Dopamine-Coated Carbon Nanodots: A Supramolecular Approach to Polydopamine Composite
Abstract
:1. Introduction
2. Results
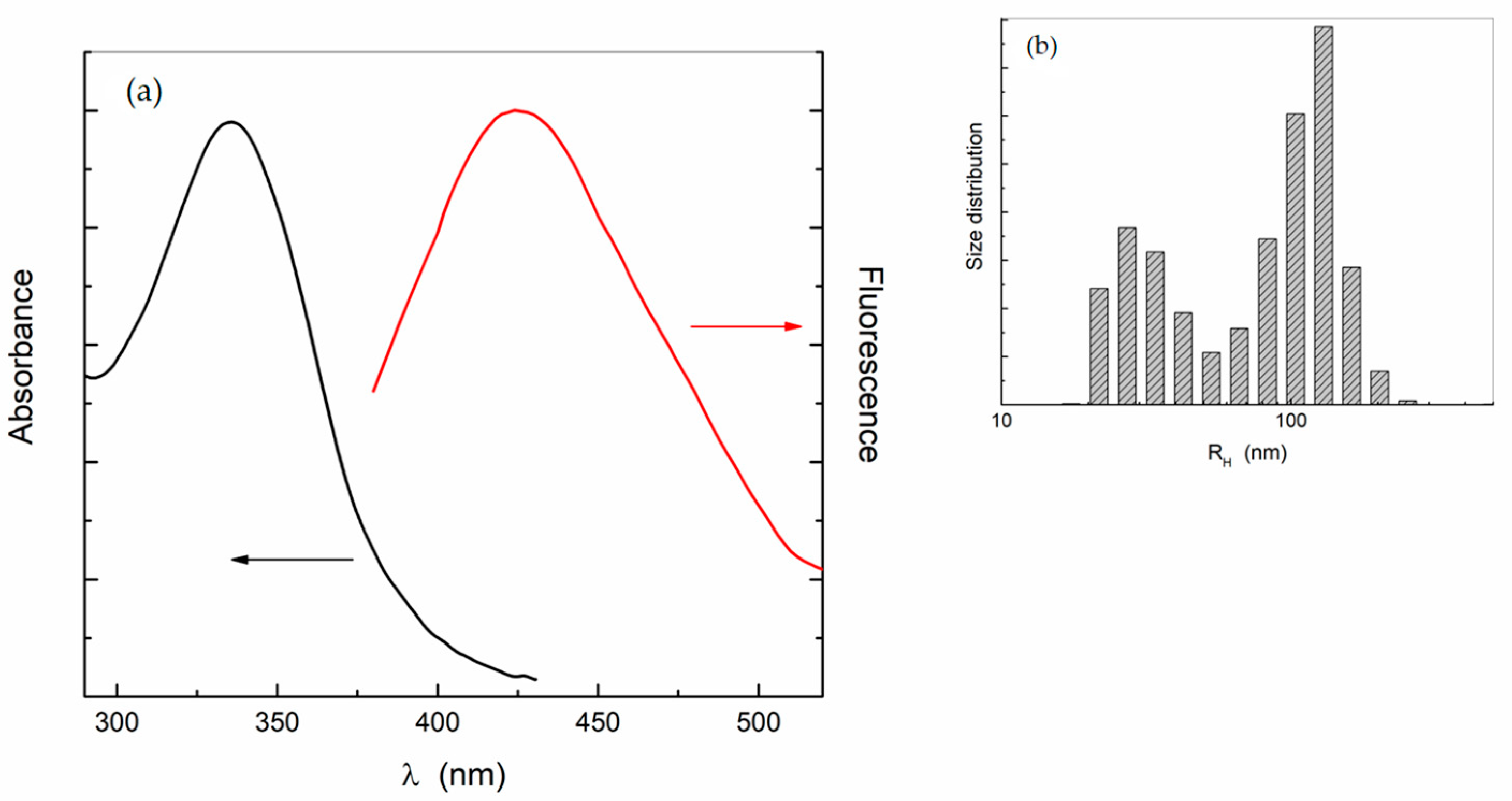
2.1. Carbon Nanodots Water Dispersion
2.2. Dopamine Aqueous Solutions
2.3. Dopamine–Nanodots Complexes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. Instrument Set-Up
4.4. Static and Dynamic Fluorescence Quenching
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of Polydopamine: A Never-Ending Story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [PubMed]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-Covalent Self-Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- d’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C.-T.; Buehler, M.J. Polydopamine and Eumelanin: From Structure–Property Relationships to a Unified Tailoring Strategy. Acc. Chem. Res. 2014, 47, 3541–3550. [Google Scholar] [CrossRef]
- Saiz-Poseu, J.; Mancebo-Aracil, J.; Nador, F.; Busqué, F.; Ruiz-Molina, D. The Chemistry behind Catechol-Based Adhesion. Angew. Chem. Int. Ed. 2019, 58, 696–714. [Google Scholar] [CrossRef]
- Khan, Z.; Shanker, R.; Um, D.; Jaiswal, A.; Ko, H. Bioinspired Polydopamine and Composites for Biomedical Applications. In Electrically Conductive Polymer and Polymer Composites: From Synthesis to Biomedical Applcations; Khan, A., Jawaid, M., Khan, A.A.P., Asiri, A.M., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2018; pp. 1–29. [Google Scholar]
- Ball, V. Polydopamine Nanomaterials: Recent Advances in Synthesis Methods and Applications. Front. Bioeng. Biotechnol. 2018, 6, 109. [Google Scholar] [CrossRef]
- Chen, R.; Lin, B.; Luo, R. Recent progress in polydopamine-based composites for the adsorption and degradation of industrial wastewater treatment. Heliyon 2022, 8, e12105. [Google Scholar] [CrossRef]
- Fredj, Z.; Sawan, M. Advanced Nanomaterials-Based Electrochemical Biosensors for Catecholamines Detection: Challenges and Trends. Biosensors 2023, 13, 211. [Google Scholar] [CrossRef]
- Yang, X.; Tian, F.; Wen, S.; Xu, H.; Zhang, L.; Zeng, J. Selective Determination of Dopamine in Pharmaceuticals and Human Urine Using Carbon Quantum Dots as a Fluorescent Probe. Processes 2021, 9, 170. [Google Scholar] [CrossRef]
- Naik, V.; Zantye, P.; Gunjal, D.; Gore, A.; Anbhule, P.; Kowshik, M.; Bhosale, S.V.; Kolekar, G. Nitrogen-Doped Carbon Dots via Hydrothermal Synthesis: Naked Eye Fluorescent Sensor for Dopamine and Used for Multicolor Cell Imaging. ACS Appl. Bio Mater. 2019, 2, 2069–2077. [Google Scholar] [CrossRef]
- Xu, J.; Tao, J.; Su, L.; Wang, J.; Jiao, T. A Critical Review of Carbon Quantum Dots: From Synthesis toward Applications in Electrochemical Biosensors for the Determination of a Depression-Related Neurotransmitter. Materials 2021, 14, 3987. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.-D.; Shi, L.; Guo, L.-H.; Wang, J.-W.; Zhang, X. Determination of dopamine hydrochloride by host-guest interaction based on water-soluble pillar[5]arene. Spectrochim. Acta Part A 2017, 173, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-X.; Ma, C.-P.; Gao, A.-J.; Wang, Y. Modification of Carbon Fiber Surfaces Using Dopamine. Fibers Polym. 2023, 24, 1003–1014. [Google Scholar] [CrossRef]
- Jayasekhar Babu, P.; Saranya, S.; Singh, Y.D.; Venkataswamy, M.; Raichur, A.M.; Doble, M. Photoluminescence carbon nano dots for the conductivity based optical sensing of dopamine and bioimaging applications. Opt. Mater. 2021, 117, 111120. [Google Scholar] [CrossRef]
- Anju; Rais, A.; Rawat, K.; Prasad, T.; Bohidar, H.B. Boron-doped carbon quantum dots: A ‘turn-off’ fluorescent probe for dopamine detection. Nanotechnology 2020, 32, 025501. [Google Scholar] [CrossRef]
- Thondaiman, P.; Manikandan, R.; Raj, C.J.; Savariraj, A.D.; Moulton, S.E.; Kim, B.C. Boron and nitrogen doped graphene quantum dots on a surface modified Cu mesh for the determination of dopamine and epinephrine. Synth. Met. 2021, 278, 116831. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Yang, M.; Yao, Z.; Zhang, B.; Liu, E.; Hu, X.; Xue, W.; Fan, J. Biocompatible sulfur nitrogen co-doped carbon quantum dots for highly sensitive and selective detection of dopamine. Colloids Surf. B 2021, 205, 111874. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Li, L.; Jiang, M.; Yang, X.; Yu, X.; Xu, L. One-pot synthesis of boron and nitrogen co-doped silicon-carbon dots for fluorescence enhancement and on-site colorimetric detection of dopamine with high selectivity. Appl. Surf. Sci. 2022, 573, 151457. [Google Scholar] [CrossRef]
- Bharathi, D.; Siddlingeshwar, B.; Krishna, R.H.; Singh, V.; Kottam, N.; Divakar, D.D.; Alkheraif, A.A. Green and Cost Effective Synthesis of Fluorescent Carbon Quantum Dots for Dopamine Detection. J. Fluoresc. 2018, 28, 573–579. [Google Scholar] [CrossRef]
- Liu, X.; Yu, W.; Mu, X.; Zhang, W.; Wang, X.; Gu, Q. A fluorescence probe based on carbon dots for determination of dopamine utilizing its self-polymerization. Spectrochim. Acta Part A 2023, 287, 122112. [Google Scholar] [CrossRef]
- Gu, B.; Pu, G.; Ding, B.; Zhang, K.; He, R.; Fan, J.; Xing, T.; Wu, J.; Yang, W. Improved interfacial bonding strength of silicone rubber/carbon fiber modified by dopamine. Polym. Compos. 2022, 43, 6975–6986. [Google Scholar] [CrossRef]
- Tian, X.; Han, S.; Zhuang, Q.; Bian, H.; Li, S.; Zhang, C.; Wang, C.; Han, W. Surface Modification of Staple Carbon Fiber by Dopamine to Reinforce Natural Latex Composite. Polymers 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Song, J.H. Improvement in the mechanical properties of carbon and aramid composites by fiber surface modification using polydopamine. Compos. Part B 2019, 160, 31–36. [Google Scholar] [CrossRef]
- Liu, Y.; Su, C.; Zu, Y.; Chen, X.; Sha, J.; Dai, J. Ultrafast deposition of polydopamine for high-performance fiber-reinforced high-temperature ceramic composites. Sci. Rep. 2022, 12, 20489. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Tian, J.; An, L.; Jiao, Y.; Yin, Q.; Tan, Y. Study on the Construction of Dopamine/Poly(ethyleneimine)/Aminoated Carbon Nanotube Multilayer Films on Aramid Fiber Surfaces to Improve the Mechanical Properties of Aramid Fibers/Epoxy Composites. ACS Omega 2022, 7, 35610–35625. [Google Scholar] [CrossRef]
- An, T.; Lee, N.; Cho, H.-J.; Kim, S.; Shin, D.-S.; Lee, S.-M. Ultra-selective detection of Fe2+ ion by redox mechanism based on fluorescent polymerized dopamine derivatives. RSC Adv. 2017, 7, 30582–30587. [Google Scholar] [CrossRef]
- Bisaglia, M.; Mammi, S.; Bubacco, L. Kinetic and Structural Analysis of the Early Oxidation Products of Dopamine. J. Biol. Chem. 2007, 282, 15597–15605. [Google Scholar] [CrossRef]
- Barreto, W.J.; Barreto, S.R.G.; Santos, M.A.; Schimidt, R.; Paschoal, F.M.M.; Mangrich, A.S.; deOliveira, L.F.C. Interruption of the MnO2 oxidative process on dopamine and l-dopa by the action of S2O32−. J. Inorg. Biochem. 2001, 84, 89–96. [Google Scholar] [CrossRef]
- Salomäki, M.; Marttila, L.; Kivelä, H.; Ouvinen, T.; Lukkari, J. Effects of pH and Oxidants on the First Steps of Polydopamine Formation: A Thermodynamic Approach. J. Phys. Chem. B 2018, 122, 6314–6327. [Google Scholar] [CrossRef]
- Jha, O.; Yadav, T.K.; Yadav, R.A. Structural and vibrational study of a neurotransmitter molecule: Dopamine [4-(2-aminoethyl) benzene-1,2-diol]. Spectrochim. Acta Part A 2018, 189, 473–484. [Google Scholar] [CrossRef]
- Figueiredo, M.L.B.; Martin, C.S.; Furini, L.N.; Rubira, R.J.G.; Batagin-Neto, A.; Alessio, P.; Constantino, C.J.L. Surface-enhanced Raman scattering for dopamine in Ag colloid: Adsorption mechanism and detection in the presence of interfering species. Appl. Surf. Sci. 2020, 522, 146466. [Google Scholar] [CrossRef]
- Barreto, W.J.; Ponzoni, S.; Sassi, P. A Raman and UV-Vis study of catecholamines oxidized with Mn(III). Spectrochim. Acta Part A 1999, 55, 65–72. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Kumar, R.T.; Ponnusamy, S. Vibrational spectra and normal coordinate analysis of adrenaline and dopamine. Indian J. Pure Appl. Phys. 2007, 45, 884–892. [Google Scholar]
- Villari, V.; Gaeta, M.; D’Urso, A.; Micali, N. Porphyrin/carbon nanodot supramolecular complexes and their optical properties. Colloids Surf. A 2022, 648, 129436. [Google Scholar] [CrossRef]
- Cadranel, A.; Strauss, V.; Margraf, J.T.; Winterfeld, K.A.; Vogl, C.; Đorđević, L.; Arcudi, F.; Hoelzel, H.; Jux, N.; Prato, M.; et al. Screening Supramolecular Interactions between Carbon Nanodots and Porphyrins. J. Am. Chem. Soc. 2018, 140, 904–907. [Google Scholar] [CrossRef]
- Phua, S.L.; Yang, L.; Huang, S.; Ding, G.; Zhou, R.; Lew, J.H.; Lau, S.K.; Yuan, X.; Lu, X. Shape memory polyurethane with polydopamine-coated nanosheets: Simultaneous enhancement of recovery stress and strain recovery ratio and the underlying mechanisms. Eur. Polym. J. 2014, 57, 11–21. [Google Scholar] [CrossRef]
- Phua, S.L.; Yang, L.; Toh, C.L.; Huang, S.; Tsakadze, Z.; Lau, S.K.; Mai, Y.-W.; Lu, X. Reinforcement of Polyether Polyurethane with Dopamine-Modified Clay: The Role of Interfacial Hydrogen Bonding. ACS Appl. Mater. Interfaces 2012, 4, 4571–4578. [Google Scholar] [CrossRef]
- Mian, S.A.; Saha, L.C.; Jang, J.; Wang, L.; Gao, X.; Nagase, S. Density Functional Theory Study of Catechol Adhesion on Silica Surfaces. J. Phys. Chem. C 2010, 114, 20793–20800. [Google Scholar] [CrossRef]
- Mian, S.A.; Yang, L.-M.; Saha, L.C.; Ahmed, E.; Ajmal, M.; Ganz, E. A Fundamental Understanding of Catechol and Water Adsorption on a Hydrophilic Silica Surface: Exploring the Underwater Adhesion Mechanism of Mussels on an Atomic Scale. Langmuir 2014, 30, 6906–6914. [Google Scholar] [CrossRef]
- Alfieri, M.; Panzella, L.; Oscurato, S.; Salvatore, M.; Avolio, R.; Errico, M.; Maddalena, P.; Napolitano, A.; d’Ischia, M. The Chemistry of Polydopamine Film Formation: The Amine-Quinone Interplay. Biomimetics 2018, 3, 26. [Google Scholar] [CrossRef]
- Lim, C.; Huang, J.; Kim, S.; Lee, H.; Zeng, H.; Hwang, D.S. Nanomechanics of Poly(catecholamine) Coatings in Aqueous Solutions. Angew. Chem. Int. Ed. 2016, 55, 3342–3346. [Google Scholar] [CrossRef] [PubMed]
- Villari, V.; Micali, N. Light Scattering as Spectroscopic Tool for the Study of Disperse Systems Useful in Pharmaceutical Sciences. J. Pharm. Sci. 2008, 97, 1703–1730. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.F.; Schätzel, K.; Vincent, B. The determination of very small electrophoretic mobilities in polar and nonpolar colloidal dispersions using phase analysis light scattering. J. Colloid Interface Sci. 1991, 143, 532–554. [Google Scholar] [CrossRef]
- Angelini, N.; Micali, N.; Villari, V.; Mineo, P.; Vitalini, D.; Scamporrino, E. Interactions between water soluble porphyrin-based star polymer and amino acids: Spectroscopic evidence of molecular binding. Phys. Rev. E 2005, 71, 021915. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicosia, A.; Mineo, P.; Micali, N.; Villari, V. Dopamine-Coated Carbon Nanodots: A Supramolecular Approach to Polydopamine Composite. Int. J. Mol. Sci. 2023, 24, 15384. https://doi.org/10.3390/ijms242015384
Nicosia A, Mineo P, Micali N, Villari V. Dopamine-Coated Carbon Nanodots: A Supramolecular Approach to Polydopamine Composite. International Journal of Molecular Sciences. 2023; 24(20):15384. https://doi.org/10.3390/ijms242015384
Chicago/Turabian StyleNicosia, Angelo, Placido Mineo, Norberto Micali, and Valentina Villari. 2023. "Dopamine-Coated Carbon Nanodots: A Supramolecular Approach to Polydopamine Composite" International Journal of Molecular Sciences 24, no. 20: 15384. https://doi.org/10.3390/ijms242015384
APA StyleNicosia, A., Mineo, P., Micali, N., & Villari, V. (2023). Dopamine-Coated Carbon Nanodots: A Supramolecular Approach to Polydopamine Composite. International Journal of Molecular Sciences, 24(20), 15384. https://doi.org/10.3390/ijms242015384








