The Dual-Mode Transition of Myofibroblasts Derived from Hepatic Stellate Cells in Liver Fibrosis
Abstract
1. Introduction
2. Results and Discussion
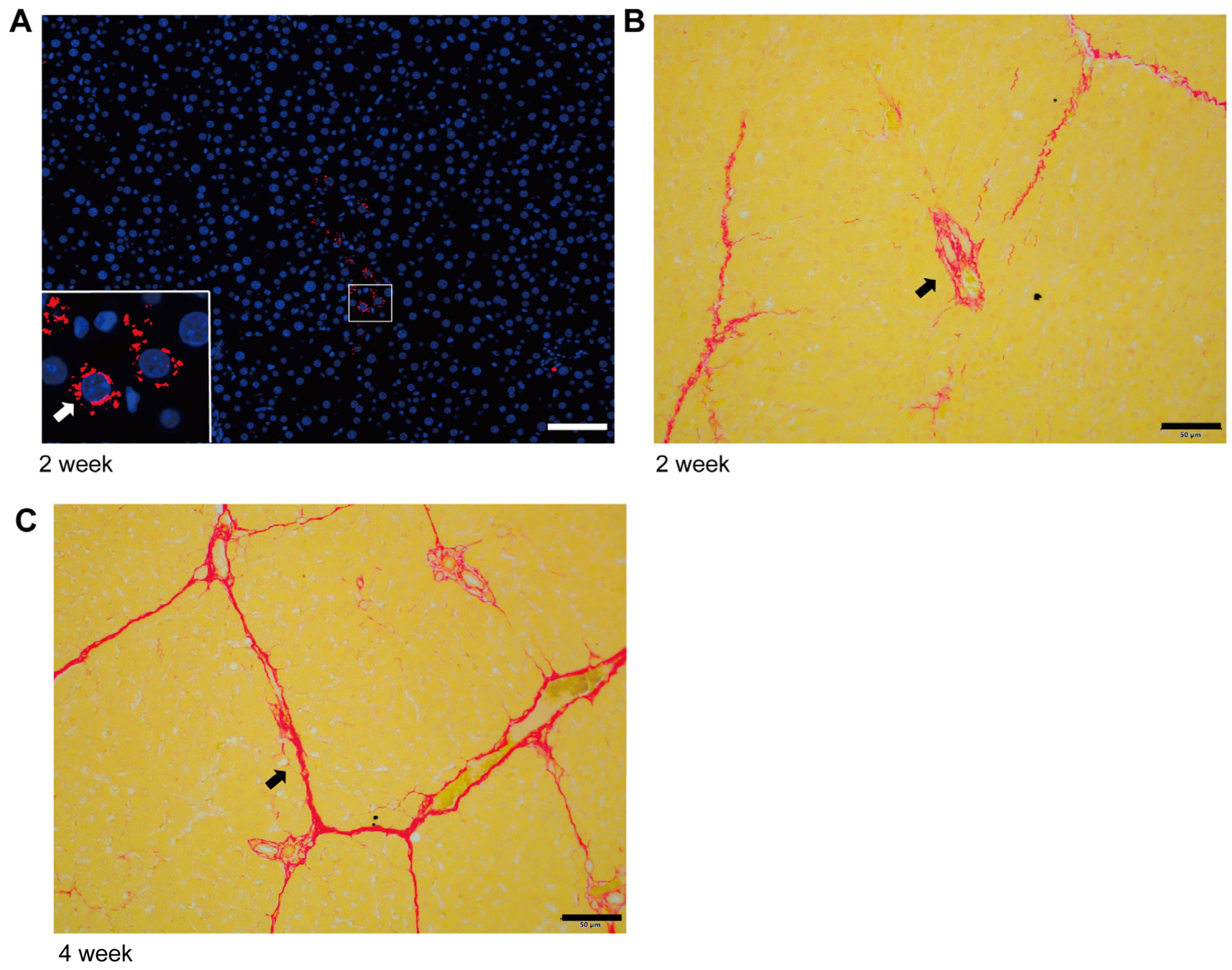
2.1. The Initiation and Progression Characteristics of Liver Fibrosis
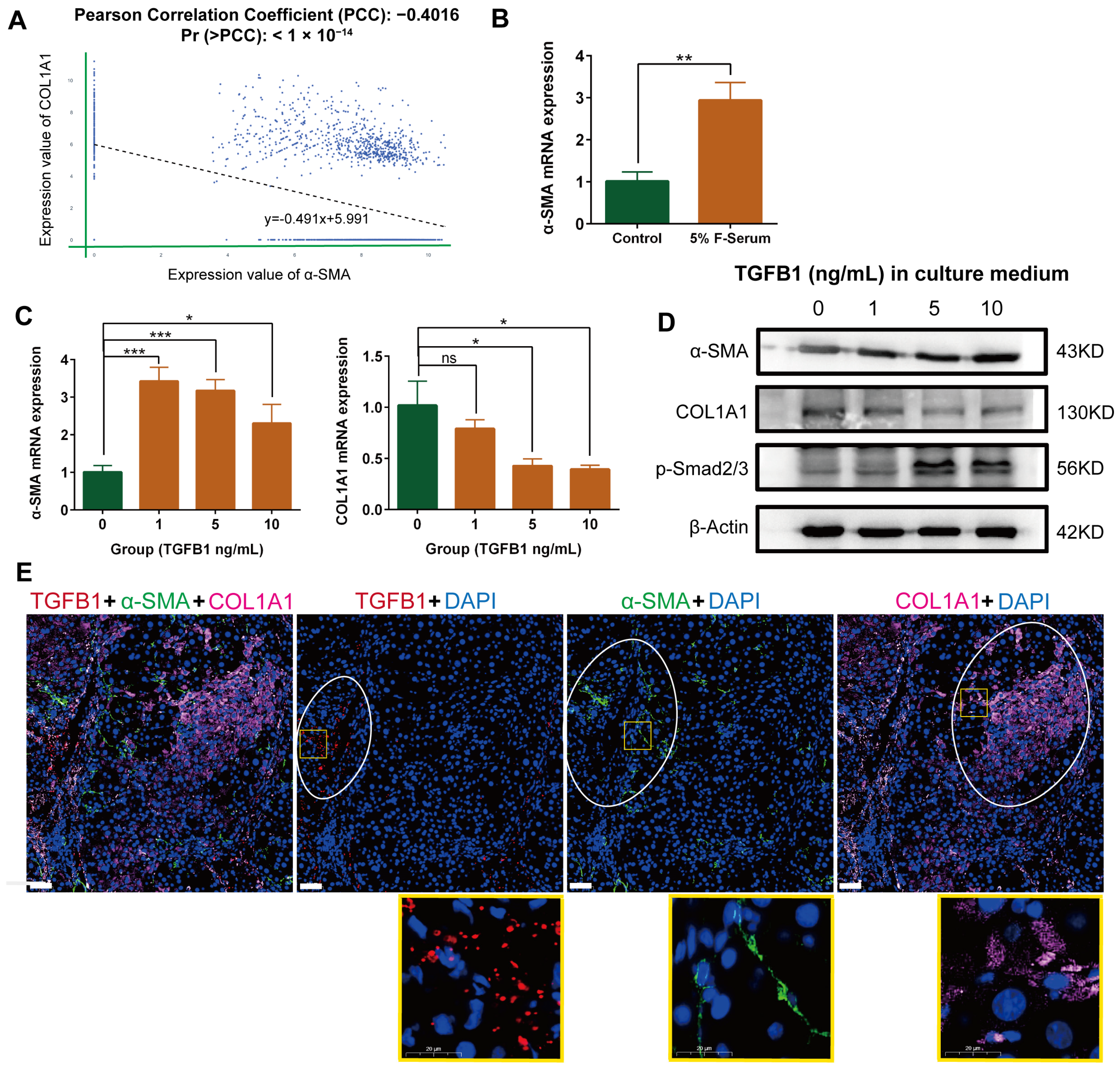
2.2. Negative Correlation between α-SMA and Type I Collagen, Alpha 1 (COL1A1) Expression in Myofibroblasts in Single Cell Database
2.3. Negative Correlation between α-SMA and COL1A1 Expression in HSC-Derived Myofibroblasts in the Presence of TGF-β1
2.4. Differential Localization of α-SMA, COL1A1, and TGF-β in Rat Fibrotic Liver
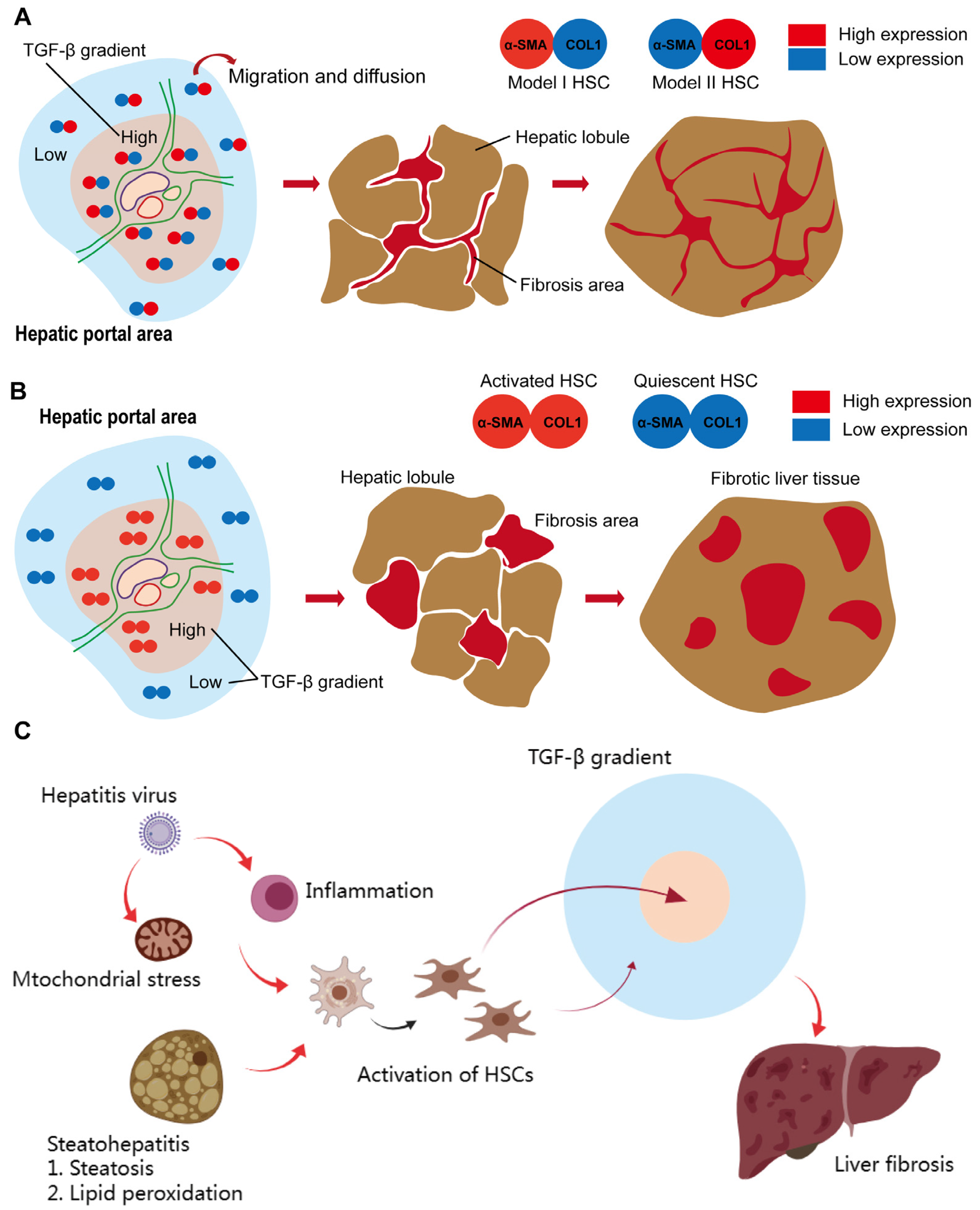
2.5. The Dual Mode Transition of Myofibroblasts in Fibrotic Liver (DMTM Model)
3. Materials and Methods
3.1. Cell Culture
3.2. Real-Time Quantitative RT-PCR (qRT-PCR)
3.3. Western Blot
3.4. Elisa
3.5. Animal Treatment
3.6. Sirius Red Staining
3.7. Immunofluorescence
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, F.D.; Zhou, J.; Chen, E.Q. Molecular Mechanisms and Potential New Therapeutic Drugs for Liver Fibrosis. Front. Pharmacol. 2022, 13, 787748. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Wei, B.; Fu, X.; Wang, C.; Wu, Y. Natural Products that Target the NLRP3 Inflammasome to Treat Fibrosis. Front. Pharmacol. 2020, 11, 591393. [Google Scholar] [CrossRef]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef] [PubMed]
- Moore-Morris, T.; Guimarães-Camboa, N.; Banerjee, I.; Zambon, A.C.; Kisseleva, T.; Velayoudon, A.; Stallcup, W.B.; Gu, Y.; Dalton, N.D.; Cedenilla, M.; et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J. Clin. Investig. 2014, 124, 2921–2934. [Google Scholar] [CrossRef] [PubMed]
- Akita, S.; Kubota, K.; Kobayashi, A.; Misawa, R.; Shimizu, A.; Nakata, T.; Yokoyama, T.; Takahashi, M.; Miyagawa, S. Role of bone marrow cells in the development of pancreatic fibrosis in a rat model of pancreatitis induced by a choline-deficient/ethionine-supplemented diet. Biochem. Biophys. Res. Commun. 2012, 420, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Haudek, S.B.; Cheng, J.; Du, J.; Wang, Y.; Hermosillo-Rodriguez, J.; Trial, J.; Taffet, G.E.; Entman, M.L. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J. Mol. Cell. Cardiol. 2010, 49, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradere, J.P.; Schwabe, R.F. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 2013, 4, 2823. [Google Scholar] [CrossRef]
- Berumen, J.; Baglieri, J.; Kisseleva, T.; Mekeel, K. Liver fibrosis: Pathophysiology and clinical implications. WIREs Mech. Dis. 2021, 13, e1499. [Google Scholar] [CrossRef]
- Kaftanovskaya, E.M.; Ng, H.H.; Soula, M.; Rivas, B.; Myhr, C.; Ho, B.A.; Cervantes, B.A.; Shupe, T.D.; Devarasetty, M.; Hu, X.; et al. Therapeutic effects of a small molecule agonist of the relaxin receptor ML290 in liver fibrosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 12435–12446. [Google Scholar] [CrossRef]
- Friedman, S.L. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. S1), 173–179. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Arnold, T.D.; Katamura, Y.; Giacomini, M.M.; Rodriguez, J.D.; McCarty, J.H.; Pellicoro, A.; Raschperger, E.; Betsholtz, C.; Ruminski, P.G.; et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. 2013, 19, 1617–1624. [Google Scholar] [CrossRef]
- Görtzen, J.; Schierwagen, R.; Bierwolf, J.; Klein, S.; Uschner, F.E.; van der Ven, P.F.; Fürst, D.O.; Strassburg, C.P.; Laleman, W.; Pollok, J.M.; et al. Interplay of Matrix Stiffness and c-SRC in Hepatic Fibrosis. Front. Physiol. 2015, 6, 359. [Google Scholar] [CrossRef]
- Mazagova, M.; Wang, L.; Anfora, A.T.; Wissmueller, M.; Lesley, S.A.; Miyamoto, Y.; Eckmann, L.; Dhungana, S.; Pathmasiri, W.; Sumner, S.; et al. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 1043–1055. [Google Scholar] [CrossRef]
- Tomita, K.; Teratani, T.; Suzuki, T.; Shimizu, M.; Sato, H.; Narimatsu, K.; Okada, Y.; Kurihara, C.; Irie, R.; Yokoyama, H.; et al. Free cholesterol accumulation in hepatic stellate cells: Mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology 2014, 59, 154–169. [Google Scholar] [CrossRef]
- Hinz, B. The myofibroblast: Paradigm for a mechanically active cell. J. Biomech. 2010, 43, 146–155. [Google Scholar] [CrossRef]
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Rønnov-Jessen, L.; Petersen, O.W. A function for filamentous alpha-smooth muscle actin: Retardation of motility in fibroblasts. J. Cell Biol. 1996, 134, 67–80. [Google Scholar] [CrossRef]
- van der Putten, C.; van den Broek, D.; Kurniawan, N.A. Myofibroblast transdifferentiation of keratocytes results in slower migration and lower sensitivity to mesoscale curvatures. Front. Cell Dev. Biol. 2022, 10, 930373. [Google Scholar] [CrossRef] [PubMed]
- Brenmoehl, J.; Miller, S.N.; Hofmann, C.; Vogl, D.; Falk, W.; Schölmerich, J.; Rogler, G. Transforming growth factor-beta 1 induces intestinal myofibroblast differentiation and modulates their migration. World J. Gastroenterol. 2009, 15, 1431–1442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chobert, M.N.; Couchie, D.; Fourcot, A.; Zafrani, E.S.; Laperche, Y.; Mavier, P.; Brouillet, A. Liver precursor cells increase hepatic fibrosis induced by chronic carbon tetrachloride intoxication in rats. Lab. Investig. J. Tech. Methods Pathol. 2012, 92, 135–150. [Google Scholar] [CrossRef]
- Ramachandran, P.; Dobie, R.; Wilson-Kanamori, J.R.; Dora, E.F.; Henderson, B.E.P.; Luu, N.T.; Portman, J.R.; Matchett, K.P.; Brice, M.; Marwick, J.A.; et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 2019, 575, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.; Lee, J.H.; Kim, K.M.; Park, E.Y.; Ku, S.K.; Cho, I.J.; Yang, J.H.; Ki, S.H. REDD1 attenuates hepatic stellate cell activation and liver fibrosis via inhibiting of TGF-β/Smad signaling pathway. Free Radic. Biol. Med. 2021, 176, 246–256. [Google Scholar] [CrossRef]
- Li, T.T.; Su, X.W.; Chen, L.L.; Zhang, W.N.; Zhang, J.P.; Wang, Y.; Xu, W.H. Roxarsone inhibits hepatic stellate cell activation and ameliorates liver fibrosis by blocking TGF-β1/Smad signaling pathway. Int. Immunopharmacol. 2023, 114, 109527. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Guerrieri, F.; Levrero, M. HBV, mitochondrial stress, and liver fibrosis: Chicken or the egg. Hepatology 2023, 77, 1088–1089. [Google Scholar] [CrossRef]
- Iannacone, M.; Guidotti, L.G. Immunobiology and pathogenesis of hepatitis B virus infection. Nat. Rev. Immunol. 2022, 22, 19–32. [Google Scholar] [CrossRef]
- Ma, B.; Ju, A.; Zhang, S.; An, Q.; Xu, S.; Liu, J.; Yu, L.; Fu, Y.; Luo, Y. Albumosomes formed by cytoplasmic pre-folding albumin maintain mitochondrial homeostasis and inhibit nonalcoholic fatty liver disease. Signal Transduct. Target. Ther. 2023, 8, 229. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, M.; Xie, Y.; Yao, J.; Li, X. The Dual-Mode Transition of Myofibroblasts Derived from Hepatic Stellate Cells in Liver Fibrosis. Int. J. Mol. Sci. 2023, 24, 15460. https://doi.org/10.3390/ijms242015460
Yan M, Xie Y, Yao J, Li X. The Dual-Mode Transition of Myofibroblasts Derived from Hepatic Stellate Cells in Liver Fibrosis. International Journal of Molecular Sciences. 2023; 24(20):15460. https://doi.org/10.3390/ijms242015460
Chicago/Turabian StyleYan, Mengchao, Ye Xie, Jia Yao, and Xun Li. 2023. "The Dual-Mode Transition of Myofibroblasts Derived from Hepatic Stellate Cells in Liver Fibrosis" International Journal of Molecular Sciences 24, no. 20: 15460. https://doi.org/10.3390/ijms242015460
APA StyleYan, M., Xie, Y., Yao, J., & Li, X. (2023). The Dual-Mode Transition of Myofibroblasts Derived from Hepatic Stellate Cells in Liver Fibrosis. International Journal of Molecular Sciences, 24(20), 15460. https://doi.org/10.3390/ijms242015460




