Mixed Connective Tissue Disease as Different Entity: Global Methylation Aspect
Abstract
1. Introduction
2. Results
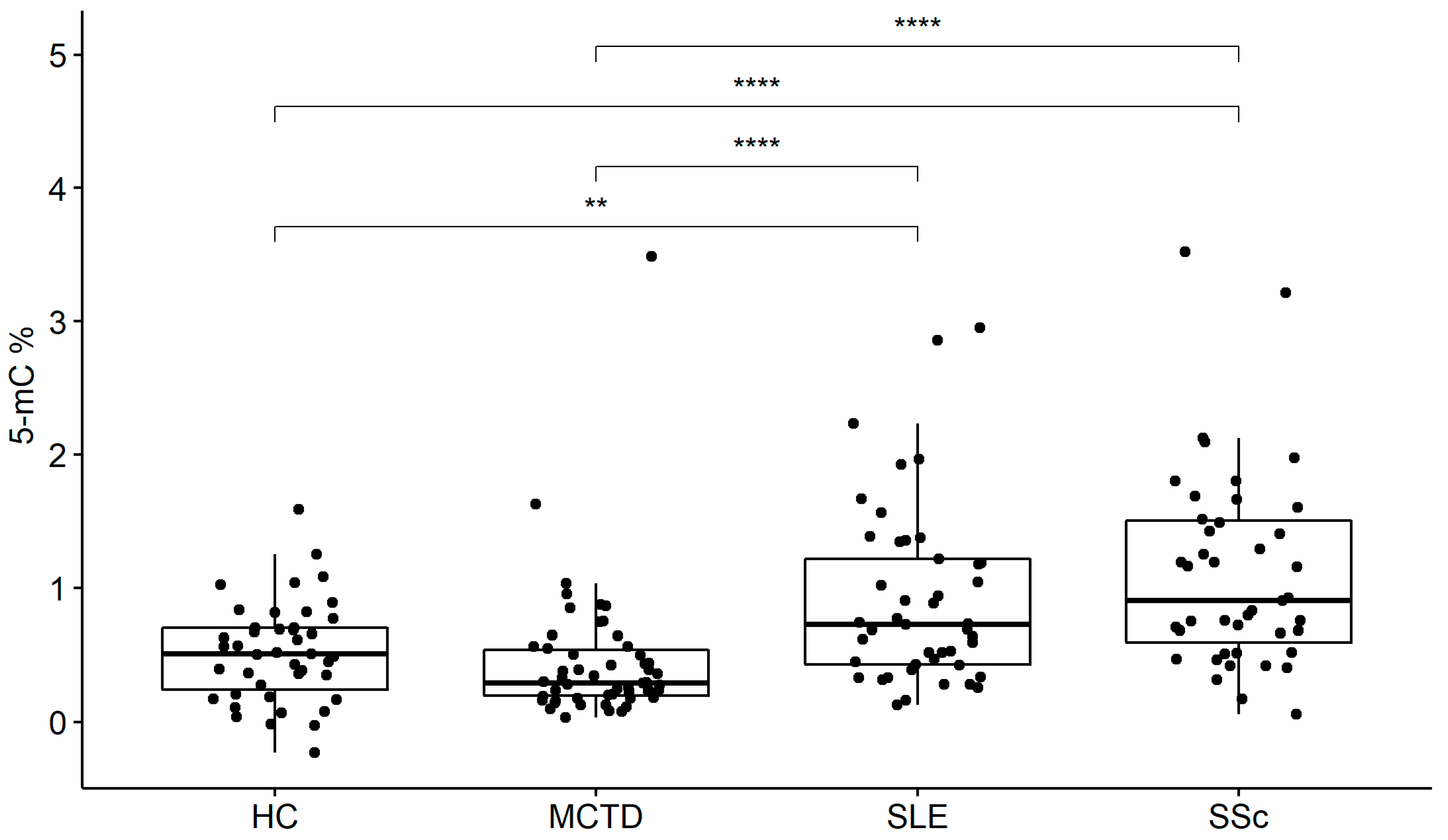
2.1. Global DNA Methylation in ACTDs
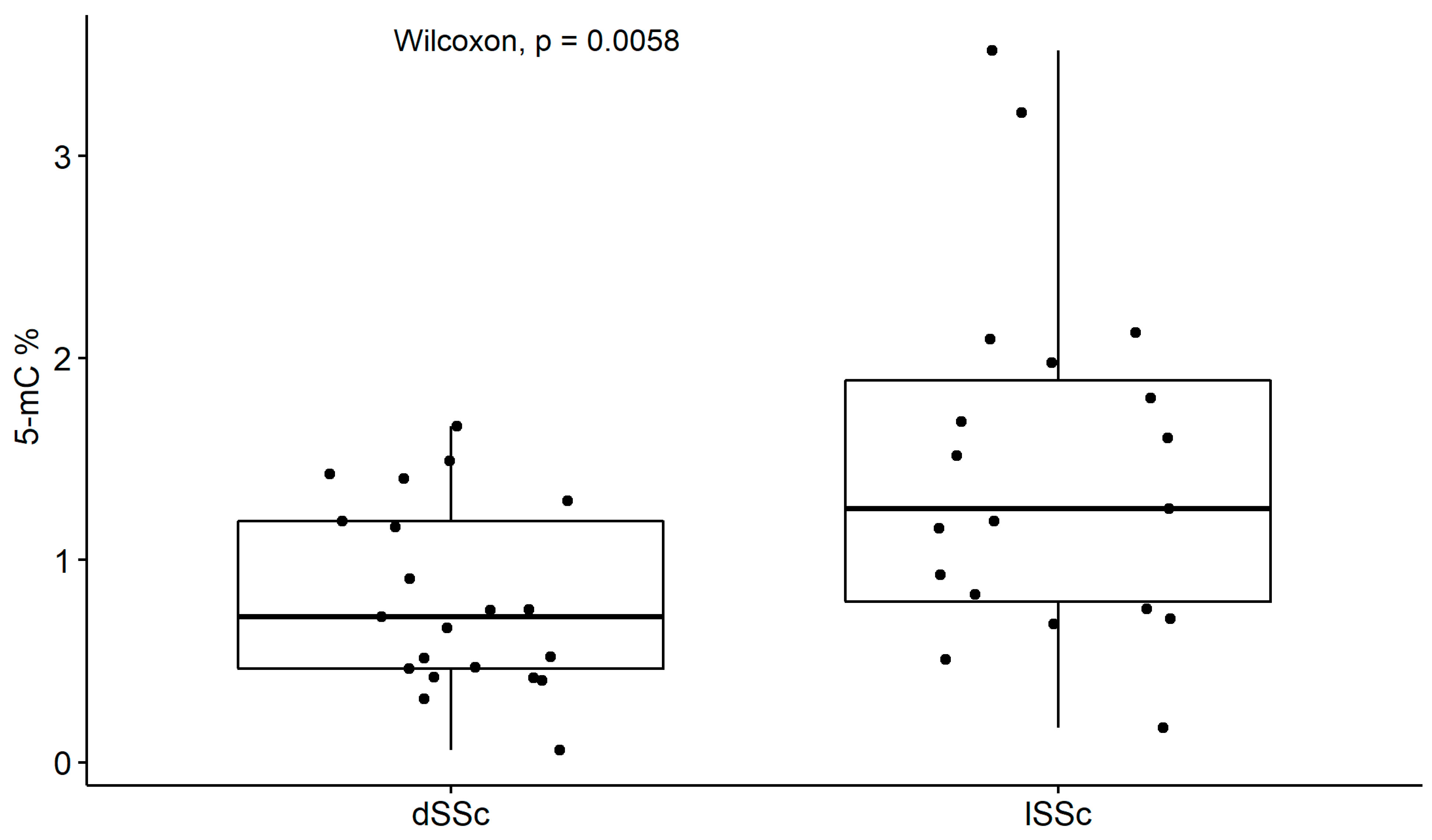
2.2. Global DNA Methylation within SSc Disease
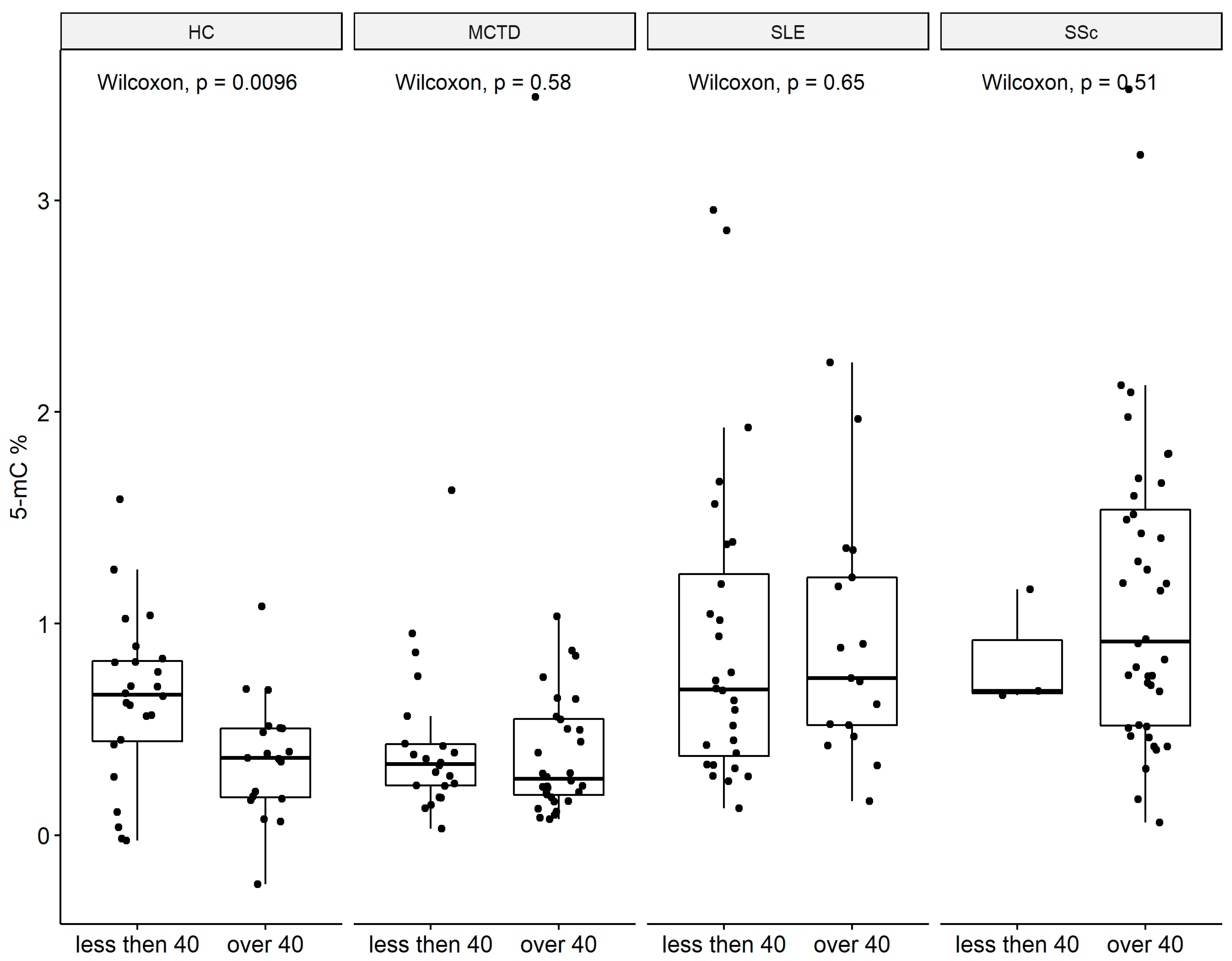
2.3. Global DNA Methylation Decrease with Age
3. Discussion
4. Materials and Methods
4.1. Patients and Clinical Characteristics
4.2. Global DNA Methylation Assessment
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-mC | 5-methylocytosine |
| ACA | Anti-centromere antibody |
| aCL IgG | Anti Cardiolipin antibody of immunoglobulin G |
| aCL IgM | Anti Cardiolipin antibody of immunoglobulin M |
| ACR | American College of Rheumatology |
| ACTDs | Autoimmune Connective Tissue Diseases |
| AMA-M2 | Anti-mitochondrial M2 antibody-positive autoimmune hepatitis |
| ANGPT2 | Angiopoietin 2 |
| Anti-CCP | anti-cyclic citrullinated peptide autoantibodies |
| Anti-CENP-A | Anti-centromere proteins A |
| Anti-CENP-B | Anti-centromere proteins B |
| Anti-dsDNA | Anti-double stranded DNA |
| Anti Jo-1 | Anti-nuclear antibody |
| Anti-La/SSB | Anti-SLE Sjogren’s syndrome or SLE-related autoantibodies |
| Anti-PCNA | Proliferating cell nuclear antigen antibody |
| Anti-Rib-P | Anti-ribosomal P |
| Anti-Ro/SSA | Anti-Sjogren’s-syndrome-related antigen A autoantibodies |
| Anti-Scl-70 | Anti-topoisomerase I |
| Anti-Sm | Anti-Smith |
| Anti-SmB | Anti-Smith B |
| Anti-SmD | Anti-Smith D |
| Anti-U1 RNP | Anti-U1RNP antibody |
| CRP | C-reactive protein |
| DLCO | Diffusing capacity of the lung of carbon monoxide |
| DNMT | DNA methyltransferase |
| DNMT3a | DNA methyltransferase 3 Alpha |
| DNMT3b | DNA methyltransferase 3 Beta |
| dSSc | diffuse systemic sclerosis |
| ESR | Erythrocyte sedimentation rate |
| EULAR | European Alliance of Associations for Rheumatology |
| FCV | Forced vital capacity |
| HDAC4 | Histone deacetylase 4 |
| HRTC | High-resolution computed tomography |
| ILD | Interstitial lung disease |
| LAC | Lupus anticoagulant antibody |
| lSSc | lLmited systemic sclerosis |
| MCTD | Mixed connective tissue disease |
| miRNA | Micro RNA |
| mRSS | Modified-Rodnan skin score |
| MTX | Methotrexate |
| MVEC | Microvascular endothelial cells |
| ncRNA | Non-coding RNA |
| NOS1 | Nictric oxide synthase 1 |
| PBMC | Peripheral blood mononuclear cell |
| RF | Rheumatoid factor |
| SLE | Systemic lupus erythematosus |
| SSc | Systemic sclerosis |
References
- Karagianni, P.; Tzioufas, A.G. Epigenetic perspectives on systemic autoimmune disease. J. Autoimmuny 2019, 104, 102315. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chang, C.; Lu, Q. The Epigenetics of Lupus Erythematosus. Adv. Exp. Med. Biol. 2020, 1253, 185–207. [Google Scholar] [PubMed]
- Venables, P.J.W. Mixed connective tissue disease. Lupus 2006, 15, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Tani, C.; Carli, L.; Vagnani, S.; Talarico, R.; Baldini, C.; Mosca, M.; Bombardieri, S. The diagnosis and classification of mixed connective tissue disease. J. Autoimmuny 2014, 48–49, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Kasukawa, R. Mixed connective tissue disease. Intern. Med. 1999, 38, 386–393. [Google Scholar] [CrossRef]
- Hurtado, C.; Acavedo Saenz, L.Y.; Vasquez Trespalacios, E.M.; Urrego, R.; Jenks, S.; Sanz, I.; Vasquez, G. DNA methylation changes on immune cells in Systemic Lupus Erythematosus. Autoimmunity 2020, 53, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Steward, J.J. The female X-inactivation mosaic in systemic lupus erythematosus. Immunol. Today 1998, 19, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Stypinska, B.; Lewandowska, A.; Felis-Giemza, A.; Olesińska, M.; Paradowska-Gorycka, A. Association study between immune-related miRNAs and mixed connective tissue disease. Arhtritis Res. Ther. 2021, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Stypinska, B.; Wajda, A.; Walczuk, B.; Olesinska, M.; Lewandowska, A.; Walczyk, M.; Paradowska-Gorycka, A. The serum Cell-Free microRNA expression profile in MCTD, SLE, SSc, and RA patients. J. Clin. Med. 2020, 9, 161. [Google Scholar] [CrossRef]
- Carnero-Montoro, E.; Barturen, G.; Povedano, E.; Kerick, M.; Martinez-Bueno, M.; Ballestar, E.; Martin, J.; Teruel, M.; Alarcon-Riquelme, M. Epigenome-Wide Comparative Study Reveals Key Differences between Mixed Connective Tissue Disease and Related Systemic Autoimmune Disease. Front. Immunol. 2019, 10, 1880. [Google Scholar] [CrossRef]
- Fouad, M.A.; Salem, S.E.; Hussein, M.M.; Zekri, A.R.; Hafez, H.F.; Desouky, E.D.; Shourman, S.A. Impact of Global DNA Methylation in Treatment Outcome of Colorectal Cancer Patients. Front. Pharmacol. 2018, 9, 1173. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Whitaker, J.W.; Boyle, D.L.; Wang, W.; Firestein, G.S. DNA methylome signature in rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Karouzakis, E.; Gay, R.E.; Michael, B.A.; Gay, S.; Neidhart, M. DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2009, 60, 3613–3622. [Google Scholar] [CrossRef]
- Szyf, M. Epigenetic therapeutics in autoimmune disease. Clin. Rev. Allergy Immunol. 2010, 39, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.M.; Fry, R.C. Environmental Influences on the Epigenome: Exposure-Associated DNA Methylation in Human Populations. Annu. Rev. Public. Health 2018, 39, 309–333. [Google Scholar] [CrossRef] [PubMed]
- Agodi, A.; Barchitta, M.; Quattrocchi, A.; Maugeri, A.; Canto, C.; Marchese, A.E.; Vinciguerra, M. Low fruit consumption and folate edficiency are associated with LINE-1 hypomethylation in women of cancer-free population. Genes. Nutr. 2015, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Breton, C.V.; Byun, H.M.; Wenten, M.; Pan, F.; Yang, A.; Gilliland, F.D. Prenatal tabacco smoke exposure affects global and gene-specific DNA methylation. Am. J. Respir. Crit. Care Med. 2014, 180, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Carmona, J.J.; Sofer, T.; Hutchinson, J.; Cantone, L.; Coull, B.; Maity, A.; Vokonas, P.; Lin, X.; Schwartz, J.; Baccarelli, A.A. Short-term airborne particulate matter exposure alters the epigenetic landscape of human genes associated with the mitogen-activated protein kinase network: A cross-sectional study. Environ. Health 2014, 13, 94. [Google Scholar]
- Beach, S.R.H.; Lei, M.K.; Ong, M.L.; Brody, G.H.; Dogan, M.V.; Philibert, R.A. MTHFR methylation moderates the impact of smoking on DNA methylation at AHRR for African American young adults. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2017, 174, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Cribbs, A.; Feldmann, M.; Oppermann, U. Towards an understanding of the role of DNA methylation in rheumatoid arthritits therepeutic and diagnostic implications. Ther. Adv. Musculoskelet. Dis. 2015, 7, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Kreuz-Imgenberg, J.; Almlof, J.C.; Leonard, D.; Alexsson, A.; Nordmak, G.; Eloranta, M.L.; Rantapaa-Dahlqvist, S.; Bengtsson, A.A.; Jonsen, A.; Padyukov, L.; et al. DNA methylation mapping identifies gene regulatory effects in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2018, 77, 736–743. [Google Scholar] [CrossRef]
- He, D.; Yang, C.X.; Sahin, B.; Shannon, C.P.; Oliveria, J.P.; Gavreau, G.M.; Tebbutt, S.J. Whole blood vs PBMC: Compartmental differences in gene expression profiling exemplified in asthma. Allergy Asthma Clin. Immunol. 2019, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Adalsteinsson, B.T.; Gudnason, H.; Aspelund, T.; Harris, T.B.; Launer, L.J.; Eiriksdottir, G.; Smith, A.V.; Gudnason, V. Heterogeneity in white blood cells has potential to confound DNA methylation measurements. PLoS ONE 2012, 7, e46705. [Google Scholar] [CrossRef] [PubMed]
- Glossop, J.R.; Nixon, N.B.; Emer, R.D.; Haworth, K.E.; Packham, J.C.; Dawes, P.T.; Fryer, A.A.; Mattey, D.L.; Farrell, W.E. Epigenome-wide profiling identifies significant differences in DNA methylation between matched-pairs of T- and B-lymphocytes from healthy individuals. Epigenetics 2013, 8, 1188–1197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Andres, M.C.; Perez-Pampin, E.; Calaza, M.; Santaclara, F.J.; Ortea, I.; Gomez-Reino, J.J.; Gonzalez, A. Assessment of global DNA methylation in peripheral blood cell subpopulations of early rheumatoid arthritis before and after methotrexate. Arthritis Res. Ther. 2015, 17, 233. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Ou, T.T.; Wu, C.C.; Li, R.N.; Lin, Y.C.; Lin, C.H.; Tsai, W.C.; Liu, H.W.; Yen, J.H. Global DNA methylation, DNMT1, and MBD2 in patients with systemic lupus erythematosus. Lupus 2011, 20, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhao, M.; Tan, L.; Lu, Q. The key culprit in the pathogenesis of systemic lupus erythematosus: Aberrant DNA methylation. Autoimmun. Rev. 2016, 15, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Dal-Bekar, N.E.; Siomek-Gorecka, A.; Gackowski, D.; Koken-Avsar, A.; Yarkan-Tugsal, H.; Birlik, M.; Islekel, H. Global hypomethylation pattern in systemic sclerosis: An application for absolute quantification of epigenetic DNA modification products by 2D-UPLC-MS/MS. Clin. Immunol. 2022, 239, 108997. [Google Scholar] [CrossRef] [PubMed]
- Matatiele, P.; Tikly, M.; Tarr, G.; Gulumian, M. DNA methylation similarities in genes of black South Africans with systemic lupus erythematosus and systemic sclerosis. J. Biomed. Sci. 2015, 22, 34. [Google Scholar] [CrossRef]
- Lei, W.; Luo, U.; Lei, W.; Luo, Y.; Yan, K.; Zhao, S.; Li, Y.; Qiu, X.; Zhou, Y.; Long, H.; et al. Abnormal DNA methylation in CD4+ T cells from patients with systemic lupus erythematosus, systemic sclerosis, and dermatomyositis. Scand. J. Rheumatol. 2009, 38, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.S.; Zimmerman, K.D.; Haddad, S.; Langefeld, C.D.; Medsger, T.A.; Feghali-Bostwick, C.A. Integrative analysis of DNA methylation in discordant twins unveils distinct architectures of systemic sclerosis subsets. Clin. Epigenetics 2019, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Frost, A.; Silveira, W.; Hazard, E.; Atanelishvili, I.; Wilson, R.; Flume, J.; Day, K.; Oates, J.; Bogathevich, G.; Feghali-Bostwick, C.; et al. Differential DNA methylation landscape in skin fibroblasts from African Americans with systemic sclerosis. Genes 2021, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Altorok, N.; Tsou, P.; Coit, P.; Khanna, D.; Sawalha, A.H. Gemone-wide DNA methylation analysis in dermal fibroblasts from patients with diffuse and limited systemic sclerosis reveals common and subset-specific DNA methylation aberrancies. Ann. Rheum. Dis. 2015, 74, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Nyce, J. Drug-induced DNA hypermethylation and drug resistance in human tumors. Cancer Res. 1989, 49, 5829–5836. [Google Scholar] [PubMed]
- Lim, U.; Song, M.A. Dietary and lifestyle factors of DNA methylation. Methods Mol. Biol. 2012, 863, 359–376. [Google Scholar] [PubMed]
- Wickham, H.; Bryan, J. Readxl: Read Excel Files. R Package Version 1.3.1. 2019. Available online: https://CRAN.R-project.org/package=readxl (accessed on 19 October 2022).
- Wickham, H.; Francois, R.; Henry, L.; Muller, K. dplyr: A Grammar of Data Manipulation. R Package Version 1.0.7. 2021. Available online: http://CRAN.R-project.org/package=dplyr (accessed on 19 October 2022).
- DeWitt, P. qwraps2: Quick Wraps 2. R Package Version 0.5.2. 2021. Available online: https://CRAN.R-project.org/package=qwraps2 (accessed on 19 October 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 0.4.0. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 19 October 2022).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 19 October 2022).
- Dinno, A. dunn.test: Dunn’s Test of Multiple Comparisons Using Rank Sums. R Package Version 1.3.5. 2017. Available online: https://CRAN.R-project.org/package=dunn.test (accessed on 19 October 2022).
| Control (N = 43) | MCTD (N = 54) | SLE (N = 45) | SSc (N = 43) | ||
|---|---|---|---|---|---|
| 5-mC (%) | median (IQR: Q1, Q3) | 0.51 (0.24, 0.70) | 0.29 (0.20, 0.54) | 0.73 (0.43, 1.22) | 0.91 (0.59, 1.50) |
| age | mean ± sd | 39.00 ± 14.76 | 43.09 ± 15.27 | 39.96 ± 13.44 | 57.28 ± 13.41 |
| Gender | |||||
| women | n (%) | 20 (46.51%) | 41 (75.93%) | 41 (91.11%) | 30 (69.77%) |
| men | n (%) | 23 (53.49%) | 13 (24.07%) | 4 (8.89%) | 13 (30.23%) |
| Parameters | MCTD (N = 51) | SLE (N = 45) | All SSc (N = 43) | dSSc (N = 21) | lSSc (N = 19) |
|---|---|---|---|---|---|
| Age mean ± sd | 44.08 ± 14.92 | 39.96 ± 13.44 | 57.00 ± 13.58 | 57.24 ± 13.41 | 56.74 ± 14.12 |
| Gender | |||||
| women | 38 (74.51%) | 41 (91.11%) | 27 (67.50%) | 15 (71.43%) | 12 (63.16%) |
| men | 13 (25.49%) | 4 (8.89%) | 13 (32.50%) | 6 (28.57%) | 7 (36.84%) |
| Disease duration (months) | 116.31 ± 102.75 | 54.09 ± 84.04 | |||
| Disease activity median (IRQ) | 7 (1.00, 17.00) N = 11 | 4.00 (2.00, 8.00) * 1.00 (0.00, 2.00) ** | |||
| ILD | 25 (64.10%) | 14 (66.67%) | 11 (61.11%) | ||
| FVC (%) mean ± sd | 77.48 ± 13.07 | 75.54 ± 13.41 | 80.00 ± 12.85 | ||
| DLCO mean ± sd | 63.00 ± 14.99 | 61.44 ± 15.10 | 64.67 ± 15.21 | ||
| HRTC 0 2 5 | 14 (38.89%) 12 (33.33%) 10 (27.78%) | 7 (35.00%) 6 (30.00%) 7 (35.00%) | 7 (43.75%) 6 (37.50%) 3 (18.75%) | ||
| mRSS median (IRQ) | 9.50 (4.00, 13.00) | 9.00 (4.75, 12.50) | 9.50 (3.50, 13.00) | ||
| CRP median (IRQ) | 5.00 (2.25, 8.23) | 8.00 (4.50,19.00) | 6.00 (4.00, 9.25) | 7.00 (4.00, 10.00) | 5.00 (3.50, 8.00) |
| ESR median (IRQ) | 15.00 (10.00, 34.75) | 19.00 (9.00, 41.50) | 17.50 (10.00, 28.25) | 16.00 (11.00, 29.00) | 18.00 (9.00, 27.50) |
| Autoantibody profile | |||||
| Anti-dsDNA | 2 (5.88%) | 29 (65.91%) | 1 (3.33%) | 1 (5.88%) | 0 (0.00%) |
| Anti-scl-70 | 1 (2.94%) | 1 (2.50%) | 20 (52.63%) | 11 (52.38%) | 9 (52.94%) |
| Anti Jo-1 | 1 (2.94%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Anti-histone | 2 (5.88%) | 5 (12.82%) | 2 (6.06%) | 2 (10.53%) | 0 (0.00%) |
| Anti-Rib-P | 2 (5.88%) | 5 (12.82%) | 4 (12.50%) | 1 (5.26%) | 3 (23.08%) |
| Anti-Ro/SSA | 17 (43.59%) | ||||
| Anti-Ro/SSA-60 | 6 (17.65%) | 3 (11.11%) | 2 (11.11%) | 1 (11.11%) | |
| Anti-Ro/SSA-52 | 8 (23.53%) | 7 (18.92%) | 5 (25.00%) | 2 (11.76%) | |
| Anti-La/SSB | 3 (8.82%) | 6 (15.38%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Anti-U1 RNP | 35 (100.00%) | 8 (20.51%) | |||
| Anti-A | 30 (88.24%) | ||||
| Anti-C | 25 (73.53%) | ||||
| Anti-70kD | 24 (70.59%) | ||||
| Anti-nucleosome | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||
| Anti-Sm | 12 (29.27%) | ||||
| Anti-SmB | 11 (32.35%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| Anti-SmD | 2 (5.88%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| Anti-CCP | 4 (8.51%) | ||||
| Anti-PCNA | 1 (2.94%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| Anti-centromere ACA | 6 (15.79%) | 5 (23.81%) | 1 (5.88%) | ||
| Anti-CENP-A | 10 (28.57%) | 6 (30.00%) | 4 (26.67%) | ||
| Anti-CENP-B | 2 (5.00%) | 11 (28.21%) | 7 (33.33%) | 4 (22.22%) | |
| aCL IgM | 5 (11.90%) | ||||
| aCL IgG | 11 (26.19%) | ||||
| LAC | 14 (35.90%) | ||||
| RF | 25 (51.02%) | 5 (14.71%) | 4 (20.00%) | 1 (7.14%) | |
| PM_Scl | 4 (11.43%) | 3 (14.29%) | 1 (7.14%) | ||
| PM-Scl-75 | 2 (5.71%) | 1 (5.00%) | 1 (6.67%) | ||
| PM_Scl_100 | 2 (5.71%) | 1 (5.00%) | 1 (6.67%) | ||
| AMA-M2 | 2 (6.45%) | 0 (0.00%) | 2 (16.67%) | ||
| RP11 | 2 (5.88%) | 0 (0.00%) | 2 (13.33%) | ||
| RP155 | 3 (8.33%) | 1 (5.00%) | 2 (12.50%) | ||
| Anti-Fibrillarin | 4 (11.43%) | 1 (5.00%) | 3 (20.00%) | ||
| Anti-NOR 90 | 1 (2.86%) | 0 (0.00%) | 1 (6.67%) | ||
| Anti-Th/To | 1 (2.86%) | 0 (0.00%) | 1 (6.67%) | ||
| Anti-Ku | 2 (5.56%) | 2 (10.00%) | 0 (0.00%) | ||
| Anti-PDGFR | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||
| Medication | Methotrexate −14% | Methotrexate −17% | Methotrexate −23% | Methotrexate −26% | |
| Steroids −97% | Steroids −14% | Steroids −15% | |||
| Immunosuppressive drugs −24% | Azathioprine −37% | Immunosuppresive drugs −95% | Immunosuppresive drugs −73% | ||
| Chloroquine −16% | Chloroquine −45% | Vasodilators −95% | Vasodilators −89% | ||
| Hydroxychlorquine −5% | Hydroxychlorquine −37% | Amlodipine—85% | Amlodipine −89% | ||
| Cyclophoshamid −9% | Cyclophoshamid −10% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipowicz, G.; Wajda, A.; Stypińska, B.; Kmiołek, T.; Felis-Giemza, A.; Stańczyk, S.; Czuszyńska, Z.; Walczyk, M.; Olesińska, M.; Paradowska-Gorycka, A. Mixed Connective Tissue Disease as Different Entity: Global Methylation Aspect. Int. J. Mol. Sci. 2023, 24, 15495. https://doi.org/10.3390/ijms242015495
Filipowicz G, Wajda A, Stypińska B, Kmiołek T, Felis-Giemza A, Stańczyk S, Czuszyńska Z, Walczyk M, Olesińska M, Paradowska-Gorycka A. Mixed Connective Tissue Disease as Different Entity: Global Methylation Aspect. International Journal of Molecular Sciences. 2023; 24(20):15495. https://doi.org/10.3390/ijms242015495
Chicago/Turabian StyleFilipowicz, Gabriela, Anna Wajda, Barbara Stypińska, Tomasz Kmiołek, Anna Felis-Giemza, Sandra Stańczyk, Zenobia Czuszyńska, Marcela Walczyk, Marzena Olesińska, and Agnieszka Paradowska-Gorycka. 2023. "Mixed Connective Tissue Disease as Different Entity: Global Methylation Aspect" International Journal of Molecular Sciences 24, no. 20: 15495. https://doi.org/10.3390/ijms242015495
APA StyleFilipowicz, G., Wajda, A., Stypińska, B., Kmiołek, T., Felis-Giemza, A., Stańczyk, S., Czuszyńska, Z., Walczyk, M., Olesińska, M., & Paradowska-Gorycka, A. (2023). Mixed Connective Tissue Disease as Different Entity: Global Methylation Aspect. International Journal of Molecular Sciences, 24(20), 15495. https://doi.org/10.3390/ijms242015495







