Effect of Mowing on Wheat Growth at Seeding Stage
Abstract
:1. Introduction
2. Results
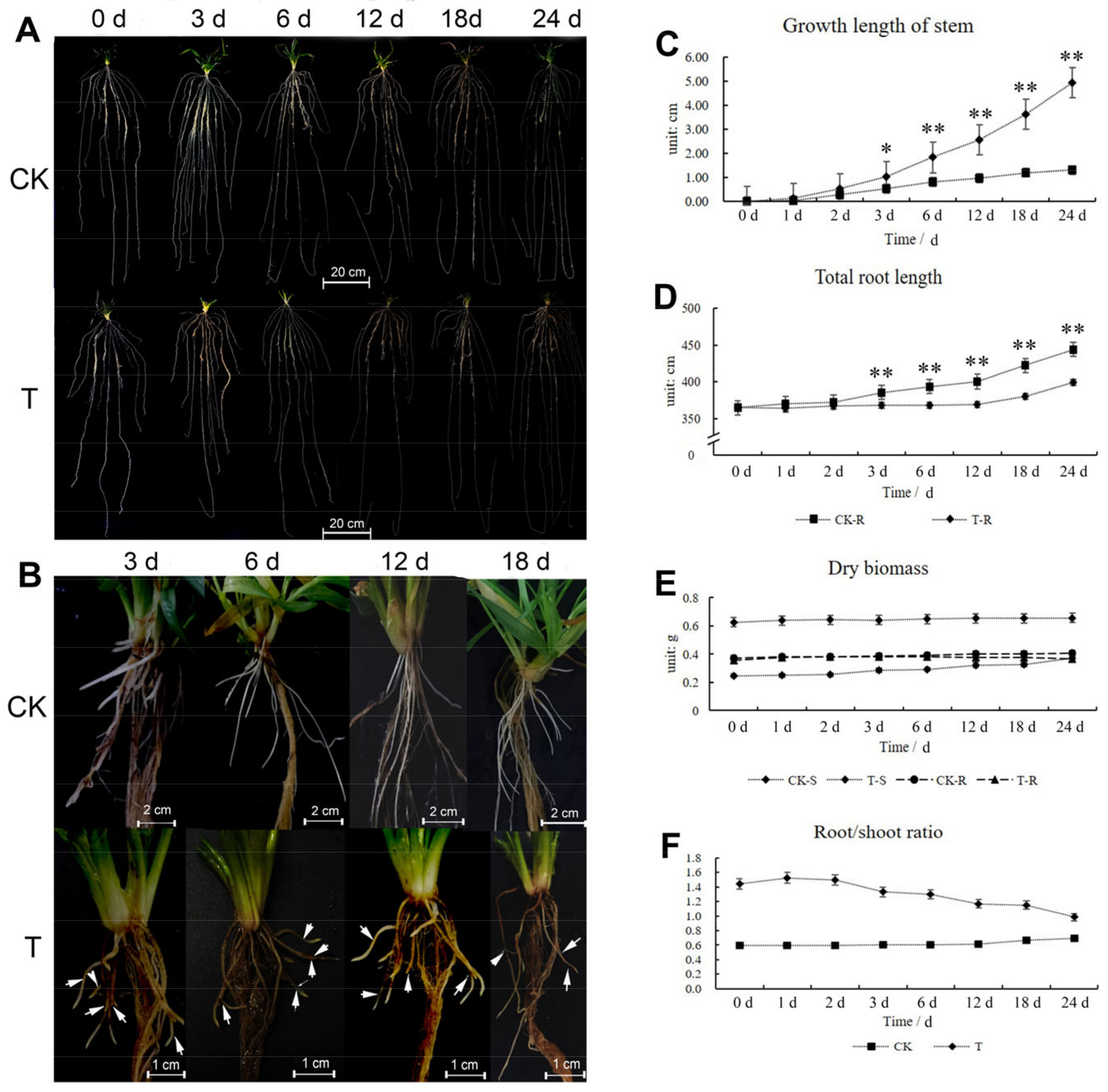
2.1. Shoot and Root Phenotype Analysis
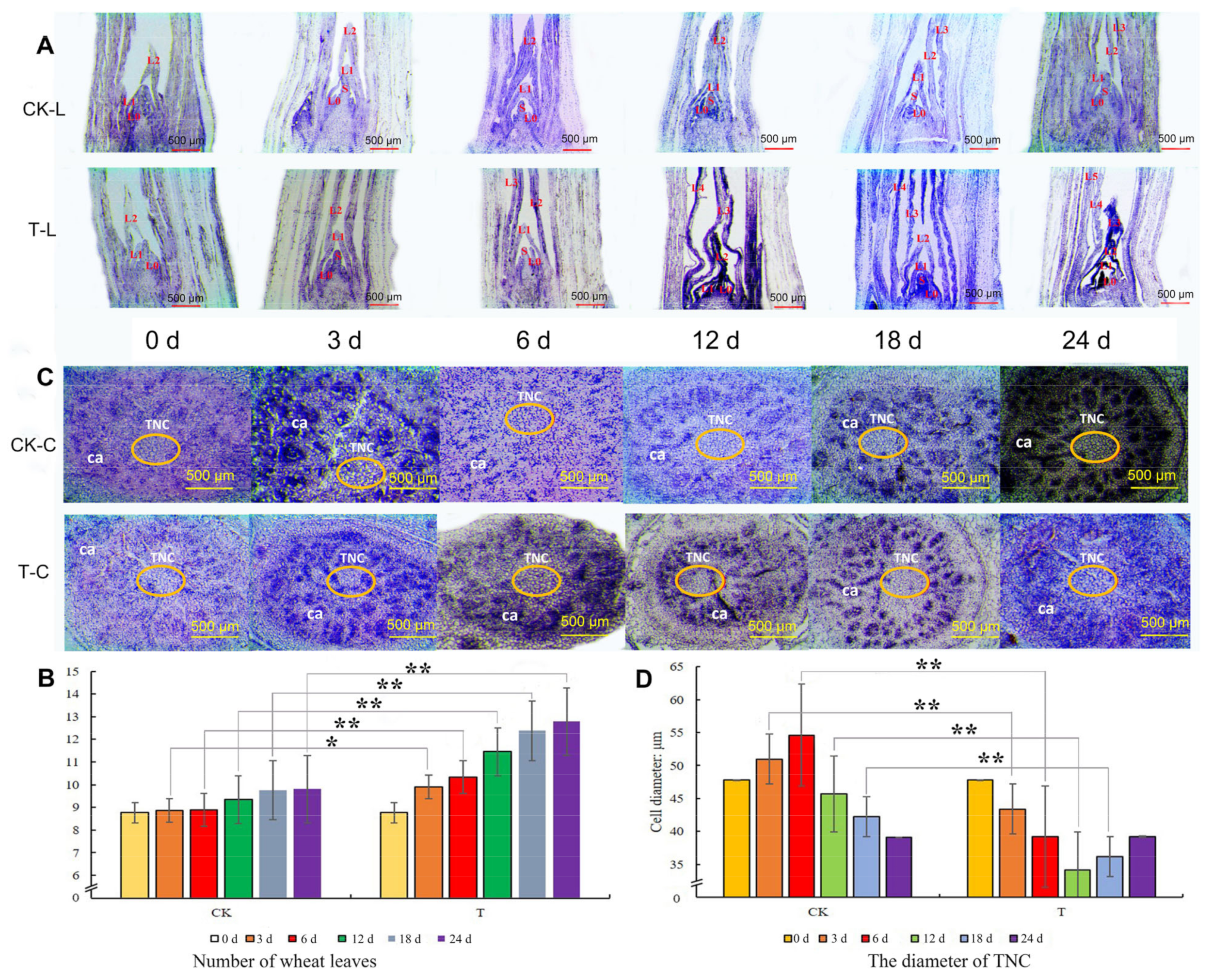
2.2. Paraffin Section Analysis of Tillering Node after Mowing
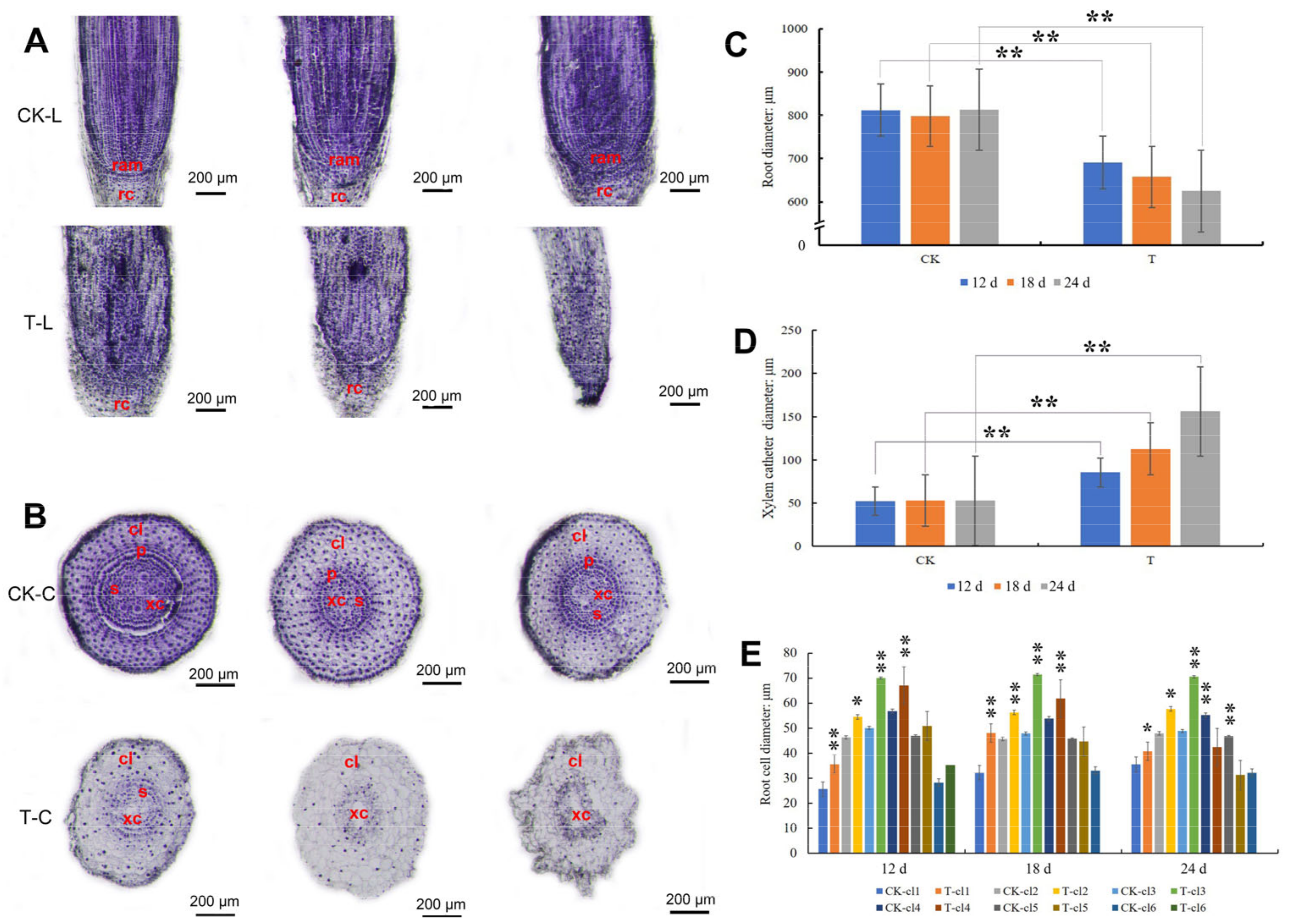
2.3. Paraffin Section Analysis of Root after Mowing
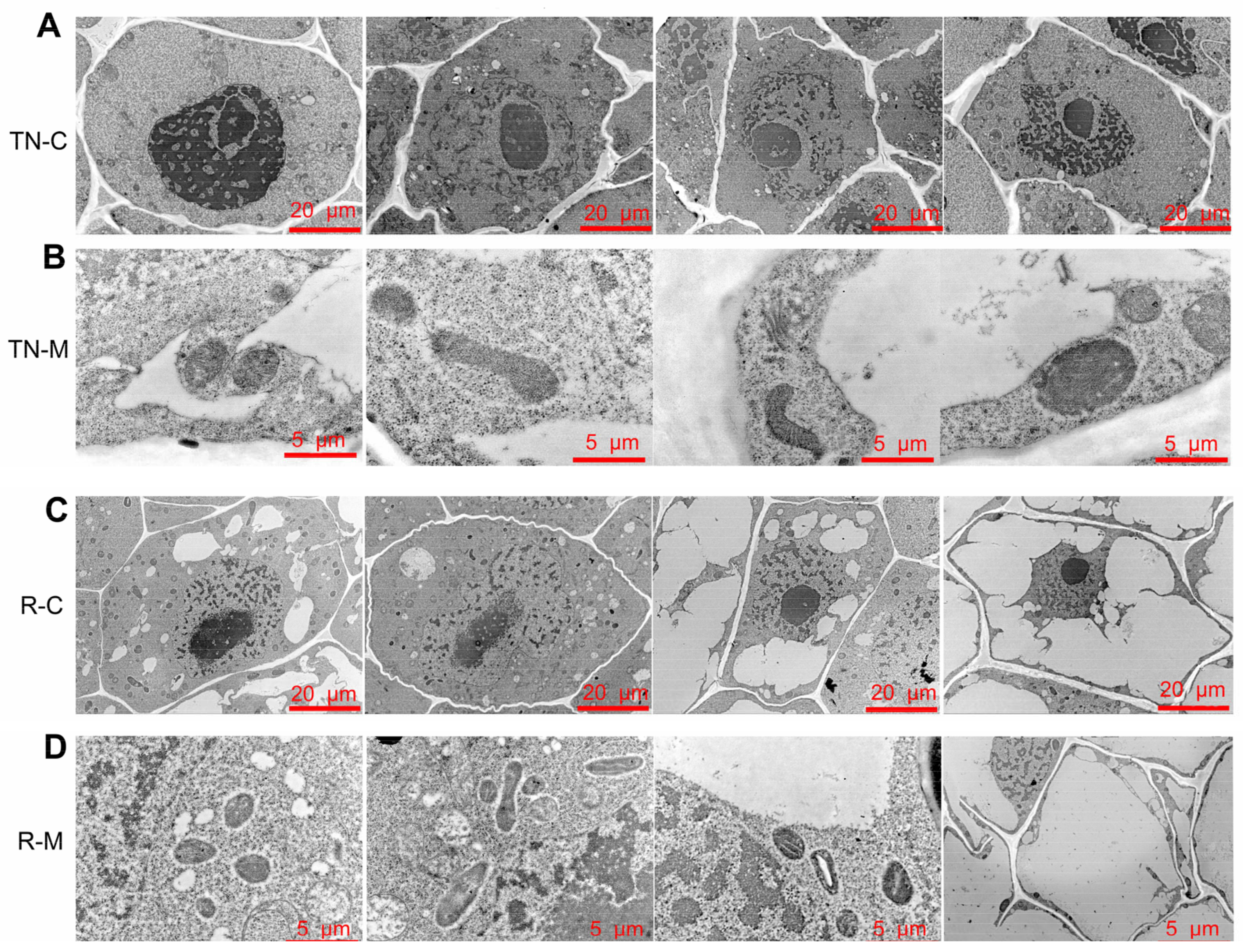
2.4. Sub-Microstructure of Wheat Tillering Node and Secondary Root after Mowing
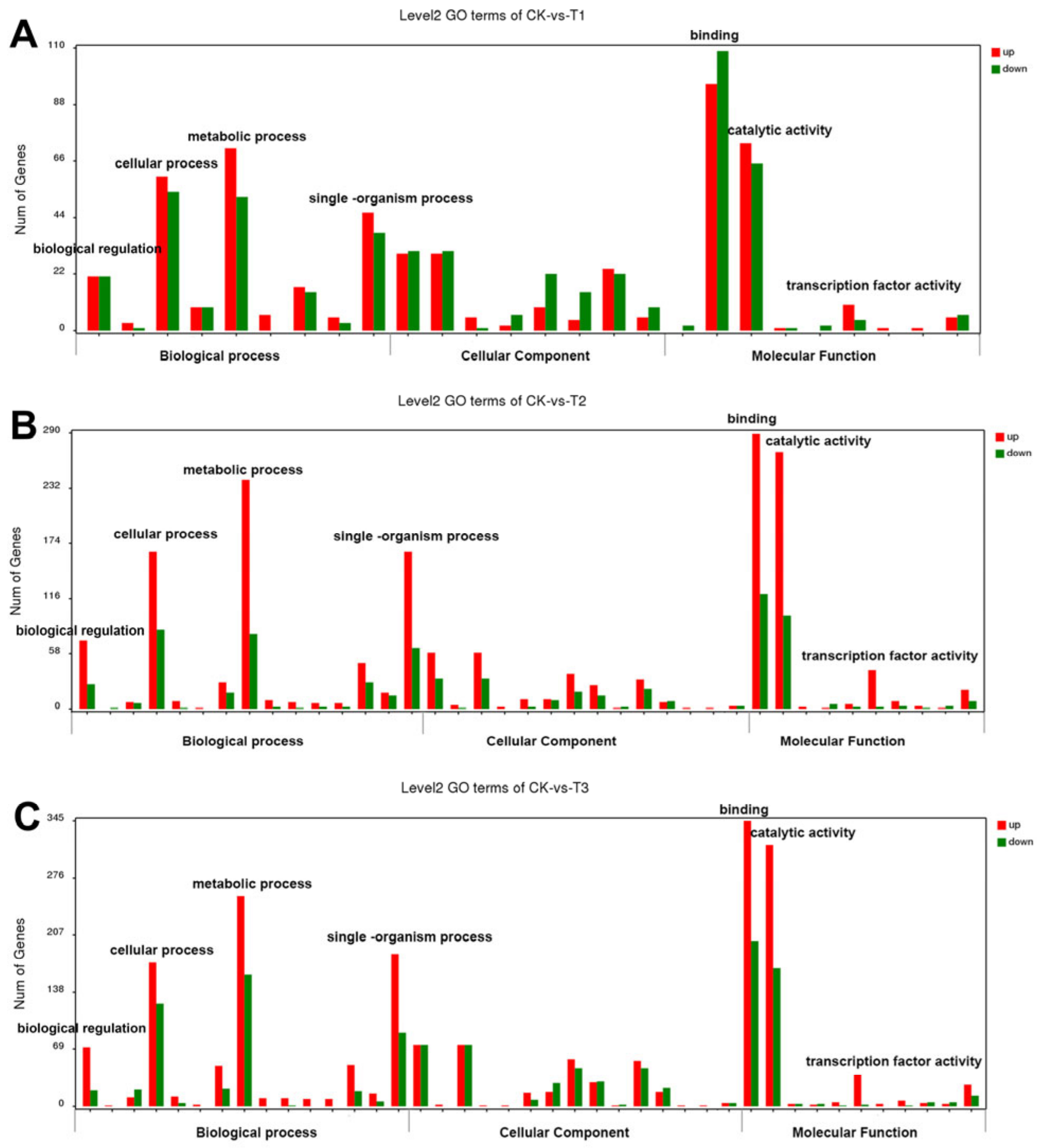
2.5. Transcriptome Analysis of the DEGs in TN
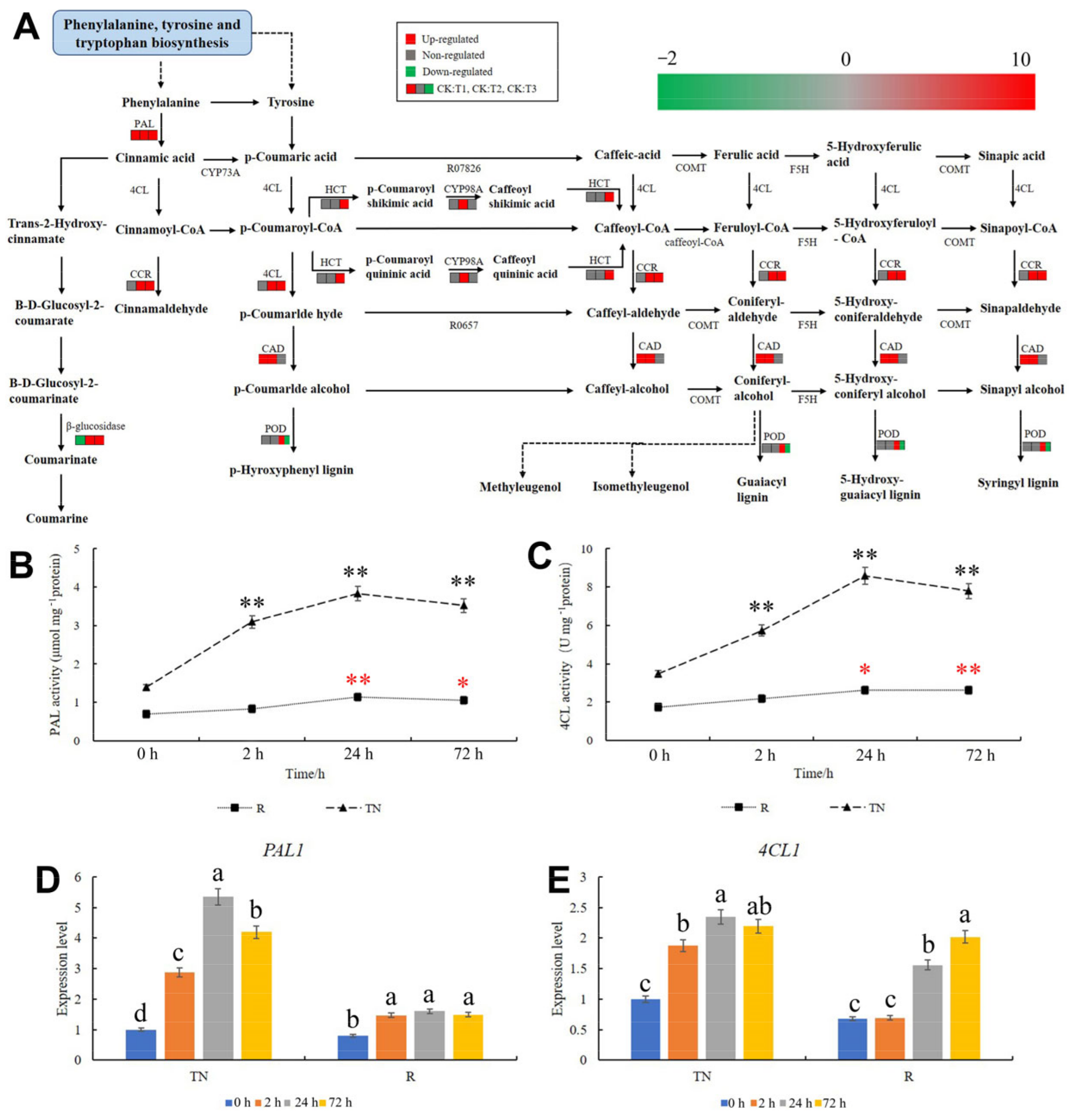
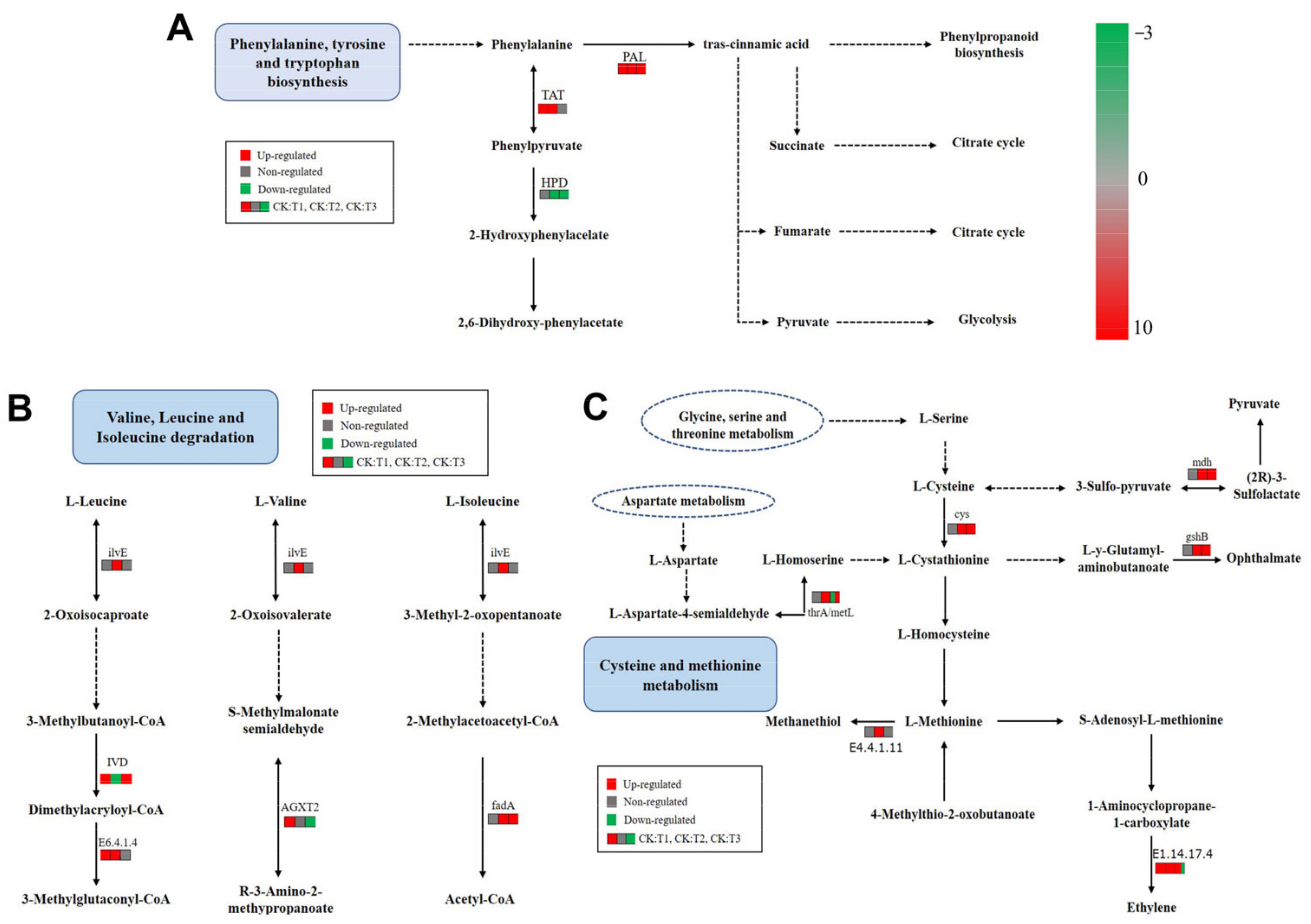
2.6. KEGG Pathway Analysis of Phenylpropanoid Biosynthesis
2.7. KEGG Pathway Analysis of Carbohydrate Metabolism
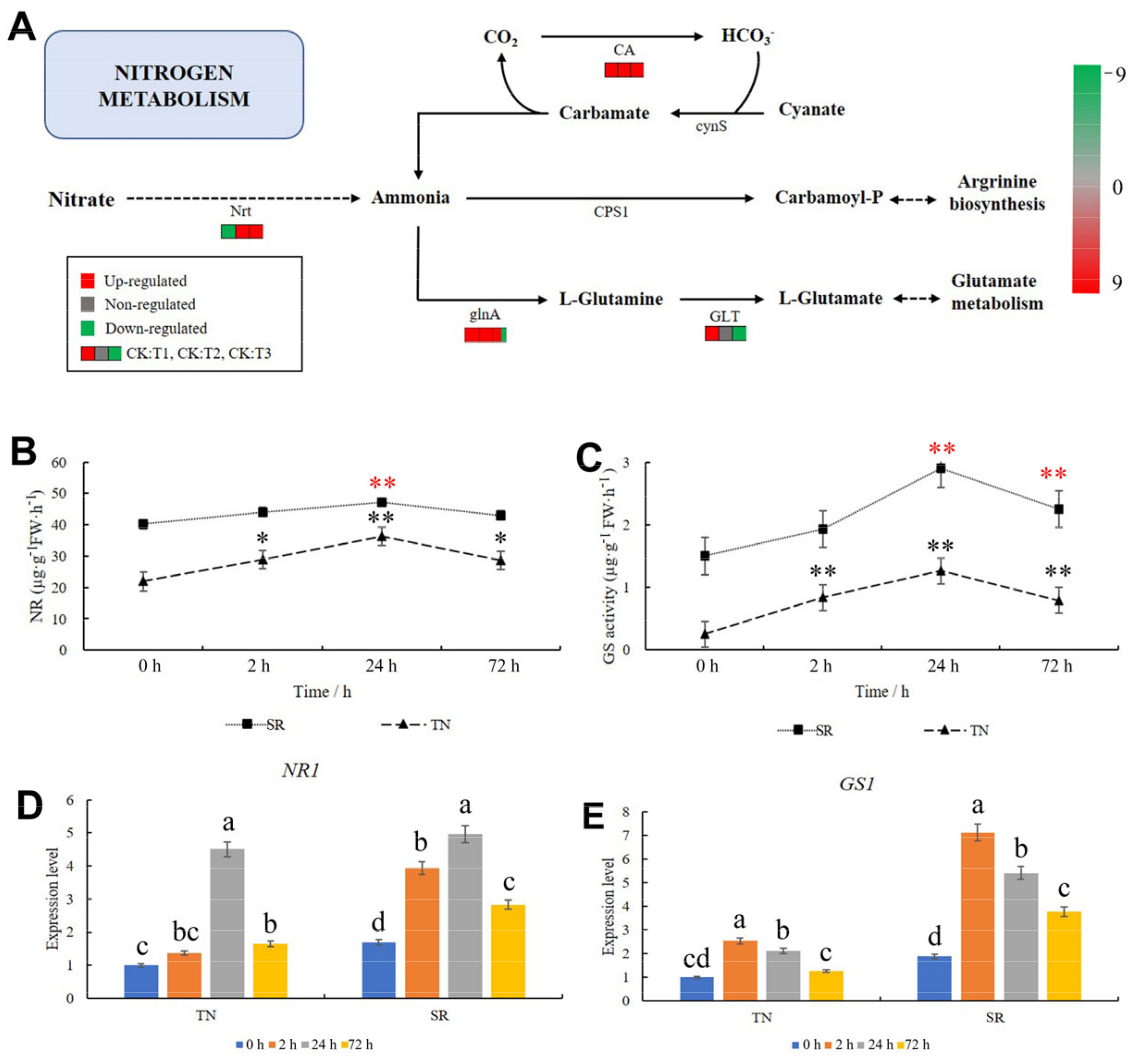
2.8. KEGG Pathway Analysis of Nitrogen Metabolism
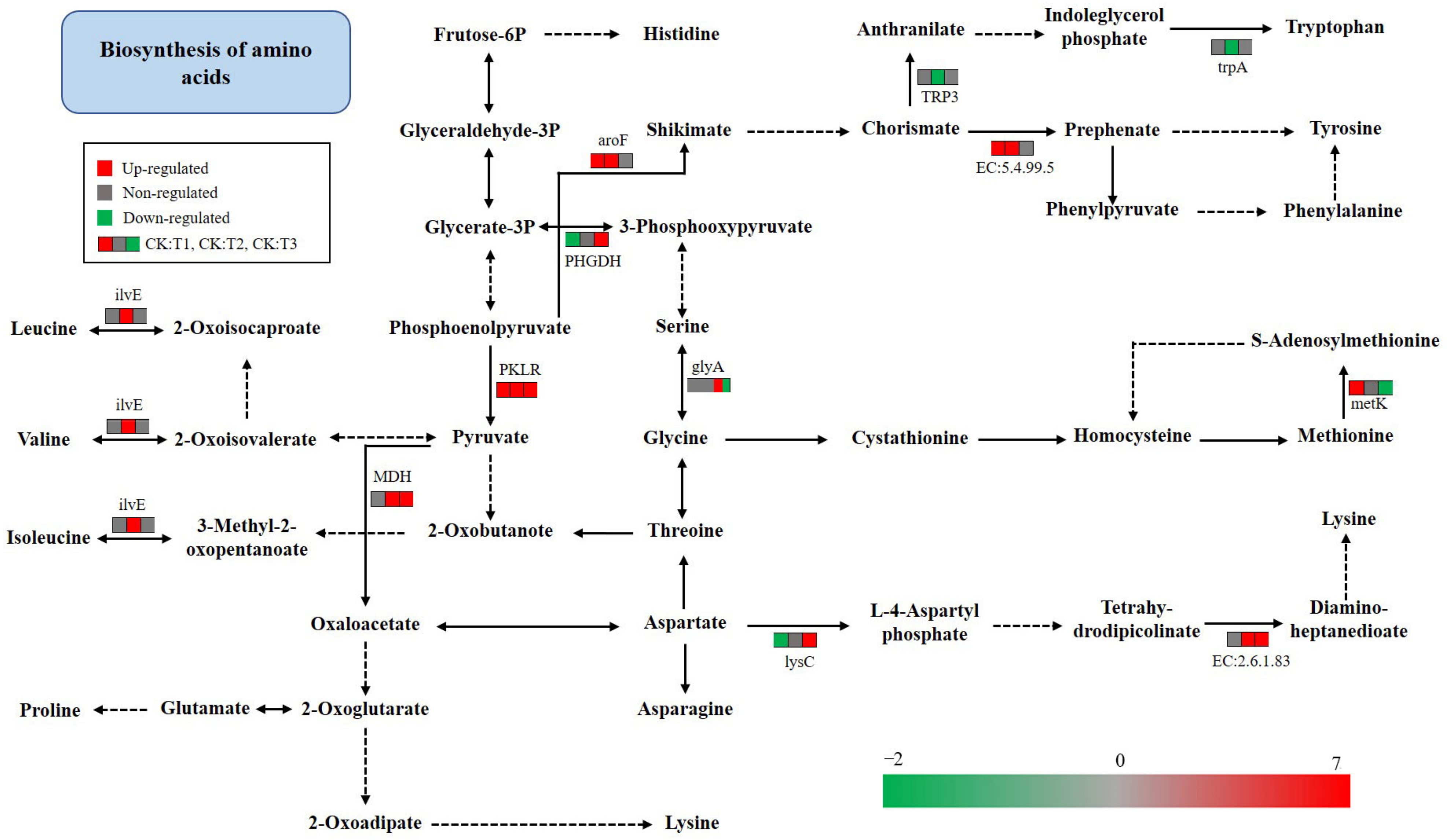
2.9. KEGG Pathway Analysis of Amino Acid Metabolism
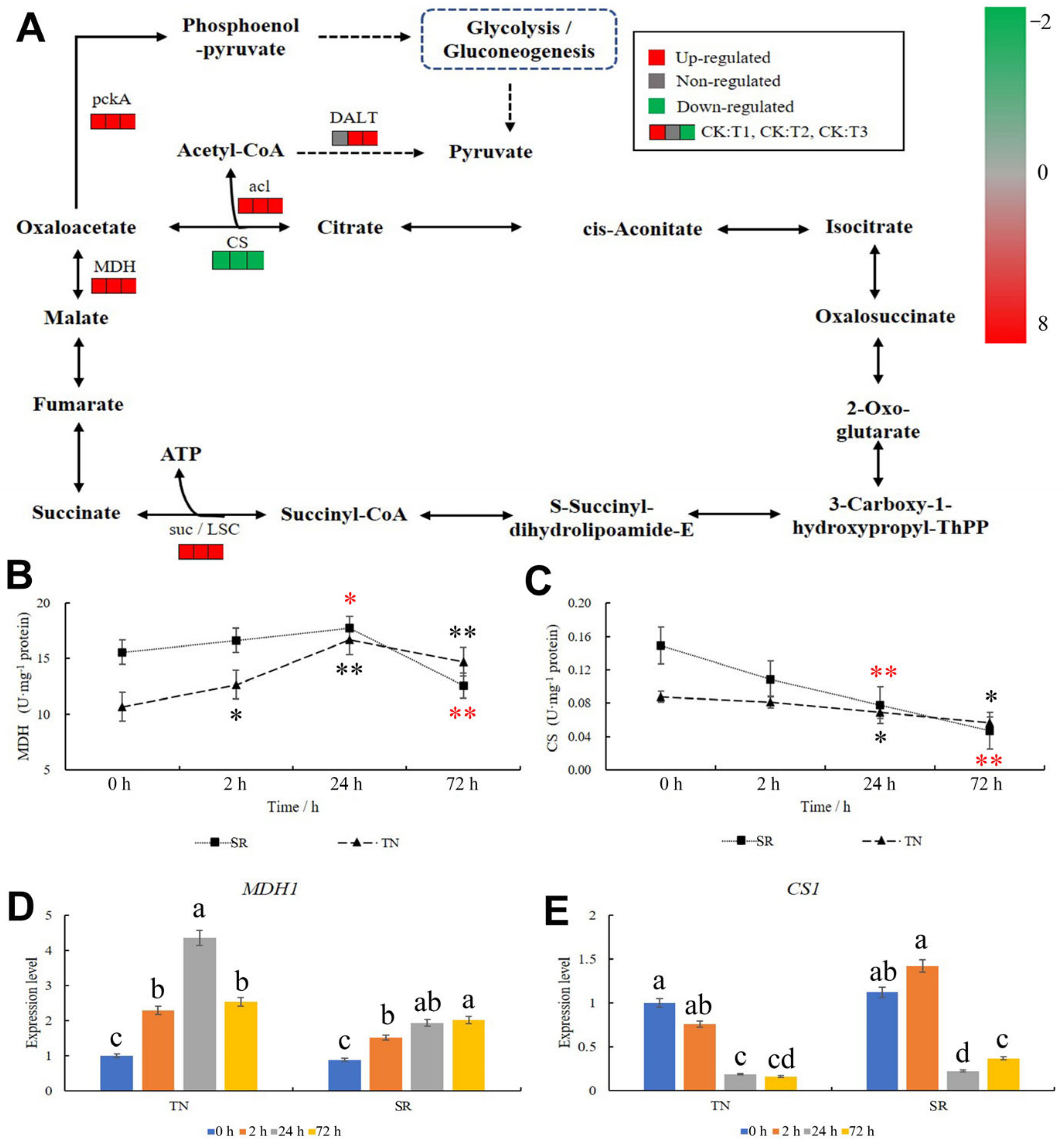
2.10. KEGG Pathway Analysis of Citrate Cycle (TCA Cycle)
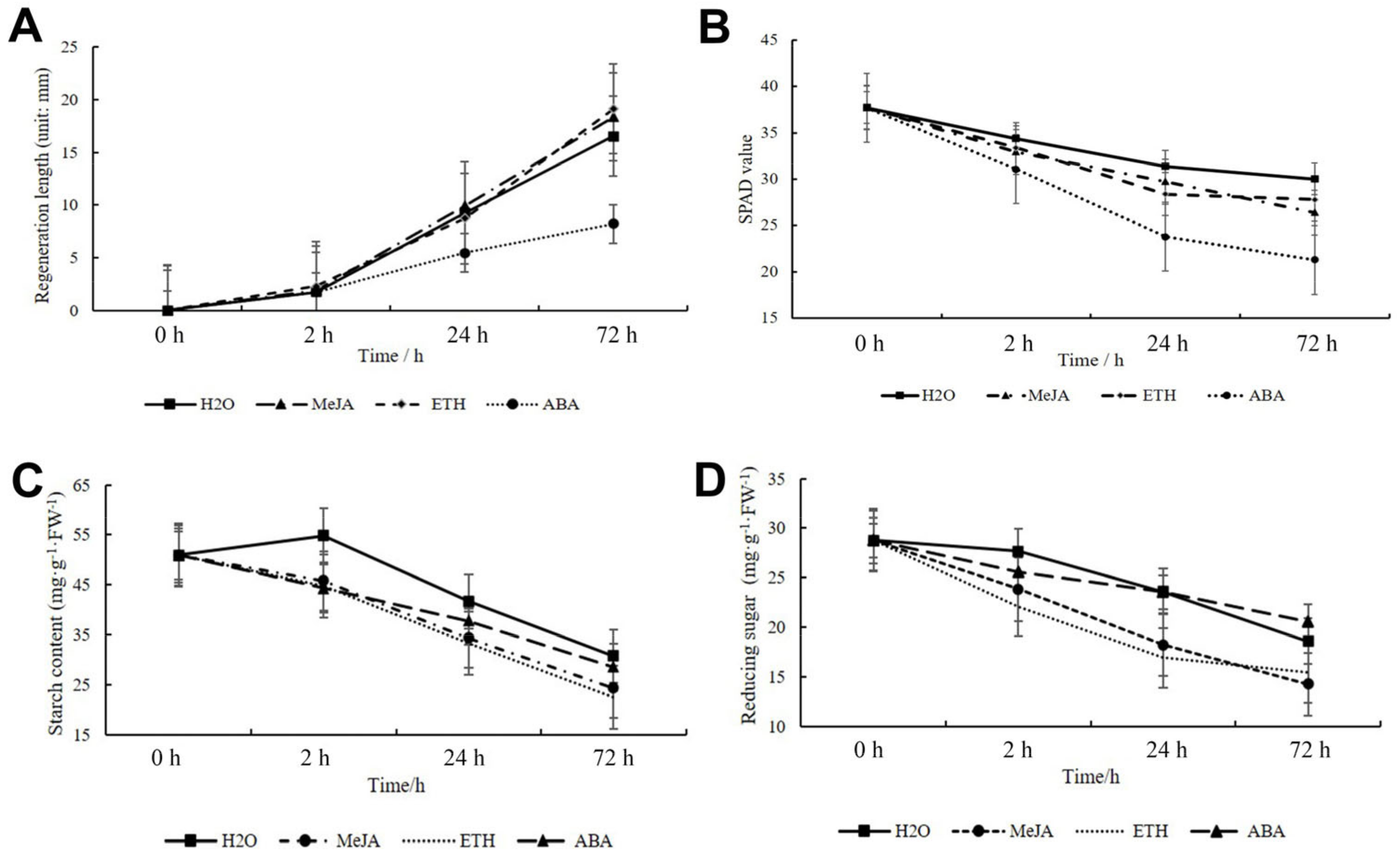
2.11. Determination of Hormone Content in TN and SR
2.12. Regeneration and Gene Expression Pattern under ETH, MeJA, and ABA Treatment
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Shoot and Root Phenotype Investigation and Analysis
4.3. Cytological Analysis of Tillering Node and Root Tip
4.4. Submicroscopic Structure of Wheat TN and SRT
4.5. RNA Extraction, mRNA Library Construction and Sequencing
4.6. Transcriptome Assembly and Annotation
4.7. Analysis of DEGs
4.8. RNA Isolates and Gene Expression Pattern Analysis
4.9. Physiological and Biochemical Measures
4.10. Wheat Endogenous Hormone Content Determination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mondal, T.; Yadav, R.P.; Meena, V.S.; Choudhury, M.; Nath, S.; Bisht, J.K.; Mishra, P.K.; Arya, S.K.; Pattanayak, A. Biomass yield and nutrient content of dual purpose wheat in the fruit based cropping system in the North-Western mid-Himalaya ecosystem, India. Field Crop. Res. 2020, 247, 107700. [Google Scholar] [CrossRef]
- Hunt, J.R. Winter wheat cultivars in Australian farming systems: A review. Crop Pasture Sci. 2017, 68, 501–515. [Google Scholar] [CrossRef]
- Dove, H.; Mcmullen, K.G. Diet selection, herbage intake and liveweight gain in young sheep grazing dual-purpose wheats and sheep responses to mineral supplements. Anim. Prod. Sci. 2009, 49, 749–758. [Google Scholar] [CrossRef]
- Tian, L.H.; Bell, L.W.; Shen, Y.Y.; Whish, J.P.M. Dual-purpose use of winter wheat in western China: Cutting time and nitrogen application effects on phenology, forage production, and grain yield. Crop Pasture Sci. 2012, 63, 520–528. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Li, F.; Lin, Q.; Dai, Q.; Sun, J.; Huang, X.; Chen, X.; Yin, Y. Effects of dietary ramie powder at various levels on carcass traits and meat quality in finishing pigs. Meat Sci. 2018, 143, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; He, Y.; Yu, L.; Tang, S.; Tan, Z.; Zhou, C.; Dong, L.; Wang, H. The potential of ramie as forage for ruminants: Impacts on growth, digestion, ruminal fermentation, carcass characteristics and meat quality of goats. Anim. Sci. J. 2019, 90, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Holman, J.D.; Thompson, C.R.; Hale, R.L.; Schlegel, A.J. Forage Yield and Nutritive Value of Hard Red and Hard White Winter Wheat. Agron. J. 2010, 102, 759–773. [Google Scholar] [CrossRef]
- Butchee, J.D.; Edwards, J.T. Dual-Purpose Wheat Grain Yield as Affected by Growth Habit and Simulated Grazing Intensity. Crop Sci. 2013, 53, 1686–1692. [Google Scholar] [CrossRef]
- Raza, G.; Singh, M.B.; Bhalla, P.L. In Vitro Plant Regeneration from Commercial Cultivars of Soybean. BioMed Res. Int. 2017, 2017, 7379693. [Google Scholar] [CrossRef]
- Chapin, F.S. Integrated responses of plants to stress: A centralized system of physiological responses. BioScience 1991, 41, 29–36. [Google Scholar] [CrossRef]
- Owen, D.F. How plants may benefit from the animals that eat them. Oikos 1980, 35, 230–235. [Google Scholar] [CrossRef]
- Evans, A.S. Whole-Plant Responses of Brassica campestris (Cruciferae) to Altered Sink-Source Relations. Am. J. Bot. 1991, 78, 394–400. [Google Scholar]
- Cheng, Y.; Dai, X.; Zhao, Y. Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 2007, 19, 2430–2439. [Google Scholar] [CrossRef] [PubMed]
- Pinon, V.; Prasad, K.; Grigg, S.P.; Sanchez-Perez, G.F.; Scheres, B. Local auxin biosynthesis regulation by PLETHORA transcription factors controls phyllotaxis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.P.; Chickarmane, V.S.; Ohno, C.; Meyerowitz, E.M. Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc. Natl. Acad. Sci. USA 2009, 106, 16529–16534. [Google Scholar] [CrossRef] [PubMed]
- Kurakawa, T.; Ueda, N.; Maekawa, M.; Kobayashi, K.; Kojima, M.; Nagato, Y.; Sakakibara, H.; Kyozuka, J. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 2007, 445, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin Localization and Transport in Plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef]
- Pena-Cortes, H.; Willmitzer, L.; Sanchez-Serrano, J.J. Abscisic Acid Mediates Wound Induction but Not Developmental-Specific Expression of the Proteinase Inhibitor II Gene Family. Plant Cell 1991, 3, 963–972. [Google Scholar] [CrossRef]
- Ge, X.; Zhang, C.; Wang, Q.; Yang, Z.; Wang, Y.; Zhang, X.; Wu, Z.; Hou, Y.; Wu, J.; Li, F. iTRAQ protein profile differential analysis between somatic globular and cotyledonary embryos reveals stress, hormone, and respiration involved in increasing plantlet regeneration of Gossypium hirsutum L. J. Proteome Res. 2015, 14, 268–278. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, W.; Gu, Z.; Wu, S.; E, Y.; Zhou, W.; Lin, J.; Xu, L. Roles of the wound hormone jasmonate in plant regeneration. J. Exp. Bot. 2021, 74, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lozano-Torres, J.L.; Blilou, I.; Zhang, X.; Zhai, Q.; Smant, G.; Li, C.; Scheres, B. A Jasmonate Signaling Network Activates Root Stem Cells and Promotes Regeneration. Cell 2019, 177, 942–956.e14. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Fan, M.; Yang, M.; Zhao, J.; Zhang, W.; Su, Y.; Xiao, L.; Deng, H.; Xie, D. Injury Activates Ca2+/Calmodulin-Dependent Phosphorylation of JAV1-JAZ8-WRKY51 Complex for Jasmonate Biosynthesis. Mol. Cell 2018, 70, 136–149.e7. [Google Scholar] [CrossRef] [PubMed]
- Mira, M.M.; Wally, O.S.; Elhiti, M.; El-Shanshory, A.; Reddy, D.S.; Hill, R.D.; Stasolla, C. Jasmonic acid is a downstream component in the modulation of somatic embryogenesis by Arabidopsis Class 2 phytoglobin. J. Exp. Bot. 2016, 67, 2231–2246. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.B.; Shang, G.D.; Pan, Y.; Xu, Z.G.; Zhou, C.M.; Mao, Y.B.; Bao, N.; Sun, L.; Xu, T.; Wang, J.W. AP2/ERF Transcription Factors Integrate Age and Wound Signals for Root Regeneration. Plant Cell 2020, 32, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Arteca, J.M.; Arteca, R.N. A multi-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase (ACS6) in mature Arabidopsis leaves. Plant Mol. Biol. 1999, 39, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Hayakawa, Y.; Hyodo, H.; Ikoma, Y.; Yano, M. Wound-induced ethylene synthesis and expression and formation of 1-aminocyclopropane-1-carboxylate (ACC) synthase, ACC oxidase, phenylalanine ammonia-lyase, and peroxidase in wounded mesocarp tissue of Cucurbita maxima. Plant Cell Rep. 2000, 41, 440–447. [Google Scholar] [CrossRef]
- Dombrowski, J.E.; Kronmiller, B.A.; Hollenbeck, V.; Martin, R.C. Transcriptome Analysis of Wounding in the Model Grass Lolium temulentum. Plants 2020, 9, 780. [Google Scholar] [CrossRef]
- Becerra-Moreno, A.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as biofactories: Glyphosate-induced production of shikimic acid and phenolic antioxidants in wounded carrot tissue. J. Agric. Food Chem. 2012, 60, 11378–11386. [Google Scholar] [CrossRef]
- Han, C.; Jin, P.; Li, M.; Wang, L.; Zheng, Y. Physiological and Transcriptomic Analysis Validates Previous Findings of Changes in Primary Metabolism for the Production of Phenolic Antioxidants in Wounded Carrots. J. Agric. Food Chem. 2017, 65, 7159–7167. [Google Scholar] [CrossRef]
- Pommerrenig, B.; Ludewig, F.; Cvetkovic, J.; Trentmann, O.; Klemens, P.A.; Neuhaus, H.E. In concert: Orchestrated changes in carbohydrate homeostasis are critical for plant abiotic stress tolerance. Plant Cell Physiol. 2018, 59, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Effect of exogenous amylolytic enzymes on the accumulation of chlorogenic acid isomers in wounded potato tubers. J. Agric. Food Chem. 2014, 62, 7671–7675. [Google Scholar] [CrossRef] [PubMed]
- Baslam, M.; Mitsui, T.; Sueyoshi, K.; Ohyama, T. Recent Advances in Carbon and Nitrogen Metabolism in C3 Plants. Int. J. Mol. Sci. 2020, 22, 318. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, F.J.; Pérez-Hernández, C.A.; Rodríguez-Murillo, M.; Moreno-Altamirano, M.M.B. The Role of Tricarboxylic Acid Cycle Metabolites in Viral Infections. Front. Cell Infect. Mi. 2021, 11, 725043. [Google Scholar] [CrossRef] [PubMed]
- Lafta, A.M.; Fugate, K.K. Metabolic profile of wound-induced changes in primary carbon metabolism in sugarbeet root. Phytochemistry 2011, 72, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Giunta, F.; Motzo, R.; Fois, G.; Bacciu, P. Developmental ideotype in the context of the dual-purpose use of triticale, barley and durum wheat. Ann. Appl. Biol. 2015, 166, 118–128. [Google Scholar] [CrossRef]
- Zeleke, K.T. Effect of grazing time and intensity on growth and yield of spring wheat (Triticum aestivum L.). J. Integr. Agr. 2019, 18, 204–213. [Google Scholar] [CrossRef]
- Anderson, W.K. Production of green feed and grain from grazed barley in Northern Syria. Field Crop. Res. 1985, 10, 57–75. [Google Scholar] [CrossRef]
- Sugimoto, K.; Gordon, S.P.; Meyerowitz, E.M. Regeneration in plants and animals: Dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol. 2011, 21, 212–218. [Google Scholar] [CrossRef]
- Grafi, G. How cells dedifferentiate: A lesson from plants. Dev. Biol. 2004, 268, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Collin, P.; Epron, D.; Alaoui-Sosse, B.; Badot, P.M. Growth Responses of Common Ash Seedlings (Fraxinus excelsior L.) to Total and Partial Defoliation. Ann. Bot. 2000, 85, 317–323. [Google Scholar] [CrossRef]
- McNaughton, S. Ecology of A Grazing Ecosystem—The Serengeti. Ecol. Monogr. 1985, 55, 259–294. [Google Scholar] [CrossRef]
- Gao, Y.H.; Chen, H.; Luo, P.; Wang, N. Effect of grazing intensity on biomass of alpine meadow and its allocation in the Northwestern Sichuan. J. Ecol. Rural. Environ. 2008, 24, 26–32. [Google Scholar]
- Paez-Garcia, A.; Liao, F.; Blancaflor, E.B. Two Wheat Cultivars with Contrasting Post-Embryonic Root Biomass Differ in Shoot Re-Growth after Defoliation: Implications for Breeding Grazing Resilient Forages. Plants 2019, 8, 470. [Google Scholar] [CrossRef] [PubMed]
- Bar, M.; Ori, N. Leaf development and morphogenesis. Development 2014, 141, 4219–4230. [Google Scholar] [CrossRef] [PubMed]
- Trumble, J.T. Plant Compensation for Arthropod Herbivory. Ann. Rev. Entomol. 1993, 38, 93–120. [Google Scholar] [CrossRef]
- Darnell, R.L.; Cruz-Huerta, N.; Williamson, J.G. Night temperature and source-sink effects on overall growth, cell number and cell size in bell pepper ovaries. Ann. Bot. 2012, 110, 987–994. [Google Scholar] [CrossRef]
- Yang, J.L.; Lu, Y.X.; Pei, L.Y. A Study on the Relationship between the Primary Root System and the Growth and the Development of Spring Wheat. Acta Agric. Boreali Sin. 1988, 3, 13–18. [Google Scholar]
- Sun, N.; Hu, J.; Li, C.; Wang, X.; Gai, Y.; Jiang, X. Fusion gene 4CL-CCR promotes lignification in tobacco suspension cells. Plant Cell Rep. 2023, 42, 939–952. [Google Scholar] [CrossRef]
- Lavhale, S.G.; Kalunke, R.M.; Giri, A.P. Structural, functional and evolutionary diversity of 4-coumarate-CoA ligase in plants. Planta 2018, 248, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Hu, W.; Xu, Y.; Yang, X.; Ji, Y.; Feng, K.; Sarengaowa. Metabolomics and physiological analyses validates previous findings on the mechanism of response to wounding stress of different intensities in broccoli. Food Res. Int. 2021, 140, 110058. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ballesta, M.T.; Lafuente, M.T.; Zacarias, L.; Granell, A. Involvement of phenylalanine ammonia-lyase in the response of Fortune mandarin fruits to cold temperature. Physiol. Plant. 2010, 108, 382–389. [Google Scholar] [CrossRef]
- Akiyama, T.; Jin, S.; Yoshida, M.; Hoshino, T.; Opassiri, R.; Ketudat Cairns, J.R. Expression of an endo-(1,3;1,4)-beta-glucanase in response to wounding, methyl jasmonate, abscisic acid and ethephon in rice seedlings. J. Plant Physiol. 2009, 166, 1814–1825. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Chaki, M.; Fernández-Ocaña, A.; Valderrama, R.; Palma, J.M.; Carreras, A.; Begara-Morales, J.C.; Airaki, M.; del Río, L.A.; Barroso, J.B. Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions. Plant Cell Physiol. 2008, 49, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Balotf, S.; Kavoosi, G.; Kholdebarin, B. Nitrate reductase, nitrite reductase, glutamine synthetase, and glutamate synthase expression and activity in response to different nitrogen sources in nitrogen-starved wheat seedlings. Biotechnol. Appl. Biochem. 2015, 63, 220–229. [Google Scholar] [CrossRef]
- Alain, O.; Jean, B.; Jean, S. Nitrogen Mobilization from Stubble and Roots during Re-growth of Defoliated Perennial Ryegrass. J. Exp. Bot. 1988, 6, 803–809. [Google Scholar]
- Corpas, F.J.; Leterrier, M.; Valderrama, R.; Airaki, M.; Chaki, M.; Palma, J.M.; Barroso, J.B. Nitric oxide imbalance provokes a nitrosative response in plants under abiotic stress. Plant Sci. 2011, 181, 604–611. [Google Scholar] [CrossRef]
- Ueno, M.; Smith, D. Growth and Carbohydrate Changes in the Root Wood and Bark of Different Sized Alfalfa Plants during Regrowth after Cutting. Crop Sci. 1970, 10, 396–399. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. Glutamate: A truly functional amino acid. Amino Acids 2013, 45, 413–418. [Google Scholar] [CrossRef]
- Toyota, M.; Spencer, D.; Sawai-Toyota, S.; Jiaqi, W.; Zhang, T.; Koo, A.J.; Howe, G.A.; Gilroy, S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 2018, 361, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Bezsudnova, E.Y.; Boyko, K.M.; Popov, V.O. Properties of Bacterial and Archaeal Branched-Chain Amino Acid Aminotransferases. Biochem. Biokhimiia 2017, 82, 1572–1591. [Google Scholar] [CrossRef] [PubMed]
- Harun, S.; Abdullah-Zawawi, M.R.; Goh, H.H.; Mohamed-Hussein, Z.A. A Comprehensive Gene Inventory for Glucosinolate Biosynthetic Pathway in Arabidopsis thaliana. J. Agric. Food Chem. 2020, 68, 7281–7297. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Han, Y.; Tao, F.; Chong, K. Knockdown of SAMS genes encoding S-adenosyl-l-methionine synthetases causes methylation alterations of DNAs and histones and leads to late flowering in rice. J. Plant. Physiol. 2011, 168, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant. Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Han, G.; Meng, Z.; Lin, L.; Sui, N. Roles of malic enzymes in plant development and stress responses. Plant Signal. Behav. 2019, 14, e1644596. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Zhao, M.; Zhang, S.; Wang, Z.; Meng, M.; Sun, F.; Zhang, C.; Xi, Y. MicroRNA and regulation of auxin and cytokinin signalling during post-mowing regeneration of winter wheat (Triticum aestivum L.). Plant Physiol. Biochem. PPB 2020, 155, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Zhao, M.; Tan, H.; Wang, Z.; Meng, M.; Sun, F.; Zhang, C.; Xi, Y. RNA Sequencing Reveals Dynamic Carbohydrate Metabolism and Phytohormone Signaling Accompanying Post-mowing Regeneration of Forage Winter Wheat (Triticum aestivum L.). Front. Plant Sci. 2021, 12, 664933. [Google Scholar] [CrossRef]
- Du, F.; Guan, C.; Jiao, Y. Molecular Mechanisms of Leaf Morphogenesis. Mol. Plant 2018, 11, 1117–1134. [Google Scholar] [CrossRef]
- Müller, D. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef]
- Swarup, R.; Perry, P.; Hagenbeek, D.; Van Der Straeten, D.; Beemster, G.T.; Sandberg, G.; Bhalerao, R.; Ljung, K.; Bennett, M.J. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 2007, 19, 2186–2196. [Google Scholar] [CrossRef]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 2017, 68, 1303–1321. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lu, W.; Wei, X.; Li, L.; Mao, L.; Zhao, Y. Proteomics analysis to understand the ABA stimulation of wound suberization in kiwifruit. J. Proteom. 2018, 173, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Park, O.S.; Bae, S.H.; Kim, S.G.; Seo, P.J. JA-pretreated hypocotyl explants potentiate de novo shoot regeneration in Arabidopsis. Plant Signal. Behav. 2019, 14, 1618180. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Dahleen, L.S.; Suttle, J.C. Ethylene influences green plant regeneration from barley callus. Plant Cell Rep. 2007, 26, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Wang, J.; Wang, W.; Wang, Z.; Zhang, C.; Sun, F.; Liu, S.; Xi, Y. Study on cultivation pattern of daul-purpose wheat Xinong 136. Seed Technol. 2022, 40, 14–21. [Google Scholar]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Kechin, A.; Boyarskikh, U.; Kel, A.; Filipenko, M. cutPrimers: A New Tool for Accurate Cutting of Primers from Reads of Targeted Next Generation Sequencing. J. Comput. Biol. 2017, 24, 1138–1143. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Marioni, J.C.; Mason, C.E.; Mane, S.M.; Stephens, M.; Gilad, Y. RNA-seq: An assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 2008, 18, 1509–1517. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Li, S.; Yao, Y.; Ye, W.; Wang, S.; Zhang, C.; Liu, S.; Sun, F.; Xi, Y. Genome-Wide Identification of Wheat KNOX Gene Family and Functional Characterization of TaKNOX14-D in Plants. Int. J. Mol. Sci. 2022, 23, 15918. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, H.S. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Almeida-Trapp, M.; Mithöfer, A. Quantification of Phytohormones by HPLC-MS/MS Including Phytoplasma-Infected Plants. Methods Mol. Biol. 2019, 1875, 345–358. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wang, S.; Ye, W.; Yao, Y.; Sun, F.; Zhang, C.; Liu, S.; Xi, Y. Effect of Mowing on Wheat Growth at Seeding Stage. Int. J. Mol. Sci. 2023, 24, 15353. https://doi.org/10.3390/ijms242015353
Li S, Wang S, Ye W, Yao Y, Sun F, Zhang C, Liu S, Xi Y. Effect of Mowing on Wheat Growth at Seeding Stage. International Journal of Molecular Sciences. 2023; 24(20):15353. https://doi.org/10.3390/ijms242015353
Chicago/Turabian StyleLi, Song, Shaoyu Wang, Wenjie Ye, Yaxin Yao, Fengli Sun, Chao Zhang, Shudong Liu, and Yajun Xi. 2023. "Effect of Mowing on Wheat Growth at Seeding Stage" International Journal of Molecular Sciences 24, no. 20: 15353. https://doi.org/10.3390/ijms242015353




