Histopathological Gap in Aortic Diseases: A Prospective Analysis
Abstract
:1. Introduction
2. Results
2.1. Clinicopathological Features and Study Design
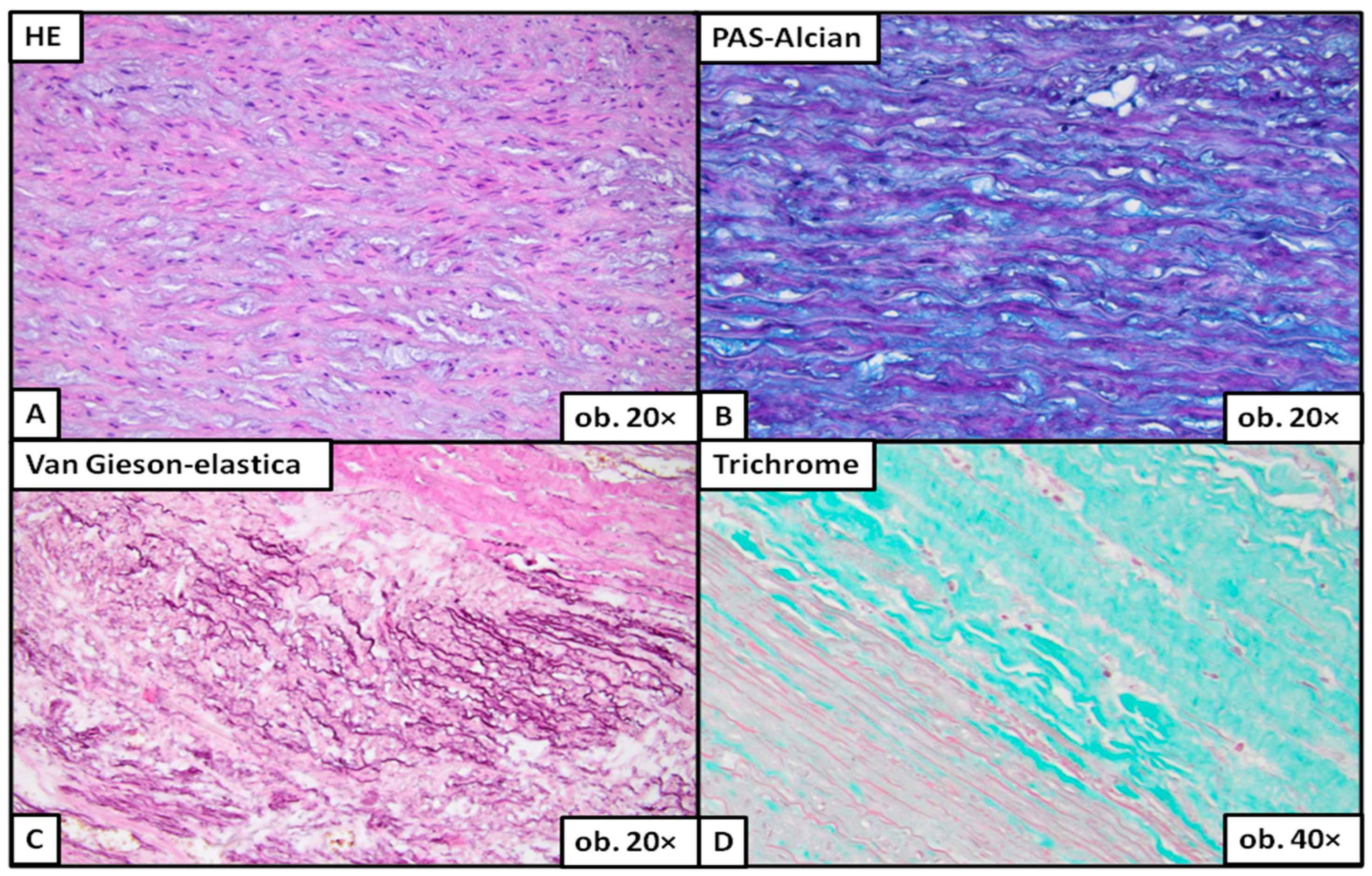
2.2. Histopathological Changes: Description
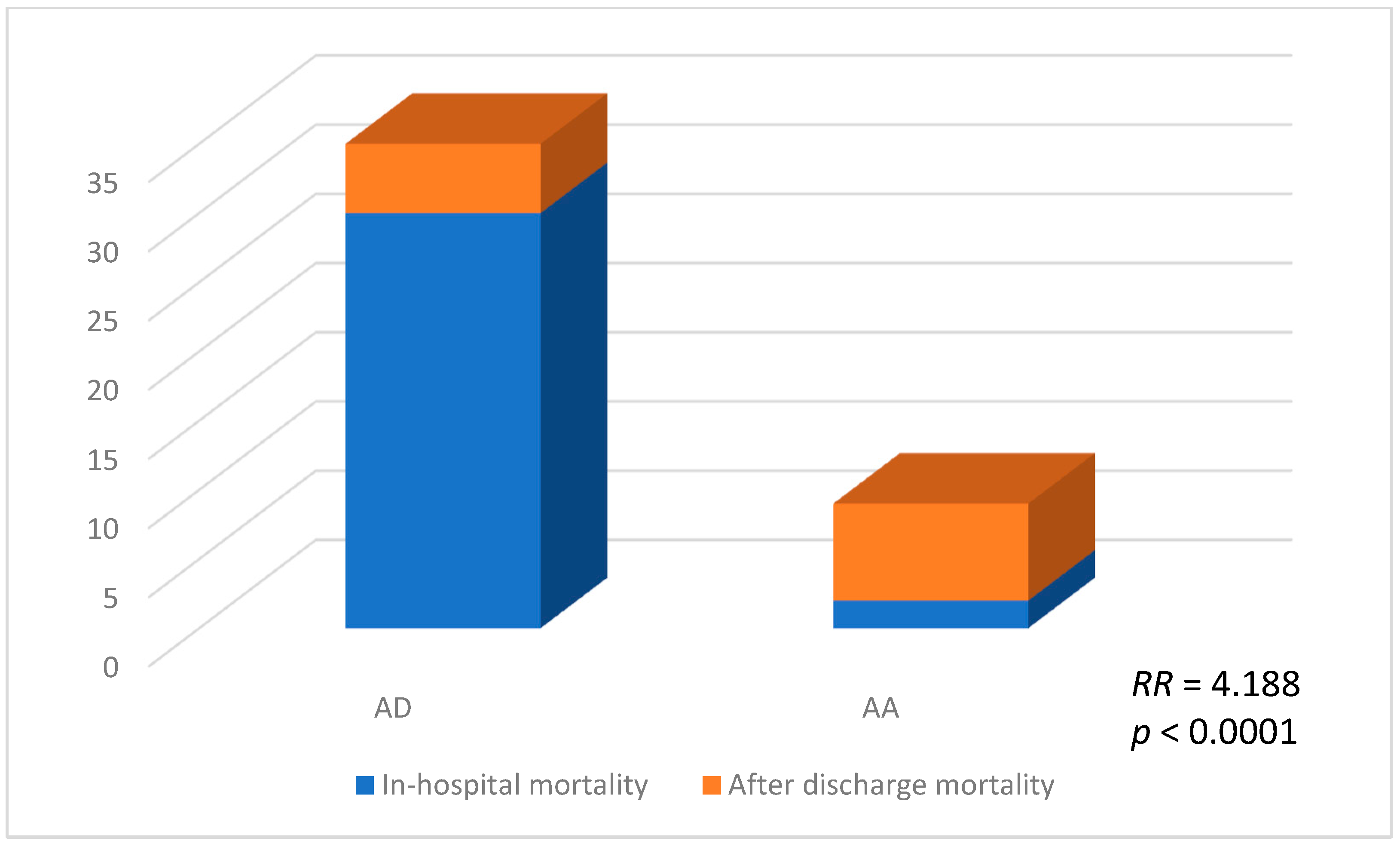
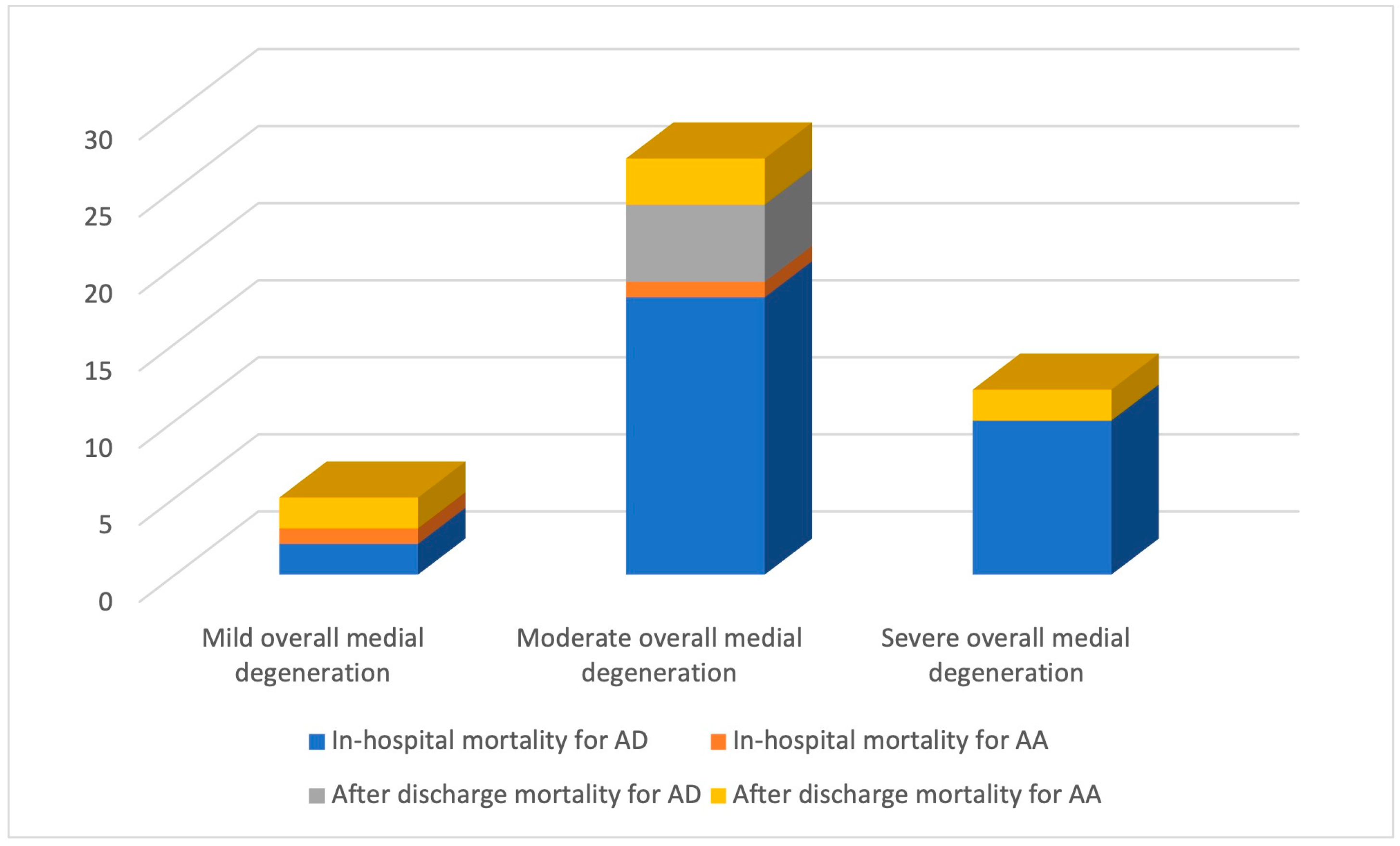
2.3. Histopathological Changes: Quantification
3. Discussion
Limitations
4. Materials and Methods
4.1. Histological Assessment
4.2. Statistical Tests and Standard Protocols
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, M.; Mestres, C.A.; Lavanchy, I.; Gerçek, M.; Van Hemelrijck, M.; Sromicki, J.; Vogt, P.; Reser, D. The impact of age and sex on in hospital outcomes in acute type A aortic dissection surgery. J. Thorac. Dis. 2022, 14, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Bawiskar, N.; Kamat, S.; Acharya, S.; Mohale, S.; Kumar, S. Type-A aortic dissection masquerading as acute inferior wall myocardial infarction—A rare case report. Med. Sci. 2022, 26, ms61e2007. [Google Scholar] [CrossRef]
- Yin, Z.Q.; Han, H.; Yan, X.; Zheng, Q.J. Research progress on the pathogenesis of aortic dissection. Curr. Probl. Cardiol. 2022, 48, 101249. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.; Rabasa, J.M.; Mosquera, V.X.; Barros, A.; Fernández-Tarrio, R.; Calvo-Iglesias, F.; Ferrera, C.; Rozado, J.; López-Ayerbe, J.; Garrote, C.; et al. Diagnosis, management and mortality in acute aortic syndrome: Results of the Spanish Registry of Acute Aortic Syndrome (RESA-II). Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Song, H.C. Role of PET/CT in the Evaluation of Aortic Disease. Chonnam Med. J. 2018, 54, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Berretta, P.; Trimarchi, S.; Patel, H.J.; Gleason, T.G.; Eagle, K.A.; Di Eusanio, M. Malperfusion syndromes in type A aortic dissection: What we have learned from IRAD. J. Vis. Surg. 2018, 4, 65. [Google Scholar] [CrossRef]
- Morello, F.; Santoro, M.; Fargion, A.T.; Grifoni, S.; Nazerian, P. Diagnosis and management of acute aortic syndromes in the emergency department. Intern. Emerg. Med. 2021, 16, 171–181. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, N.; Xu, S.; Fan, Z.; Zhu, J.; Huang, L.; Chen, D.; Sun, Z.; Sun, L. Acute type A aortic intramural hematoma and type A aortic dissection: Correlation between the intimal tear features and pathogenesis. Quant. Imaging. Med. Surg. 2020, 10, 1504–1514. [Google Scholar] [CrossRef]
- Moritz, A.R. Medionecrosis Aortae Idiopathica Cystica. Am. J. Pathol. 1932, 8, 717–734.3. [Google Scholar]
- Yuan, S.M.; Jing, H. Cystic medial necrosis: Pathological findings and clinical implications. Rev. Bras. Cir. Cardiovasc. 2011, 26, 107–115, Erratum in Rev. Bras. Cir. Cardiovasc. 2012, 27, 173. [Google Scholar] [CrossRef]
- Osada, H.; Kyogoku, M.; Matsuo, T.; Kanemitsu, N. Histopathological evaluation of aortic dissection: A comparison of congenital versus acquired aortic wall weakness. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Halushka, M.K.; Angelini, A.; Bartoloni, G.; Basso, C.; Batoroeva, L.; Bruneval, P.; Buja, L.M.; Butany, J.; D’Amati, G.; Fallon, J.T.; et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association For European Cardiovascular Pathology: II. Noninflammatory degenerative diseases—Nomenclature and diagnostic criteria. Cardiovasc. Pathol. 2016, 25, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, L.; Li, T.; Xi, Z.; Wu, H.; Li, D. Surgical treatment of type A acute aortic dissection with cerebral malperfusion: A systematic review. J. Cardiothorac. Surg. 2022, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Watanuki, H.; Tochii, M.; Futamura, Y.; Kitagawa, Y.; Makino, S.; Ohashi, W.; Matsuyama, K. Impact of GERAADA score in patients with acute type A aortic dissection. J. Cardiothorac. Surg. 2022, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Evangelista Masip, A.; Lopez-Sainz, A.; Barros Membrilla, A.J.; Iglesias, F.C.; Ayerbe, J.L.; Molluna, M.A.; Rodríguez, V.X.M.; Montoya, F.A.; Fernández, R.T.; Orodea, A.R.; et al. RESA centres. Spanish Registry of Acute Aortic Syndrome (RESA). Changes in therapeutic management and lower mortality in acute aortic syndrome. Rev. Esp. Cardiol. 2022, 75, 816–824. [Google Scholar] [PubMed]
- Leone, O.; Pacini, D.; Foà, A.; Corsini, A.; Agostini, V.; Corti, B.; Di Marco, L.; Leone, A.; Lorenzini, M.; Reggiani, L.B.; et al. Redefining the histopathologic profile of acute aortic syndromes: Clinical and prognostic implications. J. Thorac. Cardiovasc. Surg. 2018, 156, 1776–1785.e6. [Google Scholar] [CrossRef]
- Fujiyoshi, T.; Obikane, H.; Nagao, T.; Ogino, H. Relationship between the aortic root Z-score and degree of translamellar mucoid extracellular matrix accumulation. Surg. Today. 2022, 52, 408–413. [Google Scholar] [CrossRef]
- Malaisrie, S.C.; Szeto, W.Y.; Halas, M.; Girardi, L.N.; Coselli, J.S.; Sundt, T.M.; Chen, E.P.; Fischbein, M.P.; Gleason, T.G.; Okita, Y.; et al. The American Association for Thoracic Surgery expert consensus document: Surgical treatment of acute type A aortic dissection. J. Thorac. Cardiovasc. Surg. 2021, 162, 735–758.e2. [Google Scholar] [CrossRef]
- Farag, M.; Büsch, C.; Rylski, B.; Pöling, J.; Dohle, D.S.; Sarvanakis, K.; Hagl, C.; Krüger, T.; Detter, C.; Holubec, T.; et al. Early outcomes of patients with Marfan syndrome and acute aortic type A dissection. J. Thorac. Cardiovasc. Surg. 2021, 166, 25–34.e8. [Google Scholar] [CrossRef]
- Pisano, C.; Balistreri, C.R.; Nardi, P.; Altieri, C.; Bertoldo, F.; Buioni, D.; Ferrante, M.S.; Asta, L.; Trombetti, D.; Ruvolo, G. Risk of aortic dissection in patients with ascending aorta aneurysm: A new biological, morphological, and biomechanical network behind the aortic diameter. Vessel. Plus. 2020, 4, 33. [Google Scholar] [CrossRef]
- Haverich, A.; Boyle, E.C. Aortic dissection is a disease of the vasa vasorum. JTCVS Open 2021, 5, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Calafiore, A.M.; Katsavrias, K.; Di Marco, M.; Guarracini, S.; Di Mauro, M. Commentary: Vasa vasorum dysfunction and acute aortic syndromes: When guidelines do not follow the evolution of knowledge. JTCVS Open 2021, 5, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, K.; Mousseaux, E.; Ishibashi-Ueda, H.; Achouh, P.; Ochiai, M.; Bruneval, P. Impact of histopathological changes in ascending aortic diseases. Int. J. Cardiol. 2020, 311, 91–96. [Google Scholar] [CrossRef] [PubMed]
- De Martino, A.; Ballestracci, P.; Faggioni, L.; Pratali, S.; Morganti, R.; Pucci, A.; Bortolotti, U. Incidence of Aortitis in Surgical Specimens of the Ascending Aorta Clinical Implications at Follow-Up. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Juraszek, A.; Czerny, M.; Rylski, B. Update in aortic dissection. Trends. Cardiovasc. Med. 2021, 32, 456–461. [Google Scholar] [CrossRef]
- Mehta, C.K.; Son, A.Y.; Chia, M.C.; Budd, A.N.; Allen, B.D.; Vassallo, P.; Hoel, A.W.; Brady, W.J.; Nable, J.V. Management of acute aortic syndromes from initial presentation to definitive treatment. Am. J. Emerg. Med. 2022, 51, 108–113. [Google Scholar] [CrossRef]
- Yuan, X.; Clough, R.E.; Nienaber, C.A. Acute aortic syndrome. Medicine 2022, 50, 449–452. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Morrow, D.A. New clues for optimal diagnosis and treatment of an old foe: The acute aortic syndrome. Eur. Heart. J. Acute. Cardiovasc. Care. 2020, 9, S1–S2. [Google Scholar] [CrossRef]
- Pupovac, S.S.; Hemli, J.M.; Bavaria, J.E.; Patel, H.J.; Trimarchi, S.; Pacini, D.; Bekeredjian, R.; Chen, E.P.; Myrmel, T.; Ouzounian, M.; et al. Moderate Versus Deep Hypothermia in Type A Acute Aortic Dissection Repair: Insights from the International Registry of Acute Aortic Dissection. Ann. Thorac. Surg. 2021, 112, 1893–1899. [Google Scholar] [CrossRef]
- Freundt, M.; Kolat, P.; Friedrich, C.; Salem, M.; Gruenewald, M.; Elke, G.; Pühler, T.; Cremer, J.; Haneya, A. Preoperative Predictors of Adverse Clinical Outcome in Emergent Repair of Acute Type A Aortic Dissection in 15 Year Follow Up. J. Clin. Med. 2021, 10, 5370. [Google Scholar] [CrossRef]
- Vagnarelli, F.; Corsini, A.; Lorenzini, M.; Pacini, D.; Ferlito, M.; Reggiani, M.L.B.; Longhi, S.; Nanni, S.; Norscini, G.; Cinti, L.; et al. Acute heart failure in patients with acute aortic syndrome: Pathophysiology and clinical-prognostic implications. Eur. J. Heart. Fail. 2015, 17, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Milewicz, D.M.; Guo, D.; Hostetler, E.; Marin, I.; Pinard, A.C.; Cecchi, A.C. Update on the genetic risk for thoracic aortic aneurysms and acute aortic dissections: Implications for clinical care. J. Cardiovasc. Surg. 2021, 62, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Bossone, E.; Gorla, R.; Ranieri, B.; Russo, V.; Jakob, H.; Erbel, R. Initiating a New Era of Cardiovascular Diagnosis and Therapy in Acute Aortic Syndromes: The Mainz-Essen Experience (Part I)-Imaging and Biomarkers. Aorta 2021, 9, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Endo, D.; Shimada, A.; Matsushita, S.; Asai, T.; Amano, A. Surgical treatment of acute aortic dissection in a patient with SLE and prior antiphospholipid syndrome on therapy for over 30 years: A case report. BMC Cardiovasc. Disord. 2022, 22, 216. [Google Scholar] [CrossRef]
- Guimbretière, G.; Nusinovici, S.; Monnot, A.; Sobocinski, J.; Sénage, T.; Delsart, P.; Gourraud, P.-A.; Maurel, B. Impact of Relative Change in Temperature and Atmospheric Pressure on Acute Aortic Syndrome Occurrence in France. Sci. Rep. 2020, 10, 76. [Google Scholar] [CrossRef]
- Huckaby, L.V.; Sultan, I.; Trimarchi, S.; Leshnower, B.; Chen, E.P.; Brinster, D.R.; Myrmel, T.; Estrera, A.L.; Montgomery, D.G.; Korach, A.; et al. Sex-Based Aortic Dissection Outcomes From the International Registry of Acute Aortic Dissection. Ann. Thorac. Surg. 2022, 113, 498–505. [Google Scholar] [CrossRef]
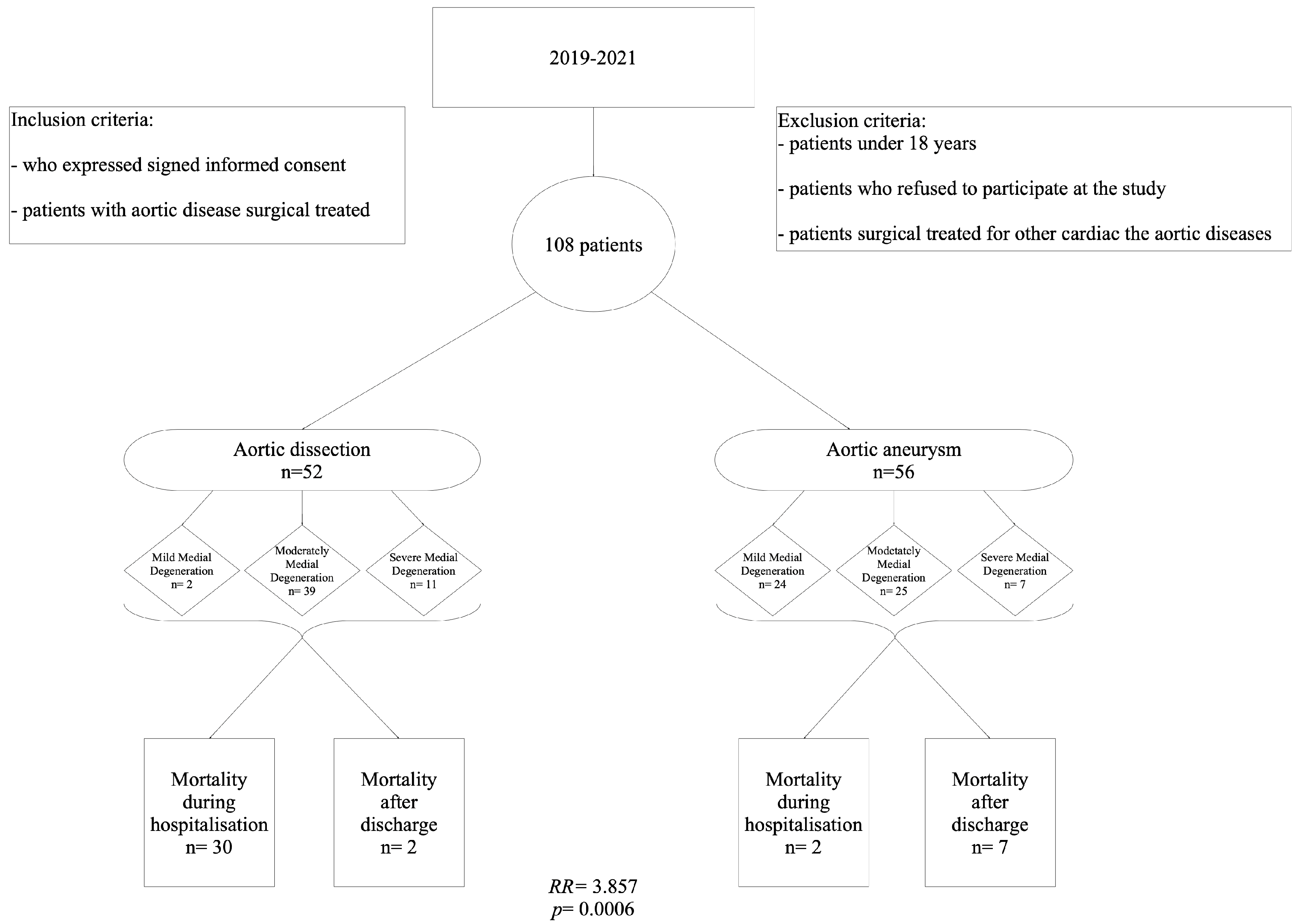


| Parameter | Aortic Dissection (n = 52) | Aortic Aneurysm (n = 56) | OR | p-Value |
|---|---|---|---|---|
| Male | 36 (69.23%) | 42 (75%) | 0.8654 | 0.5266 |
| Female | 16 (30.77%) | 14 (25%) | ||
| Age (mean ± SD) (median) | 62.42 ± 12.79 | 57.20 ± 9.572 | - | 0.0072 |
| Hospitalization days (mean ± SD) | 8.769 ± 7.722 | 11.11 ± 11.20 | - | 0.1631 |
| Parameter | Aortic Dissection (n = 52) | Aortic Aneurysm (n = 56) | OR | p-Value |
|---|---|---|---|---|
| Preoperative | ||||
| Pericardial effusion | 21 (40.38%) | 0 (0.00%) | 77.127 | <0.0001 |
| Cardiac tamponade | 10 (19.23%) | 0 (0.00%) | 27.918 | 0.0004 |
| Malperfusion syndrome | 2 (3.85%) | 0 (0.00%) | 5.594 | 0.2295 |
| Pleural effusion | 0 (0.00%) | 1 (1.79%) | 2.3186 | 0.9999 |
| Acute kidney injury | 6 (11.54%) | 7 (12.50%) | 0.9532 | 0.9999 |
| Chronic kidney diseases | 1 (1.92%) | 2 (3.57%) | 0.6863 | 0.9999 |
| Stroke | 1 (1.92%) | 0 (0.00%) | 2.098 | 0.4815 |
| Syncope | 2 (3.85%) | 0 (0.00%) | 5.594 | 0.2295 |
| Systemic hypertension | 32 (61.54%) | 18 (32.14%) | 3.378 | 0.0036 |
| Bicuspid aortic valve | 17 (30.36%) | 3 (5.77%) | 7.120 | 0.0011 |
| Aortic valve regurgitation | 32 (61.54%) | 27 (48.21%) | 1.1719 | 0.1809 |
| Aortic valve stenosis | 2 (3.85%) | 7 (12.50%) | 0.2800 | 0.1638 |
| Atrial fibrillation | 28 (50.00%) | 7 (13.46%) | 6.429 | <0.0001 |
| Postoperative | ||||
| Days in the ICU (mean ± SD) | 2.750 ± 2.317 | 4.268 ± 5.786 | - | 0.6173 |
| Intubation time-hours (mean ± SD) | 36.63 ± 51.31 | 65.90 ± 130.4 | - | 0.2969 |
| Hepatic dysfunction | 18 (34.62%) | 23 (41.07%) | 0.7596 | 0.5541 |
| Renal dysfunction | 11 (21.15%) | 15 (26.79%) | 0.7333 | 0.5100 |
| Hemofiltration | 7 (13.46%) | 11 (19.64%) | 0.6364 | 0.4461 |
| Systemic infection | 2 (3.85%) | 4 (7.14%) | 0.5200 | 0.6799 |
| Reintervention | 10 (19.23%) | 7 (12.50%) | 1.667 | 0.4304 |
| Histological Medial Degenerative Change | Degenerative Degree | Aortic Dissection (n = 52) | Aortic Aneurysm (n = 56) | p-Value |
|---|---|---|---|---|
| Interlamellar (MEMA-i) | Mild | 0 (0.00%) | 6 (10.71%) | <0.0001 |
| Moderate | 9 (17.31%) | 24 (42.86%) | ||
| Severe | 43 (82.69%) | 26 (46.43%) | ||
| Translamellar (MEMA-t) | Mild | 4 (7.69%) | 20 (40.00%) | 0.0001 |
| Moderate | 40 (79.62%) | 24 (48.00%) | ||
| Severe | 8 (15.38%) | 6 (12.00%) | ||
| Elastic fragmentation or loss | Mild | 11 (21.15%) | 29 (54.72%) | 0.0011 |
| Moderate | 37 (71.15%) | 20 (37.74%) | ||
| Severe | 4 (7.69%) | 4 (7.55%) | ||
| Smooth muscle cell nuclei loss | Mild | 26 (50.98%) | 26 (70.27%) | <0.0001 |
| Moderate | 23 (45.10%) | 10 (27.03%) | ||
| Severe | 2 (3.92%) | 1 (2.70%) | ||
| Laminar medial collapse | Mild | 22 (45.83%) | 24 (60.00%) | 0.0223 |
| Moderate | 22 (45.83%) | 14 (35.00%) | ||
| Severe | 4 (8.33%) | 2 (5.00%) |
| Histological Medial Degenerative Change | Degenerative Degree | Subdivision | Aortic Dissection (n = 52) | Aortic Aneurysm (n = 56) | p-Value |
|---|---|---|---|---|---|
| Interlamellar (MEMA-i) | Mild | Multifocal | 0 (0.00%) | 4 (100%) | 0.2378 |
| Focal | 0 (0.00%) | 2 (100%) | |||
| Moderate | Multifocal | 9 (30.00%) | 21 (70.00%) | 0.5447 | |
| Focal | 0 (0.00%) | 3 (100%) | |||
| Severe | Multifocal | 40 (60.61%) | 26 (39.39%) | 0.2852 | |
| Focal | 3 (100%) | 0 (0.00%) | |||
| Translamellar (MEMA-t) | Mild | Multifocal | 3 (23.08%) | 10 (76.92%) | 0.5963 |
| Focal | 1 (9.09%) | 10 (90.91%) | |||
| Moderate | Multifocal | 29 (69.05%) | 13 (30.95%) | 0.1768 | |
| Focal | 11 (50.00%) | 11 (50.00%) | |||
| Severe | Multifocal | 4 (44.44%) | 5 (55.56%) | 0.3007 | |
| Focal | 4 (80.00%) | 1 (20.00%) | |||
| Elastic fragmentation or loss | Mild | Multifocal | 5 (31.32%) | 11 (68.57%) | 0.7275 |
| Focal | 6 (25.00%) | 18 (75.00%) | |||
| Moderate | Multifocal | 22 (61.11%) | 14 (38.89%) | 0.5675 | |
| Focal | 15 (71.43%) | 6 (28.57%) | |||
| Severe | Multifocal | 1 (20.00%) | 4 (80.00%) | 0.1429 | |
| Focal | 3 (100%) | 0 (0.00%) | |||
| Smooth muscle cell nuclei loss | Mild | Multifocal | 12 (50.00%) | 12 (50.00%) | 0.9999 |
| Focal | 14 (50.00%) | 14 (50.00%) | |||
| Moderate | Multifocal | 12 (63.16%) | 7 (36.84%) | 0.4551 | |
| Focal | 11 (78.57%) | 3 (21.43%) | |||
| Severe | Multifocal | 2 (100%) | 0 (0.00%) | - | |
| Focal | 0 (0.00%) | 1 (100%) | |||
| Laminar medial collapse | Mild | Multifocal | 14 (73.68%) | 5 (26.32%) | 0.0063 |
| Focal | 8(29.63%) | 19(70.37%) | |||
| Moderate | Multifocal | 13 (61.90%) | 8 (38.10%) | 0.9999 | |
| Focal | 9 (60.00%) | 6 (40.00%) | |||
| Severe | Multifocal | 2 (100%) | 0 (0.00%) | 0.4667 | |
| Focal | 2 (50.00%) | 2 (50.00%) |
| Severity | Aortic Dissection | Aortic Aneurysm | p-Value |
|---|---|---|---|
| Mild (n = 26) | 2 (7.69%) | 24 (92.31%) | <0.0001 |
| Moderate (n = 64) | 39 (60.94%) | 25 (39.06%) | |
| Severe (n = 18) | 11 (61.11%) | 7 (38.89%) |
| In-Hospital Mortality | |||||
|---|---|---|---|---|---|
| Predictors | B | Exp(B) | 95% CI for EXP(B) | p-value | |
| Lower | Upper | ||||
| Histopathologic score | 1.123 | 3.073 | 1.467 | 6.439 | 0.0029 |
| Overall Mortality | |||||
| Predictors | B | Exp(B) | 95% CI for EXP(B) | p-value | |
| Lower | Upper | ||||
| Histopathologic score | 1.065 | 2.900 | 1.455 | 5.779 | 0.0025 |
| Mucoid Extracellular Matrix Accumulation, Intralamellar (MEMA-i) | Mucoid Extracellular Matrix Accumulation, Translamellar (MEMA-t) | Elastic Fiber Fragmentation and Loss | Loss of Smooth Muscle Cell Nuclei | Laminar Medial Collapse | Overall Medial Degeneration | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Absent | Absent | Absent | Absent | Absent | ABSENT (score 0) | |||||
| Mild | Mild | Mild | Mild | Mild | MILD (score 1) | |||||
| Focal | Multifocal | Focal | Multifocal | Focal | Multifocal | Focal | Multifocal | Focal | Multifocal | |
| Moderate | Moderate | Moderate | Moderate | Moderate | MODERATE (score 2) | |||||
| Focal | Multifocal | Focal | Multifocal | Focal | Multifocal | Focal | Multifocal | Focal | Multifocal | |
| Severe | Severe | Severe | Severe | Severe | SEVERE (score 3) | |||||
| Focal | Multifocal | Focal | Multifocal | Focal | Multifocal | Focal | Multifocal | Focal | Multifocal | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banceu, C.M.; Gurzu, S.; Satala, C.-B.; Ghiga, D.; Neamtu, M.H.; Voth, V.; Liebrich, M.; Suciu, H. Histopathological Gap in Aortic Diseases: A Prospective Analysis. Int. J. Mol. Sci. 2023, 24, 15470. https://doi.org/10.3390/ijms242015470
Banceu CM, Gurzu S, Satala C-B, Ghiga D, Neamtu MH, Voth V, Liebrich M, Suciu H. Histopathological Gap in Aortic Diseases: A Prospective Analysis. International Journal of Molecular Sciences. 2023; 24(20):15470. https://doi.org/10.3390/ijms242015470
Chicago/Turabian StyleBanceu, Cosmin Marian, Simona Gurzu, Catalin-Bogdan Satala, Dana Ghiga, Mihai Halic Neamtu, Vladimir Voth, Markus Liebrich, and Horatiu Suciu. 2023. "Histopathological Gap in Aortic Diseases: A Prospective Analysis" International Journal of Molecular Sciences 24, no. 20: 15470. https://doi.org/10.3390/ijms242015470
APA StyleBanceu, C. M., Gurzu, S., Satala, C.-B., Ghiga, D., Neamtu, M. H., Voth, V., Liebrich, M., & Suciu, H. (2023). Histopathological Gap in Aortic Diseases: A Prospective Analysis. International Journal of Molecular Sciences, 24(20), 15470. https://doi.org/10.3390/ijms242015470







