Renal Organic Anion Transporters 1 and 3 In Vitro: Gone but Not Forgotten
Abstract
:1. Introduction
2. Cellular and Physiological Regulation of OAT1 and OAT3 Expression and Activity
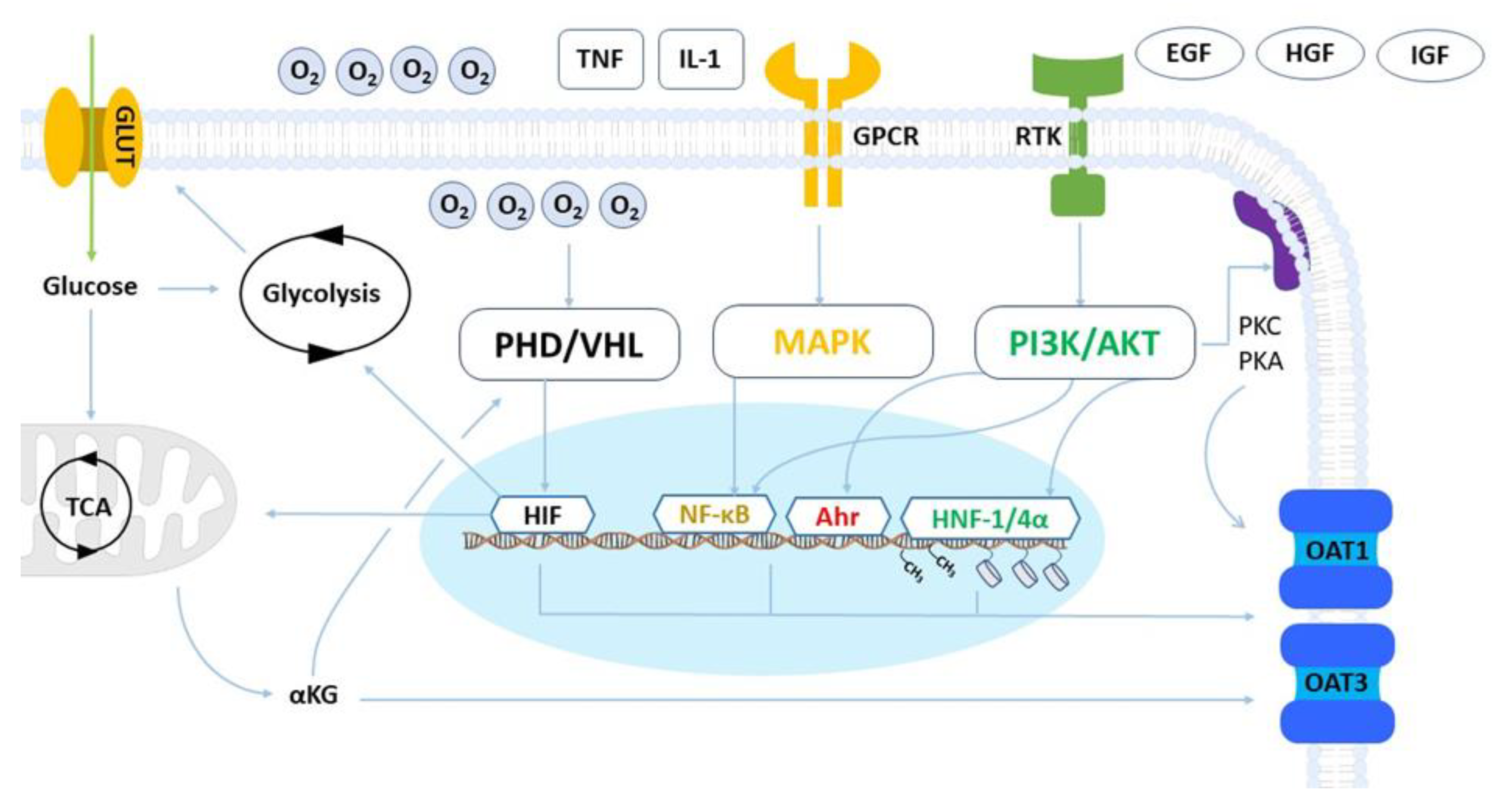
2.1. Metabolism and Hypoxia
2.2. Inflammatory and Growth Factors
2.3. MicroRNAs
2.4. Epigenetic Modifications
2.5. Cellular Adhesion
2.6. Post-Translational Regulation and Trafficking
3. OAT1 and OAT3 Expression in Kidney Cancer
4. Considerations for the Functional Loss of OAT1 and OAT3
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, W.; Bush, K.T.; Nigam, S.K. Key Role for the Organic Anion Transporters, OAT1 and OAT3, in the in vivo Handling of Uremic Toxins and Solutes. Sci. Rep. 2017, 7, 4939. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.K. What do drug transporters really do? Nat. Rev. Drug Discov. 2014, 14, 29–44. [Google Scholar] [CrossRef]
- Motohashi, H.; Sakurai, Y.; Saito, H.; Masuda, S.; Urakami, Y.; Goto, M.; Fukatsu, A.; Ogawa, O.; Inui, K.I. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J. Am. Soc. Nephrol. 2002, 13, 866–874. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Fan, Y.; Yu, Z.; You, G. Regulation of organic anion transporters: Role in physiology, pathophysiology, and drug elimination. Pharmacol. Ther. 2021, 217, 107647. [Google Scholar] [CrossRef]
- Granados, J.C.; Richelle, A.; Gutierrez, J.M.; Zhang, P.; Zhang, X.; Bhatnagar, V.; Lewis, N.E.; Nigam, S.K. Coordinate regulation of systemic and kidney tryptophan metabolism by the drug transporters OAT1 and OAT3. J. Biol. Chem. 2021, 296, 100575. [Google Scholar] [CrossRef] [PubMed]
- Hagos, Y.; Wolff, N.A. Assessment of the role of renal organic anion transporters in drug-induced nephrotoxicity. Toxins 2010, 2, 2055–2082. [Google Scholar] [CrossRef]
- Caetano-Pinto, P.; Justian, N.; Dib, M.; Fischer, J.; Somova, M.; Burchardt, M.; Wolff, I. In Vitro Characterization of Renal Drug Transporter Activity in Kidney Cancer. Int. J. Mol. Sci. 2022, 23, 10177. [Google Scholar] [CrossRef]
- Caetano-Pinto, P. Amplifying the impact of kidney microphysiological systems: Predicting renal drug clearance using mechanistic modelling based on reconstructed drug secretion. ALTEX 2022, 40, 408–424. [Google Scholar] [CrossRef]
- Pou Casellas, C.; Jansen, K.; Rookmaaker, M.B.; Clevers, H.; Verhaar, M.C.; Masereeuw, R. Regulation of Solute Carriers Oct2 and Oat1/3 in the Kidney: A Phylogenetic, Ontogenetic, and Cell Dynamic Perspective. Physiol. Rev. 2022, 102, 993–1024. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.D.A.; Sayer, R.; Windass, A.S.; Haslam, I.S.; De Broe, M.E.; D’Haese, P.C.; Verhulst, A. Characterisation of human tubular cell monolayers as a model of proximal tubular xenobiotic handling. Toxicol. Appl. Pharmacol. 2008, 233, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sweet, D.H. Renal organic anion transporters (SLC22 Family): Expression, regulation, roles in toxicity, and impact on injury and disease. AAPS J. 2013, 15, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yee, S.W.; Kim, R.B.; Giacomini, K.M. SLC transporters as therapeutic targets: Emerging opportunities. Nat. Rev. Drug Discov. 2015, 14, 543–560. [Google Scholar] [CrossRef]
- Roth, M.; Obaidat, A.; Hagenbuch, B. OATPs, OATs and OCTs: The organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br. J. Pharmacol. 2012, 165, 1260–1287. [Google Scholar] [CrossRef]
- Nigam, S.K.; Bush, K.T.; Martovetsky, G.; Ahn, S.Y.; Liu, H.C.; Richard, E.; Bhatnagar, V.; Wu, W. The organic anion transporter (OAT) family: A systems biology perspective. Physiol. Rev. 2015, 95, 83–123. [Google Scholar] [CrossRef]
- Rosner, M.H.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.J.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin. J. Am. Soc. Nephrol. 2021, 16, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; LaPerle, J.L.; Chang, G.; Varma, M.V. Renal clearance in drug discovery and development: Molecular descriptors, drug transporters and disease state. Expert Opin. Drug Metab. Toxicol. 2010, 6, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Radi, Z.A. Kidney Transporters and Drug-Induced Injury in Drug Development. Toxicol. Pathol. 2020, 48, 721–724. [Google Scholar] [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef]
- Ewart, L.; Apostolou, A.; Briggs, S.A.; Carman, C.V.; Chaff, J.T.; Heng, A.R.; Jadalannagari, S.; Janardhanan, J.; Jang, K.-J.; Joshipura, S.R.; et al. Performance assessment and economic analysis of a human Liver-Chip for predictive toxicology. Commun. Med. 2022, 2, 154. [Google Scholar] [CrossRef]
- Zou, L.; Stecula, A.; Gupta, A.; Prasad, B.; Chien, H.C.; Yee, S.W.; Wang, L.; Unadkat, J.D.; Stahl, S.H.; Fenner, K.S.; et al. Molecular mechanisms for species differences in organic anion transporter 1, OAT1: Implications for renal drug toxicity. Mol. Pharmacol. 2018, 94, 689–699. [Google Scholar] [CrossRef]
- Drozdzik, M.; Drozdzik, M.; Oswald, S. Membrane carriers and transporters in kidney physiology and disease. Biomedicines 2021, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.C.; Rönnau, C.; Burchardt, M.; Wolff, I. Kidney Cancer and Chronic Kidney Disease: Too Close for Comfort. Biomedicines 2021, 9, 1761. [Google Scholar] [CrossRef]
- Haase, V.H. Mechanisms of hypoxia responses in renal tissue. J. Am. Soc. Nephrol. 2013, 24, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Schödel, J.; Grampp, S.; Maher, E.R.; Moch, H.; Ratcliffe, P.J.; Russo, P.; Mole, D.R. Hypoxia, hypoxia-inducible transcription factors, and renal cancer. Eur. Urol. 2016, 69, 646–657. [Google Scholar] [CrossRef]
- Jamshidi, N.; Nigam, S.K. Drug transporters OAT1 and OAT3 have specific effects on multiple organs and gut microbiome as revealed by contextualized metabolic network reconstructions. Sci. Rep. 2022, 12, 18308. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): Implications for cellular physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Q.; You, G. Three ubiquitination sites of organic anion transporter-1 synergistically mediate protein kinase c-dependent endocytosis of the transporter. Mol. Pharmacol. 2013, 84, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Phatchawan, A.; Chutima, S.; Varanuj, C.; Anusorn, L. Decreased renal organic anion transporter 3 expression in type 1 diabetic rats. Am. J. Med. Sci. 2014, 347, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, Z.; You, G. Insulin-like growth factor 1 modulates the phosphorylation, expression, and activity of organic anion transporter 3 through protein kinase A signaling pathway. Acta Pharm. Sin. B 2020, 10, 186–194. [Google Scholar] [CrossRef]
- Clemmons, D.R. Role of insulin-like growth factor I in maintaining normal glucose homeostasis. Horm. Res. 2004, 62, 77–82. [Google Scholar] [CrossRef]
- Schneider, R.; Sauvant, C.; Betz, B.; Otremba, M.; Fischer, D.; Holzinger, H.; Wanner, C.; Galle, J.; Gekle, M. Downregulation of organic anion transporters OAT1 and OAT3 correlates with impaired secretion of para-aminohippurate after ischemic acute renal failure in rats. Am. J. Physiol.-Ren. Physiol. 2007, 292, F1599–F1605. [Google Scholar] [CrossRef]
- Dodd, K.M.; Yang, J.; Shen, M.H.; Sampson, J.R.; Tee, A.R. mTORC1 drives HIF-1α and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene 2015, 34, 2239–2250. [Google Scholar] [CrossRef] [PubMed]
- Hagos, Y.; Schley, G.; Scḧdel, J.; Krick, W.; Burckhardt, G.; Willam, C.; Burckhardt, B.C. α-Ketoglutarate-related inhibitors of HIF prolyl hydroxylases are substrates of renal organic anion transporters 1 (OAT1) and 4 (OAT4). Pflug. Arch. Eur. J. Physiol. 2012, 464, 367–374. [Google Scholar] [CrossRef]
- Taylor, C.T.; Colgan, S.P. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 2017, 17, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Sirijariyawat, K.; Ontawong, A.; Palee, S.; Thummasorn, S.; Maneechote, C.; Boonphang, O.; Chatsudthipong, V.; Chattipakorn, N.; Srimaroeng, C. Impaired renal organic anion transport 1 (SLC22A6) and its regulation following acute myocardial infarction and reperfusion injury in rats. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 2342–2355. [Google Scholar] [CrossRef]
- Soodvilai, S.; Wright, S.H.; Dantzler, W.H.; Chatsudthipong, V. Involvement of tyrosine kinase and PI3K in the regulation of OAT3-mediated estrone sulfate transport in isolated rabbit renal proximal tubules. Am. J. Physiol.-Ren. Physiol. 2005, 289, F1057–F1064. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Xu, L.; Shi, Y.; Fang, L.; Gu, H.; Wang, H.; Ding, X.; Zhuang, S. Pharmacologic targeting ERK1/2 attenuates the development and progression of hyperuricemic nephropathy in rats. Oncotarget 2017, 8, 33807–33826. [Google Scholar] [CrossRef]
- Li, T.T.; An, J.X.; Xu, J.Y.; Tuo, B.G. Overview of organic anion transporters and organic anion transporter polypeptides and their roles in the liver. World J. Clin. Cases 2019, 7, 3915–3933. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Pinto, P.; Jamalpoor, A.; Ham, J.; Goumenou, A.; Mommersteeg, M.; Pijnenburg, D.; Ruijtenbeek, R.; Sanchez-Romero, N.; Van Zelst, B.; Heil, S.G.; et al. Cetuximab Prevents Methotrexate-Induced Cytotoxicity in Vitro through Epidermal Growth Factor Dependent Regulation of Renal Drug Transporters. Mol. Pharm. 2017, 14, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Serocki, M.; Bartoszewska, S.; Janaszak-Jasiecka, A.; Ochocka, R.J.; Collawn, J.F.; Bartoszewski, R. miRNAs regulate the HIF switch during hypoxia: A novel therapeutic target. Angiogenesis 2018, 21, 183–202. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, H.; Zhang, K.; Ma, D.; Yang, G.; Zheng, Z.; Liu, K.; Yu, B.; Zhai, C.; Yang, S. A feedback regulatory loop between HIF-1α and miR-21 in response to hypoxia in cardiomyocytes. FEBS Lett. 2014, 588, 3137–3146. [Google Scholar] [CrossRef]
- Gomez, I.G.; MacKenna, D.A.; Johnson, B.G.; Kaimal, V.; Roach, A.M.; Ren, S.; Nakagawa, N.; Xin, C.; Newitt, R.; Pandya, S.; et al. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J. Clin. Investig. 2015, 125, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Jansen, K.; Neven, E.; Poesen, R.; Othman, A.; van Mil, A.; Sluijter, J.; Torano, J.S.; Zaal, E.A.; Berkers, C.R.; et al. Remote sensing and signaling in kidney proximal tubules stimulates gut microbiome-derived organic anion secretion. Proc. Natl. Acad. Sci. USA 2019, 116, 16105–16110. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Fisel, P.; Schaeffeler, E.; Schwab, M. DNA methylation of ADME genes. Clin. Pharmacol. Ther. 2016, 99, 512–527. [Google Scholar] [CrossRef]
- Hirota, T.; Tanaka, T.; Takesue, H.; Ieiri, I. Epigenetic regulation of drug transporter expression in human tissues. Expert Opin. Drug Metab. Toxicol. 2017, 13, 19–30. [Google Scholar] [CrossRef]
- Jin, L.; Kikuchi, R.; Saji, T.; Kusuhara, H.; Sugiyama, Y. Regulation of tissue-specific expression of renal organic anion transporters by hepatocyte nuclear factor 1 α/β and DNA methylation. J. Pharmacol. Exp. Ther. 2012, 340, 648–655. [Google Scholar] [CrossRef]
- Zhou, S.; Shu, Y. Special Section on New Era of Transporter Science: Unraveling the Functional Role of Orphan Transporters-Minireview Transcriptional Regulation of Solute Carrier Drug Transporters. Drug Metab. Dispos. 2022, 50, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Litke, C.; Hagenston, A.M.; Kenkel, A.K.; Paldy, E.; Lu, J.; Kuner, R.; Mauceri, D. Organic anion transporter 1 is an HDAC4-regulated mediator of nociceptive hypersensitivity in mice. Nat. Commun. 2022, 13, 875. [Google Scholar] [CrossRef]
- Martovetsky, G.; Tee, J.B.; Nigam, S.K. Hepatocyte nuclear factors 4α and 1α regulate kidney developmental expression of drug-metabolizing enzymes and drug transporters. Mol. Pharmacol. 2013, 84, 808–823. [Google Scholar] [CrossRef]
- Ogasawara, K.; Terada, T.; Asaka, J.I.; Katsura, T.; Inui, K.I. Hepatocyte nuclear factor-4α regulates the human organic anion transporter 1 gene in the kidney. Am. J. Physiol.-Ren. Physiol. 2007, 292, F1819–F1826. [Google Scholar] [CrossRef]
- Yang, J.; Kalogerou, M.; Gallacher, J.; Sampson, J.R.; Shen, M.H. Renal tumours in a Tsc1+/− mouse model show epigenetic suppression of organic cation transporters Slc22a1, Slc22a2 and Slc22a3, and do not respond to metformin. Eur. J. Cancer 2013, 49, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643. [Google Scholar] [CrossRef]
- Duan, P.; You, G. Short-term regulation of organic anion transporters. Pharmacol. Ther. 2010, 125, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Epstein, F.H.; Fish, E.M.; Molitoris, B.A. Alterations in Epithelial Polarity and the Pathogenesis of Disease States. N. Engl. J. Med. 1994, 330, 1580–1588. [Google Scholar] [CrossRef]
- Pou Casellas, C.; Rookmaaker, M.B.; Verhaar, M.C. Controlling cellular plasticity to improve in vitro models for kidney regeneration. Curr. Opin. Biomed. Eng. 2021, 20, 100345. [Google Scholar] [CrossRef]
- Jansen, J.; Fedecostante, M.; Wilmer, M.J.; Peters, J.G.; Kreuser, U.M.; Van Den Broek, P.H.; Mensink, R.A.; Boltje, T.J.; Stamatialis, D.; Wetzels, J.F.; et al. Bioengineered kidney tubules efficiently excrete uremic toxins. Sci. Rep. 2016, 6, 26715. [Google Scholar] [CrossRef]
- Rougerie, P.; Pieuchot, L.; dos Santos, R.S.; Marteau, J.; Bigerelle, M.; Chauvy, P.F.; Farina, M.; Anselme, K. Topographical curvature is sufficient to control epithelium elongation. Sci. Rep. 2020, 10, 14784. [Google Scholar] [CrossRef] [PubMed]
- van Genderen, A.M.; Jansen, K.; Kristen, M.; van Duijn, J.; Li, Y.; Schuurmans, C.C.L.; Malda, J.; Vermonden, T.; Jansen, J.; Masereeuw, R.; et al. Topographic Guidance in Melt-Electrowritten Tubular Scaffolds Enhances Engineered Kidney Tubule Performance. Front. Bioeng. Biotechnol. 2021, 8, 617364. [Google Scholar] [CrossRef]
- Garreta, E.; Prado, P.; Tarantino, C.; Oria, R.; Fanlo, L.; Martí, E.; Zalvidea, D.; Trepat, X.; Roca-Cusachs, P.; Gavaldà-Navarro, A.; et al. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat. Mater. 2019, 18, 397–405. [Google Scholar] [CrossRef]
- Batchelder, C.A.; Martinez, M.L.; Tarantal, A.F. Natural scaffolds for renal differentiation of human embryonic stem cells for kidney tissue engineering. PLoS ONE 2015, 10, e0143849. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Verhaar, M.C. Editorial overview: Kidney regeneration; cells, organoids and whole organ engineering? Curr. Opin. Biomed. Eng. 2022, 23, 100403. [Google Scholar] [CrossRef]
- Ohi, M.D.; Kenworthy, A.K. Emerging Insights into the Molecular Architecture of Caveolin-1. J. Membr. Biol. 2022, 255, 375–383. [Google Scholar] [CrossRef]
- Fridolfsson, H.N.; Roth, D.M.; Insel, P.A.; Patel, H.H. Regulation of intracellular signaling and function by caveolin. FASEB J. 2014, 28, 3823–3831. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.A.; Srimaroeng, C.; Perry, J.L.; Walden, R.; Dembla-Rajpal, N.; Sweet, D.H.; Pritchard, J.B. Activation of protein kinase Cζ increases OAT1 (SLC22A6)-and OAT3 (SLC22A8)-mediated transport. J. Biol. Chem. 2009, 284, 2672–2679. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.; You, G. Posttranslational Regulation of Organic Anion Transporters by Ubiquitination: Known and Novel. Med. Res. Rev. 2016, 36, 964–979. [Google Scholar] [CrossRef]
- Preising, C.; Schneider, R.; Bucher, M.; Gekle, M.; Sauvant, C. Regulation of expression of renal organic anion transporters OAT1 and OAT3 in a model of ischemia/reperfusion injury. Cell. Physiol. Biochem. 2015, 37, 1–13. [Google Scholar] [CrossRef]
- Tanaka, K.; Xu, W.; Zhou, F.; You, G. Role of Glycosylation in the Organic Anion Transporter OAT1. J. Biol. Chem. 2004, 279, 14961–14966. [Google Scholar] [CrossRef] [PubMed]
- Celen, A.B.; Sahin, U. Sumoylation on its 25th anniversary: Mechanisms, pathology, and emerging concepts. FEBS J. 2020, 287, 3110–3140. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.; You, G. Activation of Protein Kinase A Stimulates SUMOylation, Expression, and Transport Activity of Organic Anion Transporter 3. AAPS J. 2019, 21, 30. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, C.; Zhang, J.; Liang, Z.; You, G. Protein kinase C regulates organic anion transporter 1 through phosphorylating ubiquitin ligase Nedd4–2. BMC Mol. Cell Biol. 2021, 22, 53. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.; You, G. An Essential Role of Nedd4-2 in the Ubiquitination, Expression, and Function of Organic Anion Transporter-3. Mol. Pharm. 2016, 13, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, H.; Yu, Z.; Liang, Z.; Li, Y.; You, G. Inhibition of proteasome, but not lysosome, upregulates organic anion transporter 3 in vitro and in vivo. Biochem. Pharmacol. 2023, 208, 115387. [Google Scholar] [CrossRef]
- Fan, Y.; Liang, Z.; Zhang, J.; You, G. Oral proteasomal inhibitors ixazomib, oprozomib, and delanzomib upregulate the function of organic anion transporter 3 (OAT3): Implications in OAT3-mediated drug-drug interactions. Pharmaceutics 2021, 13, 314. [Google Scholar] [CrossRef]
- Kim, H.; Shim, B.Y.; Lee, S.-J.; Lee, J.Y.; Lee, H.-J.; Kim, I.-H. Loss of Von Hippel–Lindau (VHL) Tumor Suppressor Gene Function: VHL–HIF Pathway and Advances in Treatments for Metastatic Renal Cell Carcinoma (RCC). Int. J. Mol. Sci. 2021, 22, 9795. [Google Scholar] [CrossRef]
- Guida, M.; Casamassima, A.; Monticelli, G.; Quaranta, M.; Colucci, G. Basal cytokines profile in metastatic renal cell carcinoma patients treated with subcutaneous IL-2-based therapy compared with that of healthy donors. J. Transl. Med. 2007, 5, 51. [Google Scholar] [CrossRef]
- Ozerlat, I. Kidney cancer: Targeted therapy of glucose uptake via GLUT1 kills RCC cells. Nat. Rev. Urol. 2011, 8, 471. [Google Scholar] [CrossRef]
- Li, Q.; Shu, Y. Role of solute carriers in response to anticancer drugs. Mol. Cell. Ther. 2014, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Whisenant, T.C.; Nigam, S.K. Organic Anion Transporters (OAT) and Other SLC22 Transporters in Progression of Renal Cell Carcinoma. Cancers 2022, 14, 4772. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.; Fisel, P.; Büttner, F.; Rausch, S.; D’Amico, D.; Hennenlotter, J.; Kruck, S.; Nies, A.T.; Stenzl, A.; Junker, K.; et al. Methylomes of renal cell lines and tumors or metastases differ significantly with impact on pharmacogenes. Sci. Rep. 2016, 6, 29930. [Google Scholar] [CrossRef]
- Aguilar, A. Kidney cancer: OCT2 demethylation cracks open oxaliplatin resistance. Nat. Rev. Nephrol. 2016, 12, 581. [Google Scholar] [CrossRef]
- Verhulst, A.; Sayer, R.; De Broe, M.E.; D’Haese, P.C.; Brown, C.D.A. Human proximal tubular epithelium actively secretes but does not retain rosuvastatin. Mol. Pharmacol. 2008, 74, 1084–1091. [Google Scholar] [CrossRef]
- Bajaj, P.; Chung, G.; Pye, K.; Yukawa, T.; Imanishi, A.; Takai, Y.; Brown, C.; Wagoner, M.P. Freshly isolated primary human proximal tubule cells as an in vitro model for the detection of renal tubular toxicity. Toxicology 2020, 442, 152535. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Shi, B.; Zeng, T.; Zhang, Y.; Huang, B.; Ouyang, B.; Cai, Z.; Liu, M. Drug Transporters in the Kidney: Perspectives on Species Differences, Disease Status, and Molecular Docking. Front. Pharmacol. 2021, 12, 746208. [Google Scholar] [CrossRef] [PubMed]
- Dickman, K.G.; Mandel, L.J. Glycolytic and oxidative metabolism in primary renal proximal tubule cultures. Am. J. Physiol.-Cell Physiol. 1989, 257, C333–C340. [Google Scholar] [CrossRef]
- Vazquez, A.; Liu, J.; Zhou, Y.; Oltvai, Z.N. Catabolic efficiency of aerobic glycolysis: The Warburg effect revisited. BMC Syst. Biol. 2010, 4, 58. [Google Scholar] [CrossRef]
- Brand, M.D.; Orr, A.L.; Perevoshchikova, I.V.; Quinlan, C.L. The role of mitochondrial function and cellular bioenergetics in ageing and disease. Br. J. Dermatol. 2013, 169, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Balan, M.; Sabarwal, A.; Choueiri, T.K.; Pal, S. Metabolic reprogramming in renal cancer: Events of a metabolic disease. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1876, 188559. [Google Scholar] [CrossRef]
- Vriend, J.; Hoogstraten, C.A.; Schirris, T.J.J.; Russel, F.G.M.; Venrooij, K.R.; van den Berge, B.T.; Govers, L.P.; Wilmer, M.J.; van Rooij, A.; Huigen, M.C.D.G.; et al. Organic anion transporters 1 and 3 influence cellular energy metabolism in renal proximal tubule cells. Biol. Chem. 2020, 400, 1347–1358. [Google Scholar] [CrossRef]
- Abla, H.; Sollazzo, M.; Gasparre, G.; Iommarini, L.; Porcelli, A.M. The multifaceted contribution of α-ketoglutarate to tumor progression: An opportunity to exploit? Semin. Cell Dev. Biol. 2020, 98, 26–33. [Google Scholar] [CrossRef]
- Wu, N.; Yang, M.; Gaur, U.; Xu, H.; Yao, Y.; Li, D. Alpha-ketoglutarate: Physiological functions and applications. Biomol. Ther. 2016, 24, 1–8. [Google Scholar] [CrossRef]
- Weber, E.J.; Chapron, A.; Chapron, B.D.; Voellinger, J.L.; Lidberg, K.A.; Yeung, C.K.; Wang, Z.; Yamaura, Y.; Hailey, D.W.; Neumann, T.; et al. Development of a microphysiological model of human kidney proximal tubule function. Kidney Int. 2016, 90, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, T.; Huang, W.; Shum, S.; Hailey, D.W.; Chang, S.-Y.; Chapron, A.; Yeung, C.K.; Himmelfarb, J.; Isoherranen, N.; Kelly, E.J. Bridging the gap between in silico and in vivo by modeling opioid disposition in a kidney proximal tubule microphysiological system. Sci. Rep. 2021, 11, 21356. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, M.; Robinson, S.; Alaei, B.; Clausen, M.; Hicks, R.; Belfield, G.; Althage, M.; Bak, A.; Lewis, J.A.; Hansen, P.B.L.; et al. 3D vascularised proximal tubules-on-a-multiplexed chip model for enhanced cell phenotypes. Lab Chip 2023. [Google Scholar] [CrossRef] [PubMed]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Little, M.H. Generation of kidney organoids from human pluripotent stem cells. Nat. Protoc. 2016, 11, 1681–1692. [Google Scholar] [CrossRef]
- Rizki-Safitri, A.; Gupta, N.; Hiratsuka, K.; Kobayashi, K.; Zhang, C.; Ida, K.; Satlin, L.M.; Morizane, R. Live functional assays reveal longitudinal maturation of transepithelial transport in kidney organoids. Front. Cell Dev. Biol. 2022, 10, 978888. [Google Scholar] [CrossRef]
- Soo, J.Y.C.; Jansen, J.; Masereeuw, R.; Little, M.H. Advances in predictive in vitro models of drug-induced nephrotoxicity. Nat. Rev. Nephrol. 2018, 14, 378–393. [Google Scholar] [CrossRef]
- Jenkinson, S.E.; Chung, G.W.; van Loon, E.; Bakar, N.S.; Dalzell, A.M.; Brown, C.D.A. The limitations of renal epithelial cell line HK-2 as a model of drug transporter expression and function in the proximal tubule. Pflug. Arch. 2012, 464, 601–611. [Google Scholar] [CrossRef]
- Chandrasekaran, V.; Carta, G.; da Costa Pereira, D.; Gupta, R.; Murphy, C.; Feifel, E.; Kern, G.; Lechner, J.; Cavallo, A.L.; Gupta, S.; et al. Generation and characterization of iPSC-derived renal proximal tubule-like cells with extended stability. Sci. Rep. 2021, 11, 11575. [Google Scholar] [CrossRef] [PubMed]
- Nookala, A.R.; Ronxhi, J.; Sliz, J.; Jeanty, S.; Manatakis, D.V.; Jadalannagari, S.; Hamilton, G.; Park, H.; He, Y.; Lavarias, M.; et al. Assessment of Human Renal Transporter Based Drug-Drug Interactions Using Proximal Tubule Kidney-Chip. bioRxiv 2022, 2022-05. [Google Scholar] [CrossRef]
- Bolon, C.; Gauthier, C.; Simonnet, H. Glycolysis inhibition by palmitate in renal cells cultured in a two-chamber system. Am. J. Physiol.-Cell Physiol. 1997, 273, C1732–C1738. [Google Scholar] [CrossRef] [PubMed]
- Darshi, M.; Tumova, J.; Saliba, A.; Kim, J.; Baek, J.; Pennathur, S.; Sharma, K. Crabtree effect in kidney proximal tubule cells via late-stage glycolytic intermediates. iScience 2023, 26, 4. [Google Scholar] [CrossRef]
- Nagao, A.; Kobayashi, M.; Koyasu, S.; Chow, C.C.T.; Harada, H. HIF-1-dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance. Int. J. Mol. Sci. 2019, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, X.; Yu, Q.; Wang, H.; Tan, F.; Zhu, Q.; Yuan, L.; Jiang, H.; Yu, L.; Zeng, S. Epigenetic activation of the drug transporter OCT2 sensitizes renal cell carcinoma to oxaliplatin. Sci. Transl. Med. 2016, 8, 348ra97. [Google Scholar] [CrossRef]
- Conte, F.; van Buuringen, N.; Voermans, N.C.; Lefeber, D.J. Galactose in human metabolism, glycosylation and congenital metabolic diseases: Time for a closer look. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2021, 1865, 129898. [Google Scholar] [CrossRef] [PubMed]
| Factors | Cellular Pathway | Process | Regulation | Effect | |
|---|---|---|---|---|---|
| OAT1 | OAT3 | ||||
| αKG | HIF-1α/VHL-PHD/TCA | Metabolism | Transcriptional | −/+ | −/+ |
| Glucose | HIF-1α/NF-kB/AMPK | − | − | ||
| TNF-α | NF-kB/MAPK | Inflammation | + | + | |
| IL-1β | NF-kB/MAPK | + | + | ||
| DNMTs | − | Epigenetic modification | − | − | |
| HDACs | − | − | − | ||
| IGF-1 | PI3K/AKT/mTOR | Cell proliferation/ differentiation | + | + | |
| HGF | c-Met/PI3K/AKT | + | + | ||
| HNF-1α | EGF/PI3K/AKT | + | + | ||
| HNF-4α | EGF/PI3K/AKT | + | + | ||
| IS | EGFR/AhR/ARNT | + | nd | ||
| miR-223 | EGFR/AhR/ARNT | + | nd | ||
| EGF | EGFR/ERK | Post-transcriptional | + | + | |
| Cav | − | Cell adhesion | Post-translational | −/+ | −/+ |
| PKA | SUMO | Signal transduction | −/+ | −/+ | |
| PKC | Ubiquitin | −/+ | −/+ | ||
| GTs | − | Glycosylation | + | + | |
| Cell Lines | Reference | ||||
|---|---|---|---|---|---|
| Model | OAT1 | OAT3 | |||
| Expression | Activity | Expression | Activity | ||
| Cryopreserved RPTEC | − | − | − | − | [7] |
| Fresh RPTEC | + | + | + | + | [10] |
| RPTEC-TERT1 | − | − | − | − | [7] |
| ciPTEC | − | − | − | − | [39] |
| HK-2 | − | − | − | − | [98] |
| iPSC derived RPTEC | − | − | − | − | [99] |
| Advanced models | |||||
| Nortis-ParVivo * | + | + | − | − | [8] |
| Emulate Kidney-chip * | + | + | − | − | [100] |
| iPSC derived renal organoids | + | + | + | + | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caetano-Pinto, P.; Stahl, S.H. Renal Organic Anion Transporters 1 and 3 In Vitro: Gone but Not Forgotten. Int. J. Mol. Sci. 2023, 24, 15419. https://doi.org/10.3390/ijms242015419
Caetano-Pinto P, Stahl SH. Renal Organic Anion Transporters 1 and 3 In Vitro: Gone but Not Forgotten. International Journal of Molecular Sciences. 2023; 24(20):15419. https://doi.org/10.3390/ijms242015419
Chicago/Turabian StyleCaetano-Pinto, Pedro, and Simone H. Stahl. 2023. "Renal Organic Anion Transporters 1 and 3 In Vitro: Gone but Not Forgotten" International Journal of Molecular Sciences 24, no. 20: 15419. https://doi.org/10.3390/ijms242015419






