The OSR9 Regimen: A New Augmentation Strategy for Osteosarcoma Treatment Using Nine Older Drugs from General Medicine to Inhibit Growth Drive
Abstract
1. Introduction
Preface: In trying to hold onto everything, one holds onto nothing.King Fredrick the Great, 1712–1786
- Patchy cortical bone destruction
- Areas of sclerotic bone
- Wide and gradual transitions from normal-appearing trabecular bone to clearly abnormal, moth eaten, and irregularly mineralized bone
- Peritumoral soft tissue edema
- Codman’s triangle (irregular periosteum that has lifted off where advancing tumor margins destroy periosteal new bone formation faster than it can ossify)
2. The Need for Both Repurposed and Multidrug Regimens in OS
2.1. Why Many Drugs Are Needed
- Absent a “silver bullet”, many drugs may be needed to address tumors’ readily evolving and multiple resistance pathways and their ability to shift reliance to alternate growth drives when the one in use becomes blocked.
- The common metastatic deadly cancers have metabolic flexibility, shifting between aerobic oxphos, aerobic glycolysis, anaerobic glycolysis, beta-oxidation, autophagy, glutamine, and other energy sources as circumstances dictate.
- The common metastatic deadly cancers are hardy tissues with growth drive flexibility. The many alternate growth driving pathways can cross-cover for any one or two that become blocked.
- Inherent multiple subpopulations exist in these cancers, even before any treatment. Each subpopulation has its own particular set of growth drives and inhibition susceptibilities and resistances. Treatment-induced selection pressure favors the development of resistant clones.
- Ionizing irradiation and many common cytotoxic chemotherapies that are currently used to treat cancer are mutagens that hasten responses to selection pressure.
2.2. Why Repurposed Non-Oncology Drugs Are Needed
- We are constrained in treating today’s illnesses with today’s tools.
- Directly cytotoxic and genotoxic drugs have limits on how many, how much, and how often they can be used without destroying bone marrow or other essential body systems.
- Cancers, generally, and OS, specifically, use normal physiological growth drive pathways, but they pathologically and exaggeratedly engage them. We have many drugs in general medical practice with established safety for influencing those core mammalian systems.
- Repurposed drugs from general medical practice are cheap, generic, readily available, and well-tolerated, and GPs worldwide are already familiar with their use.
3. The OSR9 Drugs
3.1. The Anti-Nausea Drug Aprepitant
3.2. The Analgesic Celecoxib
3.3. The Antimalarial Drug Chloroquine
3.4. The Antibiotic Dapsone
3.5. The Alcoholism Treatment Drug Disulfiram
3.6. The Antifungal Drug Itraconazole
3.7. The Anti-Diabetes Drug Linagliptin
- OS overexpress RUNX-2, yet the malignant clone fails to mature
- OS overexpress DPP-4
- P-AMPK is required for RUNX-2 to function in osteoblast maturation
- gliptins promote AMPK phosphorylation
3.8. The Antihypertension Drug Propranolol
3.9. The Psychiatric Drug Quetiapine
4. TICO
5. Discussion and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panez-Toro, I.; Muñoz-García, J.; Vargas-Franco, J.W.; Renodon-Cornière, A.; Heymann, M.-F.; Lézot, F.; Heymann, D. Advances in Osteosarcoma. Curr. Osteoporos. Rep. 2023, 21, 330–343. [Google Scholar] [CrossRef]
- Mortus, J.R.; Zhang, Y.; Hughes, D.P.M. Developmental Pathways Hijacked by Osteosarcoma. Adv. Exp. Med. Biol. 2014, 804, 93–118. [Google Scholar] [CrossRef]
- Lindsey, B.A.; Markel, J.E.; Kleinerman, E.S. Osteosarcoma Overview. Rheumatol. Ther. 2017, 4, 25–43. [Google Scholar] [CrossRef]
- Thomas, D.M.; Johnson, S.A.; Sims, N.A.; Trivett, M.K.; Slavin, J.L.; Rubin, B.P.; Waring, P.; McArthur, G.A.; Walkley, C.R.; Holloway, A.J. Terminal osteoblast differentiation, mediated by runx2 and p27KIP1, is disrupted in osteosarcoma. J. Cell Biol. 2004, 167, 925–934. [Google Scholar] [CrossRef]
- Martin, J.W.; Zielenska, M.; Stein, G.S.; van Wijnen, A.J.; Squire, J.A. The Role of RUNX2 in Osteosarcoma Oncogenesis. Sarcoma 2011, 2011, 282745. [Google Scholar] [CrossRef]
- Cole, S.; Gianferante, D.M.; Zhu, B.; Mirabello, L. Osteosarcoma: A Surveillance, Epidemiology, and End Results program-based analysis from 1975 to 2017. Cancer 2022, 128, 2107–2118. [Google Scholar] [CrossRef]
- Mirabello, L.; Zhu, B.; Koster, R.; Karlins, E.; Dean, M.; Yeager, M.; Gianferante, M.; Spector, L.G.; Morton, L.M.; Karyadi, D.; et al. Frequency of Pathogenic Germline Variants in Cancer-Susceptibility Genes in Patients With Osteosarcoma. JAMA Oncol. 2020, 6, 724–734. [Google Scholar] [CrossRef]
- Kast, R.E.; Alfieri, A.; Assi, H.I.; Burns, T.C.; Elyamany, A.M.; Gonzalez-Cao, M.; Karpel-Massler, G.; Marosi, C.; Salacz, M.E.; Sardi, I.; et al. MDACT: A New Principle of Adjunctive Cancer Treatment Using Combinations of Multiple Repurposed Drugs, with an Example Regimen. Cancers 2022, 14, 2563. [Google Scholar] [CrossRef]
- Halatsch, M.-E.; Kast, R.E.; Karpel-Massler, G.; Mayer, B.; Zolk, O.; Schmitz, B.; Scheuerle, A.; Maier, L.; Bullinger, L.; Mayer-Steinacker, R.; et al. A phase Ib/IIa trial of 9 repurposed drugs combined with temozolomide for the treatment of recurrent glioblastoma: CUSP9v3. Neuro-Oncol. Adv. 2021, 3, vdab075. [Google Scholar] [CrossRef]
- Kast, R.E.; Karpel-Massler, G.; Halatsch, M.-E. CUSP9* treatment protocol for recurrent glioblastoma: Aprepitant, artesunate, auranofin, captopril, celecoxib, disulfiram, itraconazole, ritonavir, sertraline augmenting continuous low dose temozolomide. Oncotarget 2014, 5, 8052–8082. [Google Scholar] [CrossRef]
- Kast, R.E.; Boockvar, J.A.; Brüning, A.; Cappello, F.; Chang, W.-W.; Cvek, B.; Dou, Q.P.; Duenas-Gonzalez, A.; Efferth, T.; Focosi, D.; et al. A conceptually new treatment approach for relapsed glioblastoma: Coordinated undermining of survival paths with nine repurposed drugs (CUSP9) by the International Initiative for Accelerated Improvement of Glioblastoma Care. Oncotarget 2013, 4, 502–530. [Google Scholar] [CrossRef]
- Asija, S.; Chatterjee, A.; Yadav, S.; Chekuri, G.; Karulkar, A.; Jaiswal, A.K.; Goda, J.S.; Purwar, R. Combinatorial approaches to effective therapy in glioblastoma (GBM): Current status and what the future holds. Int. Rev. Immunol. 2022, 41, 582–605. [Google Scholar] [CrossRef]
- Dahlberg, D.; Rummel, J.; Distante, S.; De Souza, G.A.; Stensland, M.E.; Mariussen, E.; Rootwelt, H.; Voie, Ø.; Hassel, B. Glioblastoma microenvironment contains multiple hormonal and non-hormonal growth-stimulating factors. Fluids Barriers CNS 2022, 19, 45. [Google Scholar] [CrossRef]
- Kilmister, E.J.; Koh, S.P.; Weth, F.R.; Gray, C.; Tan, S.T. Cancer Metastasis and Treatment Resistance: Mechanistic Insights and Therapeutic Targeting of Cancer Stem Cells and the Tumor Microenvironment. Biomedicines 2022, 10, 2988. [Google Scholar] [CrossRef]
- Martell, E.; Kuzmychova, H.; Senthil, H.; Kaul, E.; Chokshi, C.R.; Venugopal, C.; Anderson, C.M.; Singh, S.K.; Sharif, T. Compensatory cross-talk between autophagy and glycolysis regulates senescence and stemness in heterogeneous glioblastoma tumor subpopulations. Acta Neuropathol. Commun. 2023, 11, 110. [Google Scholar] [CrossRef]
- Nguyen, H.S.; Shabani, S.; Awad, A.J.; Kaushal, M.; Doan, N. Molecular Markers of Therapy-Resistant Glioblastoma and Potential Strategy to Combat Resistance. Int. J. Mol. Sci. 2018, 19, 1765. [Google Scholar] [CrossRef]
- Palmer, A.C.; Chidley, C.; Sorger, P.K. A curative combination cancer therapy achieves high fractional cell killing through low cross-resistance and drug additivity. eLife 2019, 8, e50036. [Google Scholar] [CrossRef]
- Shafi, O.; Siddiqui, G. Tracing the origins of glioblastoma by investigating the role of gliogenic and related neurogenic genes/signaling pathways in GBM development: A systematic review. World J. Surg. Oncol. 2022, 20, 146. [Google Scholar] [CrossRef]
- Westermarck, J. Inhibition of adaptive therapy tolerance in cancer: Is triplet mitochondrial targeting the key? Mol. Oncol. 2023, 17, 537–540. [Google Scholar] [CrossRef]
- Pongratz, P.; Kurth, F.; Ngoma, G.M.; Basra, A.; Ramharter, M. In vitro activity of antifungal drugs against Plasmodium falciparum field isolates. Wien. Klin. Wochenschr. 2011, 123 (Suppl. S1), 26–30. [Google Scholar] [CrossRef]
- Aapro, M.; Carides, A.; Rapoport, B.L.; Schmoll, H.-J.; Zhang, L.; Warr, D. Aprepitant and Fosaprepitant: A 10-Year Review of Efficacy and Safety. Oncologist 2015, 20, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Padilla, A.; Habib, A.S. A pharmacological overview of aprepitant for the prevention of postoperative nausea and vomiting. Expert Rev. Clin. Pharmacol. 2023, 16, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E.; Ramiro, S.; Lladó, S.; Toro, S.; Coveñas, R.; Muñoz, M. Antitumor action of temozolomide, ritonavir and aprepitant against human glioma cells. J. Neuro-Oncol. 2016, 126, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E. Glioblastoma: Synergy of growth promotion between CCL5 and NK-1R can be thwarted by blocking CCL5 with miraviroc, an FDA approved anti-HIV drug and blocking NK-1R with aprepitant, an FDA approved anti-nausea drug. J. Clin. Pharm. Ther. 2010, 35, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E. Why cerebellar glioblastoma is rare and how that indicates adjunctive use of the FDA-approved anti-emetic aprepitant might retard cerebral glioblastoma growth: A new hypothesis to an old question. Clin. Transl. Oncol. 2009, 11, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Coveñas, R. The Neurokinin-1 Receptor Antagonist Aprepitant: An Intelligent Bullet against Cancer? Cancers 2020, 12, 2682. [Google Scholar] [CrossRef]
- Muñoz, M.; Parrilla, J.; Rosso, M.; Coveñas, R. Antipruritic vs. Antitumour Action of Aprepitant: A Question of Dose. Acta Derm. Venereol. 2019, 99, 620–621. [Google Scholar] [CrossRef]
- Beirith, I.; Renz, B.W.; Mudusetti, S.; Ring, N.S.; Kolorz, J.; Koch, D.; Bazhin, A.V.; Berger, M.; Wang, J.; Angele, M.K.; et al. Identification of the Neurokinin-1 Receptor as Targetable Stratification Factor for Drug Repurposing in Pancreatic Cancer. Cancers 2021, 13, 2703. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Dong, D.; Wang, Z.; Ji, W.; Yu, M.; Zhang, F.; Niu, R.; Zhou, Y. MiR-34b/c-5p and the neurokinin-1 receptor regulate breast cancer cell proliferation and apoptosis. Cell Prolif. 2019, 52, e12527. [Google Scholar] [CrossRef]
- Garnier, A.; Ilmer, M.; Becker, K.; Häberle, B.; VON Schweinitz, D.; Kappler, R.; Berger, M. Truncated neurokinin-1 receptor is an ubiquitous antitumor target in hepatoblastoma, and its expression is independent of tumor biology and stage. Oncol. Lett. 2016, 11, 870–878. [Google Scholar] [CrossRef][Green Version]
- Ebrahimi, S.; Erfani, B.; Alalikhan, A.; Ghorbani, H.; Farzadnia, M.; Afshari, A.R.; Mashkani, B.; Hashemy, S.I. The In Vitro Pro-inflammatory Functions of the SP/NK1R System in Prostate Cancer: A Focus on Nuclear Factor-Kappa B (NF-κB) and Its Pro-inflammatory Target Genes. Appl. Biochem. Biotechnol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Korfi, F.; Javid, H.; Darban, R.A.; Hashemy, S.I. The Effect of SP/NK1R on the Expression and Activity of Catalase and Superoxide Dismutase in Glioblastoma Cancer Cells. Biochem. Res. Int. 2021, 2021, 6620708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-W.; Li, L.; Hu, W.-Q.; Hu, M.-N.; Tao, Y.; Hu, H.; Miao, X.-K.; Yang, W.-L.; Zhu, Q.; Mou, L.-Y. Neurokinin-1 receptor promotes non-small cell lung cancer progression through transactivation of EGFR. Cell Death Dis. 2022, 13, 6620708. [Google Scholar] [CrossRef] [PubMed]
- Javid, H.; Asadi, J.; Avval, F.Z.; Afshari, A.R.; Hashemy, S.I. The role of substance P/neurokinin 1 receptor in the pathogenesis of esophageal squamous cell carcinoma through constitutively active PI3K/Akt/NF-κB signal transduction pathways. Mol. Biol. Rep. 2020, 47, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Ilmer, M.; Garnier, A.; Vykoukal, J.; Alt, E.; von Schweinitz, D.; Kappler, R.; Berger, M. Targeting the Neurokinin-1 Receptor Compromises Canonical Wnt Signaling in Hepatoblastoma. Mol. Cancer Ther. 2015, 14, 2712–2721. [Google Scholar] [CrossRef]
- Golestaneh, M.; Firoozrai, M.; Javid, H.; Hashemy, S.I. The substance P/neurokinin-1 receptor signaling pathway mediates metastasis in human colorectal SW480 cancer cells. Mol. Biol. Rep. 2022, 49, 4893–4900. [Google Scholar] [CrossRef]
- Henssen, A.G.; Odersky, A.; Szymansky, A.; Seiler, M.; Althoff, K.; Beckers, A.; Speleman, F.; Schäfers, S.; De Preter, K.; Astrahanseff, K.; et al. Targeting tachykinin receptors in neuroblastoma. Oncotarget 2017, 8, 430–443. [Google Scholar] [CrossRef]
- Kolorz, J.; Demir, S.; Gottschlich, A.; Beirith, I.; Ilmer, M.; Lüthy, D.; Walz, C.; Dorostkar, M.M.; Magg, T.; Hauck, F.; et al. The Neurokinin-1 Receptor Is a Target in Pediatric Rhabdoid Tumors. Curr. Oncol. 2021, 29, 94–110. [Google Scholar] [CrossRef]
- Mohammadi, F.; Javid, H.; Afshari, A.R.; Mashkani, B.; Hashemy, S.I. Substance P accelerates the progression of human esophageal squamous cell carcinoma via MMP-2, MMP-9, VEGF-A, and VEGFR1 overexpression. Mol. Biol. Rep. 2020, 47, 4263–4272. [Google Scholar] [CrossRef]
- Berger, M.; Neth, O.; Ilmer, M.; Garnier, A.; Salinas-Martín, M.V.; de Agustín Asencio, J.C.; von Schweinitz, D.; Kappler, R.; Muñoz, M. Hepatoblastoma cells express truncated neurokinin-1 receptor and can be growth inhibited by aprepitant in vitro and in vivo. J. Hepatol. 2014, 60, 985–994. [Google Scholar] [CrossRef]
- Akbari, S.; Darban, R.A.; Javid, H.; Esparham, A.; Hashemy, S.I. The anti-tumoral role of Hesperidin and Aprepitant on prostate cancer cells through redox modifications. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dikmen, M.; Gökhaner, G.; Cantürk, Z. Evaluation of the antileukemic effects of neurokinin-1 receptor antagonists, aprepitant, and L-733,060, in chronic and acute myeloid leukemic cells. Anti-Cancer Drugs 2019, 30, 693–705. [Google Scholar] [CrossRef]
- Muñoz, M.; Rosso, M.; Robles-Frias, M.J.; Salinas-Martín, M.V.; Rosso, R.; González-Ortega, A.; Coveñas, R. The NK-1 receptor is expressed in human melanoma and is involved in the antitumor action of the NK-1 receptor antagonist aprepitant on melanoma cell lines. Lab. Investig. 2010, 90, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E. High Neutrophil-to-Lymphocyte Ratio Facilitates Cancer Growth—Currently Marketed Drugs Tadalafil, Isotretinoin, Colchicine, and Omega-3 to Reduce It: The TICO Regimen. Cancers 2022, 14, 4965. [Google Scholar] [CrossRef]
- Bayati, S.; Bashash, D.; Ahmadian, S.; Safaroghli-Azar, A.; Alimoghaddam, K.; Ghavamzadeh, A.; Ghaffari, S.H. Inhibition of tachykinin NK1 receptor using aprepitant induces apoptotic cell death and G1 arrest through Akt/p53 axis in pre-B acute lymphoblastic leukemia cells. Eur. J. Pharmacol. 2016, 791, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Molinos-Quintana, A.; Trujillo-Hacha, P.; Piruat, J.I.; Bejarano-García, J.A.; García-Guerrero, E.; Pérez-Simón, J.A.; Muñoz, M. Human acute myeloid leukemia cells express Neurokinin-1 receptor, which is involved in the antileukemic effect of Neurokinin-1 receptor antagonists. Investig. New Drugs 2019, 37, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.; Mirzavi, F.; Hashemy, S.I.; Ghadiri, M.K.; Stummer, W.; Gorji, A. The in vitro anti-cancer synergy of neurokinin-1 receptor antagonist, aprepitant, and 5-aminolevulinic acid in glioblastoma. Biofactors 2023, 49, 900–911. [Google Scholar] [CrossRef]
- Garnier, A.; Vykoukal, J.; Hubertus, J.; Alt, E.; VON Schweinitz, D.; Kappler, R.; Berger, M.; Ilmer, M. Targeting the neurokinin-1 receptor inhibits growth of human colon cancer cells. Int. J. Oncol. 2015, 47, 151–160. [Google Scholar] [CrossRef]
- Halik, P.K.; Lipiński, P.F.J.; Matalińska, J.; Koźmiński, P.; Misicka, A.; Gniazdowska, E. Radiochemical Synthesis and Evaluation of Novel Radioconjugates of Neurokinin 1 Receptor Antagonist Aprepitant Dedicated for NK1R-Positive Tumors. Molecules 2020, 25, 3756. [Google Scholar] [CrossRef]
- Cao, X.; Yang, Y.; Zhou, W.; Wang, Y.; Wang, X.; Ge, X.; Wang, F.; Zhou, F.; Deng, X.; Miao, L. Aprepitant inhibits the development and metastasis of gallbladder cancer via ROS and MAPK activation. BMC Cancer 2023, 23, 471. [Google Scholar] [CrossRef]
- Zheng, Y.; Sang, M.; Liu, F.; Gu, L.; Li, J.; Wu, Y.; Shan, B. Aprepitant inhibits the progression of esophageal squamous cancer by blocking the truncated neurokinin-1 receptor. Oncol. Rep. 2023, 50, 131. [Google Scholar] [CrossRef]
- Muñoz, M.; González-Ortega, A.; Coveñas, R. The NK-1 receptor is expressed in human leukemia and is involved in the antitumor action of aprepitant and other NK-1 receptor antagonists on acute lymphoblastic leukemia cell lines. Investig. New Drugs 2012, 30, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Berger, M.; Rosso, M.; Gonzalez-Ortega, A.; Carranza, A.; Coveñas, R. Antitumor activity of neurokinin-1 receptor antagonists in MG-63 human osteosarcoma xenografts. Int. J. Oncol. 2014, 44, 137–146. [Google Scholar] [CrossRef]
- Robinson, P.; Rosso, M.; Muñoz, M. Neurokinin-1 Receptor Antagonists as a Potential Novel Therapeutic Option for Osteosarcoma Patients. J. Clin. Med. 2023, 12, 2135. [Google Scholar] [CrossRef] [PubMed]
- Alsaeed, M.A.; Ebrahimi, S.; Alalikhan, A.; Hashemi, S.F.; Hashemy, S.I. The Potential In Vitro Inhibitory Effects of Neurokinin-1 Receptor (NK-1R) Antagonist, Aprepitant, in Osteosarcoma Cell Migration and Metastasis. BioMed Res. Int. 2022, 2022, 8082608. [Google Scholar] [CrossRef] [PubMed]
- Togari, A.; Arai, M.; Mizutani, S.; Mizutani, S.; Koshihara, Y.; Nagatsu, T. Expression of mRNAs for neuropeptide receptors and β-adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci. Lett. 1997, 233, 125–128. [Google Scholar] [CrossRef]
- Krasselt, M.; Baerwald, C. Celecoxib for the treatment of musculoskeletal arthritis. Expert Opin. Pharmacother. 2019, 20, 1689–1702. [Google Scholar] [CrossRef]
- Tive, L. Celecoxib clinical profile. Rheumatology 2000, 39 (Suppl. S2), 21–28. [Google Scholar] [CrossRef]
- Teerawattananon, C.; Tantayakom, P.; Suwanawiboon, B.; Katchamart, W. Risk of perioperative bleeding related to highly selective cyclooxygenase-2 inhibitors: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2017, 46, 520–528. [Google Scholar] [CrossRef]
- Ho, K.Y.; Gwee, K.A.; Cheng, Y.K.; Yoon, K.H.; Hee, H.T.; Omar, A.R. Nonsteroidal anti-inflammatory drugs in chronic pain: Implications of new data for clinical practice. J. Pain Res. 2018, 11, 1937–1948. [Google Scholar] [CrossRef]
- Walker, C. Are All Oral COX-2 Selective Inhibitors the Same? A Consideration of Celecoxib, Etoricoxib, and Diclofenac. Int. J. Rheumatol. 2018, 2018, 1302835. [Google Scholar] [CrossRef]
- Tołoczko-Iwaniuk, N.; Dziemiańczyk-Pakieła, D.; Nowaszewska, B.K.; Celińska-Janowicz, K.; Miltyk, W. Celecoxib in Cancer Therapy and Prevention—Review. Curr. Drug Targets 2019, 20, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Sharma, P.K.; Purohit, P. A journey of celecoxib from pain to cancer. Prostaglandins Other Lipid Mediat. 2020, 147, 106379. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E. Adding high-dose celecoxib to increase effectiveness of standard glioblastoma chemoirradiation. Ann. Pharm. Fr. 2021, 79, 481–488. [Google Scholar] [CrossRef] [PubMed]
- North, G.L.T. Celecoxib as Adjunctive Therapy for Treatment of Colorectal Cancer. Ann. Pharmacother. 2001, 35, 1638–1643. [Google Scholar] [CrossRef]
- Li, J.; Hao, Q.; Cao, W.; Vadgama, J.V.; Wu, Y. Celecoxib in breast cancer prevention and therapy. Cancer Manag. Res. 2018, 10, 4653–4667. [Google Scholar] [CrossRef]
- Liu, R.; Xu, K.-P.; Tan, G.-S. Cyclooxygenase-2 inhibitors in lung cancer treatment: Bench to bed. Eur. J. Pharmacol. 2015, 769, 127–133. [Google Scholar] [CrossRef]
- Tudor, D.V.; Bâldea, I.; Lupu, M.; Kacso, T.; Kutasi, E.; Hopârtean, A.; Stretea, R.; Filip, A.G. COX-2 as a potential biomarker and therapeutic target in melanoma. Cancer Biol. Med. 2020, 17, 20–31. [Google Scholar] [CrossRef]
- Gore, E. Celecoxib and radiation therapy in non-small-cell lung cancer. Oncology 2004, 18 (Suppl. S14), 10–14. [Google Scholar]
- Futagami, S.; Suzuki, K.; Hiratsuka, T.; Shindo, T.; Hamamoto, T.; Ueki, N.; Kusunoki, M.; Miyake, K.; Gudis, K.; Tsukui, T.; et al. Chemopreventive effect of celecoxib in gastric cancer. Inflammopharmacology 2007, 15, 1–4. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, N.; Tiwari, R.P.; Sah, N.K.; Prasad, G.B.; Bisen, P.S. Biology of Cox-2: An Application in Cancer Therapeutics. Curr. Drug Targets 2011, 12, 1082–1093. [Google Scholar] [CrossRef]
- Liu, G.; Yu, M.-Y.; Huang, X.; Zhu, D.; Cheng, S.; Ma, R.; Gu, G. Synergistic effect of celecoxib in tumor necrosis factor-related apoptosis-inducing ligand treatment in osteosarcoma cells. Mol. Med. Rep. 2014, 10, 2198–2202. [Google Scholar] [CrossRef]
- Liu, J.; Wu, J.; Zhou, L.; Pan, C.; Zhou, Y.; Du, W.; Chen, J.-M.; Zhu, X.; Shen, J.; Chen, S.; et al. ZD6474, a new treatment strategy for human osteosarcoma, and its potential synergistic effect with celecoxib. Oncotarget 2015, 6, 21341–21352. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, X.; Ren, K.; Fan, G.-T.; Wu, S.J.; Zhao, J.-N. Celecoxib inhibits cell growth and modulates the expression of matrix metalloproteinases in human osteosarcoma MG-63 cell line. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4087–4097. [Google Scholar]
- Liu, B.; Yan, S.; Qu, L.; Zhu, J. Celecoxib enhances anticancer effect of cisplatin and induces anoikis in osteosarcoma via PI3K/Akt pathway. Cancer Cell Int. 2017, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, X.; Jeschke, U.; von Schönfeldt, V. COX-2-PGE2-EPs in gynecological cancers. Arch. Gynecol. Obstet. 2020, 301, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; White, A.C.; Borowsky, A.D. New insights into the functions of Cox-2 in skin and esophageal malignancies. Exp. Mol. Med. 2020, 52, 538–547. [Google Scholar] [CrossRef]
- Zmigrodzka, M.; Rzepecka, A.; Krzyzowska, M.; Witkowska-Pilaszewicz, O.; Cywinska, A.; Winnicka, A. The cyclooxygenase-2/prostaglandin E2 pathway and its role in the pathogenesis of human and dog hematological malignancies. J. Physiol. Pharmacol. 2018, 69, 653–661. [Google Scholar] [CrossRef]
- Sheng, J.; Sun, H.; Yu, F.-B.; Li, B.; Zhang, Y.; Zhu, Y.-T. The Role of Cyclooxygenase-2 in Colorectal Cancer. Int. J. Med. Sci. 2020, 17, 1095–1101. [Google Scholar] [CrossRef]
- Pu, D.; Yin, L.; Huang, L.; Qin, C.; Zhou, Y.; Wu, Q.; Li, Y.; Zhou, Q.; Li, L. Cyclooxygenase-2 Inhibitor: A Potential Combination Strategy With Immunotherapy in Cancer. Front. Oncol. 2021, 11, 637504. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Guo, W.; Ren, T.; Huang, Y.; Wang, S.; Liu, K.; Zheng, B.; Yang, K.; Zhang, H.; Liang, X. Tumor-associated macrophages promote lung metastasis and induce epithelial-mesenchymal transition in osteosarcoma by activating the COX-2/STAT3 axis. Cancer Lett. 2019, 440–441, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, A.; Yin, B.; Wu, D.; Han, S.; Zhang, W.; Liu, J.; Sun, K. SND1 promotes the proliferation of osteosarcoma cells by upregulating COX-2/PGE2 expression via activation of NF-κB. Oncol. Rep. 2019, 41, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Naruse, T.; Nishida, Y.; Hosono, K.; Ishiguro, N. Meloxicam inhibits osteosarcoma growth, invasiveness and metastasis by COX-2-dependent and independent routes. Carcinogenesis 2006, 27, 584–592. [Google Scholar] [CrossRef]
- Qian, M.; Yang, X.; Li, Z.; Jiang, C.; Song, D.; Yan, W.; Liu, T.; Wu, Z.; Kong, J.; Wei, H.; et al. P50-associated COX-2 extragenic RNA (PACER) overexpression promotes proliferation and metastasis of osteosarcoma cells by activating COX-2 gene. Tumor Biol. 2016, 37, 3879–3886. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cai, M.; Ji, F.; Lou, L.-M. The impact of COX-2 on invasion of osteosarcoma cell and its mechanism of regulation. Cancer Cell Int. 2014, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, M.; Xiao, Z.; Wu, H.; Wu, Y. Quantitative Assessment of the Association of COX-2 (Cyclooxygenase-2) Immunoexpression with Prognosis in Human Osteosarcoma: A Meta-Analysis. PLoS ONE 2013, 8, e82907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qu, P.; Zhao, H.; Zhao, T.; Cao, N. COX-2 promotes epithelial-mesenchymal transition and migration in osteosarcoma MG-63 cells via PI3K/AKT/NF-κB signaling. Mol. Med. Rep. 2019, 20, 3811–3819. [Google Scholar] [CrossRef]
- Masi, L.; Recenti, R.; Silvestri, S.; Pinzani, P.; Pepi, M.; Paglierani, M.; Brandi, M.L.; Franchi, A. Expression of Cyclooxygenase-2 in Osteosarcoma of Bone. Appl. Immunohistochem. Mol. Morphol. 2007, 15, 70–76. [Google Scholar] [CrossRef]
- Lee, E.J.; Choi, E.M.; Kim, S.R.; Park, J.H.; Kim, H.; Ha, K.S.; Kim, Y.M.; Kim, S.S.; Choe, M.; Kim, J.-I.; et al. Cyclooxygenase-2 promotes cell proliferation, migration and invasion in U2OS human osteosarcoma cells. Exp. Mol. Med. 2007, 39, 469–476. [Google Scholar] [CrossRef]
- Reckamp, K.L.; Krysan, K.; Morrow, J.D.; Milne, G.L.; Newman, R.A.; Tucker, C.; Elashoff, R.M.; Dubinett, S.M.; Figlin, R.A. A Phase I Trial to Determine the Optimal Biological Dose of Celecoxib when Combined with Erlotinib in Advanced Non–Small Cell Lung Cancer. Clin. Cancer Res. 2006, 12 Pt 1, 3381–3388. [Google Scholar] [CrossRef]
- Knudsen, J.F.; Carlsson, U.; Hammarström, P.; Sokol, G.H.; Cantilena, L.R. The Cyclooxygenase-2 Inhibitor Celecoxib Is a Potent Inhibitor of Human Carbonic Anhydrase II. Inflammation 2004, 28, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Alper, A.B., Jr.; Tomlin, H.; Sadhwani, U.; Whelton, A.; Puschett, J. Effects of the Selective Cyclooxygenase-2 Inhibitor Analgesic Celecoxib on Renal Carbonic Anhydrase Enzyme Activity: A Randomized, Controlled Trial. Am. J. Ther. 2006, 13, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Casini, A.; Heine, A.; Kuhn, D.; Supuran, C.T.; Scozzafava, A.; Klebe, G. Unexpected Nanomolar Inhibition of Carbonic Anhydrase by COX-2-Selective Celecoxib: New Pharmacological Opportunities Due to Related Binding Site Recognition. J. Med. Chem. 2004, 47, 550–557. [Google Scholar] [CrossRef] [PubMed]
- De Monte, C.; Carradori, S.; Gentili, A.; Mollica, A.; Trisciuoglio, D.; Supuran, C.T. Dual Cyclooxygenase and Carbonic Anhydrase Inhibition by Nonsteroidal Anti-Inflammatory Drugs for the Treatment of Cancer. Curr. Med. Chem. 2015, 22, 2812–2818. [Google Scholar] [CrossRef]
- Mahboubi-Rabbani, M.; Zarghi, A. Dual Human Carbonic Anhydrase/Cyclooxygenase-2 Inhibitors: A Promising Approach for Cancer Treatment. Anti-Cancer Agents Med. Chem. 2021, 21, 2163–2180. [Google Scholar] [CrossRef]
- Pastorek, J.; Pastorekova, S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: From biology to clinical use. Semin. Cancer Biol. 2015, 31, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Takacova, M.; Kajanova, I.; Kolarcikova, M.; Lapinova, J.; Zatovicova, M.; Pastorekova, S. Understanding metabolic alterations and heterogeneity in cancer progression through validated immunodetection of key molecular components: A case of carbonic anhydrase IX. Cancer Metastasis Rev. 2021, 40, 1035–1053. [Google Scholar] [CrossRef]
- Potter, C.; Harris, A.L. Hypoxia Inducible Carbonic Anhydrase IX, Marker of Tumour: Hypoxia, Survival Pathway and Therapy Target. Cell Cycle 2004, 3, 159–162. [Google Scholar] [CrossRef]
- Andreucci, E.; Peppicelli, S.; Carta, F.; Brisotto, G.; Biscontin, E.; Ruzzolini, J.; Bianchini, F.; Biagioni, A.; Supuran, C.T.; Calorini, L. Carbonic anhydrase IX inhibition affects viability of cancer cells adapted to extracellular acidosis. J. Mol. Med. 2017, 95, 1341–1353. [Google Scholar] [CrossRef]
- Ward, C.; Meehan, J.; E Gray, M.; Murray, A.F.; Argyle, D.J.; Kunkler, I.H.; Langdon, S.P. The impact of tumour pH on cancer progression: Strategies for clinical intervention. Explor. Target. Anti-Tumor Ther. 2020, 1, 71–100. [Google Scholar] [CrossRef]
- Avnet, S.; Lemma, S.; Cortini, M.; Di Pompo, G.; Perut, F.; Lipreri, M.V.; Roncuzzi, L.; Columbaro, M.; Errani, C.; Longhi, A.; et al. The Release of Inflammatory Mediators from Acid-Stimulated Mesenchymal Stromal Cells Favours Tumour Invasiveness and Metastasis in Osteosarcoma. Cancers 2021, 13, 5855. [Google Scholar] [CrossRef] [PubMed]
- Avnet, S.; Di Pompo, G.; Chano, T.; Errani, C.; Ibrahim-Hashim, A.; Gillies, R.J.; Donati, D.M.; Baldini, N. Cancer-associated mesenchymal stroma fosters the stemness of osteosarcoma cells in response to intratumoral acidosis via NF-κB activation. Int. J. Cancer 2017, 140, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Di Pompo, G.; Cortini, M.; Baldini, N.; Avnet, S. Acid Microenvironment in Bone Sarcomas. Cancers 2021, 13, 3848. [Google Scholar] [CrossRef]
- Feng, Z.; Ou, Y.; Hao, L. The roles of glycolysis in osteosarcoma. Front. Pharmacol. 2022, 13, 950886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, J.; Zhang, X.; Cao, L.; Wu, Y.; Miao, X. Transcription factor ELK1 accelerates aerobic glycolysis to enhance osteosarcoma chemoresistance through miR-134/PTBP1 signaling cascade. Aging 2021, 13, 6804–6819. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, E.; Tsukahara, T.; Emori, M.; Murata, K.; Akamatsu, A.; Shibayama, Y.; Hamada, S.; Watanabe, Y.; Kaya, M.; Hirohashi, Y.; et al. Osteosarcoma-initiating cells show high aerobic glycolysis and attenuation of oxidative phosphorylation mediated by LIN28B. Cancer Sci. 2020, 111, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Griffiths, J.R. How and Why Are Cancers Acidic? Carbonic Anhydrase IX and the Homeostatic Control of Tumour Extracellular pH. Cancers 2020, 12, 1616. [Google Scholar] [CrossRef]
- Okuno, K.; Matsubara, T.; Nakamura, T.; Iino, T.; Kakimoto, T.; Asanuma, K.; Matsumine, A.; Sudo, A. Carbonic anhydrase IX enhances tumor cell proliferation and tumor progression in osteosarcoma. OncoTargets Ther. 2018, 11, 6879–6886. [Google Scholar] [CrossRef]
- Perut, F.; Carta, F.; Bonuccelli, G.; Grisendi, G.; Di Pompo, G.; Avnet, S.; Sbrana, F.V.; Hosogi, S.; Dominici, M.; Kusuzaki, K.; et al. Carbonic anhydrase IX inhibition is an effective strategy for osteosarcoma treatment. Expert Opin. Ther. Targets 2015, 19, 1593–1605. [Google Scholar] [CrossRef]
- Matsubara, T.; DiResta, G.R.; Kakunaga, S.; Li, D.; Healey, J.H. Additive Influence of Extracellular pH, Oxygen Tension, and Pressure on Invasiveness and Survival of Human Osteosarcoma Cells. Front. Oncol. 2013, 3, 199. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, G.; Sun, C.; Lei, L.; Williamson, R.A.; Wang, Y.; Zhang, J.; Chen, P.; Wang, A.; Fan, R.; et al. Hypoxia promotes osteosarcoma cell proliferation and migration through enhancing platelet-derived growth factor-BB/platelet-derived growth factor receptor-β axis. Biochem. Biophys. Res. Commun. 2019, 512, 360–366. [Google Scholar] [CrossRef]
- Nazon, C.; Pierrevelcin, M.; Willaume, T.; Lhermitte, B.; Weingertner, N.; Di Marco, A.; Bund, L.; Vincent, F.; Bierry, G.; Gomez-Brouchet, A.; et al. Together Intra-Tumor Hypoxia and Macrophagic Immunity Are Driven Worst Outcome in Pediatric High-Grade Osteosarcomas. Cancers 2022, 14, 1482. [Google Scholar] [CrossRef]
- Anai, S.; Tanaka, M.; Shiverick, K.T.; Kim, W.; Takada, S.; Boehlein, S.; Goodison, S.; Mizokami, A.; Rosser, C.J. Increased Expression of Cyclooxygenase-2 Correlates With Resistance to Radiation in Human Prostate Adenocarcinoma Cells. J. Urol. 2007, 177, 1913–1917. [Google Scholar] [CrossRef] [PubMed]
- Ihnatko, R.; Kubes, M.; Takacova, M.; Sedlakova, O.; Sedlak, J.; Pastorek, J.; Kopacek, J.; Pastorekova, S. Extracellular acidosis elevates carbonic anhydrase IX in human glioblastoma cells via transcriptional modulation that does not depend on hypoxia. Int. J. Oncol. 2006, 29, 1025–1033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rezuchova, I.; Bartosova, M.; Belvoncikova, P.; Takacova, M.; Zatovicova, M.; Jelenska, L.; Csaderova, L.; Meciarova, I.; Pohlodek, K. Carbonic Anhydrase IX in Tumor Tissue and Plasma of Breast Cancer Patients: Reliable Biomarker of Hypoxia and Prognosis. Int. J. Mol. Sci. 2023, 24, 4325. [Google Scholar] [CrossRef] [PubMed]
- Slater, A.F. Chloroquine: Mechanism of drug action and resistance in Plasmodium falciparum. Pharmacol. Ther. 1993, 57, 203–235. [Google Scholar] [CrossRef]
- Nirk, E.L.; Reggiori, F.; Mauthe, M. Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO Mol. Med. 2020, 12, e12476. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Ferreira, P.M.P.; de Sousa, R.W.R.; Ferreira, J.R.d.O.; Militão, G.C.G.; Bezerra, D.P. Chloroquine and hydroxychloroquine in antitumor therapies based on autophagy-related mechanisms. Pharmacol. Res. 2021, 168, 105582. [Google Scholar] [CrossRef]
- Allemailem, K.S.; Almatroudi, A.; Alrumaihi, F.; Almatroodi, S.A.; Alkurbi, M.O.; Basfar, G.T.; Rahmani, A.H.; Khan, A.A. Novel Approaches of Dysregulating Lysosome Functions in Cancer Cells by Specific Drugs and Its Nanoformulations: A Smart Approach of Modern Therapeutics. Int. J. Nanomed. 2021, 16, 5065–5098. [Google Scholar] [CrossRef]
- Niemann, B.; Puleo, A.; Stout, C.; Markel, J.; Boone, B.A. Biologic Functions of Hydroxychloroquine in Disease: From COVID-19 to Cancer. Pharmaceutics 2022, 14, 2551. [Google Scholar] [CrossRef] [PubMed]
- Hraběta, J.; Belhajová, M.; Šubrtová, H.; Rodrigo, M.A.M.; Heger, Z.; Eckschlager, T. Drug Sequestration in Lysosomes as One of the Mechanisms of Chemoresistance of Cancer Cells and the Possibilities of Its Inhibition. Int. J. Mol. Sci. 2020, 21, 4392. [Google Scholar] [CrossRef]
- Jogalekar, M.P.; Veerabathini, A.; Gangadaran, P. Recent developments in autophagy-targeted therapies in cancer. Exp. Biol. Med. 2021, 246, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Liu, Y.; Huang, T.; Wei, Y. Autophagy and its role in osteosarcoma. Cancer Med. 2023, 12, 5676–5687. [Google Scholar] [CrossRef]
- Xu, R.; Ji, Z.; Xu, C.; Zhu, J. The clinical value of using chloroquine or hydroxychloroquine as autophagy inhibitors in the treatment of cancers: A systematic review and meta-analysis. Medicine 2018, 97, e12912. [Google Scholar] [CrossRef] [PubMed]
- Dalpke, A.; Zimmermann, S.; Heeg, K. Immunopharmacology of CpG DNA. Biol. Chem. 2002, 383, 1491–1500. [Google Scholar] [CrossRef]
- Torigoe, M.; Sakata, K.; Ishii, A.; Iwata, S.; Nakayamada, S.; Tanaka, Y. Hydroxychloroquine efficiently suppresses inflammatory responses of human class-switched memory B cells via Toll-like receptor 9 inhibition. Clin. Immunol. 2018, 195, 1–7. [Google Scholar] [CrossRef]
- Kužnik, A.; Benčina, M.; Švajger, U.; Jeras, M.; Rozman, B.; Jerala, R. Mechanism of Endosomal TLR Inhibition by Antimalarial Drugs and Imidazoquinolines. J. Immunol. 2011, 186, 4794–4804. [Google Scholar] [CrossRef]
- Zhu, X.; Pan, Y.; Li, Y.; Jiang, Y.; Shang, H.; Gowda, D.C.; Cui, L.; Cao, Y. Targeting Toll-like receptors by chloroquine protects mice from experimental cerebral malaria. Int. Immunopharmacol. 2012, 13, 392–397. [Google Scholar] [CrossRef]
- Han, J.; Li, X.; Luo, X.; He, J.; Huang, X.; Zhou, Q.; Han, Y.; Jie, H.; Zhuang, J.; Li, Y.; et al. The mechanisms of hydroxychloroquine in rheumatoid arthritis treatment: Inhibition of dendritic cell functions via Toll like receptor 9 signaling. Biomed. Pharmacother. 2020, 132, 110848. [Google Scholar] [CrossRef]
- Marastoni, S.; Madariaga, A.; Pesic, A.; Nair, S.N.; Li, Z.J.; Shalev, Z.; Ketela, T.; Colombo, I.; Mandilaras, V.; Cabanero, M.; et al. Repurposing Itraconazole and Hydroxychloroquine to Target Lysosomal Homeostasis in Epithelial Ovarian Cancer. Cancer Res. Commun. 2022, 2, 293–306. [Google Scholar] [CrossRef]
- Yasuda, H.; Leelahavanichkul, A.; Tsunoda, S.; Dear, J.W.; Takahashi, Y.; Ito, S.; Hu, X.; Zhou, H.; Doi, K.; Childs, R.; et al. Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am. J. Physiol. Renal Physiol. 2008, 294, F1050–F1058. [Google Scholar] [CrossRef]
- Zou, W.; Amcheslavsky, A.; Bar-Shavit, Z. CpG Oligodeoxynucleotides Modulate the Osteoclastogenic Activity of Osteoblasts via Toll-like Receptor 9. J. Biol. Chem. 2003, 278, 16732–16740. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Liang, H.; Zhang, Y.; Cleveland, J.; Yan, J.; Zhang, D. Up-regulation of Toll-like Receptor 9 in Osteosarcoma. Anticancer Res. 2015, 35, 5839–5843. [Google Scholar] [PubMed]
- Jing, Y.; Jia, M.; Zhuang, J.; Han, D.; Zhou, C.; Yan, J. TLR9 Exerts an Oncogenic Role in Promoting Osteosarcoma Progression Depending on the Regulation of NF-κB Signaling Pathway. Biol. Pharm. Bull. 2022, 45, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.E.Z.; Jalan, R.; Minogue, S.; Andreola, F.; Habtesion, A.; Hall, A.; Winstanley, A.; Damink, S.O.; Malagó, M.; Davies, N.; et al. Inhibition of TLR7 and TLR9 Reduces Human Cholangiocarcinoma Cell Proliferation and Tumor Development. Dig. Dis. Sci. 2022, 67, 1806–1821. [Google Scholar] [CrossRef]
- González-Reyes, S.; Marín, L.; González, L.; O González, L.; del Casar, J.M.; Lamelas, M.L.; González-Quintana, J.M.; Vizoso, F.J. Study of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasis. BMC Cancer 2010, 10, 665. [Google Scholar] [CrossRef]
- Sheyhidin, I.; Nabi, G.; Hasim, A.; Zhang, R.-P.; Ainiwaer, J.; Ma, H.; Wang, H. Overexpression of TLR3, TLR4, TLR7 and TLR9 in esophageal squamous cell carcinoma. World J. Gastroenterol. 2011, 17, 3745–3751. [Google Scholar] [CrossRef]
- Diaz-Ruiz, A.; Nader-Kawachi, J.; Calderón-Estrella, F.; Bermudez, A.M.; Alvarez-Mejia, L.; Ríos, C. Dapsone, More than an Effective Neuro and Cytoprotective Drug. Curr. Neuropharmacol. 2022, 20, 194–210. [Google Scholar] [CrossRef]
- Ghaoui, N.; Hanna, E.; Abbas, O.; Kibbi, A.; Kurban, M. Update on the use of dapsone in dermatology. Int. J. Dermatol. 2020, 59, 787–795. [Google Scholar] [CrossRef]
- Wozel, G.; Blasum, C. Dapsone in dermatology and beyond. Arch. Dermatol. Res. 2014, 306, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, K.; Shichino, S.; Ueha, S. Thirty-five years since the discovery of chemotactic cytokines, interleukin-8 and MCAF: A historical overview. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2023, 99, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Karpel-Massler, G.; Kast, R.E.; Siegelin, M.D.; Dwucet, A.; Schneider, E.; Westhoff, M.-A.; Wirtz, C.R.; Chen, X.Y.; Halatsch, M.-E.; Bolm, C. Anti-glioma Activity of Dapsone and Its Enhancement by Synthetic Chemical Modification. Neurochem. Res. 2017, 42, 3382–3389. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Estrella, F.; Franco-Bourland, R.E.; Rios, C.; de Jesús-Nicolás, D.; Pineda, B.; Méndez-Armenta, M.; Mata-Bermúdez, A.; Diaz-Ruiz, A. Early treatment with dapsone after spinal cord injury in rats decreases the inflammatory response and promotes long-term functional recovery. Heliyon 2023, 9, e14687. [Google Scholar] [CrossRef] [PubMed]
- Geyfman, M.; Debabov, D.; Poloso, N.; Alvandi, N. Mechanistic insight into the activity of a sulfone compound dapsone on Propionibacterium (Newly Reclassified as Cutibacterium) Acnes-mediated cytokine production. Exp. Dermatol. 2019, 28, 190–197. [Google Scholar] [CrossRef]
- Kast, R.E. Research Supporting a Pilot Study of Metronomic Dapsone during Glioblastoma Chemoirradiation. Med. Sci. 2021, 9, 12. [Google Scholar] [CrossRef]
- A Booth, S.; E Moody, C.; Dahl, M.V.; Herron, M.J.; Nelson, R.D. Dapsone Suppresses Integrin-Mediated Neutrophil Adherence Function. J. Investig. Dermatol. 1992, 98, 135–140. [Google Scholar] [CrossRef]
- Schmidt, E.; Reimer, S.; Kruse, N.; Bröcker, E.B.; Zillikens, D. The IL-8 release from cultured human keratinocytes, mediated by antibodies to bullous pemphigoid autoantigen 180, is inhibited by dapsone. Clin. Exp. Immunol. 2001, 124, 157–162. [Google Scholar] [CrossRef]
- Gremese, E.; Tolusso, B.; Bruno, D.; Perniola, S.; Ferraccioli, G.; Alivernini, S. The forgotten key players in rheumatoid arthritis: IL-8 and IL-17—Unmet needs and therapeutic perspectives. Front. Med. 2023, 10, 956127. [Google Scholar] [CrossRef]
- Kast, R.E. Dapsone as treatment adjunct in ARDS. Exp. Lung Res. 2020, 46, 157–161. [Google Scholar] [CrossRef]
- Kanwar, B.A.; Khattak, A.; Balentine, J.; Lee, J.H.; Kast, R.E. Benefits of Using Dapsone in Patients Hospitalized with COVID-19. Vaccines 2022, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Han, X.-G.; Tu, B.; Wang, M.-Q.; Qiao, H.; Zhang, S.-H.; Fan, Q.-M.; Tang, T.-T. CXCR1/Akt signaling activation induced by mesenchymal stem cell-derived IL-8 promotes osteosarcoma cell anoikis resistance and pulmonary metastasis. Cell Death Dis. 2018, 9, 714. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Yang, X.; Lu, W.; Chen, Y.; Lin, Y.; Wang, J.; Lin, S.; Yun, J.-P. H3K27 acetylation activated-COL6A1 promotes osteosarcoma lung metastasis by repressing STAT1 and activating pulmonary cancer-associated fibroblasts. Theranostics 2021, 11, 1473–1492. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X.; Miao, W.; Wang, B.; Qiu, Y. CXCL8 promotes the invasion of human osteosarcoma cells by regulation of PI3K/Akt signaling pathway. Apmis 2017, 125, 773–780. [Google Scholar] [CrossRef]
- Liu, T.; Ma, Q.; Zhang, Y.; Wang, X.; Xu, K.; Yan, K.; Dong, W.; Fan, Q.; Zhang, Y.; Qiu, X. Self-seeding circulating tumor cells promote the proliferation and metastasis of human osteosarcoma by upregulating interleukin-8. Cell Death Dis. 2019, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Kamińska, J.; Kowalska, M.; Ruka, W.; Steffen, J. Cytokine and cytokine receptor serum levels in adult bone sarcoma patients: Correlations with local tumor extent and prognosis. J. Surg. Oncol. 2003, 84, 151–159. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, L.; Luo, G.; Son, H.; Prectoni, J.H.; Zheng, W. Effect of the cytokine levels in serum on osteosarcoma. Tumor Biol. 2014, 35, 1023–1028. [Google Scholar] [CrossRef]
- Reinecke, J.B.; Roberts, R.D. Targetable Intercellular Signaling Pathways Facilitate Lung Colonization in Osteosarcoma. Adv. Exp. Med. Biol. 2020, 1258, 111–123. [Google Scholar] [CrossRef]
- Gross, A.C.; Cam, H.; Phelps, D.A.; Saraf, A.J.; Bid, H.K.; Cam, M.; London, C.A.; Winget, S.A.; Arnold, M.A.; Brandolini, L.; et al. IL-6 and CXCL8 mediate osteosarcoma-lung interactions critical to metastasis. J. Clin. Investig. 2018, 3, e99791. [Google Scholar] [CrossRef]
- Allende, C.; Higgins, B.; Johns, J. Comparison of serum cytokine concentrations between healthy dogs and canine osteosarcoma patients at the time of diagnosis. Veter. Immunol. Immunopathol. 2020, 227, 110084. [Google Scholar] [CrossRef]
- Zha, Z.; Su, A.; Huo, S. Activation of GPER suppresses the malignancy of osteosarcoma cells via down regulation of IL-6 and IL-8. Arch. Biochem. Biophys. 2018, 660, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-G.; Du, L.; Qiao, H.; Tu, B.; Wang, Y.-G.; Qin, A.; Dai, K.-R.; Fan, Q.-M.; Tang, T.-T. CXCR1 knockdown improves the sensitivity of osteosarcoma to cisplatin. Cancer Lett. 2015, 369, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Tanaka, K.; Itonaga, I.; Iwasaki, T.; Tsumura, H. Interaction between human osteosarcoma and mesenchymal stem cells via an interleukin-8 signaling loop in the tumor microenvironment. Cell Commun. Signal. 2018, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Pietrovito, L.; Leo, A.; Gori, V.; Lulli, M.; Parri, M.; Becherucci, V.; Piccini, L.; Bambi, F.; Taddei, M.L.; Chiarugi, P. Bone marrow-derived mesenchymal stem cells promote invasiveness and transendothelial migration of osteosarcoma cells via a mesenchymal to amoeboid transition. Mol. Oncol. 2018, 12, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E. Erlotinib augmentation with dapsone for rash mitigation and increased anti-cancer effectiveness. Springerplus 2015, 4, 638. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Guzmán, M.E.; Hernández, M.I.; Gómez-Gallegos, A.; Ortiz-Sánchez, E. ALDH as a Stem Cell Marker in Solid Tumors. Curr. Stem Cell Res. Ther. 2019, 14, 375–388. [Google Scholar] [CrossRef]
- Kang, X.; Jadhav, S.; Annaji, M.; Huang, C.-H.; Amin, R.; Shen, J.; Ashby, C.R., Jr.; Tiwari, A.K.; Babu, R.J.; Chen, P. Advancing Cancer Therapy with Copper/Disulfiram Nanomedicines and Drug Delivery Systems. Pharmaceutics 2023, 15, 1567. [Google Scholar] [CrossRef]
- Chico, M.A.; Mesas, C.; Doello, K.; Quiñonero, F.; Perazzoli, G.; Ortiz, R.; Prados, J.; Melguizo, C. Cancer Stem Cells in Sarcomas: In Vitro Isolation and Role as Prognostic Markers: A Systematic Review. Cancers 2023, 15, 2449. [Google Scholar] [CrossRef]
- Mu, X.; Patel, S.; Mektepbayeva, D.; Mahjoub, A.; Huard, J.; Weiss, K. Retinal Targets ALDH Positive Cancer Stem Cell and Alters the Phenotype of Highly Metastatic Osteosarcoma Cells. Sarcoma 2015, 2015, 784954. [Google Scholar] [CrossRef]
- Brown, H.K.; Tellez-Gabriel, M.; Heymann, D. Cancer stem cells in osteosarcoma. Cancer Lett. 2017, 386, 189–195. [Google Scholar] [CrossRef]
- Yu, S.; Fourman, M.S.; Mahjoub, A.; Mandell, J.B.; Crasto, J.A.; Greco, N.G.; Weiss, K.R. Lung cells support osteosarcoma cell migration and survival. BMC Cancer 2017, 17, 78. [Google Scholar] [CrossRef] [PubMed]
- Avdonkina, N.; Danilova, A.; Misyurin, V.; Prosekina, E.; Girdyuk, D.; Emelyanova, N.; Nekhaeva, T.; Gafton, G.; Baldueva, I. Biological features of tissue and bone sarcomas investigated using an in vitro model of clonal selection. Pathol. Res. Pract. 2020, 217, 153214. [Google Scholar] [CrossRef] [PubMed]
- Mandell, J.B.; Douglas, N.; Ukani, V.; Beumer, J.H.; Guo, J.; Payne, J.; Newman, R.; Mancinelli, L.; Intini, G.; Anderson, C.J.; et al. ALDH1A1 Gene Expression and Cellular Copper Levels between Low and Highly Metastatic Osteosarcoma Provide a Case for Novel Repurposing with Disulfiram and Copper. Sarcoma 2022, 2022, 7157507. [Google Scholar] [CrossRef] [PubMed]
- Belayneh, R.; Weiss, K. The Role of ALDH in the Metastatic Potential of Osteosarcoma Cells and Potential ALDH Targets. Adv. Exp. Med. Biol. 2020, 1258, 157–166. [Google Scholar] [CrossRef]
- Chu, M.; An, X.; Fu, C.; Yu, H.; Zhang, D.; Li, Q.; Man, X.; Dai, X.; Li, Z. Disulfiram/Copper Induce Ferroptosis in Triple-Negative Breast Cancer Cell Line MDA-MB-231. Front. Biosci. 2023, 28, 186. [Google Scholar] [CrossRef]
- Zheng, P.; Wu, Y.; Wang, Y.; Hu, F. Disulfiram suppresses epithelial-mesenchymal transition (EMT), migration and invasion in cervical cancer through the HSP90A/NDRG1 pathway. Cell. Signal. 2023, 109, 110771. [Google Scholar] [CrossRef]
- Zhang, S.; Zong, Y.; Chen, L.; Li, Q.; Li, Z.; Meng, R. The immunomodulatory function and antitumor effect of disulfiram: Paving the way for novel cancer therapeutics. Discov. Oncol. 2023, 14, 103. [Google Scholar] [CrossRef]
- Huang, S.; Xie, P.; Huang, X.; Chen, Z.; Yang, J.; Wang, J.; Liu, C.; Li, H.; Zhou, B. Disulfiram combined with chemoimmunotherapy potentiates pancreatic cancer treatment efficacy through the activation of cGAS-STING signaling pathway via suppressing PARP1 expression. Am. J. Cancer Res. 2023, 13, 2055–2065. [Google Scholar]
- Gan, Y.; Liu, T.; Feng, W.; Wang, L.; Li, L.; Ning, Y. Drug repositioning of disulfiram induces endometrioid epithelial ovarian cancer cell death via the both apoptosis and cuproptosis pathways. Oncol. Res. 2023, 31, 333–343. [Google Scholar] [CrossRef]
- Bermudez, N.M.; Rodríguez-Tamez, G.; Perez, S.; Tosti, A. Onychomycosis: Old and New. J. Fungi 2023, 9, 559. [Google Scholar] [CrossRef]
- Lestner, J.; Hope, W.W. Itraconazole: An update on pharmacology and clinical use for treatment of invasive and allergic fungal infections. Expert Opin. Drug Metab. Toxicol. 2013, 9, 911–926. [Google Scholar] [CrossRef] [PubMed]
- McKinsey, D.S. Treatment and Prevention of Histoplasmosis in Adults Living with HIV. J. Fungi 2021, 7, 429. [Google Scholar] [CrossRef]
- Rahi, M.S.; Jindal, V.; Pednekar, P.; Parekh, J.; Gunasekaran, K.; Sharma, S.; Stender, M.; Jaiyesimi, I.A. Fungal infections in hematopoietic stem-cell transplant patients: A review of epidemiology, diagnosis, and management. Ther. Adv. Infect. Dis. 2021, 8, 20499361211039050. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lan, C.; Qin, S.; Qin, Z.; Zhang, Z.; Zhang, P.; Cao, W. Efficacy of anti-fungal agents for invasive fungal infection prophylaxis in liver transplant recipients: A network meta-analysis. Mycoses 2022, 65, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-L.; Fang, Z.-X.; Wu, Z.; Hou, Y.-Y.; Wu, H.-T.; Liu, J. Repurposed itraconazole for use in the treatment of malignancies as a promising therapeutic strategy. Biomed. Pharmacother. 2022, 154, 113616. [Google Scholar] [CrossRef]
- Wei, X.; Liu, W.; Wang, J.Q.; Tang, Z. “Hedgehog pathway”: A potential target of itraconazole in the treatment of cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 297–304. [Google Scholar] [CrossRef]
- Lin, M.; Chuang, Y.; Wu, H.; Hsu, C.; Lin, N.; Huang, M.; Lou, P. Targeting tumor O-glycosylation modulates cancer–immune-cell crosstalk and enhances anti-PD-1 immunotherapy in head and neck cancer. Mol. Oncol. 2023. [Google Scholar] [CrossRef]
- Lam, A.; Hoang, J.D.; Singleton, A.; Han, X.; Bleier, B.S. Itraconazole and clarithromycin inhibit P-glycoprotein activity in primary human sinonasal epithelial cells. Int. Forum Allergy Rhinol. 2015, 5, 477–480. [Google Scholar] [CrossRef]
- Steinhilber, D.; Jaschonek, K.; Knospe, J.; Morof, O.; Roth, H.J. Effects of novel antifungal azole derivatives on the 5-lipoxygenase and cyclooxygenase pathway. Arzneimittelforschung 1990, 40, 1260–1263. [Google Scholar]
- Saitoh, Y.; Setoguchi, T.; Nagata, M.; Tsuru, A.; Nakamura, S.; Nagano, S.; Ishidou, Y.; Nagao-Kitamoto, H.; Yokouchi, M.; Maeda, S.; et al. Combination of Hedgehog inhibitors and standard anticancer agents synergistically prevent osteosarcoma growth. Int. J. Oncol. 2016, 48, 235–242. [Google Scholar] [CrossRef]
- Lézot, F.; Corre, I.; Morice, S.; Rédini, F.; Verrecchia, F. SHH Signaling Pathway Drives Pediatric Bone Sarcoma Progression. Cells 2020, 9, 536. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.W.; Wunder, J.S.; Dickson, B.C.; Campbell, V.; McGovern, K.; Alman, B.A.; Andrulis, I.L. Involvement and targeted intervention of dysregulated Hedgehog signaling in osteosarcoma. Cancer 2014, 120, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.W.; Pinnaduwage, D.; Gokgoz, N.; Wunder, J.S.; Andrulis, I.L. Aberrant Hedgehog Signaling and Clinical Outcome in Osteosarcoma. Sarcoma 2014, 2014, 261804. [Google Scholar] [CrossRef]
- Chan, L.H.; Wang, W.; Yeung, W.; Deng, Y.; Yuan, P.; Mak, K.K.-L. Hedgehog signaling induces osteosarcoma development through Yap1 and H19 overexpression. Oncogene 2014, 33, 4857–4866. [Google Scholar] [CrossRef]
- Yang, W.; Liu, X.; Choy, E.; Mankin, H.; Hornicek, F.J.; Duan, Z. Targeting hedgehog-GLI-2 pathway in osteosarcoma. J. Orthop. Res. 2013, 31, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Han, L.; Chen, Y.; He, F.; Sun, B.; Kamar, S.; Zhang, Y.; Yang, Y.; Wang, C.; Yang, Z. Hedgehog signalling in the tumourigenesis and metastasis of osteosarcoma, and its potential value in the clinical therapy of osteosarcoma. Cell Death Dis. 2018, 9, 701. [Google Scholar] [CrossRef] [PubMed]
- Martins-Neves, S.R.; Sampaio-Ribeiro, G.; Gomes, C.M.F. Self-Renewal and Pluripotency in Osteosarcoma Stem Cells’ Chemoresistance: Notch, Hedgehog, and Wnt/β-Catenin Interplay with Embryonic Markers. Int. J. Mol. Sci. 2023, 24, 8401. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Li, J.; Li, S.; Yang, Q.; Fan, C. CNOT1 cooperates with LMNA to aggravate osteosarcoma tumorigenesis through the Hedgehog signaling pathway. Mol. Oncol. 2017, 11, 388–404. [Google Scholar] [CrossRef]
- Kumar, R.M.R.; Fuchs, B. Hedgehog Signaling Inhibitors as Anti-Cancer Agents in Osteosarcoma. Cancers 2015, 7, 784–794. [Google Scholar] [CrossRef]
- Wang, C.; Jing, J.; Hu, X.; Yu, S.; Yao, F.; Li, Z.; Cheng, L. Gankyrin activates the hedgehog signalling to drive metastasis in osteosarcoma. J. Cell. Mol. Med. 2021, 25, 6232–6241. [Google Scholar] [CrossRef]
- Qu, W.; Li, D.; Wang, Y.; Wu, Q.; Hao, D. Activation of Sonic Hedgehog Signaling Is Associated with Human Osteosarcoma Cells Radioresistance Characterized by Increased Proliferation, Migration, and Invasion. Med. Sci. Monit. 2018, 24, 3764–3771. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kang, X.; Li, Z.; Chen, L.; Ma, Q.; Fan, P. Hedgehog/GLI1 signaling pathway regulates the resistance to cisplatin in human osteosarcoma. J. Cancer 2021, 12, 6676–6684. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.-W.; Chou, Y.-M.; Li, C.-L.; Liao, E.-C.; Huang, H.-S.; Yin, C.-H.; Chen, C.-L.; Yu, S.-J. Itraconazole improves survival outcomes in patients with colon cancer by inducing autophagic cell death and inhibiting transketolase expression. Oncol. Lett. 2021, 22, 768. [Google Scholar] [CrossRef] [PubMed]
- Sawasaki, M.; Tsubamoto, H.; Sugihara, A.; Ikuta, S.; Sakai, Y.; Osaki, Y.; Sonoda, T. First-Line Gemcitabine, Nab-Paclitaxel, and Oxaliplatin Chemotherapy With Itraconazole in Patients With Metastatic Pancreatic Cancer: A Single Institution Experience. Anticancer Res. 2022, 42, 6063–6069. [Google Scholar] [CrossRef] [PubMed]
- Sawasaki, M.; Tsubamoto, H.; Nakamoto, Y.; Kakuno, A.; Sonoda, T. S-1, Oxaliplatin, Nab-paclitaxel and Itraconazole for Conversion Surgery for Advanced or Recurrent Gastric Cancer. Anticancer Res. 2020, 40, 991–997. [Google Scholar] [CrossRef]
- Rudin, C.M.; Brahmer, J.R.; Juergens, R.A.; Hann, C.L.; Ettinger, D.S.; Sebree, R.; Smith, R.; Aftab, B.T.; Huang, P.; Liu, J.O. Phase 2 Study of Pemetrexed and Itraconazole as Second-Line Therapy for Metastatic Nonsquamous Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2013, 8, 619–623. [Google Scholar] [CrossRef]
- Tsubamoto, H.; Sonoda, T.; Yamasaki, M.; Inoue, K. Impact of combination chemotherapy with itraconazole on survival of patients with refractory ovarian cancer. Anticancer Res. 2014, 34, 2481–2487. [Google Scholar]
- Vreugdenhil, G.; Raemaekers, J.M.M.; van Dijke, B.J.; de Pauw, B.E. Itraconazole and multidrug resistance: Possible effects on remission rate and disease-free survival in acute leukemia. Ann. Hematol. 1993, 67, 107–109. [Google Scholar] [CrossRef]
- Gupta, S.; Kim, J.; Gollapudi, S. Reversal of daunorubicin resistance in P388/ADR cells by itraconazole. J. Clin. Investig. 1991, 87, 1467–1469. [Google Scholar] [CrossRef]
- Warzecha, J.; Göttig, S.; Chow, K.; Brüning, C.; Percic, D.; Boehrer, S.; Brude, E.; Kurth, A. Inhibition of Osteosarcoma Cell Proliferation by the Hedgehog-Inhibitor Cyclopamine. J. Chemother. 2007, 19, 554–561. [Google Scholar] [CrossRef]
- Aftab, B.T.; Dobromilskaya, I.; Liu, J.O.; Rudin, C.M. Itraconazole Inhibits Angiogenesis and Tumor Growth in Non–Small Cell Lung Cancer. Cancer Res. 2011, 71, 6764–6772. [Google Scholar] [CrossRef] [PubMed]
- Nacev, B.A.; Grassi, P.; Dell, A.; Haslam, S.M.; Liu, J.O. The Antifungal Drug Itraconazole Inhibits Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) Glycosylation, Trafficking, and Signaling in Endothelial Cells*. J. Biol. Chem. 2011, 286, 44045–44056. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Abe, Y.; Kawai, A.; Furihata, T.; Endo, T.; Takeda, H. Pharmacokinetic Drug Interactions of an Orally Available TRH Analog (Rovatirelin) with a CYP3A4/5 and P-Glycoprotein Inhibitor (Itraconazole). J. Clin. Pharmacol. 2020, 60, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Unadkat, J.D.; Mao, Q. Interactions of azole antifungal agents with the human breast cancer resistance protein. J. Pharm. Sci. 2007, 96, 3226–3235. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, L.M.; Isringhausen, C.D.; Ogilvie, B.W.; Buckley, D.B. Evaluation of Ketoconazole and Its Alternative Clinical CYP3A4/5 Inhibitors as Inhibitors of Drug Transporters: The In Vitro Effects of Ketoconazole, Ritonavir, Clarithromycin, and Itraconazole on 13 Clinically-Relevant Drug Transporters. Drug Metab. Dispos. 2016, 44, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Lempers, V.J.C.; van den Heuvel, J.J.M.W.; Russel, F.G.M.; Aarnoutse, R.E.; Burger, D.M.; Brüggemann, R.J.; Koenderink, J.B. Inhibitory Potential of Antifungal Drugs on ATP-Binding Cassette Transporters P-Glycoprotein, MRP1 to MRP5, BCRP, and BSEP. Antimicrob. Agents Chemother. 2016, 60, 3372–3379. [Google Scholar] [CrossRef]
- Li, C.; Horton, J.K.; Sale, M.; Curd, L.; Goti, V.; Tao, W.; Beelen, A. Pharmacokinetic Drug–Drug Interaction Studies Between Trilaciclib and Midazolam, Metformin, Rifampin, Itraconazole, and Topotecan in Healthy Volunteers and Patients with Extensive-Stage Small-Cell Lung Cancer. Clin. Drug Investig. 2022, 42, 679–692. [Google Scholar] [CrossRef]
- Kurosawa, M.; Okabe, M.; Hara, N.; Kawamura, K.; Suzuki, S.; Sakurada, K.; Asaka, M. Reversal effect of itraconazole on adriamycin and etoposide resistance in human leukemia cells. Ann. Hematol. 1996, 72, 17–21. [Google Scholar] [CrossRef]
- Takimoto, Y.; Tsubamoto, H.; Taniguchi, R.; Sakata, K.; Takada, Y.; Adachi, J.; Tomonaga, T.; Ueda, T.; Nakagawa, K.; Narita, S.; et al. Itraconazole Repolarizes Tumor-associated Macrophages and Suppresses Cervical Cancer Cell Growth. Anticancer Res. 2023, 43, 569–580. [Google Scholar] [CrossRef]
- Weng, N.; Zhang, Z.; Tan, Y.; Zhang, X.; Wei, X.; Zhu, Q. Repurposing antifungal drugs for cancer therapy. J. Adv. Res. 2023, 48, 259–273. [Google Scholar] [CrossRef]
- Wu, H.-T.; Li, C.-L.; Fang, Z.-X.; Chen, W.-J.; Lin, W.-T.; Liu, J. Induced Cell Cycle Arrest in Triple-Negative Breast Cancer by Combined Treatment of Itraconazole and Rapamycin. Front. Pharmacol. 2022, 13, 873131. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, Y.; Tsubamoto, H.; Isono-Taniguchi, R.; Ueda, T.; Sakata, K.; Nakagawa, K.; Narita, S.; Wakimoto, Y.; Shibahara, H.; Nishiumi, S. Itraconazole Modulates Phospholipid Levels in Tumor-associated Macrophages. Anticancer Res. 2023, 43, 1981–1984. [Google Scholar] [CrossRef]
- Remm, F.; Franz, W.-M.; Brenner, C. Gliptins and their target dipeptidyl peptidase 4: Implications for the treatment of vascular disease. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Yurttas, A.G.; Dasci, M.F. Exploring the molecular mechanism of linagliptin in osteosarcoma cell lines for anti-cancer activity. Pathol. Res. Pract. 2023, 248, 154640. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Fu, B.; Luo, D.; Wang, H.; Cao, H.; Chen, X.; Tian, L.; Yu, X. The Multiple Biological Functions of Dipeptidyl Peptidase-4 in Bone Metabolism. Front. Endocrinol. 2022, 13, 856954. [Google Scholar] [CrossRef]
- Won, K.Y.; Park, H.-R.; Park, Y.-K. Prognostic Implication of Immunohistochemical Runx2 Expression in Osteosarcoma. Tumori J. 2009, 95, 311–316. [Google Scholar] [CrossRef]
- Kurek, K.C.; Del Mare, S.; Salah, Z.; Abdeen, S.; Sadiq, H.; Lee, S.-H.; Gaudio, E.; Zanesi, N.; Jones, K.B.; DeYoung, B.; et al. Frequent Attenuation of the WWOX Tumor Suppressor in Osteosarcoma Is Associated with Increased Tumorigenicity and Aberrant RUNX2 Expression. Cancer Res. 2010, 70, 5577–5586. [Google Scholar] [CrossRef]
- Sadikovic, B.; Thorner, P.; Chilton-MacNeill, S.; Martin, J.W.; Cervigne, N.K.; Squire, J.; Zielenska, M. Expression analysis of genes associated with human osteosarcoma tumors shows correlation of RUNX2 overexpression with poor response to chemotherapy. BMC Cancer 2010, 10, 202. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, H.; Mo, X.; Chen, G.; Lin, L. Synergistic relationship between dipeptidyl peptidase IV and neutral endopeptidase expression and the combined prognostic significance in osteosarcoma patients. Med. Oncol. 2013, 30, 608. [Google Scholar] [CrossRef]
- Dohi, O.; Ohtani, H.; Hatori, M.; Sato, E.; Hosaka, M.; Nagura, H.; Itoi, E.; Kokubun, S. Histogenesis-specific expression of fibroblast activation protein and dipeptidylpeptidase-IV in human bone and soft tissue tumours. Histopathology 2009, 55, 432–440. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Chung, H.S.; Jung, T.W.; Ryu, J.Y.; Hong, H.C.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Choi, K.M.; Choi, D.S.; et al. The dipeptidyl peptidase-IV inhibitor inhibits the expression of vascular adhesion molecules and inflammatory cytokines in HUVECs via Akt- and AMPK-dependent mechanisms. Mol. Cell. Endocrinol. 2015, 405, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Nirwan, N.; Vohora, D. Linagliptin in Combination With Metformin Ameliorates Diabetic Osteoporosis Through Modulating BMP-2 and Sclerostin in the High-Fat Diet Fed C57BL/6 Mice. Front. Endocrinol. 2022, 13, 944323. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Hu, X.; Chen, R. The effects of Omarigliptin on promoting osteoblastic differentiation. Bioengineered 2021, 12, 11837–11846. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Yang, H.; Wang, Y.; Yan, X.; Li, D.; Cao, Z.; Ning, Y.; Zhang, C. Anagliptin stimulates osteoblastic cell differentiation and mineralization. Biomed. Pharmacother. 2020, 129, 109796. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Wu, R.; Cao, L.; Gu, H.; Chai, F. Trelagliptin stimulates osteoblastic differentiation by increasing runt-related transcription factor 2 (RUNX2): A therapeutic implication in osteoporosis. Bioengineered 2021, 12, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qian, K.; Sun, Y.; Zhao, Y.; Zhou, Y.; Xue, Y.; Hong, X. Omarigliptin ameliorated high glucose-induced nucleotide oligomerization domain-like receptor protein 3 (NLRP3) inflammasome activation through activating adenosine monophosphate-activated protein kinase α (AMPKα) in renal glomerular endothelial cells. Bioengineered 2021, 12, 4805–4815. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, J.; Wang, J.; Zhu, L.; Wang, J.; Zhang, Q. DPP-4 Inhibitors Suppress Tau Phosphorylation and Promote Neuron Autophagy through the AMPK/mTOR Pathway to Ameliorate Cognitive Dysfunction in Diabetic Mellitus. ACS Chem. Neurosci. 2023. [Google Scholar] [CrossRef]
- Al-Damry, N.T.; Attia, H.A.; Al-Rasheed, N.M.; Al-Rasheed, N.M.; Mohamad, R.A.; Al-Amin, M.A.; Dizmiri, N.; Atteya, M. Sitagliptin attenuates myocardial apoptosis via activating LKB-1/AMPK/Akt pathway and suppressing the activity of GSK-3β and p38α/MAPK in a rat model of diabetic cardiomyopathy. Biomed. Pharmacother. 2018, 107, 347–358. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, C.; Guan, M.; Zheng, Z.; Li, J.; Xu, W.; Wang, L.; He, F.; Xue, Y. The DPP-4 inhibitor sitagliptin attenuates the progress of atherosclerosis in apolipoprotein-E-knockout mice via AMPK- and MAPK-dependent mechanisms. Cardiovasc. Diabetol. 2014, 13, 32. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, J.; Fu, M.; Dong, R.; Yang, Y.; Luo, J.; Hu, S.; Li, W.; Xu, X.; Tu, L. Dipeptidyl peptidase-4 inhibition improves endothelial senescence by activating AMPK/SIRT1/Nrf2 signaling pathway. Biochem. Pharmacol. 2020, 177, 113951. [Google Scholar] [CrossRef]
- Ideta, T.; Shirakami, Y.; Miyazaki, T.; Kochi, T.; Sakai, H.; Moriwaki, H.; Shimizu, M. The Dipeptidyl Peptidase-4 Inhibitor Teneligliptin Attenuates Hepatic Lipogenesis via AMPK Activation in Non-Alcoholic Fatty Liver Disease Model Mice. Int. J. Mol. Sci. 2015, 16, 29207–29218. [Google Scholar] [CrossRef] [PubMed]
- Monami, M.; Dicembrini, I.; Antenore, A.; Mannucci, E. Dipeptidyl Peptidase-4 Inhibitors and Bone Fractures: A meta-analysis of randomized clinical trials. Diabetes Care 2011, 34, 2474–2476. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xie, X.; Zhang, D.; Mao, F.; Wang, S.; Liao, Y. Saxagliptin enhances osteogenic differentiation in MC3T3-E1 cells, dependent on the activation of AMP-activated protein kinase α (AMPKα)/runt-related transcription factor-2 (Runx-2). Bioengineered 2022, 13, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Cordero, O.J. CD26 and Cancer. Cancers 2022, 14, 5194. [Google Scholar] [CrossRef]
- Cruickshank, J.M. The Role of Beta-Blockers in the Treatment of Hypertension. Adv. Exp. Med. Biol. 2016, 956, 149–166. [Google Scholar] [CrossRef]
- MacCormack, J.K.; Armstrong-Carter, E.L.B.; Gaudier-Diaz, M.M.; Meltzer-Brody, S.; Sloan, E.K.; Lindquist, K.A.; Muscatell, K.A. β-Adrenergic Contributions to Emotion and Physiology During an Acute Psychosocial Stressor. Psychosom. Med. 2021, 83, 959–968. [Google Scholar] [CrossRef]
- Brantigan, C.; Brantigan, T.; Joseph, N. Effect of beta blockade and beta stimulation on stage fright. Am. J. Med. 1982, 72, 88–94. [Google Scholar] [CrossRef]
- Qiao, G.; Bucsek, M.J.; Winder, N.M.; Chen, M.; Giridharan, T.; Olejniczak, S.H.; Hylander, B.L.; Repasky, E.A. β-Adrenergic signaling blocks murine CD8+ T-cell metabolic reprogramming during activation: A mechanism for immunosuppression by adrenergic stress. Cancer Immunol. Immunother. 2019, 68, 11–22. [Google Scholar] [CrossRef]
- Peixoto, R.; Pereira, M.d.L.; Oliveira, M. Beta-Blockers and Cancer: Where Are We? Pharmaceuticals 2020, 13, 105. [Google Scholar] [CrossRef]
- Vojvodic, A.; Vojvodic, P.; Vlaskovic-Jovicevic, T.; Sijan, G.; Dimitrijevic, S.; Peric-Hajzler, Z.; Matovic, D.; Wollina, U.; Tirant, M.; Thuong, N.V.; et al. Beta Blockers and Melanoma. Open Access Maced. J. Med. Sci. 2019, 7, 3110–3112. [Google Scholar] [CrossRef]
- Phadke, S.; Clamon, G. Beta blockade as adjunctive breast cancer therapy: A review. Crit. Rev. Oncol. Hematol. 2019, 138, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Pantziarka, P.; Bouche, G.; Sukhatme, V.; Meheus, L.; Rooman, I.; Sukhatme, V.P. Repurposing Drugs in Oncology (ReDO)—Propranolol as an anti-cancer agent. Ecancermedicalscience 2016, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.J.; Cranmer, L.D.; Loggers, E.T.; Pollack, S.M. Propranolol for the treatment of vascular sarcomas. J. Exp. Pharmacol. 2018, 10, 51–58. [Google Scholar] [CrossRef]
- Cole, S.W.; Sood, A.K. Molecular Pathways: Beta-Adrenergic Signaling in Cancer. Clin. Cancer Res. 2012, 18, 1201–1206. [Google Scholar] [CrossRef]
- Yan, J.; Chen, Y.; Luo, M.; Hu, X.; Li, H.; Liu, Q.; Zou, Z. Chronic stress in solid tumor development: From mechanisms to interventions. J. Biomed. Sci. 2023, 30, 8. [Google Scholar] [CrossRef] [PubMed]
- Ammons, D.T.; MacDonald, C.R.; Chow, L.; Repasky, E.A.; Dow, S. Chronic adrenergic stress and generation of myeloid-derived suppressor cells: Implications for cancer immunotherapy in dogs. Veter. Comp. Oncol. 2023, 21, 159–165. [Google Scholar] [CrossRef]
- An, J.; Feng, L.; Ren, J.; Li, Y.; Li, G.; Liu, C.; Yao, Y.; Yao, Y.; Jiang, Z.; Gao, Y.; et al. Chronic stress promotes breast carcinoma metastasis by accumulating myeloid-derived suppressor cells through activating β-adrenergic signaling. OncoImmunology 2021, 10, 2004659. [Google Scholar] [CrossRef]
- Cao, M.; Huang, W.; Chen, Y.; Li, G.; Liu, N.; Wu, Y.; Wang, G.; Li, Q.; Kong, D.; Xue, T.; et al. Chronic restraint stress promotes the mobilization and recruitment of myeloid-derived suppressor cells through β-adrenergic-activated CXCL5-CXCR2-Erk signaling cascades. Int. J. Cancer 2021, 149, 460–472. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, S.; Ning, B.; Huang, T.; Li, Y.; Wei, Y. Stress and cancer: The mechanisms of immune dysregulation and management. Front. Immunol. 2022, 13, 1032294. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Y.; Li, Z.-Z.; Sun, J.; Li, J.-W.; Wei, W.; Li, L.; Zhang, C.; Huang, C.; Yang, S.-Y.; et al. Chronic restraint stress promotes hepatocellular carcinoma growth by mobilizing splenic myeloid cells through activating β-adrenergic signaling. Brain Behav. Immun. 2019, 80, 825–838. [Google Scholar] [CrossRef]
- Ieguchi, K.; Funakoshi, M.; Mishima, T.; Takizawa, K.; Omori, T.; Nakamura, F.; Watanabe, M.; Tsuji, M.; Kiuchi, Y.; Kobayashi, S.; et al. The Sympathetic Nervous System Contributes to the Establishment of Pre-Metastatic Pulmonary Microenvironments. Int. J. Mol. Sci. 2022, 23, 10652. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, D.; Guo, L.; Cheng, X.; Guo, N.; Shi, M. Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating β-adrenergic signaling. J. Pathol. 2018, 244, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-K.; Liu, J.-X.; Zheng, C.-X.; Liu, L.; Ma, C.; Tian, J.-Y.; Yuan, Y.; Cao, Y.; Xing, S.-J.; Liu, S.-Y.; et al. Region-specific sympatho-adrenergic regulation of specialized vasculature in bone homeostasis and regeneration. iScience 2023, 26, 107455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yin, H.; Yang, M.; Lei, X.; Zhen, D.; Zhang, Z. Advanced Progress of the Relationship Between Antihypertensive Drugs and Bone Metabolism. Hypertension 2023, 80, 2255–2264. [Google Scholar] [CrossRef]
- Bandala, C.; Ávila-Luna, A.; Gómez-López, M.; Estrada-Villaseñor, E.; Montes, S.; Alfaro-Rodríguez, A.; Miliar-García, Á.; Cortés-Altamirano, J.L.; Peralta, R.; Ibáñez-Cervantes, G.; et al. Catecholamine levels and gene expression of their receptors in tissues of adults with osteosarcoma. Arch. Physiol. Biochem. 2021, 127, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G.; Khosla, S. The crosstalk between bone remodeling and energy metabolism: A translational perspective. Cell Metab. 2022, 34, 805–817. [Google Scholar] [CrossRef]
- Conceição, F.; Sousa, D.M.; Tojal, S.; Lourenço, C.; Carvalho-Maia, C.; Estevão-Pereira, H.; Lobo, J.; Couto, M.; Rosenkilde, M.M.; Jerónimo, C.; et al. The Secretome of Parental and Bone Metastatic Breast Cancer Elicits Distinct Effects in Human Osteoclast Activity after Activation of β2 Adrenergic Signaling. Biomolecules 2023, 13, 622. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, N.; Qian, C.; Qi, C.; Noller, K.; Wan, M.; Liu, X.; Zhang, W.; Cahan, P.; Cao, X. Sympathetic Innervation Regulates Osteocyte-Mediated Cortical Bone Resorption during Lactation. Adv. Sci. 2023, 10, e2207602. [Google Scholar] [CrossRef]
- Worton, L.E.; Srinivasan, S.; Threet, D.; Ausk, B.J.; Huber, P.; Kwon, R.Y.; Bain, S.D.; Gross, T.S.; Gardiner, E.M. Beta 2 Adrenergic Receptor Selective Antagonist Enhances Mechanically Stimulated Bone Anabolism in Aged Mice. JBMR Plus 2022, 7, e10712. [Google Scholar] [CrossRef]
- Koh, S.P.; Leadbitter, P.; Smithers, F.; Tan, S.T. β-blocker therapy for infantile hemangioma. Expert Rev. Clin. Pharmacol. 2020, 13, 899–915. [Google Scholar] [CrossRef]
- Solernó, L.M.; Sobol, N.T.; Gottardo, M.F.; Capobianco, C.S.; Ferrero, M.R.; Vásquez, L.; Alonso, D.F.; Garona, J. Propranolol blocks osteosarcoma cell cycle progression, inhibits angiogenesis and slows xenograft growth in combination with cisplatin-based chemotherapy. Sci. Rep. 2022, 12, 15058. [Google Scholar] [CrossRef] [PubMed]
- Duckett, M.M.; Phung, S.K.; Nguyen, L.; Khammanivong, A.; Dickerson, E.B.; Dusenbery, K.; Lawrence, J. The adrenergic receptor antagonists propranolol and carvedilol decrease bone sarcoma cell viability and sustained carvedilol reduces clonogenic survival and increases radiosensitivity in canine osteosarcoma cells. Veter. Comp. Oncol. 2020, 18, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Majeska, R.J.; Minkowitz, B.; Bastian, W.; Einhorn, T.A. Effects of β-adrenergic blockade in an osteoblast-like cell line. J. Orthop. Res. 1992, 10, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Golden, D.; Saria, E.A.; Hansen, M.F. Regulation of Osteoblast Migration Involving Receptor Activator of Nuclear Factor-kappa B (RANK) Signaling. J. Cell. Physiol. 2015, 230, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- González-Suárez, E.; Sanz-Moreno, A. RANK as a therapeutic target in cancer. FEBS J. 2016, 283, 2018–2033. [Google Scholar] [CrossRef]
- Kitazawa, S.; Kitazawa, R. RANK ligand is a prerequisite for cancer-associated osteolytic lesions. J. Pathol. 2002, 198, 228–236. [Google Scholar] [CrossRef]
- Roodman, G.D. Biology of Osteoclast Activation in Cancer. J. Clin. Oncol. 2001, 19, 3562–3571. [Google Scholar] [CrossRef]
- Branstetter, D.; Rohrbach, K.; Huang, L.-Y.; Soriano, R.; Tometsko, M.; Blake, M.; Jacob, A.P.; Dougall, W.C. RANK and RANK ligand expression in primary human osteosarcoma. J. Bone Oncol. 2015, 4, 59–68. [Google Scholar] [CrossRef]
- Bago-Horvath, Z.; Schmid, K.; Rössler, F.; Nagy-Bojarszky, K.; Funovics, P.; Sulzbacher, I. Impact of RANK signalling on survival and chemotherapy response in osteosarcoma. Pathology 2014, 46, 411–415. [Google Scholar] [CrossRef]
- Zeng, J.; Peng, Y.; Wang, D.; Ayesha, K.; Chen, S. The interaction between osteosarcoma and other cells in the bone microenvironment: From mechanism to clinical applications. Front. Cell Dev. Biol. 2023, 11, 1123065. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Y.; Zhu, Y.; Zeng, M.; Xie, J.; Lei, P.; Li, K.; Hu, Y. Role of RANK and Akt1 activation in human osteosarcoma progression: A clinicopathological study. Exp. Ther. Med. 2017, 13, 2862–2866. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Navet, B.; Ando, K.; Vargas-Franco, J.W.; Brion, R.; Amiaud, J.; Mori, K.; Yagita, H.; Mueller, C.G.; Verrecchia, F.; Dumars, C.; et al. The Intrinsic and Extrinsic Implications of RANKL/RANK Signaling in Osteosarcoma: From Tumor Initiation to Lung Metastases. Cancers 2018, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Trieb, K.; Windhager, R. Receptor activator of nuclear factor κB expression is a prognostic factor in human osteosarcoma. Oncol. Lett. 2015, 10, 1813–1815. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shen, W.; Hu, X.; Zhang, Y.; Zhuo, Y.; Li, T.; Mei, F.; Li, X.; Xiao, L.; Chu, T. Quetiapine inhibits osteoclastogenesis and prevents human breast cancer-induced bone loss through suppression of the RANKL-mediated MAPK and NF-κB signaling pathways. Breast Cancer Res. Treat. 2015, 149, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Mi, G.; Wang, Y.; Ye, E.; Gao, Y.; Liu, Q.; Chen, P.; Zhu, Y.; Yang, H.; Yang, Z. The antipsychotic drug quetiapine stimulates oligodendrocyte differentiation by modulating the cell cycle. Neurochem. Int. 2018, 118, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Cardona, J.G.; Smith, M.D.; Wang, J.; Kirby, L.; Schott, J.T.; Davidson, T.; Karnell, J.L.; Whartenby, K.A.; Calabresi, P.A. Quetiapine has an additive effect to triiodothyronine in inducing differentiation of oligodendrocyte precursor cells through induction of cholesterol biosynthesis. PLoS ONE 2019, 14, e0221747. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, N.; Li, H.; Liu, S.; Chen, X.; Yu, S.; Wu, N.; Bian, X.W.; Shen, H.Y.; Li, C.; et al. Promoting oligodendroglial-oriented differentiation of glioma stem cell: A repurposing of quetiapine for the treatment of malignant glioma. Oncotarget 2017, 8, 37511–37524. [Google Scholar] [CrossRef]
- Wang, X.; Su, Y.; Li, T.; Yu, G.; Wang, Y.; Chen, X.; Yin, C.; Tang, Z.; Yi, C.; Xiao, L.; et al. Quetiapine promotes oligodendroglial process outgrowth and membrane expansion by orchestrating the effects of Olig1. Glia 2021, 69, 1709–1722. [Google Scholar] [CrossRef]
- Bi, X.; Zhang, Y.; Yan, B.; Fang, S.; He, J.; Zhang, D.; Zhang, Z.; Kong, J.; Tan, Q.; Li, X. Quetiapine prevents oligodendrocyte and myelin loss and promotes maturation of oligodendrocyte progenitors in the hippocampus of global cerebral ischemia mice. J. Neurochem. 2012, 123, 14–20. [Google Scholar] [CrossRef]
- Ma, L.; Yang, F.; Zhao, R.; Li, L.; Kang, X.; Xiao, L.; Jiang, W. Quetiapine attenuates cognitive impairment and decreases seizure susceptibility possibly through promoting myelin development in a rat model of malformations of cortical development. Brain Res. 2015, 1622, 443–451. [Google Scholar] [CrossRef]
- Dandash, O.; Yücel, M.; Daglas, R.; Pantelis, C.; McGorry, P.; Berk, M.; Fornito, A. Differential effect of quetiapine and lithium on functional connectivity of the striatum in first episode mania. Transl. Psychiatry 2018, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Lin, S.; Wang, H.; Hsu, F.; Chung, J.G.; Hsu, L. The inhibitory effect and mechanism of quetiapine on tumor progression in hepatocellular carcinoma in vivo. Environ. Toxicol. 2022, 37, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Cupp, M.A.; Cariolou, M.; Tzoulaki, I.; Aune, D.; Evangelou, E.; Berlanga-Taylor, A.J. Neutrophil to lymphocyte ratio and cancer prognosis: An umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020, 18, 360. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xiang, D.; Wan, J.; Yang, L.; Zheng, C. Focus on the Dynamics of Neutrophil-to-Lymphocyte Ratio in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Meta-Analysis and Systematic Review. Cancers 2022, 14, 5297. [Google Scholar] [CrossRef]
- Wu, L.; Saxena, S.; Singh, R.K. Neutrophils in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1224, 1–20. [Google Scholar] [CrossRef]
- Xia, W.-K.; Liu, Z.-L.; Shen, D.; Lin, Q.-F.; Su, J.; Mao, W.-D. Prognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcoma. World J. Surg. Oncol. 2016, 14, 127. [Google Scholar] [CrossRef]
- Yapar, A.; Tokgöz, M.A.; Yapar, D.; Atalay, I.B.; Ulucaköy, C.; Güngör, B. Diagnostic and prognostic role of neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and lymphocyte/monocyte ratio in patients with osteosarcoma. Jt. Dis. Relat. Surg. 2021, 32, 489–496. [Google Scholar] [CrossRef]
- Tang, H.; Liu, D.; Lu, J.; He, J.; Ji, S.; Liao, S.; Wei, Q.; Lu, S.; Liu, Y. Significance of the neutrophil-to-lymphocyte ratio in predicting the response to neoadjuvant chemotherapy in extremity osteosarcoma: A multicentre retrospective study. BMC Cancer 2022, 22, 33. [Google Scholar] [CrossRef]
- Peng, L.-P.; Li, J.; Li, X.-F. Prognostic value of neutrophil/lymphocyte, platelet/lymphocyte, lymphocyte/monocyte ratios and Glasgow prognostic score in osteosarcoma: A meta-analysis. World J. Clin. Cases 2022, 10, 2194–2205. [Google Scholar] [CrossRef]
- Vasquez, L.; León, E.; Beltran, B.; Maza, I.; Oscanoa, M.; Geronimo, J. Pretreatment Neutrophil-to-Lymphocyte Ratio and Lymphocyte Recovery: Independent Prognostic Factors for Survival in Pediatric Sarcomas. J. Pediatr. Hematol. Oncol. 2017, 39, 538–546. [Google Scholar] [CrossRef]
- Yang, S.; Wu, C.; Wang, L.; Shan, D.; Chen, B. Pretreatment inflammatory indexes as prognostic predictors for survival in osteosarcoma patients. Int. J. Clin. Exp. Pathol. 2020, 13, 515–524. [Google Scholar]
- Huang, X.; Liu, Y.; Liang, W.; Luo, K.; Qin, Y.; Li, F.; Xie, T.; Qin, H.; He, J.; Wei, Q. A new model of preoperative systemic inflammatory markers predicting overall survival of osteosarcoma: A multicenter retrospective study. BMC Cancer 2022, 22, 1370. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, Y.; Ye, Q.; Wang, Y.; Min, L.; Luo, Y.; Zhou, Y.; Tu, C. Lung Immune Prognostic Index Could Predict Metastasis in Patients with Osteosarcoma. Front. Surg. 2022, 9, 923427. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Huang, Y.; Sun, Y.; Zhang, J.; Yao, Y.; Shen, Z.; Xiang, D.; He, A. Prognostic value of inflammation-based scores in patients with osteosarcoma. Sci. Rep. 2016, 6, 39862. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Fang, X.; Xu, Y.; Wei, L.; Qin, Y.; Yang, F.; Lu, S.; Zhao, J. Prognostic value of preoperative P-CRP in patients with osteosarcoma: A retrospective study of 101 cases. Medicine 2022, 101, e30382. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, T.; Yao, Z.; Zhang, X. Prognostic value of pre-treatment Naples prognostic score (NPS) in patients with osteosarcoma. World J. Surg. Oncol. 2020, 18, 24. [Google Scholar] [CrossRef]
- Ouyang, H.; Wang, Z. Predictive value of the systemic immune inflammation index for cancer-specific survival of osteosarcoma in children. Front. Public Health 2022, 10, 879523. [Google Scholar] [CrossRef]
- Tian, K.; Li, P.J.; Zhang, Y. Preoperative Predictors of Early Mortality Risk in People with Osteosarcoma of the Extremities Treated with Standard Therapy. Cancer Manag. Res. 2022, 14, 437–447. [Google Scholar] [CrossRef]
- Nirala, B.K.; Yamamichi, T.; Yustein, J.T. Deciphering the Signaling Mechanisms of Osteosarcoma Tumorigenesis. Int. J. Mol. Sci. 2023, 24, 11367. [Google Scholar] [CrossRef]
- Adamopoulos, C.; Gargalionis, A.N.; Basdra, E.K.; Papavassiliou, A.G. Deciphering signaling networks in osteosarcoma pathobiology. Exp. Biol. Med. 2016, 241, 1296–1305. [Google Scholar] [CrossRef]
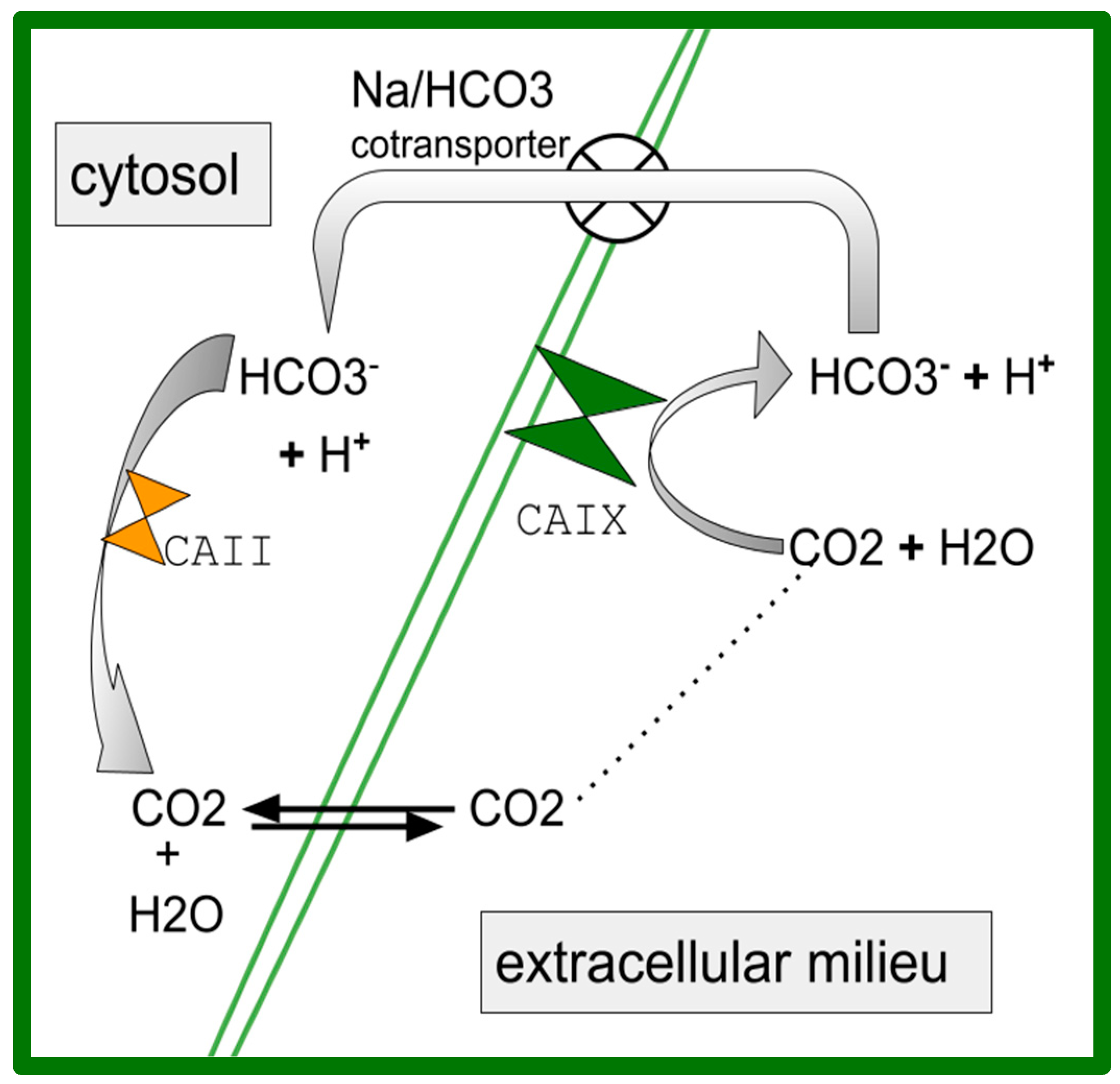
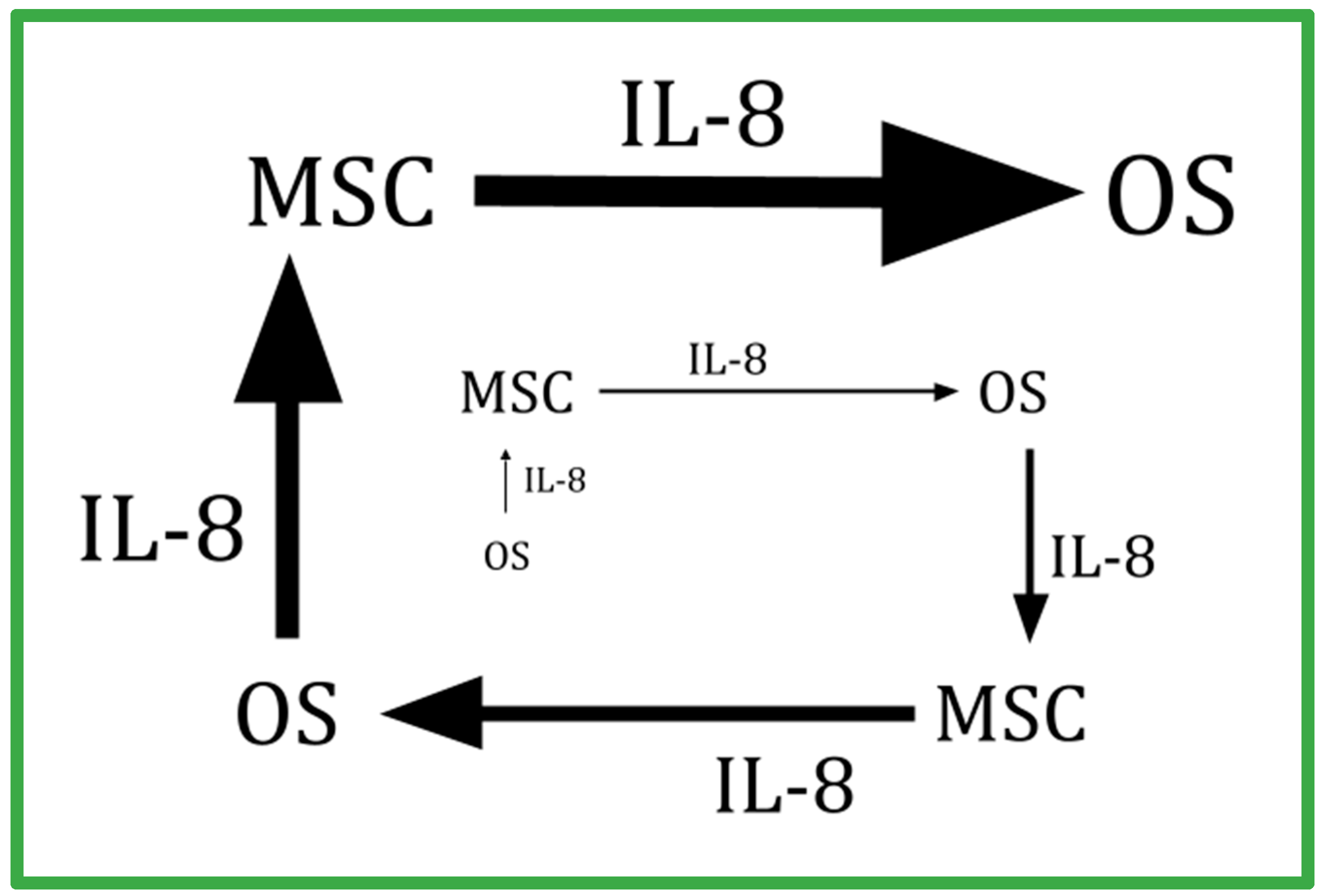
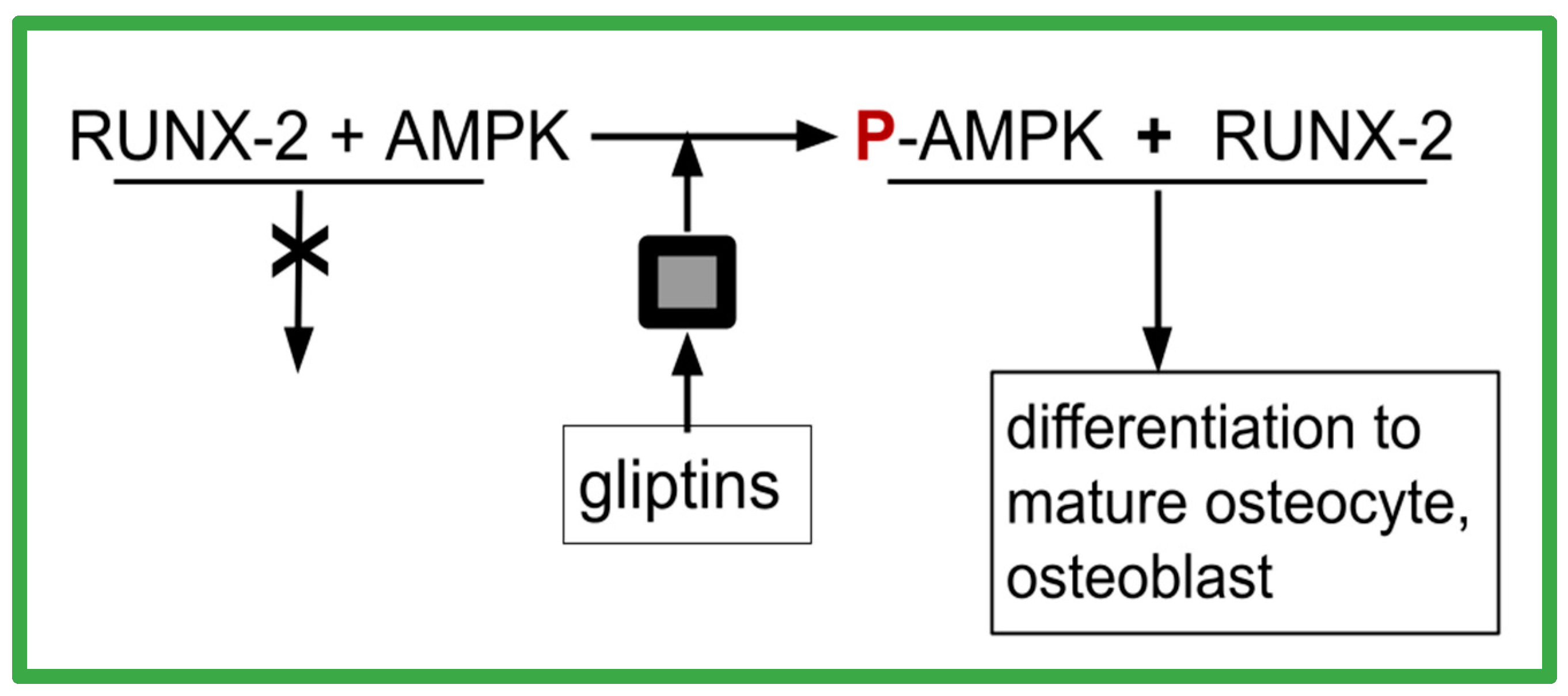
| Drug | Common Use | Targets in OSR9 |
|---|---|---|
| aprepitant # | nausea | NK-1 receptor |
| celecoxib # | analgesia | CA-2-9 and COX-2 |
| chloroquine | malaria and rheumatoid arthritis | TLR-9 |
| dapsone | malaria and Hansen’s disease | IL-8 and neutrophils |
| disulfiram # | alcoholism | ALDH |
| itraconazole # | fungal infections | Hh and 5-LO |
| linagliptin | diabetes | GLP-1 and DPP-4 |
| propranolol | hypertension | beta adrenergic receptor |
| quetiapine | psychosis | RANK-RANKL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kast, R.E. The OSR9 Regimen: A New Augmentation Strategy for Osteosarcoma Treatment Using Nine Older Drugs from General Medicine to Inhibit Growth Drive. Int. J. Mol. Sci. 2023, 24, 15474. https://doi.org/10.3390/ijms242015474
Kast RE. The OSR9 Regimen: A New Augmentation Strategy for Osteosarcoma Treatment Using Nine Older Drugs from General Medicine to Inhibit Growth Drive. International Journal of Molecular Sciences. 2023; 24(20):15474. https://doi.org/10.3390/ijms242015474
Chicago/Turabian StyleKast, Richard E. 2023. "The OSR9 Regimen: A New Augmentation Strategy for Osteosarcoma Treatment Using Nine Older Drugs from General Medicine to Inhibit Growth Drive" International Journal of Molecular Sciences 24, no. 20: 15474. https://doi.org/10.3390/ijms242015474
APA StyleKast, R. E. (2023). The OSR9 Regimen: A New Augmentation Strategy for Osteosarcoma Treatment Using Nine Older Drugs from General Medicine to Inhibit Growth Drive. International Journal of Molecular Sciences, 24(20), 15474. https://doi.org/10.3390/ijms242015474






