The Application of MicroRNAs in Glaucoma Research: A Bibliometric and Visualized Analysis
Abstract
1. Introduction
2. Results
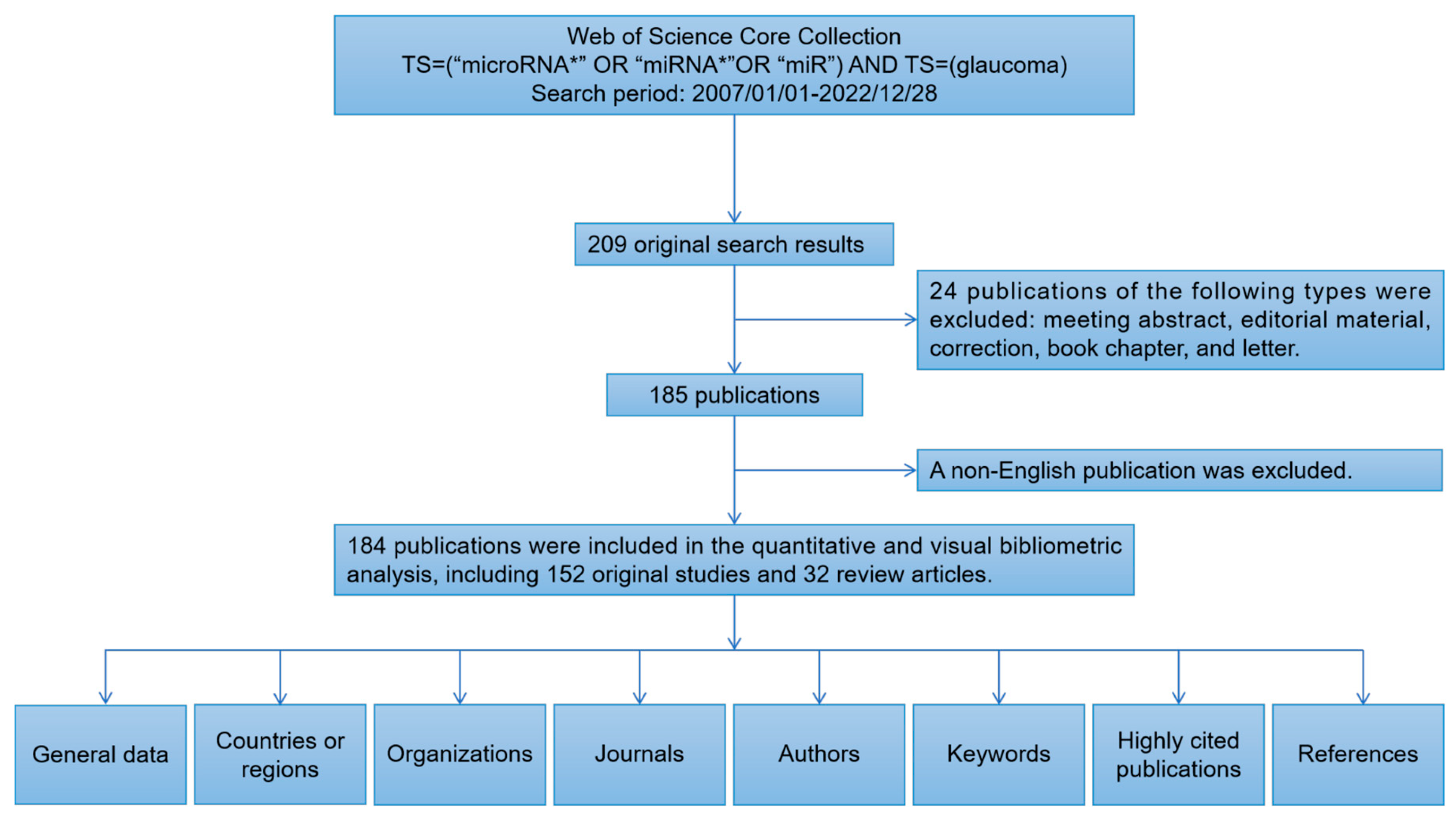
2.1. General Data
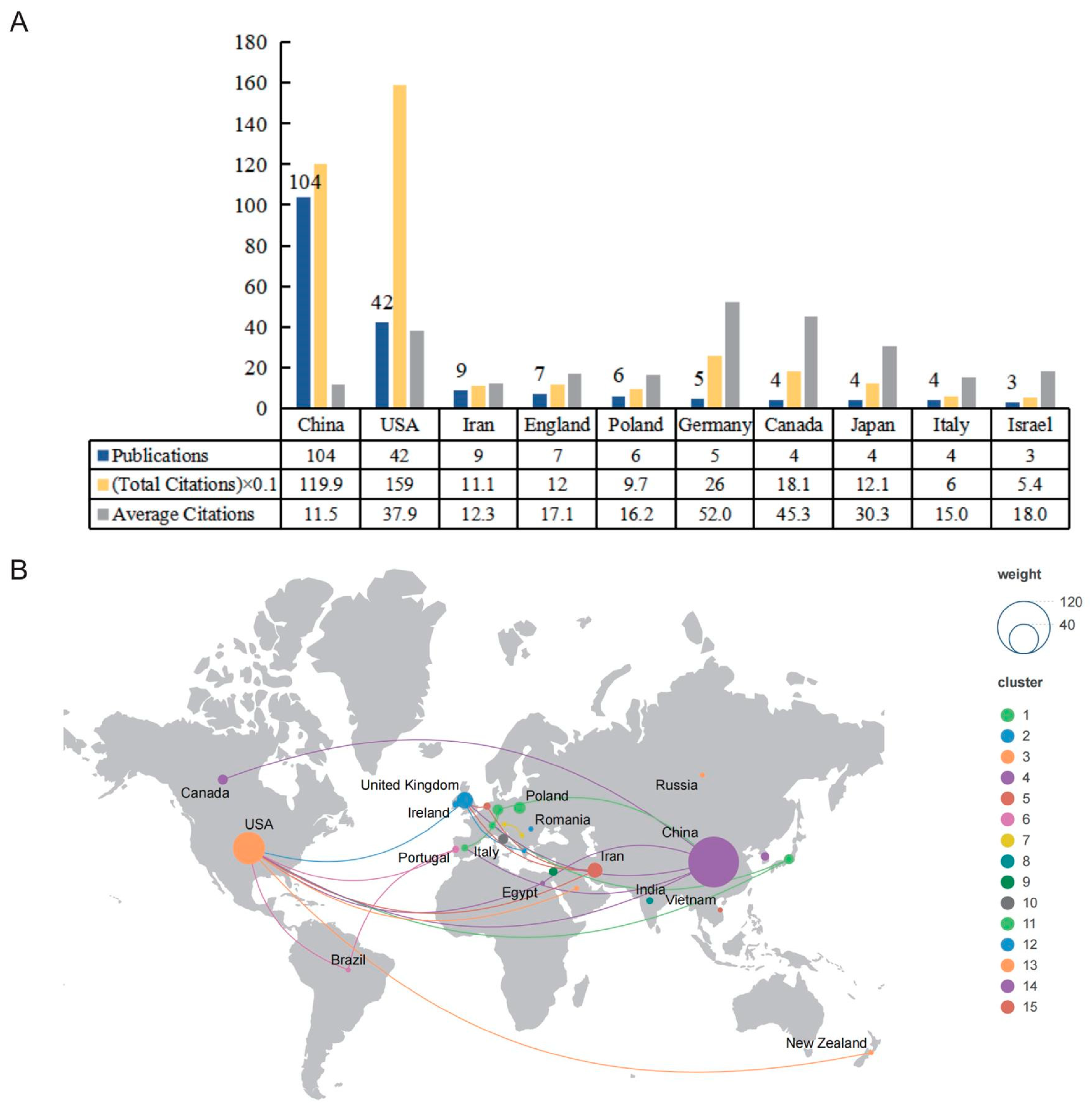
2.2. Active Countries or Regions
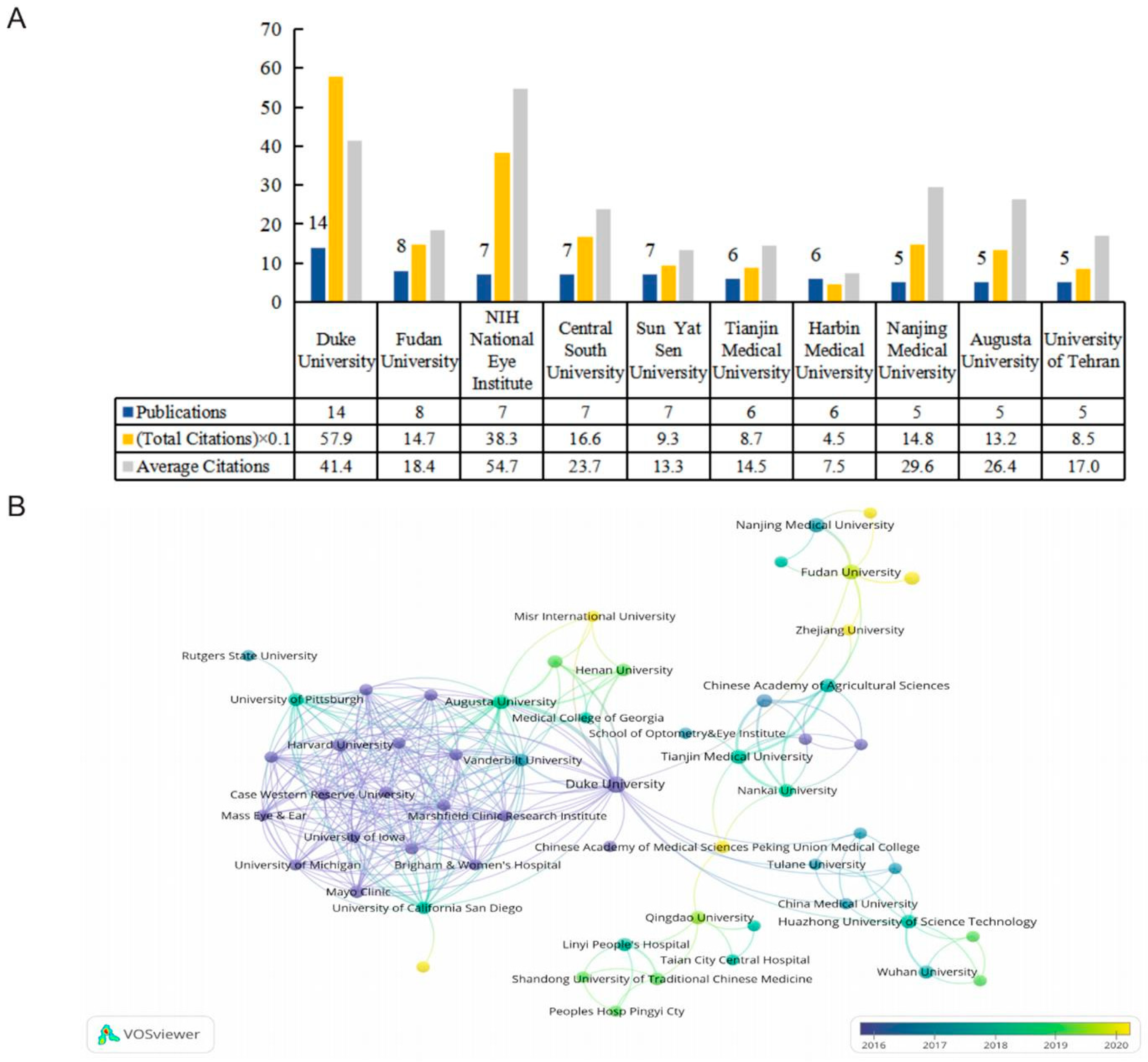
2.3. Active Organizations
2.4. Top 10 Prolific Journals and Co-Cited Journals
2.5. Active Authors
2.6. Analysis of Keywords
2.7. Cited Publications and References
| Rank | Title | Type | Citations | Journal | IF (2022) | Corresponding Author | Affiliation | Year |
|---|---|---|---|---|---|---|---|---|
| 1 | Optineurin negatively regulates TNF alpha-induced NF-kappa B activation by competing with NEMO for ubiquitinated RIP [25] | Article | 212 | Current Biology | 9.2 | Jonathan D. Ashwell | NIH National Cancer Institute | 2007 |
| 2 | Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms [30] | Article | 206 | Stem Cells Translational Medicine | 6 | Ben Mead | NIH National Eye Institute | 2017 |
| 3 | The role of TGF-beta in the pathogenesis of primary open-angle glaucoma [31] | Review | 190 | Cell and Tissue Research | 3.6 | Ernst R. Tamm | University of Regensburg | 2012 |
| 4 | MicroRNA dysregulation in neurodegenerative diseases: A systematic review [26] | Review | 174 | Progress in Neurobiology | 6.7 | Alyson E. Fournier | McGill University | 2019 |
| 5 | Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress [32] | Article | 132 | Molecular Vision | 2.2 | Pedro Gonzalez | Duke University | 2009 |
| 6 | Human aqueous humor exosomes [18] | Article | 79 | Experimental Eye Research | 3.4 | Yutao Liu | University System of Georgia | 2015 |
| 7 | Coordinated Regulation of Extracellular Matrix Synthesis by the MicroRNA-29 Family in the Trabecular Meshwork [33] | Article | 78 | Investigative Ophthalmology and Visual Science | 4.4 | Douglas J. Rhee | Harvard University | 2011 |
| 8 | Profiles of Extracellular miRNAs in the Aqueous Humor of Glaucoma Patients Assessed with a Microarray System [29] | Article | 72 | Scientific Reports | 4.6 | Toru Nakazawa | Tohoku University | 2014 |
| 9 | MicroRNA-24 Regulates the Processing of Latent TGF beta 1 During Cyclic Mechanical Stress in Human Trabecular Meshwork Cells Through Direct Targeting of FURIN [34] | Article | 70 | Journal of Cellular Physiology | 5.6 | Pedro Gonzalez | Duke University | 2011 |
| 10 | Suppression of Type I Collagen Expression by miR-29b via PI3K, Akt, and Sp1 Pathway in Human Tenon’s Fibroblasts [35] | Article | 67 | Investigative Ophthalmology and Visual Science | 4.4 | Xuanchu Duan | Central South University | 2012 |
3. Discussion
3.1. MicroRNAs
3.2. MicroRNAs in Neurodegenerative Diseases (NDs)
3.3. MicroRNAs in Glaucoma
3.3.1. Glaucoma
3.3.2. MicroRNAs in Aqueous Humor (AH)
3.3.3. MicroRNAs in Trabecular Meshwork (TM)
3.3.4. Manipulating microRNA Expressions
3.3.5. MicroRNAs in Mouse Models of Glaucoma
3.4. Other Non-Coding RNAs
3.4.1. Circular RNAs
3.4.2. Long Non-Coding RNAs
3.4.3. Competing Endogenous RNAs
3.5. MicroRNAs in Exosomes
3.6. Research Frontiers
3.7. Limitations
4. Materials and Methods
4.1. Search Strategy and Data Collection
4.2. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Sharoukhov, D.; Bucinca-Cupallari, F.; Lim, H. Microtubule Imaging Reveals Cytoskeletal Deficit Predisposing the Retinal Ganglion Cell Axons to Atrophy in DBA/2J. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5292–5300. [Google Scholar] [CrossRef]
- Martinez, B.; Peplow, P.V. MicroRNAs as biomarkers in glaucoma and potential therapeutic targets. Neural Regen. Res. 2022, 17, 2368–2375. [Google Scholar] [CrossRef]
- Lusthaus, J.; Goldberg, I. Current management of glaucoma. Med. J. Aust. 2019, 210, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K., 2nd; Wilson, M.R.; Gordon, M.O. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 701–713. [Google Scholar] [CrossRef]
- Kang, J.M.; Tanna, A.P. Glaucoma. Med. Clin. N. Am. 2021, 105, 493–510. [Google Scholar] [CrossRef]
- Kubelick, K.P.; Snider, E.J.; Ethier, C.R.; Emelianov, S. Development of a stem cell tracking platform for ophthalmic applications using ultrasound and photoacoustic imaging. Theranostics 2019, 9, 3812–3824. [Google Scholar] [CrossRef]
- Gao, X.R.; Huang, H.; Kim, H. Polygenic Risk Score Is Associated with Intraocular Pressure and Improves Glaucoma Prediction in the UK Biobank Cohort. Transl. Vis. Sci. Technol. 2019, 8, 10. [Google Scholar] [CrossRef]
- Backes, C.; Meese, E.; Keller, A. Specific miRNA Disease Biomarkers in Blood, Serum and Plasma: Challenges and Prospects. Mol. Diagn. Ther. 2016, 20, 509–518. [Google Scholar] [CrossRef]
- Vishnoi, A.; Rani, S. MiRNA Biogenesis and Regulation of Diseases: An Overview. Methods Mol. Biol. 2017, 1509, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, A.; Braniewska, A.; Kozar-Kaminska, K. MicroRNA in cardiovascular biology and disease. Adv. Clin. Exp. Med. 2017, 26, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, L.; Zhao, J.; Wang, M.; Li, K.; Zheng, Y. An update: Mechanisms of microRNA in primary open-angle glaucoma. Brief. Funct. Genom. 2021, 20, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Bagga, S.; Bracht, J.; Hunter, S.; Massirer, K.; Holtz, J.; Eachus, R.; Pasquinelli, A.E. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell 2005, 122, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Meister, G. miRNAs get an early start on translational silencing. Cell 2007, 131, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, A.; Pirtoli, L.; Giordano, A.; Dotta, F. Crosstalk between MicroRNA and Oxidative Stress in Physiology and Pathology. Int. J. Mol. Sci. 2020, 21, 1270. [Google Scholar] [CrossRef] [PubMed]
- Molasy, M.; Walczak, A.; Szaflik, J.; Szaflik, J.P.; Majsterek, I. MicroRNAs in glaucoma and neurodegenerative diseases. J. Hum. Genet. 2017, 62, 105–112. [Google Scholar] [CrossRef]
- Dismuke, W.M.; Challa, P.; Navarro, I.; Stamer, W.D.; Liu, Y. Human aqueous humor exosomes. Exp. Eye Res. 2015, 132, 73–77. [Google Scholar] [CrossRef]
- Gonzalez, P.; Li, G.; Qiu, J.; Wu, J.; Luna, C. Role of microRNAs in the trabecular meshwork. J. Ocul. Pharmacol. Ther. 2014, 30, 128–137. [Google Scholar] [CrossRef]
- Liu, Y.; Bailey, J.C.; Helwa, I.; Dismuke, W.M.; Cai, J.; Drewry, M.; Brilliant, M.H.; Budenz, D.L.; Christen, W.G.; Chasman, D.I.; et al. A Common Variant in MIR182 Is Associated with Primary Open-Angle Glaucoma in the NEIGHBORHOOD Consortium. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4528–4535. [Google Scholar] [CrossRef]
- Guo, R.; Shen, W.; Su, C.; Jiang, S.; Wang, J. Relationship between the Pathogenesis of Glaucoma and miRNA. Ophthalmic Res. 2017, 57, 194–199. [Google Scholar] [CrossRef]
- Agarwal, A.; Durairajanayagam, D.; Tatagari, S.; Esteves, S.C.; Harlev, A.; Henkel, R.; Roychoudhury, S.; Homa, S.; Puchalt, N.G.; Ramasamy, R.; et al. Bibliometrics: Tracking research impact by selecting the appropriate metrics. Asian J. Androl. 2016, 18, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.C.; Wang, H.S.; Yang, X.; Xu, C.Q.; Wang, T.; Yan, Y.J.; Fan, Z.X.; Ma, J.M.; Ye, J.; Mo, W. A Bibliometric Analysis and Visualization of Current Research Trends in Chinese Medicine for Osteosarcoma. Chin. J. Integr. Med. 2022, 28, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhang, Q.; Meng, M.; Huang, J. A bibliometric analysis of the application of stem cells in glaucoma research from 1999 to 2022. Front. Cell Dev. Biol. 2023, 11, 1081898. [Google Scholar] [CrossRef]
- Zhu, G.; Wu, C.J.; Zhao, Y.; Ashwell, J.D. Optineurin negatively regulates TNFalpha- induced NF-kappaB activation by competing with NEMO for ubiquitinated RIP. Curr. Biol. 2007, 17, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Juzwik, C.A.; Drake, S.S.; Zhang, Y.; Paradis-Isler, N.; Sylvester, A.; Amar-Zifkin, A.; Douglas, C.; Morquette, B.; Moore, C.S.; Fournier, A.E. microRNA dysregulation in neurodegenerative diseases: A systematic review. Prog. Neurobiol. 2019, 182, 101664. [Google Scholar] [CrossRef]
- Drewry, M.D.; Challa, P.; Kuchtey, J.G.; Navarro, I.; Helwa, I.; Hu, Y.; Mu, H.; Stamer, W.D.; Kuchtey, R.W.; Liu, Y. Differentially expressed microRNAs in the aqueous humor of patients with exfoliation glaucoma or primary open-angle glaucoma. Hum. Mol. Genet. 2018, 27, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Hindle, A.G.; Thoonen, R.; Jasien, J.V.; Grange, R.M.H.; Amin, K.; Wise, J.; Ozaki, M.; Ritch, R.; Malhotra, R.; Buys, E.S. Identification of Candidate miRNA Biomarkers for Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 134–146. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tsuda, S.; Kunikata, H.; Sato, J.; Kokubun, T.; Yasuda, M.; Nishiguchi, K.M.; Inada, T.; Nakazawa, T. Profiles of extracellular miRNAs in the aqueous humor of glaucoma patients assessed with a microarray system. Sci. Rep. 2014, 4, 5089. [Google Scholar] [CrossRef]
- Mead, B.; Tomarev, S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl. Med. 2017, 6, 1273–1285. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Tamm, E.R. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012, 347, 279–290. [Google Scholar] [CrossRef]
- Luna, C.; Li, G.; Qiu, J.; Epstein, D.L.; Gonzalez, P. Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Mol. Vis. 2009, 15, 2488–2497. [Google Scholar]
- Villarreal, G., Jr.; Oh, D.J.; Kang, M.H.; Rhee, D.J. Coordinated regulation of extracellular matrix synthesis by the microRNA-29 family in the trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 2011, 52, 3391–3397. [Google Scholar] [CrossRef]
- Luna, C.; Li, G.; Qiu, J.; Epstein, D.L.; Gonzalez, P. MicroRNA-24 regulates the processing of latent TGFbeta1 during cyclic mechanical stress in human trabecular meshwork cells through direct targeting of FURIN. J. Cell Physiol. 2011, 226, 1407–1414. [Google Scholar] [CrossRef]
- Li, N.; Cui, J.; Duan, X.; Chen, H.; Fan, F. Suppression of type I collagen expression by miR-29b via PI3K, Akt, and Sp1 pathway in human Tenon’s fibroblasts. Invest. Ophthalmol. Vis. Sci. 2012, 53, 1670–1678. [Google Scholar] [CrossRef]
- Dunmire, J.J.; Lagouros, E.; Bouhenni, R.A.; Jones, M.; Edward, D.P. MicroRNA in aqueous humor from patients with cataract. Exp. Eye Res. 2013, 108, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.; Li, G.; Huang, J.; Qiu, J.; Wu, J.; Yuan, F.; Epstein, D.L.; Gonzalez, P. Regulation of trabecular meshwork cell contraction and intraocular pressure by miR-200c. PLoS ONE 2012, 7, e51688. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Lu, X.; Li, B. Downregulation of microRNA-100 protects apoptosis and promotes neuronal growth in retinal ganglion cells. BMC Mol. Biol. 2014, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, H.; Cepurna, W.O.; Johnson, E.C.; Morrison, J.C. MicroRNA Expression in the Glaucomatous Retina. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7971–7982. [Google Scholar] [CrossRef]
- Romano, G.L.; Platania, C.B.; Forte, S.; Salomone, S.; Drago, F.; Bucolo, C. MicroRNA target prediction in glaucoma. Prog. Brain Res. 2015, 220, 217–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, F.; Wang, S. MicroRNA-93 is overexpressed and induces apoptosis in glaucoma trabecular meshwork cells. Mol. Med. Rep. 2016, 14, 5746–5750. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; He, M.; Cutrona, S.L.; Kiefe, C.I.; Liu, F.; Wang, Z. Theme Trends and Knowledge Structure on Mobile Health Apps: Bibliometric Analysis. JMIR Mhealth Uhealth 2020, 8, e18212. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wang, J.; Bucan, K.; Theodoratou, E.; Rudan, I.; Chan, K.Y. National and subnational prevalence and burden of glaucoma in China: A systematic analysis. J. Glob. Health 2017, 7, 020705. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Yang, D.; Tan, X.; Wang, H. Computational approaches for microRNA studies: A review. Mamm. Genome 2010, 21, 1–12. [Google Scholar] [CrossRef]
- Etheridge, A.; Lee, I.; Hood, L.; Galas, D.; Wang, K. Extracellular microRNA: A new source of biomarkers. Mutat. Res. 2011, 717, 85–90. [Google Scholar] [CrossRef]
- Bentwich, I.; Avniel, A.; Karov, Y.; Aharonov, R.; Gilad, S.; Barad, O.; Barzilai, A.; Einat, P.; Einav, U.; Meiri, E.; et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat. Genet. 2005, 37, 766–770. [Google Scholar] [CrossRef]
- Baek, D.; Villen, J.; Shin, C.; Camargo, F.D.; Gygi, S.P.; Bartel, D.P. The impact of microRNAs on protein output. Nature 2008, 455, 64–71. [Google Scholar] [CrossRef]
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Vernooy, S.Y.; Guo, M.; Hay, B.A. The Drosophila microRNA Mir-14 suppresses cell death and is required for normal fat metabolism. Curr. Biol. 2003, 13, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C. OncomiRs: The discovery and progress of microRNAs in cancers. Mol. Cancer 2007, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, J.; Hipfner, D.R.; Stark, A.; Russell, R.B.; Cohen, S.M. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 2003, 113, 25–36. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Chen, C.Z.; Li, L.; Lodish, H.F.; Bartel, D.P. MicroRNAs modulate hematopoietic lineage differentiation. Science 2004, 303, 83–86. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Sen, S. MicroRNA as Biomarkers and Diagnostics. J. Cell Physiol. 2016, 231, 25–30. [Google Scholar] [CrossRef]
- Ardekani, A.M.; Naeini, M.M. The Role of MicroRNAs in Human Diseases. Avicenna J. Med. Biotechnol. 2010, 2, 161–179. [Google Scholar]
- Shalgi, R.; Lieber, D.; Oren, M.; Pilpel, Y. Global and local architecture of the mammalian microRNA-transcription factor regulatory network. PLoS Comput. Biol. 2007, 3, e131. [Google Scholar] [CrossRef]
- Nelson, P.T.; Wang, W.X.; Rajeev, B.W. MicroRNAs (miRNAs) in neurodegenerative diseases. Brain Pathol. 2008, 18, 130–138. [Google Scholar] [CrossRef]
- Liang, H.; Li, W.H. MicroRNA regulation of human protein protein interaction network. RNA 2007, 13, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.P.; Lau, N.C.; Garrett-Engele, P.; Grimson, A.; Schelter, J.M.; Castle, J.; Bartel, D.P.; Linsley, P.S.; Johnson, J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005, 433, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Juzwik, C.A.; Drake, S.; Lecuyer, M.A.; Johnson, R.M.; Morquette, B.; Zhang, Y.; Charabati, M.; Sagan, S.M.; Bar-Or, A.; Prat, A.; et al. Neuronal microRNA regulation in Experimental Autoimmune Encephalomyelitis. Sci. Rep. 2018, 8, 13437. [Google Scholar] [CrossRef] [PubMed]
- Bredesen, D.E.; Rao, R.V.; Mehlen, P. Cell death in the nervous system. Nature 2006, 443, 796–802. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Maciotta, S.; Meregalli, M.; Torrente, Y. The involvement of microRNAs in neurodegenerative diseases. Front. Cell Neurosci. 2013, 7, 265. [Google Scholar] [CrossRef]
- Henshall, D.C.; Hamer, H.M.; Pasterkamp, R.J.; Goldstein, D.B.; Kjems, J.; Prehn, J.H.M.; Schorge, S.; Lamottke, K.; Rosenow, F. MicroRNAs in epilepsy: Pathophysiology and clinical utility. Lancet Neurol. 2016, 15, 1368–1376. [Google Scholar] [CrossRef]
- Swarbrick, S.; Wragg, N.; Ghosh, S.; Stolzing, A. Systematic Review of miRNA as Biomarkers in Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 6156–6167. [Google Scholar] [CrossRef]
- Dolati, S.; Marofi, F.; Babaloo, Z.; Aghebati-Maleki, L.; Roshangar, L.; Ahmadi, M.; Rikhtegar, R.; Yousefi, M. Dysregulated Network of miRNAs Involved in the Pathogenesis of Multiple Sclerosis. Biomed. Pharmacother. 2018, 104, 280–290. [Google Scholar] [CrossRef]
- Perdicchi, A.; Iester, M.; Iacovello, D.; Cutini, A.; Balestrieri, M.; Mutolo, M.G.; Ferreras, A.; Contestabile, M.T.; Recupero, S.M. Evaluation of Agreement between HRT III and iVue OCT in Glaucoma and Ocular Hypertension Patients. J. Ophthalmol. 2015, 2015, 691031. [Google Scholar] [CrossRef][Green Version]
- Kwon, Y.H.; Fingert, J.H.; Kuehn, M.H.; Alward, W.L. Primary open-angle glaucoma. N. Engl. J. Med. 2009, 360, 1113–1124. [Google Scholar] [CrossRef]
- Sommer, A. Intraocular pressure and glaucoma. Am. J. Ophthalmol. 1989, 107, 186–188. [Google Scholar] [CrossRef]
- Tielsch, J.M.; Sommer, A.; Katz, J.; Royall, R.M.; Quigley, H.A.; Javitt, J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA 1991, 266, 369–374. [Google Scholar] [CrossRef]
- Klein, B.E.; Klein, R.; Sponsel, W.E.; Franke, T.; Cantor, L.B.; Martone, J.; Menage, M.J. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology 1992, 99, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Smith, W.; Attebo, K.; Healey, P.R. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology 1996, 103, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Kovalyk, O.; Morales-Sanchez, J.; Verdu-Monedero, R.; Selles-Navarro, I.; Palazon-Cabanes, A.; Sancho-Gomez, J.L. PAPILA: Dataset with fundus images and clinical data of both eyes of the same patient for glaucoma assessment. Sci. Data 2022, 9, 291. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Ritch, R. Exfoliation syndrome-the most common identifiable cause of open-angle glaucoma. J. Glaucoma 1994, 3, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, B.; Tielsch, J.M.; Katz, J.; Gottsch, J.; Quigley, H.; Javitt, J.; Sommer, A. The cause-specific prevalence of visual impairment in an urban population. The Baltimore Eye Survey. Ophthalmology 1996, 103, 1721–1726. [Google Scholar] [CrossRef]
- Quigley, H.A.; Vitale, S. Models of open-angle glaucoma prevalence and incidence in the United States. Investig. Ophthalmol. Vis. Sci. 1997, 38, 83–91. [Google Scholar]
- Xu, B.Y.; Liang, S.; Pardeshi, A.A.; Lifton, J.; Moghimi, S.; Lewinger, J.P.; Varma, R. Differences in Ocular Biometric Measurements among Subtypes of Primary Angle Closure Disease: The Chinese American Eye Study. Ophthalmol. Glaucoma 2021, 4, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.S.; Wilson, M.R.; Liebmann, J.M.; Fechtner, R.D.; Weinreb, R.N. An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am. J. Ophthalmol. 2004, 138, S19–S31. [Google Scholar] [CrossRef] [PubMed]
- Leske, M.C.; Heijl, A.; Hussein, M.; Bengtsson, B.; Hyman, L.; Komaroff, E.; Early Manifest Glaucoma Trial, G. Factors for glaucoma progression and the effect of treatment: The early manifest glaucoma trial. Arch. Ophthalmol. 2003, 121, 48–56. [Google Scholar] [CrossRef]
- Lobo, J.; Gillis, A.J.M.; van den Berg, A.; Dorssers, L.C.J.; Belge, G.; Dieckmann, K.P.; Roest, H.P.; van der Laan, L.J.W.; Gietema, J.; Hamilton, R.J.; et al. Identification and Validation Model for Informative Liquid Biopsy-Based microRNA Biomarkers: Insights from Germ Cell Tumor In Vitro, In Vivo and Patient-Derived Data. Cells 2019, 8, 1637. [Google Scholar] [CrossRef]
- Callaghan, B.; Lester, K.; Lane, B.; Fan, X.; Goljanek-Whysall, K.; Simpson, D.A.; Sheridan, C.; Willoughby, C.E. Genome-wide transcriptome profiling of human trabecular meshwork cells treated with TGF-beta2. Sci. Rep. 2022, 12, 9564. [Google Scholar] [CrossRef]
- Seong, H.; Cho, H.K.; Kee, C.; Song, D.H.; Cho, M.C.; Kang, S.S. Profiles of microRNA in aqueous humor of normal tension glaucoma patients using RNA sequencing. Sci. Rep. 2021, 11, 19024. [Google Scholar] [CrossRef]
- Smyth, A.; Callaghan, B.; Willoughby, C.E.; O’Brien, C. The Role of miR-29 Family in TGF-beta Driven Fibrosis in Glaucomatous Optic Neuropathy. Int. J. Mol. Sci. 2022, 23, 10216. [Google Scholar] [CrossRef]
- Tektas, O.Y.; Lutjen-Drecoll, E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp. Eye Res. 2009, 88, 769–775. [Google Scholar] [CrossRef]
- Tabak, S.; Schreiber-Avissar, S.; Beit-Yannai, E. Crosstalk between MicroRNA and Oxidative Stress in Primary Open-Angle Glaucoma. Int. J. Mol. Sci. 2021, 22, 2421. [Google Scholar] [CrossRef]
- Vranka, J.A.; Kelley, M.J.; Acott, T.S.; Keller, K.E. Extracellular matrix in the trabecular meshwork: Intraocular pressure regulation and dysregulation in glaucoma. Exp. Eye Res. 2015, 133, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Medina-Ortiz, W.E.; Belmares, R.; Neubauer, S.; Wordinger, R.J.; Clark, A.F. Cellular fibronectin expression in human trabecular meshwork and induction by transforming growth factor-beta2. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6779–6788. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Millar, J.C.; Wang, W.H.; Silverman, S.M.; Liu, Y.; Wordinger, R.J.; Rubin, J.S.; Pang, I.H.; Clark, A.F. Existence of the canonical Wnt signaling pathway in the human trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7043–7051. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Tang, P.M.; Li, J.; Lan, H.Y. TGF-beta/Smad signaling in renal fibrosis. Front. Physiol. 2015, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Acott, T.S.; Kelley, M.J. Extracellular matrix in the trabecular meshwork. Exp. Eye Res. 2008, 86, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Tanito, M.; Ohira, A. Multiplex cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma, exfoliation glaucoma, and cataract. Investig. Ophthalmol. Vis. Sci. 2012, 53, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Pervan, C.L. Smad-independent TGF-beta2 signaling pathways in human trabecular meshwork cells. Exp. Eye Res. 2017, 158, 137–145. [Google Scholar] [CrossRef]
- Li, X.; Wang, J. Comparison of MicroRNA Expression in Aqueous Humor of Normal and Primary Open-Angle Glaucoma Patients Using PCR Arrays: A Pilot Study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4988. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Tian, Y.; Cao, Y.; Ma, Q.; Zhao, S. MiR-137 promotes cell growth and inhibits extracellular matrix protein expression in H2O2-induced human trabecular meshwork cells by targeting Src. Neurosci. Lett. 2021, 755, 135902. [Google Scholar] [CrossRef]
- Yin, R.; Chen, X. Regulatory effect of miR-144-3p on the function of human trabecular meshwork cells and fibronectin-1. Exp. Ther. Med. 2019, 18, 647–653. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Long, H.; Zhou, B.; Jiang, H. miR-486-5p Restrains Extracellular Matrix Production and Oxidative Damage in Human Trabecular Meshwork Cells by Targeting TGF-beta/SMAD2 Pathway. J. Ophthalmol. 2022, 2022, 3584192. [Google Scholar] [CrossRef] [PubMed]
- Maurer, B.; Stanczyk, J.; Jungel, A.; Akhmetshina, A.; Trenkmann, M.; Brock, M.; Kowal-Bielecka, O.; Gay, R.E.; Michel, B.A.; Distler, J.H.; et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010, 62, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Gallant-Behm, C.L.; Piper, J.; Lynch, J.M.; Seto, A.G.; Hong, S.J.; Mustoe, T.A.; Maari, C.; Pestano, L.A.; Dalby, C.M.; Jackson, A.L.; et al. A MicroRNA-29 Mimic (Remlarsen) Represses Extracellular Matrix Expression and Fibroplasia in the Skin. J. Investig. Dermatol. 2019, 139, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Junn, E.; Mouradian, M.M. MicroRNAs in neurodegenerative diseases and their therapeutic potential. Pharmacol. Ther. 2012, 133, 142–150. [Google Scholar] [CrossRef]
- Orom, U.A.; Kauppinen, S.; Lund, A.H. LNA-modified oligonucleotides mediate specific inhibition of microRNA function. Gene 2006, 372, 137–141. [Google Scholar] [CrossRef]
- Yue, X.; Yu, X.; Petersen, F.; Riemekasten, G. Recent advances in mouse models for systemic sclerosis. Autoimmun. Rev. 2018, 17, 1225–1234. [Google Scholar] [CrossRef]
- Tan, C.; Jia, F.; Zhang, P.; Sun, X.; Qiao, Y.; Chen, X.; Wang, Y.; Chen, J.; Lei, Y. A miRNA stabilizing polydopamine nano-platform for intraocular delivery of miR-21-5p in glaucoma therapy. J. Mater. Chem. B 2021, 9, 3335–3345. [Google Scholar] [CrossRef]
- Yu, Z.; Wen, Y.; Jiang, N.; Li, Z.; Guan, J.; Zhang, Y.; Deng, C.; Zhao, L.; Zheng, S.G.; Zhu, Y.; et al. TNF-alpha stimulation enhances the neuroprotective effects of gingival MSCs derived exosomes in retinal ischemia-reperfusion injury via the MEG3/miR-21a-5p axis. Biomaterials 2022, 284, 121484. [Google Scholar] [CrossRef]
- Su, W.; Li, Z.; Jia, Y.; Zhu, Y.; Cai, W.; Wan, P.; Zhang, Y.; Zheng, S.G.; Zhuo, Y. microRNA-21a-5p/PDCD4 axis regulates mesenchymal stem cell-induced neuroprotection in acute glaucoma. J. Mol. Cell Biol. 2017, 9, 289–301. [Google Scholar] [CrossRef]
- Nie, X.G.; Fan, D.S.; Huang, Y.X.; He, Y.Y.; Dong, B.L.; Gao, F. Downregulation of microRNA-149 in retinal ganglion cells suppresses apoptosis through activation of the PI3K/Akt signaling pathway in mice with glaucoma. Am. J. Physiol. Cell Physiol. 2018, 315, C839–C849. [Google Scholar] [CrossRef]
- Guo, J.; Liu, H.; Fu, L. MicroRNA-124 ameliorates autophagic dysregulation in glaucoma via regulation of P2X7-mediated Akt/mTOR signaling. Cutan. Ocul. Toxicol. 2022, 41, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Sun, Y.B.; Hao, J.L.; Lu, C.W.; Bi, M.C.; Song, E. Neuroprotective effects of overexpressed microRNA-200a on activation of glaucoma-related retinal glial cells and apoptosis of ganglion cells via downregulating FGF7-mediated MAPK signaling pathway. Cell Signal 2019, 54, 179–190. [Google Scholar] [CrossRef]
- Li, X.; Zhao, F.; Xin, M.; Li, G.; Luna, C.; Li, G.; Zhou, Q.; He, Y.; Yu, B.; Olson, E.; et al. Regulation of intraocular pressure by microRNA cluster miR-143/145. Sci. Rep. 2017, 7, 915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, C.; Li, R.; Ke, Y.; Sun, K.; Wang, J. miR-708 and miR-335-3p Inhibit the Apoptosis of Retinal Ganglion Cells Through Suppressing Autophagy. J. Mol. Neurosci. 2021, 71, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Ahmed, Z.; Tomarev, S. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Neuroprotection in a Genetic DBA/2J Mouse Model of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5473–5480. [Google Scholar] [CrossRef]
- Mak, H.K.; Yung, J.S.Y.; Weinreb, R.N.; Ng, S.H.; Cao, X.; Ho, T.Y.C.; Ng, T.K.; Chu, W.K.; Yung, W.H.; Choy, K.W.; et al. MicroRNA-19a-PTEN Axis Is Involved in the Developmental Decline of Axon Regenerative Capacity in Retinal Ganglion Cells. Mol. Ther. Nucleic Acids 2020, 21, 251–263. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Cui, H.; Yu, Y.B.; Shi, H.Q.; Zhou, Y.; Liu, M.J. MicroRNA-141-3p inhibits retinal neovascularization and retinal ganglion cell apoptosis in glaucoma mice through the inactivation of Docking protein 5-dependent mitogen-activated protein kinase signaling pathway. J. Cell Physiol. 2019, 234, 8873–8887. [Google Scholar] [CrossRef]
- Palazzo, A.F.; Lee, E.S. Non-coding RNA: What is functional and what is junk? Front. Genet. 2015, 6, 2. [Google Scholar] [CrossRef]
- Hombach, S.; Kretz, M. Non-coding RNAs: Classification, Biology and Functioning. Adv. Exp. Med. Biol. 2016, 937, 3–17. [Google Scholar] [CrossRef]
- Yu, C.Y.; Kuo, H.C. The emerging roles and functions of circular RNAs and their generation. J. Biomed. Sci. 2019, 26, 29. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, T.; Xiao, J. Circular RNAs: Promising Biomarkers for Human Diseases. eBioMedicine 2018, 34, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Verduci, L.; Strano, S.; Yarden, Y.; Blandino, G. The circRNA-microRNA code: Emerging implications for cancer diagnosis and treatment. Mol. Oncol. 2019, 13, 669–680. [Google Scholar] [CrossRef]
- Yang, L.; Han, B.; Zhang, Z.; Wang, S.; Bai, Y.; Zhang, Y.; Tang, Y.; Du, L.; Xu, L.; Wu, F.; et al. Extracellular Vesicle-Mediated Delivery of Circular RNA SCMH1 Promotes Functional Recovery in Rodent and Nonhuman Primate Ischemic Stroke Models. Circulation 2020, 142, 556–574. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Xu, M.; Zhou, J.; Kang, G.; Li, K. Circ_0080940 Regulates miR-139-5p/CTGF Pathway to Promote the Proliferation, Migration, Extracellular Matrix Deposition of Human Tenon’s Capsule Fibroblasts. Curr. Eye Res. 2023, 48, 34–43. [Google Scholar] [CrossRef]
- Moazzeni, H.; Khani, M.; Elahi, E. Insights into the regulatory molecules involved in glaucoma pathogenesis. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 782–827. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, J.; Yu, Y. CircRNA Is a Rising Star in Researches of Ocular Diseases. Front. Cell Dev. Biol. 2020, 8, 850. [Google Scholar] [CrossRef]
- Charteris, D.G.; Sethi, C.S.; Lewis, G.P.; Fisher, S.K. Proliferative vitreoretinopathy-developments in adjunctive treatment and retinal pathology. Eye 2002, 16, 369–374. [Google Scholar] [CrossRef]
- Liu, C.; Yao, M.D.; Li, C.P.; Shan, K.; Yang, H.; Wang, J.J.; Liu, B.; Li, X.M.; Yao, J.; Jiang, Q.; et al. Silencing of Circular RNA-ZNF609 Ameliorates Vascular Endothelial Dysfunction. Theranostics 2017, 7, 2863–2877. [Google Scholar] [CrossRef]
- Wang, J.J.; Liu, C.; Shan, K.; Liu, B.H.; Li, X.M.; Zhang, S.J.; Zhou, R.M.; Dong, R.; Yan, B.; Sun, X.H. Circular RNA-ZNF609 regulates retinal neurodegeneration by acting as miR-615 sponge. Theranostics 2018, 8, 3408–3415. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, R.; Shan, K.; Sun, Y.; Yan, B.; Sun, X.; Wang, J. Circular RNA Expression Profiling Identifies Glaucoma-Related Circular RNAs in Various Chronic Ocular Hypertension Rat Models. Front. Genet. 2020, 11, 556712. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Shan, K.; Liu, B.H.; Liu, C.; Zhou, R.M.; Li, X.M.; Dong, R.; Zhang, S.J.; Zhang, S.H.; Wu, J.H.; et al. Targeting circular RNA-ZRANB1 for therapeutic intervention in retinal neurodegeneration. Cell Death Dis. 2018, 9, 540. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lee, Y.C.; Chang, K.L.; Hsiao, K.Y. Analysis of common targets for circular RNAs. BMC Bioinform. 2019, 20, 372. [Google Scholar] [CrossRef]
- Ma, C.; Yao, M.D.; Han, X.Y.; Shi, Z.H.; Yan, B.; Du, J.L. Silencing of circular RNA-ZYG11B exerts a neuroprotective effect against retinal neurodegeneration. Int. J. Mol. Med. 2022, 50, 106. [Google Scholar] [CrossRef]
- Chen, L.L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020, 21, 475–490. [Google Scholar] [CrossRef]
- Han, B.; Chao, J.; Yao, H. Circular RNA and its mechanisms in disease: From the bench to the clinic. Pharmacol. Ther. 2018, 187, 31–44. [Google Scholar] [CrossRef]
- Lee, E.C.S.; Elhassan, S.A.M.; Lim, G.P.L.; Kok, W.H.; Tan, S.W.; Leong, E.N.; Tan, S.H.; Chan, E.W.L.; Bhattamisra, S.K.; Rajendran, R.; et al. The roles of circular RNAs in human development and diseases. Biomed. Pharmacother. 2019, 111, 198–208. [Google Scholar] [CrossRef]
- Silva, A.; Bullock, M.; Calin, G. The Clinical Relevance of Long Non-Coding RNAs in Cancer. Cancers 2015, 7, 2169–2182. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Wang, Y.; Gao, J.; Lv, J.; Sun, J.; Li, M.; Wang, M.; Zhao, Z.; Wang, J.; et al. Long non-coding RNAs in ocular diseases: New and potential therapeutic targets. FEBS J. 2019, 286, 2261–2272. [Google Scholar] [CrossRef]
- Xie, L.; Mao, M.; Wang, C.; Zhang, L.; Pan, Z.; Shi, J.; Duan, X.; Jia, S.; Jiang, B. Potential Biomarkers for Primary Open-Angle Glaucoma Identified by Long Noncoding RNA Profiling in the Aqueous Humor. Am. J. Pathol. 2019, 189, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, L.R.; Loomis, S.J.; Kang, J.H.; Yaspan, B.L.; Abdrabou, W.; Budenz, D.L.; Chen, T.C.; Delbono, E.; Friedman, D.S.; Gaasterland, D.; et al. CDKN2B-AS1 genotype-glaucoma feature correlations in primary open-angle glaucoma patients from the United States. Am. J. Ophthalmol. 2013, 155, 342–353 e345. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Tam, A.L.C.; Campbell, R.; Renwick, N. Emerging Evidence of Noncoding RNAs in Bleb Scarring after Glaucoma Filtration Surgery. Cells 2022, 11, 1301. [Google Scholar] [CrossRef]
- Militello, G.; Weirick, T.; John, D.; Doring, C.; Dimmeler, S.; Uchida, S. Screening and validation of lncRNAs and circRNAs as miRNA sponges. Brief. Bioinform. 2017, 18, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Smillie, C.L.; Sirey, T.; Ponting, C.P. Complexities of post-transcriptional regulation and the modeling of ceRNA crosstalk. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 231–245. [Google Scholar] [CrossRef]
- Yan, Z.; Lai, M.; Jia, Y.; Deng, C.; Zhuo, Y. CircXPO5 Plays a Neuroprotective Function in the Lateral Geniculate Nucleus of Glaucoma by Regulating GRIN2A. Brain Sci. 2022, 12, 780. [Google Scholar] [CrossRef]
- Li, F.; Huang, C.; Li, Q.; Wu, X. Construction and Comprehensive Analysis for Dysregulated Long Non-Coding RNA (lncRNA)-Associated Competing Endogenous RNA (ceRNA) Network in Gastric Cancer. Med. Sci. Monit. 2018, 24, 37–49. [Google Scholar] [CrossRef]
- Huang, Y.; Xiang, B.; Liu, Y.; Wang, Y.; Kan, H. LncRNA CDKN2B-AS1 promotes tumor growth and metastasis of human hepatocellular carcinoma by targeting let-7c-5p/NAP1L1 axis. Cancer Lett. 2018, 437, 56–66. [Google Scholar] [CrossRef]
- Paraskevopoulou, M.D.; Hatzigeorgiou, A.G. Analyzing MiRNA-LncRNA Interactions. Methods Mol. Biol. 2016, 1402, 271–286. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, Z.; Xing, X.; Liu, A. lncRNA TGFbeta2-AS1 promotes ECM production via TGF-beta2 in human trabecular meshwork cells. Biochem. Biophys. Res. Commun. 2020, 527, 881–888. [Google Scholar] [CrossRef]
- Su, Y.; Yi, Y.; Li, L.; Chen, C. circRNA-miRNA-mRNA network in age-related macular degeneration: From construction to identification. Exp. Eye Res. 2021, 203, 108427. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, T.; Zhang, X.; Cai, X.; Sun, H. Network Integration Analysis and Immune Infiltration Analysis Reveal Potential Biomarkers for Primary Open-Angle Glaucoma. Front. Cell Dev. Biol. 2021, 9, 793638. [Google Scholar] [CrossRef]
- Li, H.; Ye, Z.; Li, Z. Identification of the potential biological target molecules related to primary open-angle glaucoma. BMC Ophthalmol. 2022, 22, 188. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Wang, T.; Zhou, F.; Yao, Y.; He, J.; Xu, D. Bioinformatics-based identification of miRNA-, lncRNA-, and mRNA-associated ceRNA networks and potential biomarkers for preeclampsia. Medicine 2020, 99, e22985. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Wan, Q.; Huang, J.; Han, L.; Chen, X.; Chen, G.; Olsen, N.; Zheng, S.G.; Liang, D. Culture medium from TNF-alpha-stimulated mesenchymal stem cells attenuates allergic conjunctivitis through multiple antiallergic mechanisms. J. Allergy Clin. Immunol. 2015, 136, 423–432 e428. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Tomarev, S. Extracellular vesicle therapy for retinal diseases. Prog. Retin. Eye Res. 2020, 79, 100849. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jankovic, M.G.; Fellabaum, C.; Volarevic, A.; Djonov, V.; Arsenijevic, A.; Volarevic, V. Molecular Mechanisms Responsible for Anti-inflammatory and Immunosuppressive Effects of Mesenchymal Stem Cell-Derived Factors. Adv. Exp. Med. Biol. 2019, 1084, 187–206. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Elahi, F.M.; Farwell, D.G.; Nolta, J.A.; Anderson, J.D. Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem Cells 2020, 38, 15–21. [Google Scholar] [CrossRef]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Cantaluppi, V.; Biancone, L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010, 78, 838–848. [Google Scholar] [CrossRef]
- Otero-Ortega, L.; Laso-Garcia, F.; Gomez-de Frutos, M.; Fuentes, B.; Diekhorst, L.; Diez-Tejedor, E.; Gutierrez-Fernandez, M. Role of Exosomes as a Treatment and Potential Biomarker for Stroke. Transl. Stroke Res. 2019, 10, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, H.; Ong, H.S.; Riau, A.K.; Stanzel, T.P.; Mehta, J.S.; Yam, G.H. Current Trends and Future Perspective of Mesenchymal Stem Cells and Exosomes in Corneal Diseases. Int. J. Mol. Sci. 2019, 20, 2853. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Amaral, J.; Tomarev, S. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Neuroprotection in Rodent Models of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Chen, Z.; Zheng, M.; Liu, Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 2017, 49, e346. [Google Scholar] [CrossRef]
- Shi, M.; Liu, C.; Cook, T.J.; Bullock, K.M.; Zhao, Y.; Ginghina, C.; Li, Y.; Aro, P.; Dator, R.; He, C.; et al. Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014, 128, 639–650. [Google Scholar] [CrossRef]
- Garcia-Contreras, M.; Brooks, R.W.; Boccuzzi, L.; Robbins, P.D.; Ricordi, C. Exosomes as biomarkers and therapeutic tools for type 1 diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2940–2956. [Google Scholar]
- Zhang, Q.; Sun, J.; Huang, Y.; Bu, S.; Guo, Y.; Gu, T.; Li, B.; Wang, C.; Lai, D. Human Amniotic Epithelial Cell-Derived Exosomes Restore Ovarian Function by Transferring MicroRNAs against Apoptosis. Mol. Ther. Nucleic Acids 2019, 16, 407–418. [Google Scholar] [CrossRef]
- Chen, Q.L.; Xie, C.F.; Feng, K.L.; Cui, D.Y.; Sun, S.L.; Zhang, J.C.; Xiong, C.M.; Huang, J.H.; Chong, Z. microRNAs carried by exosomes promote epithelial-mesenchymal transition and metastasis of liver cancer cells. Am. J. Transl. Res. 2020, 12, 6811–6826. [Google Scholar]
- Kosaka, N.; Yoshioka, Y.; Fujita, Y.; Ochiya, T. Versatile roles of extracellular vesicles in cancer. J. Clin. Investig. 2016, 126, 1163–1172. [Google Scholar] [CrossRef]
- Chen, T.S.; Lai, R.C.; Lee, M.M.; Choo, A.B.; Lee, C.N.; Lim, S.K. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010, 38, 215–224. [Google Scholar] [CrossRef]
- Katsuda, T.; Ochiya, T. Molecular signatures of mesenchymal stem cell-derived extracellular vesicle-mediated tissue repair. Stem Cell Res. Ther. 2015, 6, 212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chopp, M.; Liu, X.S.; Katakowski, M.; Wang, X.; Tian, X.; Wu, D.; Zhang, Z.G. Exosomes Derived from Mesenchymal Stromal Cells Promote Axonal Growth of Cortical Neurons. Mol. Neurobiol. 2017, 54, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Synnestvedt, M.B.; Chen, C.; Holmes, J.H. CiteSpace II: Visualization and knowledge discovery in bibliographic databases. AMIA Annu. Symp. Proc. 2005, 2005, 724–728. [Google Scholar] [PubMed]



| Rank | Journal | Publications | Citations | Average Citations | IF (2022) | Co-Cited Journal | Co-Citations | IF (2022) |
|---|---|---|---|---|---|---|---|---|
| 1 | Investigative Ophthalmology and Visual Science | 23 | 750 | 32.6 | 4.4 | Investigative Ophthalmology and Visual Science | 1107 | 4.4 |
| 2 | Experimental Eye Research | 10 | 211 | 21.1 | 3.4 | Experimental Eye Research | 414 | 3.4 |
| 3 | Scientific Reports | 6 | 115 | 19.2 | 4.6 | PloS One | 332 | 3.7 |
| 4 | Molecular Medicine Reports | 5 | 56 | 11.2 | 3.4 | Molecular Vision | 249 | 2.2 |
| 5 | International Journal of Molecular Sciences | 5 | 16 | 3.2 | 5.6 | Scientific Reports | 197 | 4.6 |
| 6 | Journal of Cellular Physiology | 4 | 107 | 26.8 | 5.6 | Journal of Biological Chemistry | 190 | 4.8 |
| 7 | Biomedicine and Pharmacotherapy | 4 | 86 | 21.5 | 7.5 | Proceedings of the National Academy of Sciences of the United States of America | 175 | 11.1 |
| 8 | International Journal of Molecular Medicine | 4 | 25 | 6.3 | 5.4 | Progress in Retinal and Eye Research | 151 | 17.8 |
| 9 | Molecular Vision | 3 | 157 | 52.3 | 2.2 | Nucleic Acids Research | 138 | 14.9 |
| 10 | PloS One | 3 | 100 | 33.3 | 3.7 | Human Molecular Genetics | 137 | 3.5 |
| Rank | Author | Publications | Citations | Average Citations | Country | Co-Cited Author | Co-Citations | Country |
|---|---|---|---|---|---|---|---|---|
| 1 | Pedro Gonzalez | 6 | 300 | 50.0 | USA | Coralia Luna | 109 | USA |
| 2 | Coralia Luna | 6 | 300 | 50.0 | USA | Harry A.Quigley | 56 | USA |
| 3 | Xinghuai Sun | 6 | 117 | 19.5 | China | Ben Mead | 54 | Wales |
| 4 | Guorong Li | 5 | 297 | 59.4 | China | Guorong Li | 50 | China |
| 5 | Yutao Liu | 5 | 177 | 35.4 | USA | Hari Jayaram | 46 | UK |
| 6 | William Daniel Stamer | 5 | 145 | 29.0 | USA | Yutao Liu | 39 | USA |
| 7 | David Lee Epstein | 4 | 326 | 81.5 | USA | Yuji Tanaka | 38 | Japan |
| 8 | Biao Yan | 4 | 91 | 22.8 | China | Robert N.Weinreb | 35 | USA |
| 9 | Pratap Challa | 3 | 126 | 42.0 | USA | Guadalupe Villarreal | 34 | USA |
| 10 | Michelle Dianne Drewry | 3 | 97 | 32.3 | USA | Rudolf Fuchshofer | 30 | Germany |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Tao, Y.; Huang, J. The Application of MicroRNAs in Glaucoma Research: A Bibliometric and Visualized Analysis. Int. J. Mol. Sci. 2023, 24, 15377. https://doi.org/10.3390/ijms242015377
Zhang R, Tao Y, Huang J. The Application of MicroRNAs in Glaucoma Research: A Bibliometric and Visualized Analysis. International Journal of Molecular Sciences. 2023; 24(20):15377. https://doi.org/10.3390/ijms242015377
Chicago/Turabian StyleZhang, Ruqi, Yuanyuan Tao, and Jufang Huang. 2023. "The Application of MicroRNAs in Glaucoma Research: A Bibliometric and Visualized Analysis" International Journal of Molecular Sciences 24, no. 20: 15377. https://doi.org/10.3390/ijms242015377
APA StyleZhang, R., Tao, Y., & Huang, J. (2023). The Application of MicroRNAs in Glaucoma Research: A Bibliometric and Visualized Analysis. International Journal of Molecular Sciences, 24(20), 15377. https://doi.org/10.3390/ijms242015377








