivo Animal Models to Test the Performance of Intravitreal Liposomal Drug Delivery Systems. Pharmaceutics 2021, 13, 1013. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolone, A.; Haq, W.; Fachinger, A.; Roy, A.; Kesh, S.; Rentsch, A.; Wucherpfennig, S.; Zhu, Y.; Groten, J.; Schwede, F.; et al. The PKG Inhibitor CN238 Affords Functional Protection of Photoreceptors and Ganglion Cells against Retinal Degeneration. Int. J. Mol. Sci. 2023, 24, 15277. https://doi.org/10.3390/ijms242015277
Tolone A, Haq W, Fachinger A, Roy A, Kesh S, Rentsch A, Wucherpfennig S, Zhu Y, Groten J, Schwede F, et al. The PKG Inhibitor CN238 Affords Functional Protection of Photoreceptors and Ganglion Cells against Retinal Degeneration. International Journal of Molecular Sciences. 2023; 24(20):15277. https://doi.org/10.3390/ijms242015277
Chicago/Turabian StyleTolone, Arianna, Wadood Haq, Alexandra Fachinger, Akanksha Roy, Sandeep Kesh, Andreas Rentsch, Sophie Wucherpfennig, Yu Zhu, John Groten, Frank Schwede, and et al. 2023. "The PKG Inhibitor CN238 Affords Functional Protection of Photoreceptors and Ganglion Cells against Retinal Degeneration" International Journal of Molecular Sciences 24, no. 20: 15277. https://doi.org/10.3390/ijms242015277
APA StyleTolone, A., Haq, W., Fachinger, A., Roy, A., Kesh, S., Rentsch, A., Wucherpfennig, S., Zhu, Y., Groten, J., Schwede, F., Tomar, T., Herberg, F. W., Nache, V., & Paquet-Durand, F. (2023). The PKG Inhibitor CN238 Affords Functional Protection of Photoreceptors and Ganglion Cells against Retinal Degeneration. International Journal of Molecular Sciences, 24(20), 15277. https://doi.org/10.3390/ijms242015277






