Effects of C-Peptide on Dexamethasone-Induced In Vitro and In Vivo Models as a Potential Therapeutic Agent for Muscle Atrophy
Abstract
1. Introduction
2. Results
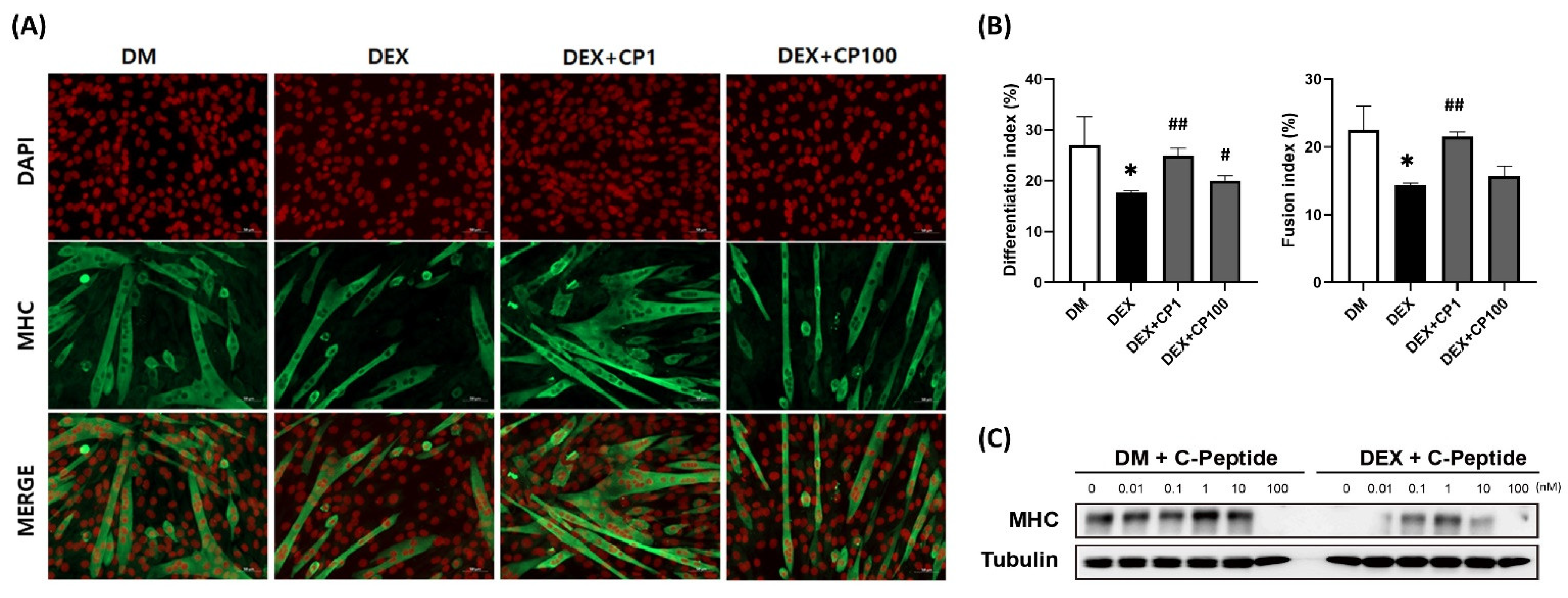
2.1. Effects of Various Concentrations of C-Peptide on C2C12 Myotubes in DEX-Induced Muscle Atrophy
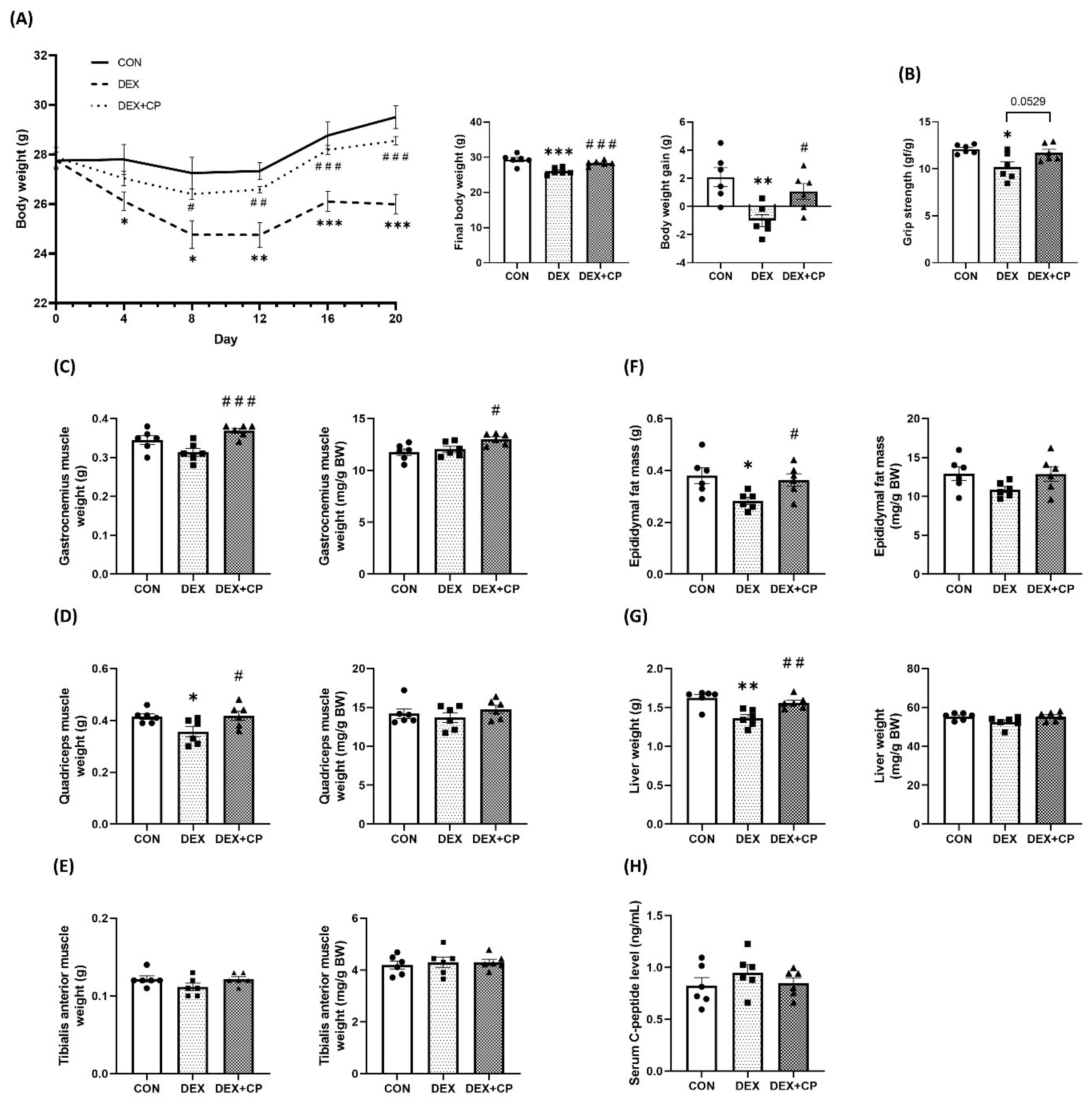
2.2. C-Peptide Co-Treatment Inhibits DEX-Induced Muscle Atrophy
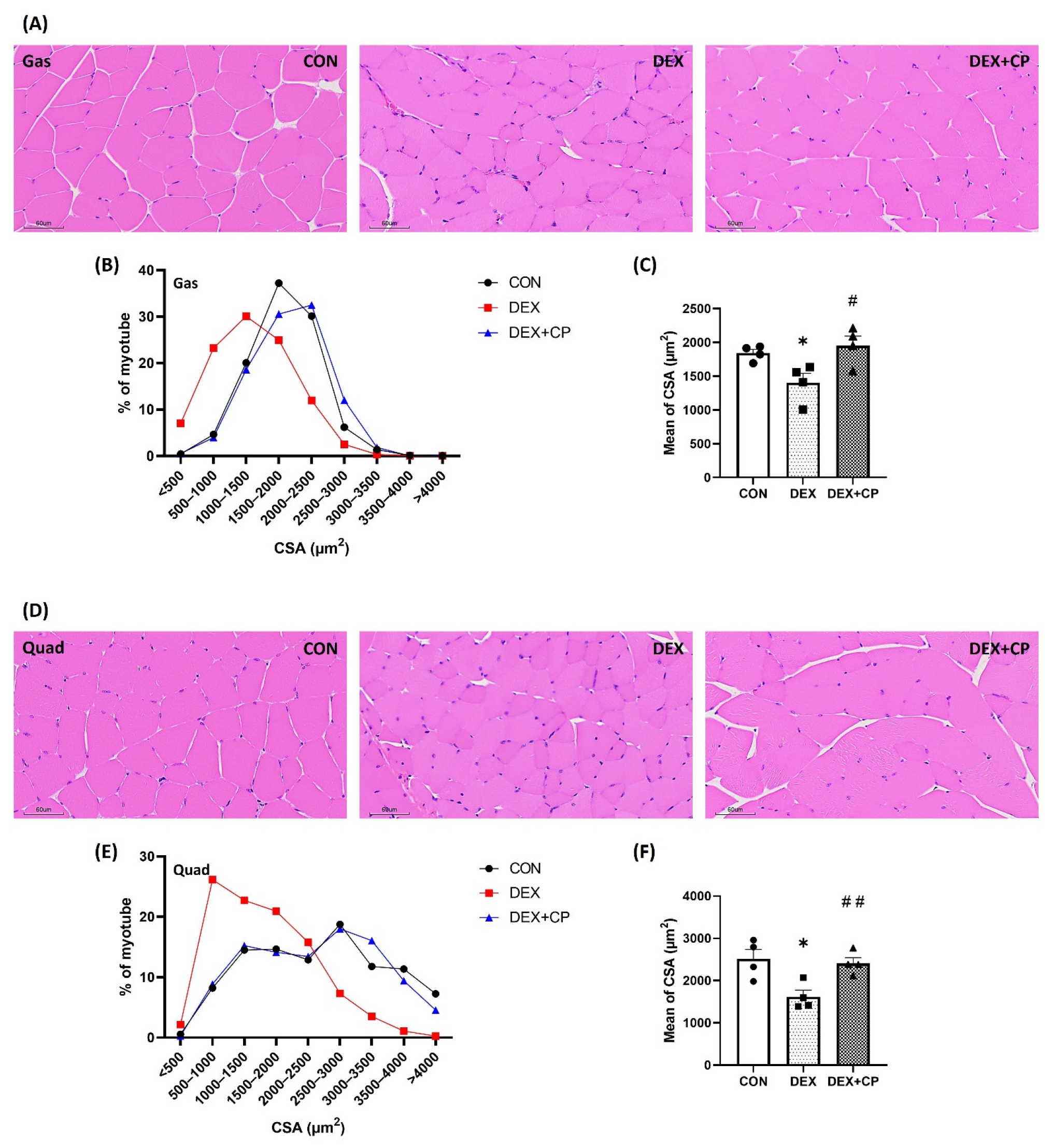
2.3. C-Peptide Attenuates the Reduction in Muscle Fiber Size in Mice with Muscle Atrophy
2.4. C-Peptide Regulates the mRNA and Protein Levels in Gastrocnemius Muscles
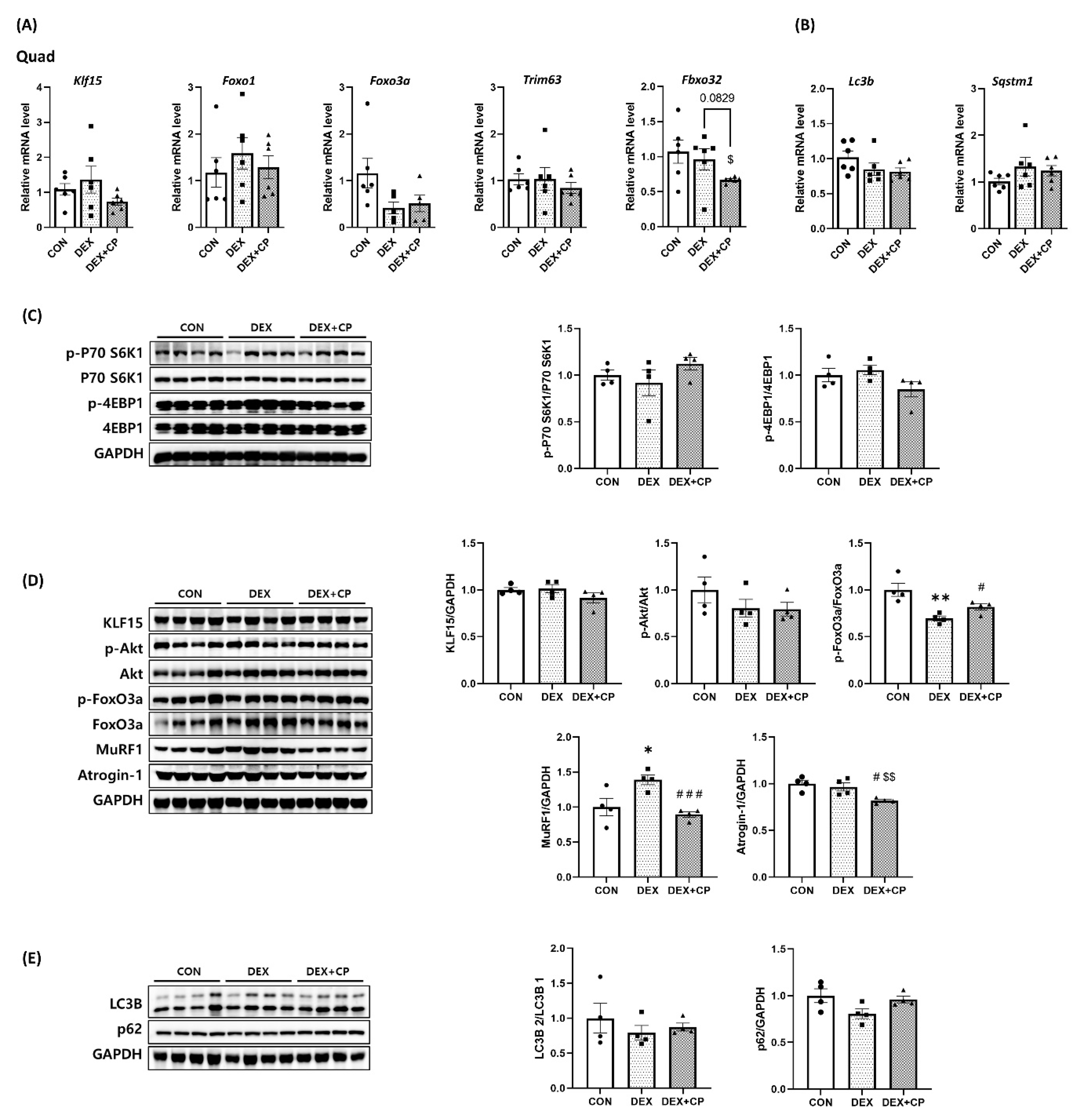
2.5. C-Peptide Modulates the mRNA and Protein Levels in Quadriceps Muscles
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Differentiation
4.2. Immunofluorescence and Determination of the Differentiation and Fusion Indices
4.3. Animals
4.4. Hematoxylin and Eosin Staining and Determination of the Cross-Sectional Area
4.5. RNA Isolation and Reverse Transcription-Quantitative Polymerase Chain Reaction
4.6. Protein Isolation and Western Blotting
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSA | cross-sectional area |
| DEX | dexamethasone |
| Fbxo32 | forkhead box protein 32 |
| FoxO | forkhead box protein O |
| Gas | gastrocnemius |
| MHC | myosin heavy chain |
| MuRF1 | muscle RING-finger protein-1 |
| TA | tibialis anterior |
| Trim63 | tripartite motif-containing 63 |
| Quad | quadriceps |
References
- Janssen, I.; Heymsfield, S.B.; Wang, Z.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef]
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef]
- Bodine, S.C. Disuse-induced muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2200–2208. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Quan, H.; Kang, S.-G.; Huang, K.; Tong, T. Nutraceuticals in the Prevention and Treatment of the Muscle Atrophy. Nutrients 2021, 13, 1914. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; MacLeod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Kitajima, Y.; Yoshioka, K.; Suzuki, N. The ubiquitin–proteasome system in regulation of the skeletal muscle homeostasis and atrophy: From basic science to disorders. J. Physiol. Sci. 2020, 70, 1–12. [Google Scholar] [CrossRef]
- Rom, O.; Reznick, A.Z. The role of E3 ubiquitin-ligases MuRF-1 and MAFbx in loss of skeletal muscle mass. Free Radic. Biol. Med. 2016, 98, 218–230. [Google Scholar] [CrossRef]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef]
- McKinnell, I.W.; Rudnicki, M.A. Molecular Mechanisms of Muscle Atrophy. Cell 2004, 119, 907–910. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Sandri, M.; Nichenko, A.S.; Southern, W.M.; Atuan, M.; Luan, J.; Peissig, K.B.; Foltz, S.J.; Beedle, A.M.; Warren, G.L.; Call, J.A.; et al. Autophagy in health and disease. 3. Involvement of autophagy in muscle atrophy. Am. J. Physiol. Physiol. 2010, 298, C1291–C1297. [Google Scholar] [CrossRef]
- Schakman, O.; Kalista, S.; Barbé, C.; Loumaye, A.; Thissen, J. Glucocorticoid-induced skeletal muscle atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Schakman, O.; Gilson, H.; Kalista, S.; Thissen, J. Mechanisms of Muscle Atrophy Induced by Glucocorticoids. Horm. Res. Paediatr. 2009, 72, 36–41. [Google Scholar] [CrossRef]
- Fanzani, A.; Conraads, V.M.; Penna, F.; Martinet, W. Molecular and cellular mechanisms of skeletal muscle atrophy: An update. J. Cachexia Sarcopenia Muscle 2012, 3, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Wahren, J.; Ekberg, K.; Jörnvall, H. C-peptide is a bioactive peptide. Diabetologia 2007, 50, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Block, M.B.; Mako, M.E.; Steiner, D.F.; Rubenstein, A.H. Circulating C-peptide Immunoreactivity: Studies in Normals and Diabetic Patients. Diabetes 1972, 21, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, K.S.; Licinio-Paixao, J.; Given, B.D.; Pugh, W.; Rue, P.; Galloway, J.; Karrison, T.; Frank, B. Use of biosynthetic human C-peptide in the measurement of insulin secretion rates in normal volunteers and type I diabetic patients. J. Clin. Investig. 1986, 77, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Wahren, J.; Ekberg, K.; Johansson, J.; Henriksson, M.; Pramanik, A.; Johansson, B.-L.; Rigler, R.; Jörnvall, H. Role of C-peptide in human physiology. Am. J. Physiol. Metab. 2000, 278, E759–E768. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.G.; Hattersley, A.T. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet. Med. 2013, 30, 803–817. [Google Scholar] [CrossRef]
- Wallerath, T.; Kunt, T.; Forst, T.; Closs, E.I.; Lehmann, R.; Flohr, T.; Gabriel, M.; Schäfer, D.; Göpfert, A.; Pfützner, A.; et al. Stimulation of endothelial nitric oxide synthase by proinsulin C-peptide. Nitric Oxide 2003, 9, 95–102. [Google Scholar] [CrossRef]
- Grunberger, G.; Qiang, X.; Li, Z.; Mathews, S.T.; Sbrissa, D.; Shisheva, A.; Sima, A.A.F. Molecular basis for the insulinomimetic effects of C-peptide. Diabetologia 2001, 44, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Galuska, D.; Pirkmajer, S.; Barrès, R.; Ekberg, K.; Wahren, J.; Chibalin, A.V. C-Peptide Increases Na,K-ATPase Expression via PKC- and MAP Kinase-Dependent Activation of Transcription Factor ZEB in Human Renal Tubular Cells. PLoS ONE 2011, 6, e28294. [Google Scholar] [CrossRef] [PubMed]
- Sima, A.A.F.; Zhang, W.; Sugimoto, K.; Henry, D.; Li, Z.; Wahren, J.; Grunberger, G. C-peptide prevents and improves chronic Type I diabetic polyneuropathy in the BB/Wor rat. Diabetologia 2001, 44, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-C.; Bhatt, M.P.; Kwon, M.-H.; Park, D.; Na, S.; Kim, Y.-M.; Ha, K.-S. Proinsulin C-Peptide Prevents Impaired Wound Healing by Activating Angiogenesis in Diabetes. J. Investig. Dermatol. 2015, 135, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.-L.; Linde, B.; Wahren, J. Effects of C-peptide on blood flow, capillary diffusion capacity and glucose utilization in the exercising forearm of Type 1 (insulin-dependent) diabetic patients. Diabetologia 1992, 35, 1151–1158. [Google Scholar] [CrossRef]
- Kamiya, H.; Zhang, W.; Ekberg, K.; Wahren, J.; Sima, A.A. C-Peptide Reverses Nociceptive Neuropathy in Type 1 Diabetes. Diabetes 2006, 55, 3581–3587. [Google Scholar] [CrossRef]
- Johansson, B.-L.; Sjöberg, S.; Wahren, J. The influence of human C-peptide on renal function and glucose utilization in Type 1 (insulin-dependent) diabetic patients. Diabetologia 1992, 35, 121–128. [Google Scholar] [CrossRef]
- Johansson, B.-L.; Sundell, J.; Ekberg, K.; Jonsson, C.; Seppänen, M.; Raitakari, O.; Luotolahti, M.; Nuutila, P.; Wahren, J.; Knuuti, J. C-peptide improves adenosine-induced myocardial vasodilation in type 1 diabetes patients. Am. J. Physiol. Metab. 2004, 286, E14–E19. [Google Scholar] [CrossRef] [PubMed]
- Maurotti, S.; Pujia, R.; Galluccio, A.; Nucera, S.; Musolino, V.; Mare, R.; Frosina, M.; Noto, F.R.; Mollace, V.; Romeo, S.; et al. Preventing muscle wasting: Pro-insulin C-peptide prevents loss in muscle mass in streptozotocin-diabetic rats. J. Cachexia Sarcopenia Muscle 2023, 14, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Al-Rasheed, N.M.; Meakin, F.; Royal, E.L.; Lewington, A.J.; Brown, J.; Willars, G.B.; Brunskill, N.J. Potent activation of multiple signalling pathways by C-peptide in opossum kidney proximal tubular cells. Diabetologia 2004, 47, 987–997. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.-Y.; Kim, H.M.; Kim, J.H.; Guo, S.; Lee, D.H.; Lim, G.M.; Kim, W.; Kim, C.Y. Salvia plebeia R.Br. and Rosmarinic Acid Attenuate Dexamethasone-Induced Muscle Atrophy in C2C12 Myotubes. Int. J. Mol. Sci. 2023, 24, 1876. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, L.; Wan, L.; Huo, Y.; Huang, J.; Li, J.; Lu, J.; Xin, B.; Yang, Q.; Guo, C. Matrine improves skeletal muscle atrophy by inhibiting E3 ubiquitin ligases and activating the Akt/mTOR/FoxO3α signaling pathway in C2C12 myotubes and mice. Oncol. Rep. 2019, 42, 479–494. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.I.; Nirmala, F.S.; Jeong, H.Y.; Seo, H.-D.; Ha, T.Y.; Jung, C.H.; Ahn, J. Chrysanthemum zawadskil Herbich attenuates dexamethasone-induced muscle atrophy through the regulation of proteostasis and mitochondrial function. BioMedicine 2021, 136, 111226. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| Gene | Direction | Sequence (5′–3′) |
|---|---|---|
| Foxo1 | Forward | GTGAACACCAATGCCTCACAC |
| Reverse | CACAGTCCAAGCGTCAATA | |
| Foxo3a | Forward | AGCCGTGTACTGTGGAGCTT |
| Reverse | TCTTGGCGGTATATGGGAAG | |
| Fbxo32 | Forward | ATGCACACTGGTGCAGAGAG |
| Reverse | TGTAAGCACACAGGCAGGTC | |
| Trim63 | Forward | ACCTGCTGGTGGAAAACATC |
| Reverse | CTTCGTGTTCCTTGCACATC | |
| Klf15 | Forward | GAGACCTTCTCGTCACCGAAA |
| Reverse | GCTGGAGACATCGCTGTCAT | |
| Sqstm1 | Forward | AGGATGGGGACTTGGTTGC |
| Reverse | TCACAGATCACATTGGGGTGC | |
| Lc3b | Forward | CCCACCAAGATCCCAGTGAT |
| Reverse | CCAGGAACTTGGTCTTGTCCA | |
| Gapdh | Forward | TCCCACTCTTCCACCTTCGA |
| Reverse | CAGGAAATGAGCTTGACAAAGTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Yang, Y.; Choi, E.; Lee, S.; Choi, J. Effects of C-Peptide on Dexamethasone-Induced In Vitro and In Vivo Models as a Potential Therapeutic Agent for Muscle Atrophy. Int. J. Mol. Sci. 2023, 24, 15433. https://doi.org/10.3390/ijms242015433
Kim J, Yang Y, Choi E, Lee S, Choi J. Effects of C-Peptide on Dexamethasone-Induced In Vitro and In Vivo Models as a Potential Therapeutic Agent for Muscle Atrophy. International Journal of Molecular Sciences. 2023; 24(20):15433. https://doi.org/10.3390/ijms242015433
Chicago/Turabian StyleKim, Jinjoo, Youngmo Yang, Eunwon Choi, Sumin Lee, and Jiyoung Choi. 2023. "Effects of C-Peptide on Dexamethasone-Induced In Vitro and In Vivo Models as a Potential Therapeutic Agent for Muscle Atrophy" International Journal of Molecular Sciences 24, no. 20: 15433. https://doi.org/10.3390/ijms242015433
APA StyleKim, J., Yang, Y., Choi, E., Lee, S., & Choi, J. (2023). Effects of C-Peptide on Dexamethasone-Induced In Vitro and In Vivo Models as a Potential Therapeutic Agent for Muscle Atrophy. International Journal of Molecular Sciences, 24(20), 15433. https://doi.org/10.3390/ijms242015433





