A Novel Isoprene Synthase from the Monocot Tree Copernicia prunifera (Arecaceae) Confers Enhanced Drought Tolerance in Transgenic Arabidopsis
Abstract
:1. Introduction
2. Results
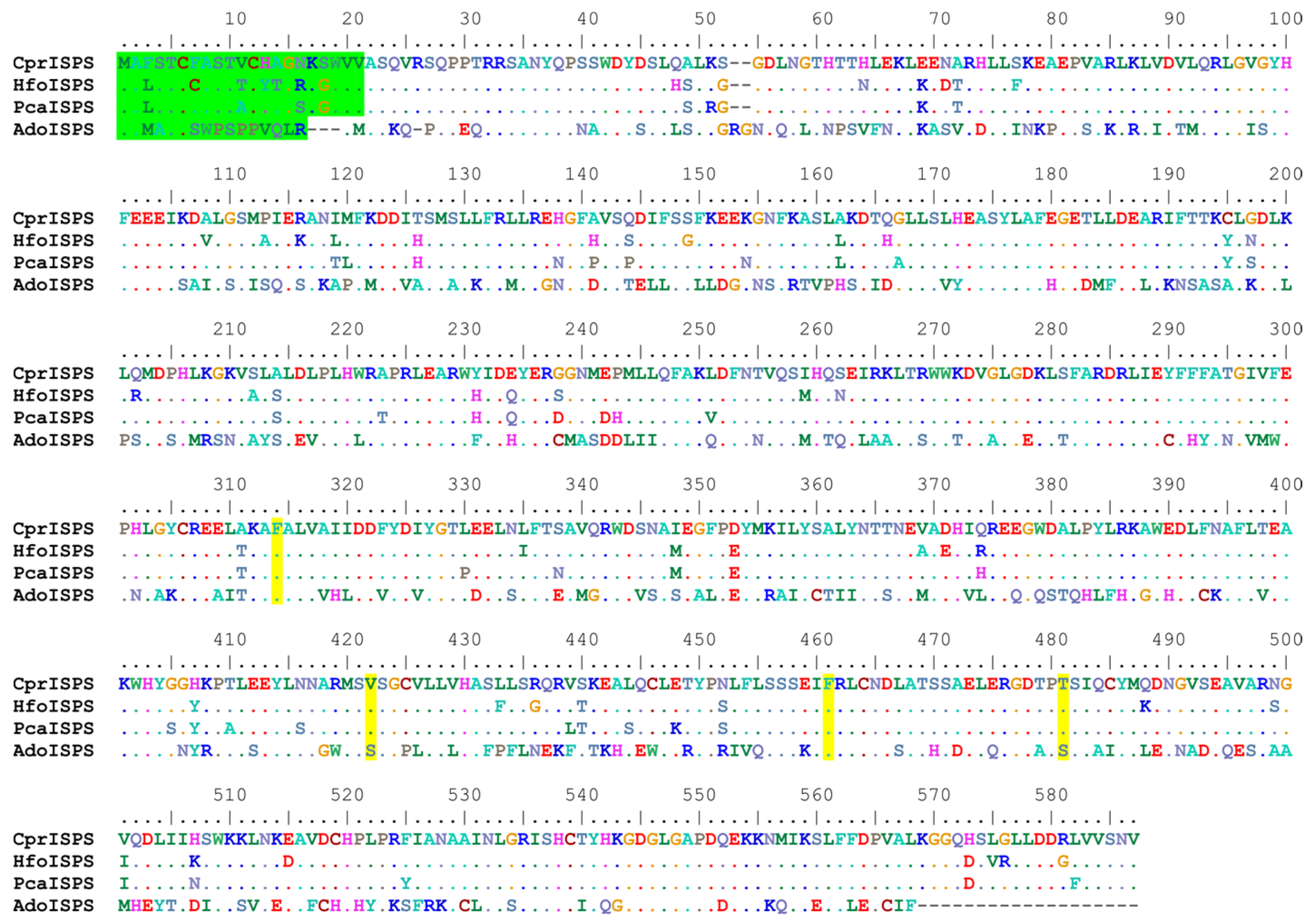
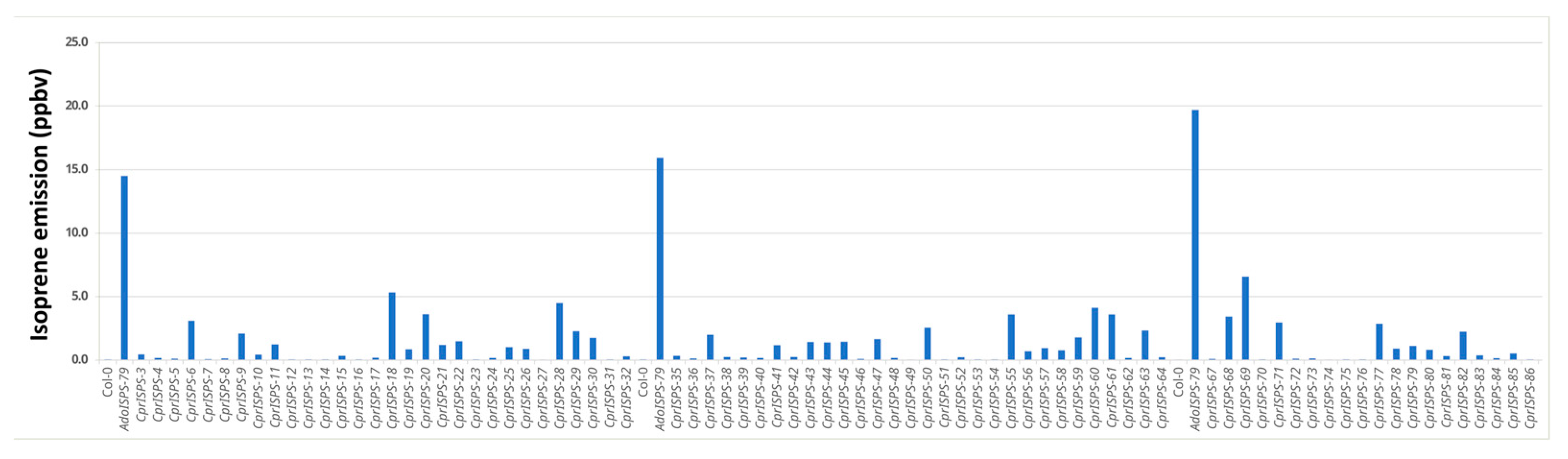
2.1. CprISPS Is a Novel, Functional Isoprene Synthase Gene from C. prunifera
2.2. Overexpression of CprISPS Enhances Seeds Germination and Cotyledon Greening Rate
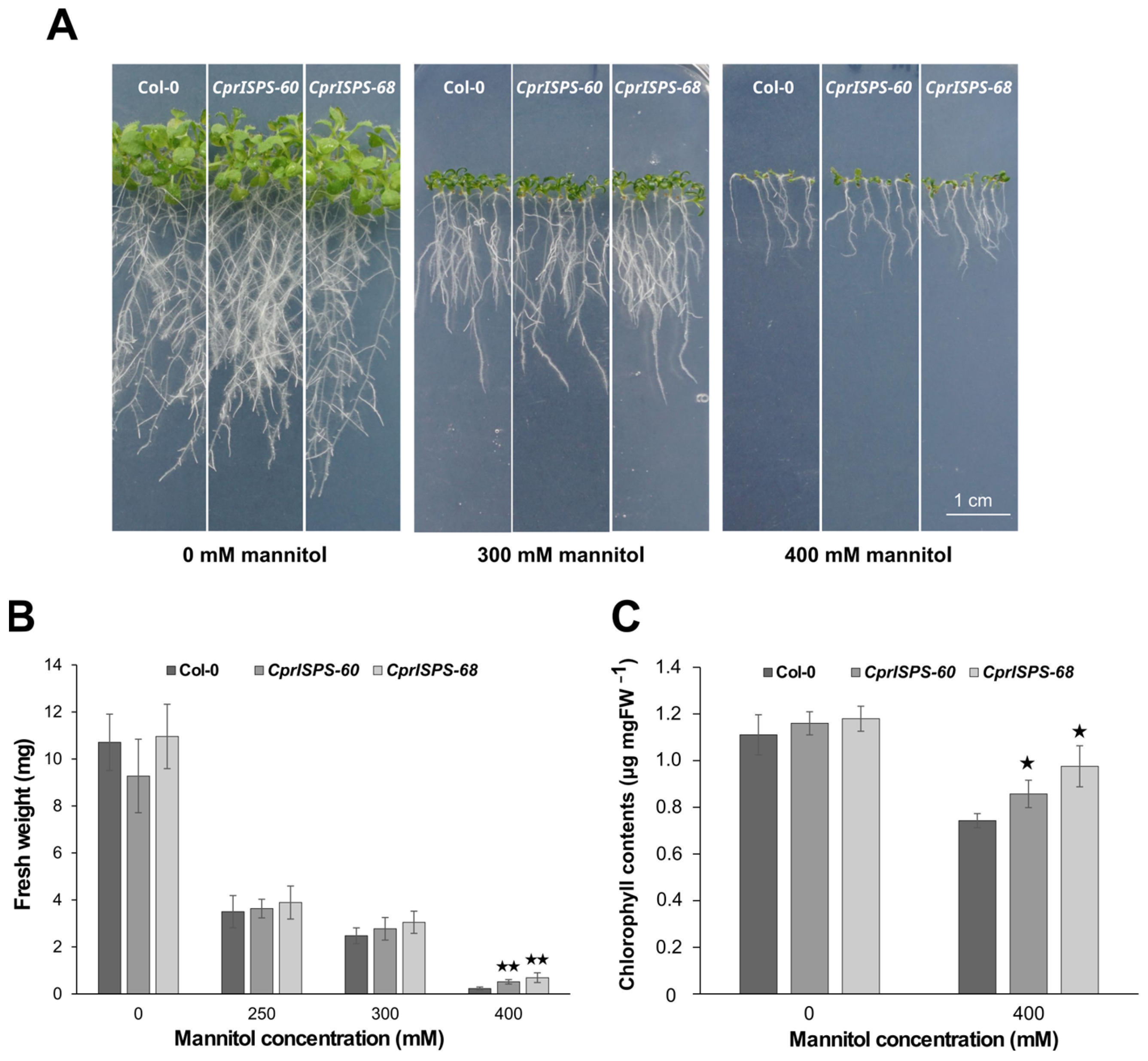
2.3. Overexpression of CprISPS Enhances Drought Stress Tolerance at Post-Germination Stage
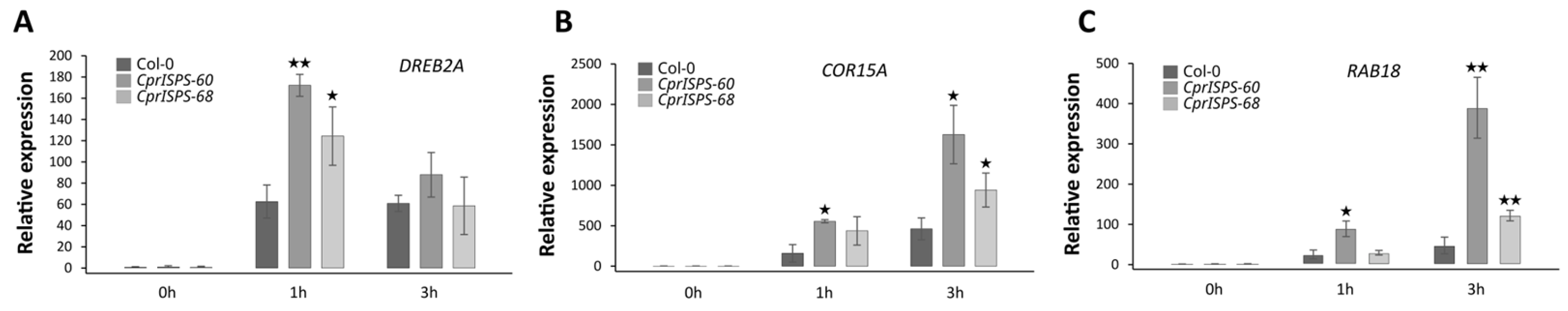
2.4. Overexpression of CprISPS Induces the Expression of Stress-Related Genes
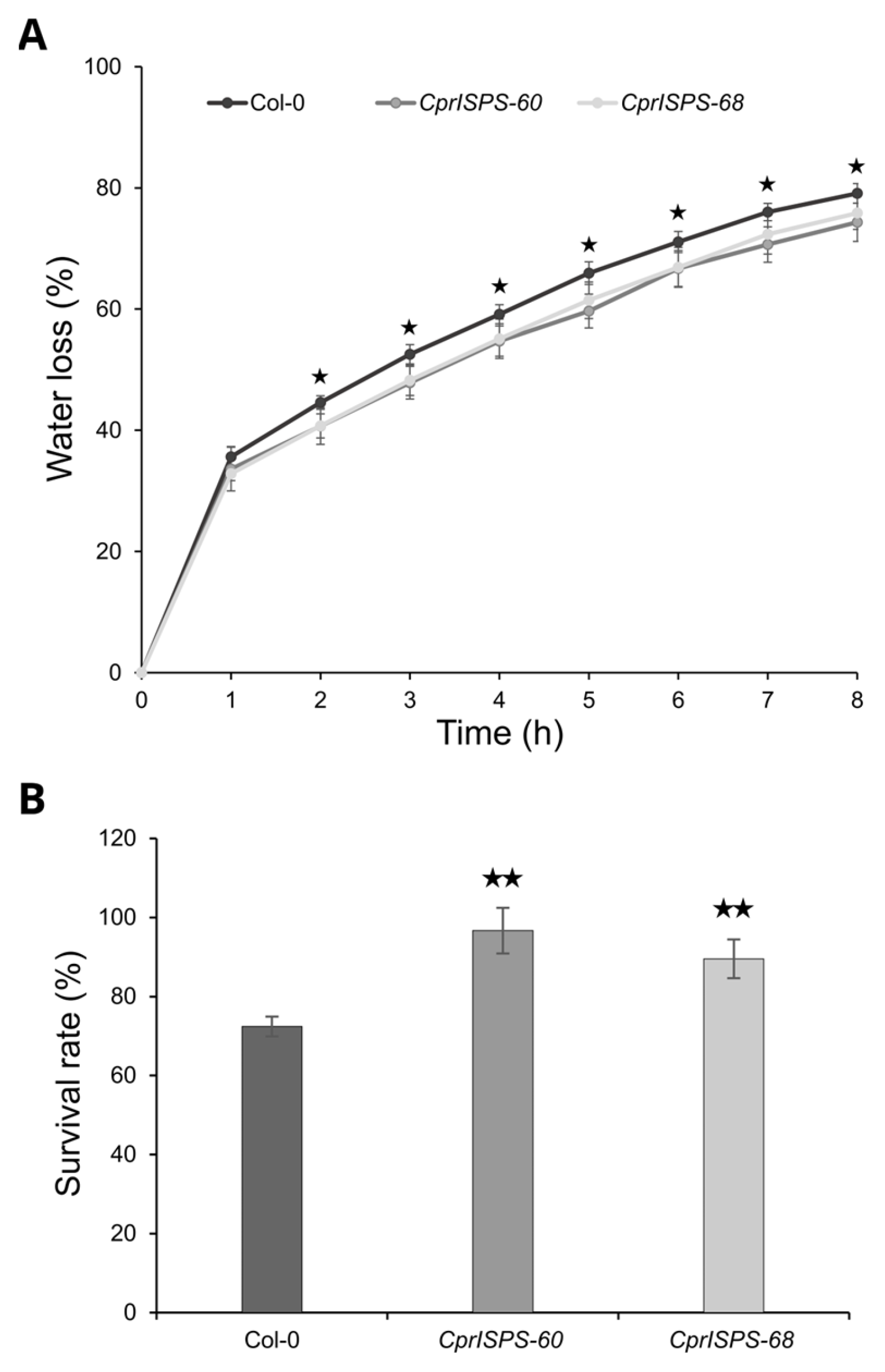
2.5. Overexpression of CprISPS Increases Drought Tolerance at the Adult Stage
3. Discussion
3.1. Common and Special Features of CprISPS
3.2. Isoprene-Induced Increase of Drought Tolerance Persists throughout Plant Growth and Development
3.3. Induction of Expression Levels of Key Drought Regulators Are Mediated by Isoprene
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Genomic DNA Purification, Total RNA Isolation and cDNA Synthesis
4.3. Cloning, Plasmid Constructs, Plant Transformation, and Screening
4.4. PTR-MS Measurement
4.5. Seed Germination and Green Cotyledon Assays
4.6. Young Seedling Fresh Weight Measurement
4.7. Chlorophyll Content Analysis
4.8. qRT-PCR Analysis
4.9. Water Loss Rate Evaluation
4.10. Survival Rate Assay
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velikova, V.; Fares, S.; Loreto, F. Isoprene and Nitric Oxide Reduce Damages in Leaves Exposed to Oxidative Stress. Plant Cell Environ. 2008, 31, 1882–1894. [Google Scholar] [CrossRef]
- Schnitzler, J.-P.; Louis, S.; Behnke, K.; Loivamäki, M. Poplar Volatiles—Biosynthesis, Regulation and (Eco)Physiology of Isoprene and Stress-Induced Isoprenoids. Plant Biol. 2010, 12, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Kuzma, J.; Nemecek-Marshall, M.; Pollock, W.H.; Fall, R. Bacteria Produce the Volatile Hydrocarbon Isoprene. Curr. Microbiol. 1995, 30, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P.I.; Geron, C. Estimates of Global Terrestrial Isoprene Emissions Using MEGAN Model of Emissions of Gases and Aerosols from Nature. Atmos. Chem. Phys. Discuss. 2006, 6, 107–173. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Chen, H.; Deng, L.; Zhang, H.; Nian, R.; Xian, M. Biochemical Characterization of Isoprene Synthase from Ipomoea Batatas. J. Biosci. Bioeng. 2019, 127, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Lantz, A.T.; Cardiello, J.F.; Gee, T.A.; Richards, M.G.; Rosenstiel, T.N.; Fisher, A.J. Biochemical Characterization of an Isoprene Synthase from Campylopus Introflexus (Heath Star Moss). Plant Physiol. Biochem. 2015, 94, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Wiberley, A.E.; Donohue, A.R.; Westphal, M.M.; Sharkey, T.D. Regulation of Isoprene Emission from Poplar Leaves throughout a Day. Plant Cell Environ. 2009, 32, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Rasulov, B.; Hüve, K.; Bichele, I.; Laisk, A.; Niinemets, Ü. Temperature Response of Isoprene Emission In Vivo Reflects a Combined Effect of Substrate Limitations and Isoprene Synthase Activity: A Kinetic Analysis. Plant Physiol. 2010, 154, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Rosenstiel, T.N.; Potosnak, M.J.; Griffin, K.L.; Fall, R.; Monson, R.K. Increased CO2 Uncouples Growth from Isoprene Emission in an Agriforest Ecosystem. Nature 2003, 421, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Wolfertz, M.; Sharkey, T.D.; Boland, W.; Kühnemann, F. Rapid Regulation of the Methylerythritol 4-Phosphate Pathway during Isoprene Synthesis. Plant Physiol. 2004, 135, 1939–1945. [Google Scholar] [CrossRef]
- Brüggemann, N.; Schnitzler, J.-P. Diurnal Variation of Dimethylallyl Diphosphate Concentrations in Oak (Quercus Robur) Leaves. Physiol. Plant. 2002, 115, 190–196. [Google Scholar] [CrossRef]
- Ahrar, M.; Doneva, D.; Koleva, D.; Romano, A.; Rodeghiero, M.; Tsonev, T.; Biasioli, F.; Stefanova, M.; Peeva, V.; Wohlfahrt, G.; et al. Isoprene Emission in the Monocot Arundineae Tribe in Relation to Functional and Structural Organization of the Photosynthetic Apparatus. Environ. Exp. Bot. 2015, 119, 87–95. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Yeh, S. Isoprene Emission from Plants. Annu. Rev. Plant Mol. Biol. 2001, 52, 407–436. [Google Scholar] [CrossRef]
- Cinege, G.; Louis, S.; Hänsch, R.; Schnitzler, J.P. Regulation of Isoprene Synthase Promoter by Environmental and Internal Factors. Plant Mol. Biol. 2009, 69, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Loivamäki, M.; Louis, S.; Cinege, G.; Zimmer, I.; Fischbach, R.J.; Schnitzler, J.-P. Circadian Rhythms of Isoprene Biosynthesis in Grey Poplar Leaves. Plant Physiol. 2007, 143, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, J.; Algarra Alarcon, A.; Carlin, S.; Barbaro, E.; Cappellin, L.; Velikova, V.; Vrhovsek, U.; Loreto, F.; Varotto, C. In Planta Recapitulation of Isoprene Synthase Evolution from Ocimene Synthases. Mol. Biol. Evol. 2017, 34, 2583–2599. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Iqbal, M.A.; Mutanda, I.; Rashid, M.H.U.; Inafuku, M.; Oku, H. Plant Hormone Effects on Isoprene Emission from Tropical Tree in Ficus Septica. Plant Cell Environ. 2019, 42, 1715–1728. [Google Scholar] [CrossRef]
- Mutanda, I.; Inafuku, M.; Saitoh, S.; Iwasaki, H.; Fukuta, M.; Watanabe, K.; Oku, H. Temperature Controls on the Basal Emission Rate of Isoprene in a Tropical Tree Ficus Septica: Exploring Molecular Regulatory Mechanisms. Plant Cell Environ. 2016, 39, 2260–2275. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Miyamoto, K.; Yumoto, E.; Parveen, S.; Mutanda, I.; Inafuku, M.; Oku, H. Plant Hormone Profile and Control over Isoprene Biosynthesis in a Tropical Tree Ficus Septica. Plant Biol. 2022, 24, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, S.; Teuber, M.; Zimmer, I.; Louis, S.; Fischbach, R.J.; Schnitzler, J.P. Diurnal and Seasonal Variation of Isoprene Biosynthesis-Related Genes in Grey Poplar Leaves. Plant Physiol. 2005, 139, 474–484. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Loreto, F. Water Stress, Temperature, and Light Effects on the Capacity for Isoprene Emission and Photosynthesis of Kudzu Leaves. Oecologia 1993, 95, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Way, D.A.; Schnitzler, J.-P.; Monson, R.K.; Jackson, R.B. Enhanced Isoprene-Related Tolerance of Heat- and Light-Stressed Photosynthesis at Low, but Not High, CO2 Concentrations. Oecologia 2011, 166, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Way, D.A.; Ghirardo, A.; Kanawati, B.; Esperschütz, J.; Monson, R.K.; Jackson, R.B.; Schmitt-Kopplin, P.; Schnitzler, J.-P. Increasing Atmospheric CO2 Reduces Metabolic and Physiological Differences between Isoprene- and Non-Isoprene-Emitting Poplars. New Phytol. 2013, 200, 534–546. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Wiberley, A.E.; Donohue, A.R. Isoprene Emission from Plants: Why and How. Ann. Bot. 2008, 101, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Monson, R.K.; Jones, R.T.; Rosenstiel, T.N.; Schnitzler, J.P. Why Only Some Plants Emit Isoprene. Plant Cell Environ. 2013, 36, 503–516. [Google Scholar] [CrossRef]
- Sharkey, T.D. Is It Useful to Ask Why Plants Emit Isoprene? Plant Cell Environ. 2013, 36, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Laothawornkitkul, J.; Paul, N.D.; Vickers, C.E.; Possell, M.; Taylor, J.E.; Mullineaux, P.M.; Hewitt, C.N. Isoprene Emissions Influence Herbivore Feeding Decisions. Plant Cell Environ. 2008, 31, 1410–1415. [Google Scholar] [CrossRef]
- Frank, L.; Wenig, M.; Ghirardo, A.; van der Krol, A.; Vlot, A.C.; Schnitzler, J.-P.; Rosenkranz, M. Isoprene and β-Caryophyllene Confer Plant Resistance via Different Plant Internal Signalling Pathways. Plant Cell Environ. 2021, 44, 1151–1164. [Google Scholar] [CrossRef]
- Loivamäki, M.; Mumm, R.; Dicke, M.; Schnitzler, J.-P. Isoprene Interferes with the Attraction of Bodyguards by Herbaceous Plants. Proc. Natl. Acad. Sci. USA 2008, 105, 17430–17435. [Google Scholar] [CrossRef]
- Monson, R.K.; Weraduwage, S.M.; Rosenkranz, M.; Schnitzler, J.-P.; Sharkey, T.D. Leaf Isoprene Emission as a Trait That Mediates the Growth-Defense Tradeoff in the Face of Climate Stress. Oecologia 2021, 197, 885–902. [Google Scholar] [CrossRef]
- Vickers, C.E.; Possell, M.; Cojocariu, C.I.; Velikova, V.B.; Laothawornkitkul, J.; Ryan, A.; Mullineaux, P.M.; Nicholas Hewitt, C. Isoprene Synthesis Protects Transgenic Tobacco Plants from Oxidative Stress. Plant Cell Environ. 2009, 32, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Schnitzler, J.P. Abiotic Stresses and Induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Centritto, M.; Haworth, M.; Marino, G.; Pallozzi, E.; Tsonev, T.; Velikova, V.; Nogues, I.; Loreto, F. Isoprene Emission Aids Recovery of Photosynthetic Performance in Transgenic Nicotiana Tabacum Following High Intensity Acute UV-B Exposure. Plant Sci. 2014, 226, 82–91. [Google Scholar] [CrossRef]
- Singsaas, L.; Lerdau, M.; Winter, K.; Sharkey, T.D. Lsoprene Lncreases Thermotolerance of Isoprene-Emitting Species. Plant Physiol. 1997, 115, 1413–1420. [Google Scholar] [CrossRef]
- Loreto, F.; Mannozzi, M.; Maris, C.; Nascetti, P.; Ferranti, F.; Pasqualini, S. Ozone Quenching Properties of Isoprene and Its Antioxidant Role in Leaves. Plant Physiol. 2001, 126, 993–1000. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Chen, X.; Yeh, S. Isoprene Increases Thermotolerance of Fosmidomycin-Fed Leaves. Plant Physiol. 2001, 125, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Brunetti, C.; Tattini, M.; Doneva, D.; Ahrar, M.; Tsonev, T.; Stefanova, M.; Ganeva, T.; Gori, A.; Ferrini, F.; et al. Physiological Significance of Isoprenoids and Phenylpropanoids in Drought Response of Arundinoideae Species with Contrasting Habitats and Metabolism. Plant Cell Environ. 2016, 39, 2185–2197. [Google Scholar] [CrossRef]
- Ryan, A.C.; Hewitt, C.N.; Possell, M.; Vickers, C.E.; Purnell, A.; Mullineaux, P.M.; Davies, W.J.; Dodd, I.C. Isoprene Emission Protects Photosynthesis but Reduces Plant Productivity during Drought in Transgenic Tobacco (Nicotiana Tabacum) Plants. New Phytol. 2014, 201, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, F.; Harrison, S.P.; Jones, C.D.; Sitch, S. Isoprene Emissions and Climate. Atmos. Environ. 2009, 43, 6121–6135. [Google Scholar] [CrossRef]
- Tattini, M.; Velikova, V.; Vickers, C.; Brunetti, C.; Di Ferdinando, M.; Trivellini, A.; Fineschi, S.; Agati, G.; Ferrini, F.; Loreto, F. Isoprene Production in Transgenic Tobacco Alters Isoprenoid, Non-Structural Carbohydrate and Phenylpropanoid Metabolism, and Protects Photosynthesis from Drought Stress. Plant Cell Environ. 2014, 37, 1950–1964. [Google Scholar] [CrossRef] [PubMed]
- Mancini, I.; Domingo, G.; Bracale, M.; Loreto, F.; Pollastri, S. Isoprene Emission Influences the Proteomic Profile of Arabidopsis Plants under Well-Watered and Drought-Stress Conditions. Int. J. Mol. Sci. 2022, 23, 3836. [Google Scholar] [CrossRef]
- Lantz, A.T.; Allman, J.; Weraduwage, S.M.; Sharkey, T.D. Isoprene: New Insights into the Control of Emission and Mediation of Stress Tolerance by Gene Expression. Plant Cell Environ. 2019, 42, 2808–2826. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Weraduwage, S.M.; Lantz, A.T.; Sanchez, L.M.; Weise, S.E.; Wang, J.; Childs, K.L.; Sharkey, T.D. Isoprene Acts as a Signaling Molecule in Gene Networks Important for Stress Responses and Plant Growth. Plant Physiol. 2019, 180, 124–152. [Google Scholar] [CrossRef]
- Pollastri, S.; Baccelli, I.; Loreto, F. Isoprene: An Antioxidant Itself or a Molecule with Multiple Regulatory Functions in Plants? Antioxidants 2021, 10, 684. [Google Scholar] [CrossRef]
- Faralli, M.; Li, M.; Varotto, C. Shoot Characterization of Isoprene and Ocimene-Emitting Transgenic Arabidopsis Plants under Contrasting Environmental Conditions. Plants 2020, 9, 477. [Google Scholar] [CrossRef]
- Xu, J.; Trainotti, L.; Li, M.; Varotto, C. Overexpression of Isoprene Synthase Affects ABA- and Drought-Related Gene Expression and Enhances Tolerance to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 4276. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, J.; Barbaro, E.; Varotto, C. High-Throughput, Robust and Highly Time-Flexible Method for Surface Sterilization of Arabidopsis Seeds. J. Vis. Exp. 2021, 2021, e62893. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.; Lyu, F.; Khomenko, I.; Biasioli, F.; Villani, M.; Baldan, B.; Varotto, C. Evolution of Isoprene Emission in Arecaceae (Palms). Evol. Appl. 2020, 14, 1–13. [Google Scholar] [CrossRef]
- Ghirardo, A.; Nosenko, T.; Kreuzwieser, J.; Winkler, J.B.; Kruse, J.; Albert, A.; Merl-Pham, J.; Lux, T.; Ache, P.; Zimmer, I.; et al. Protein Expression Plasticity Contributes to Heat and Drought Tolerance of Date Palm. Oecologia 2021, 197, 903–919. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.R.M.; de Almeida Vieira, F.; Fajardo, C.G.; Brandão, M.M.; Silva, R.A.R.; Jump, A.S. Overexploitation and Anthropogenic Disturbances Threaten the Genetic Diversity of an Economically Important Neotropical Palm. Biodivers. Conserv. 2021, 30, 2395–2413. [Google Scholar] [CrossRef]
- Arruda, G.M.T.; Calbo, M.E.R. Effects of Flooding on Carnaúba Growth, Gas Exchange and Root Porosity (Copernicia Prunifera (Mill.) H.E. Moore). Acta Bot. Bras. 2004, 18, 219–224. [Google Scholar] [CrossRef]
- Emanuelsson, O.; Nielsen, H.; Heijne, G.V. ChloroP, a Neural Network-Based Method for Predicting Chloroplast Transit Peptides and Their Cleavage Sites. Protein Sci. 1999, 8, 978–984. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.-H.; Lee, S.C. Function of ABA in Stomatal Defense against Biotic and Drought Stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef]
- Yang, Y.; Nan, R.; Mi, T.; Song, Y.; Shi, F.; Liu, X.; Wang, Y.; Sun, F.; Xi, Y.; Zhang, C. Rapid and Nondestructive Evaluation of Wheat Chlorophyll under Drought Stress Using Hyperspectral Imaging. Int. J. Mol. Sci. 2023, 24, 5825. [Google Scholar] [CrossRef] [PubMed]
- Brilli, F.; Tsonev, T.; Mahmood, T.; Velikova, V.; Loreto, F.; Centritto, M. Ultradian Variation of Isoprene Emission, Photosynthesis, Mesophyll Conductance, and Optimum Temperature Sensitivity for Isoprene Emission in Water-Stressed Eucalyptus Citriodora Saplings. J. Exp. Bot. 2013, 64, 519–528. [Google Scholar] [CrossRef]
- Fang, C.; Monson, R.K.; Cowling, E.B. Isoprene Emission, Photosynthesis, and Growth in Sweetgum (Liquidambar styraciflua) Seedlings Exposed to Short- and Long-Term Drying Cycles. Tree Physiol. 1996, 16, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, A.; Barta, C.; Brilli, F.; Centritto, M.; Zimmer, I.; Schnitzler, J.P.; Loreto, F. Isoprene Emission Is Not Temperature-Dependent during and after Severe Drought-Stress: A Physiological and Biochemical Analysis. Plant J. 2008, 55, 687–697. [Google Scholar] [CrossRef]
- Guidolotti, G.; Calfapietra, C.; Loreto, F. The Relationship between Isoprene Emission, CO2 Assimilation and Water Use Efficiency across a Range of Poplar Genotypes. Physiol. Plant. 2011, 142, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Potosnak, M.J. Including the Interactive Effect of Elevated CO2 Concentration and Leaf Temperature in Global Models of Isoprene Emission. Plant Cell Environ. 2014, 37, 1723–1726. [Google Scholar] [CrossRef]
- Tani, A.; Tozaki, D.; Okumura, M.; Nozoe, S.; Hirano, T. Effect of Drought Stress on Isoprene Emission from Two Major Quercus Species Native to East Asia. Atmos. Environ. 2011, 45, 6261–6266. [Google Scholar] [CrossRef]
- Arab, L.; Kreuzwieser, J.; Kruse, J.; Zimmer, I.; Ache, P.; Alfarraj, S.; Al-Rasheid, K.A.S.; Schnitzler, J.-P.; Hedrich, R.; Rennenberg, H. Acclimation to Heat and Drought—Lessons to Learn from the Date Palm (Phoenix Dactylifera). Environ. Exp. Bot. 2016, 125, 20–30. [Google Scholar] [CrossRef]
- Marino, G.; Brunetti, C.; Tattini, M.; Romano, A.; Biasioli, F.; Tognetti, R.; Loreto, F.; Ferrini, F.; Centritto, M. Dissecting the Role of Isoprene and Stress-Related Hormones (ABA and Ethylene) in Populus Nigra Exposed to Unequal Root Zone Water Stress. Tree Physiol. 2017, 37, 1637–1647. [Google Scholar] [CrossRef]
- Dryflor; Banda-R, K.; Delgado-Salinas, A.; Dexter, K.G.; Linares-Palomino, R.; Oliveira-Filho, A.; Prado, D.; Pullan, M.; Quintana, C.; Riina, R.; et al. Plant Diversity Patterns in Neotropical Dry Forests and Their Conservation Implications. Science 2016, 353, 1383–1387. [Google Scholar] [CrossRef]
- Cássia-Silva, C.; Freitas, C.G.; Alves, D.M.C.C.; Bacon, C.D.; Collevatti, R.G. Niche Conservatism Drives a Global Discrepancy in Palm Species Richness between Seasonally Dry and Moist Habitats. Glob. Ecol. Biogeogr. 2019, 28, 814–825. [Google Scholar] [CrossRef]
- Chang, T.-W.; Okamoto, H.; Tani, A. Rapid Sampling Protocol of Isoprene Emission Rate of Palm (Arecaceae) Species Using Excised Leaves. Atmosphere 2022, 13, 778. [Google Scholar] [CrossRef]
- Loreto, F.; Ciccioli, P.; Brancaleoni, E.; Valentini, R.; De Lillis, M.; Csiky, O.; Seufert, G. A Hypothesis on the Evolution of Isoprenoid Emission by Oaks Based on the Correlation between Emission Type and Quercus Taxonomy. Oecologia 1998, 115, 302–305. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Gray, D.W.; Pell, H.K.; Breneman, S.R.; Topper, L. Isoprene Synthase Genes Form a Monophyletic Clade of Acyclic Terpene Synthases in the Tps-b Terpene Synthase Family. Evolution 2013, 67, 1026–1040. [Google Scholar] [CrossRef]
- Mu, Z.; Llusià, J.; Zeng, J.; Zhang, Y.; Asensio, D.; Yang, K.; Yi, Z.; Wang, X.; Peñuelas, J. An Overview of the Isoprenoid Emissions From Tropical Plant Species. Front. Plant Sci. 2022, 13, 833030. [Google Scholar] [CrossRef]
- Li, M.; Cappellin, L.; Xu, J.; Biasioli, F.; Varotto, C. High-Throughput Screening for in Planta Characterization of VOC Biosynthetic Genes by PTR-ToF-MS. J. Plant Res. 2020, 133, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Vanzo, E.; Jud, W.; Li, Z.; Albert, A.; Domagalska, M.A.; Ghirardo, A.; Niederbacher, B.; Frenzel, J.; Beemster, G.T.S.; Asard, H.; et al. Facing the Future: Effects of Short-Term Climate Extremes on Isoprene-Emitting and Nonemitting Poplar. Plant Physiol. 2015, 169, 560–575. [Google Scholar] [CrossRef]
- Doneva, D.; Ahrar, M.; Tsonev, T.; Loreto, F.; Varotto, C.; Velikova, V. The Role of Isoprene in Two Arundineae Species Exposed to Progressive Drought. C. R. L’Academie Bulg. Des. Sci. 2017, 70, 203–212. [Google Scholar]
- Ahrar, M.; Doneva, D.; Tattini, M.; Brunetti, C.; Gori, A.; Rodeghiero, M.; Wohlfahrt, G.; Biasioli, F.; Varotto, C.; Loreto, F.; et al. Phenotypic Differences Determine Drought Stress Responses in Ecotypes of Arundo Donax Adapted to Different Environments. J. Exp. Bot. 2017, 68, 2439–2451. [Google Scholar] [CrossRef]
- Aghighi Shahverdi, M.; Tobeh, A.; Jahanbakhsh, S.; Rastegar, Z. The Study of Germination Index of Canola Cultivars for Drought Resistance. Int. J. Agron. Plant Prod. 2011, 2, 89–95. [Google Scholar]
- Verslues, P.E.; Longkumer, T. Size and Activity of the Root Meristem: A Key for Drought Resistance and a Key Model of Drought-Related Signaling. Physiol. Plant. 2022, 174, e13622. [Google Scholar] [CrossRef] [PubMed]
- Behnke, K.; Loivamäki, M.; Zimmer, I.; Rennenberg, H.; Schnitzler, J.-P.; Louis, S. Isoprene Emission Protects Photosynthesis in Sunfleck Exposed Grey Poplar. Photosynth. Res. 2010, 104, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Ghirardo, A.; Wright, L.P.; Bi, Z.; Rosenkranz, M.; Pulido, P.; Rodríguez-Concepción, M.; Niinemets, Ü.; Brüggemann, N.; Gershenzon, J.; Schnitzler, J.-P. Metabolic Flux Analysis of Plastidic Isoprenoid Biosynthesis in Poplar Leaves Emitting and Nonemitting Isoprene. Plant Physiol. 2014, 165, 37–51. [Google Scholar] [CrossRef]
- Behnke, K.; Kaiser, A.; Zimmer, I.; Brüggemann, N.; Janz, D.; Polle, A.; Hampp, R.; Hänsch, R.; Popko, J.; Schmitt-Kopplin, P.; et al. RNAi-Mediated Suppression of Isoprene Emission in Poplar Transiently Impacts Phenolic Metabolism under High Temperature and High Light Intensities: A Transcriptomic and Metabolomic Analysis. Plant Mol. Biol. 2010, 74, 61–75. [Google Scholar] [CrossRef]
- Harvey, C.M.; Li, Z.; Tjellström, H.; Blanchard, G.J.; Sharkey, T.D. Concentration of Isoprene in Artificial and Thylakoid Membranes. J. Bioenerg. Biomembr. 2015, 47, 419–429. [Google Scholar] [CrossRef]
- Seok, H.-Y.; Nguyen, L.V.; Park, H.-Y.; Tarte, V.N.; Ha, J.; Lee, S.-Y.; Moon, Y.-H. Arabidopsis Non-TZF Gene AtC3H17 Functions as a Positive Regulator in Salt Stress Response. Biochem. Biophys. Res. Commun. 2018, 498, 954–959. [Google Scholar] [CrossRef]
- Artus, N.N.; Uemura, M.; Steponkus, P.L.; Gilmour, S.J.; Lin, C.; Thomashow, M.F. Constitutive Expression of the Cold-Regulated Arabidopsis Thaliana COR15a Gene Affects Both Chloroplast and Protoplast Freezing Tolerance. Proc. Natl. Acad. Sci. USA 1996, 93, 13404–13409. [Google Scholar] [CrossRef]
- Thalhammer, A.; Bryant, G.; Sulpice, R.; Hincha, D.K. Disordered Cold Regulated15 Proteins Protect Chloroplast Membranes during Freezing through Binding and Folding, But Do Not Stabilize Chloroplast Enzymes In Vivo. Plant Physiol. 2014, 166, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, S.; Chen, W.; Zhang, J.; Zhang, L.; Sun, W.; Wang, Z. Plant Dehydrins: Expression, Regulatory Networks, and Protective Roles in Plants Challenged by Abiotic Stress. Int. J. Mol. Sci. 2021, 22, 12619. [Google Scholar] [CrossRef] [PubMed]
- Ciereszko, I.; Kleczkowski, L.A. Effects of Phosphate Deficiency and Sugars on Expression of Rab18 in Arabidopsis: Hexokinase-Dependent and Okadaic Acid-Sensitive Transduction of the Sugar Signal. Biochim. Biophys. Acta BBA Gene Struct. Expr. 2002, 1579, 43–49. [Google Scholar] [CrossRef]
- Yang, Y.; Costa, A.; Leonhardt, N.; Siegel, R.S.; Schroeder, J.I. Isolation of a Strong Arabidopsis Guard Cell Promoter and Its Potential as a Research Tool. Plant Methods 2008, 4, 6. [Google Scholar] [CrossRef]
- Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Qin, F.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional Analysis of an Arabidopsis Transcription Factor, DREB2A, Involved in Drought-Responsive Gene Expression. Plant Cell 2006, 18, 1292–1309. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional Genomics in Plant Abiotic Stress Responses and Tolerance: From Gene Discovery to Complex Regulatory Networks and Their Application in Breeding. Proc. Jpn. Acad. Ser. B 2022, 98, 470–492. [Google Scholar] [CrossRef]
- Hernández-Sánchez, I.E.; Maruri-López, I.; Graether, S.P.; Jiménez-Bremont, J.F. In Vivo Evidence for Homo- and Heterodimeric Interactions of Arabidopsis Thaliana Dehydrins AtCOR47, AtERD10, and AtRAB18. Sci. Rep. 2017, 7, 17036. [Google Scholar] [CrossRef]
- Hernández-Sánchez, I.E.; Maruri-López, I.; Molphe-Balch, E.P.; Becerra-Flora, A.; Jaimes-Miranda, F.; Jiménez-Bremont, J.F. Evidence for In Vivo Interactions between Dehydrins and the Aquaporin AtPIP2B. Biochem. Biophys. Res. Commun. 2019, 510, 545–550. [Google Scholar] [CrossRef]
- Harvey, C.M.; Sharkey, T.D. Exogenous Isoprene Modulates Gene Expression in Unstressed Arabidopsis Thaliana Plants. Plant Cell Environ. 2016, 39, 1251–1263. [Google Scholar] [CrossRef]
- Li, M.; Barbaro, E.; Bellini, E.; Saba, A.; Sanità di Toppi, L.; Varotto, C. Ancestral Function of the Phytochelatin Synthase C-Terminal Domain in Inhibition of Heavy Metal-Mediated Enzyme Overactivation. J. Exp. Bot. 2020, 71, 6655–6669. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Inzé, D.; Depicker, A. GATEWAYTM Vectors for Agrobacterium-Mediated Plant. Trends Plant Sci. 2002, 7, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral Dip: A Simplified Method for Agrobacterium-Mediated Transformation of Arabidopsis Thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Poli, M.; Salvi, S.; Li, M.; Varotto, C. Selection of Reference Genes Suitable for Normalization of QPCR Data under Abiotic Stresses in Bioenergy Crop Arundo donax L. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Khomenko, I.; Biasioli, F.; Li, M.; Varotto, C. A Novel Isoprene Synthase from the Monocot Tree Copernicia prunifera (Arecaceae) Confers Enhanced Drought Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2023, 24, 15329. https://doi.org/10.3390/ijms242015329
Yu J, Khomenko I, Biasioli F, Li M, Varotto C. A Novel Isoprene Synthase from the Monocot Tree Copernicia prunifera (Arecaceae) Confers Enhanced Drought Tolerance in Transgenic Arabidopsis. International Journal of Molecular Sciences. 2023; 24(20):15329. https://doi.org/10.3390/ijms242015329
Chicago/Turabian StyleYu, Jiamei, Iuliia Khomenko, Franco Biasioli, Mingai Li, and Claudio Varotto. 2023. "A Novel Isoprene Synthase from the Monocot Tree Copernicia prunifera (Arecaceae) Confers Enhanced Drought Tolerance in Transgenic Arabidopsis" International Journal of Molecular Sciences 24, no. 20: 15329. https://doi.org/10.3390/ijms242015329
APA StyleYu, J., Khomenko, I., Biasioli, F., Li, M., & Varotto, C. (2023). A Novel Isoprene Synthase from the Monocot Tree Copernicia prunifera (Arecaceae) Confers Enhanced Drought Tolerance in Transgenic Arabidopsis. International Journal of Molecular Sciences, 24(20), 15329. https://doi.org/10.3390/ijms242015329







