Radiocarbon Flux Measurements Reveal Mechanistic Insight into Heat-Stress Induction of Nicotine Biosynthesis in Nicotiana attenuata
Abstract
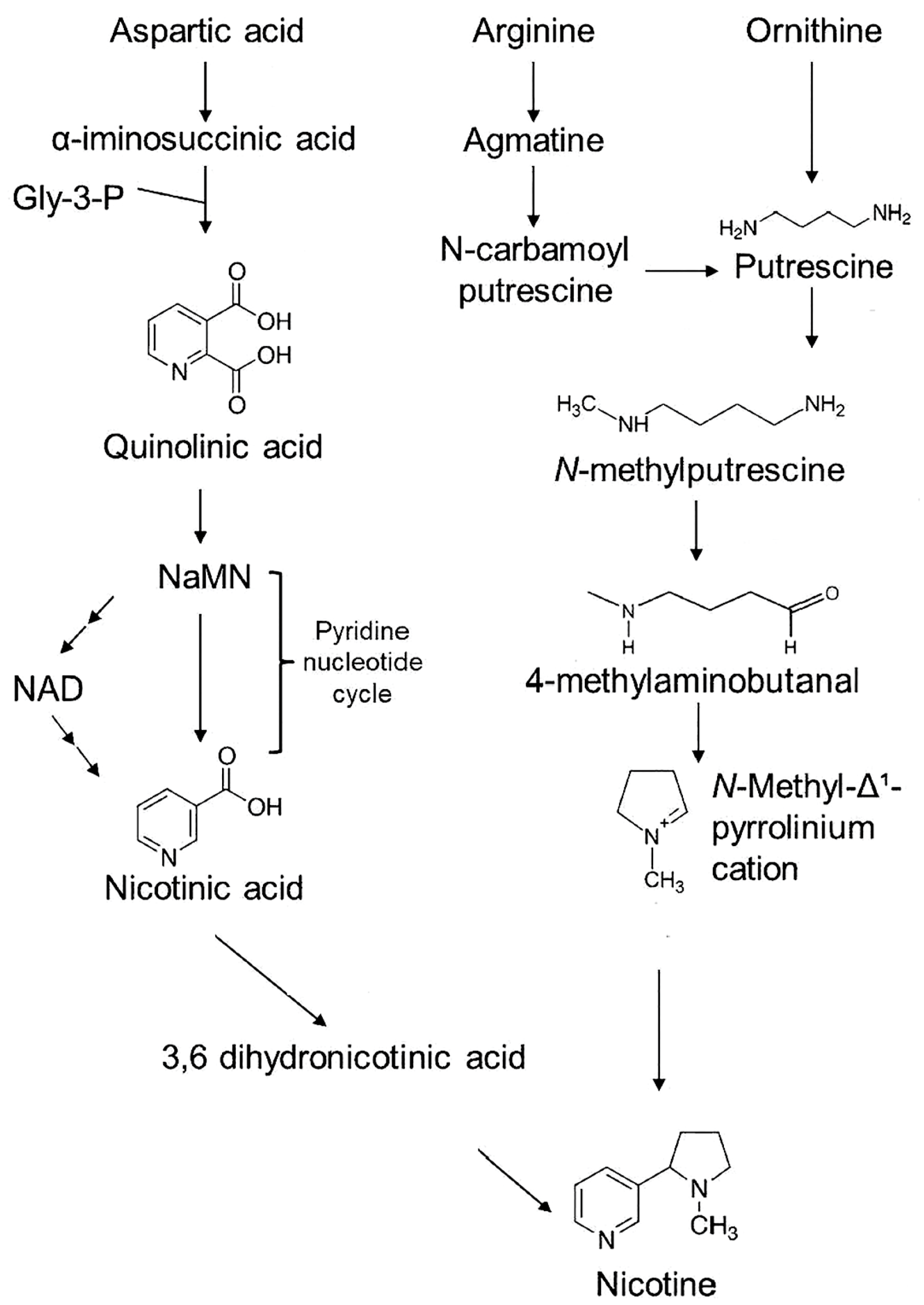
1. Introduction
2. Results
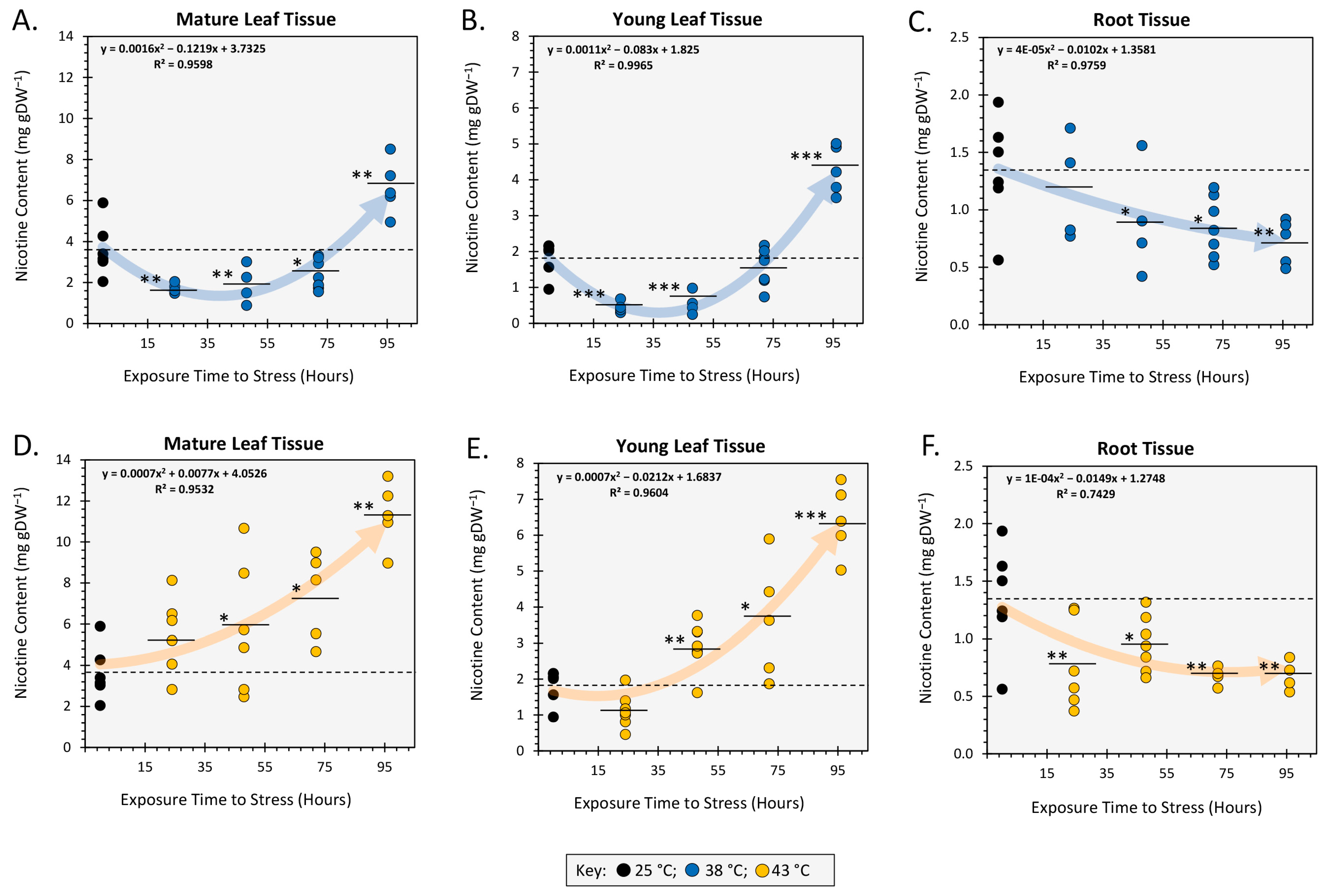
2.1. Acute and Chronic Heat Stress Alters Tissue Nicotine Content
2.2. Effect of Heat Stress on 11CO2 Fixation and 13NO3− Uptake
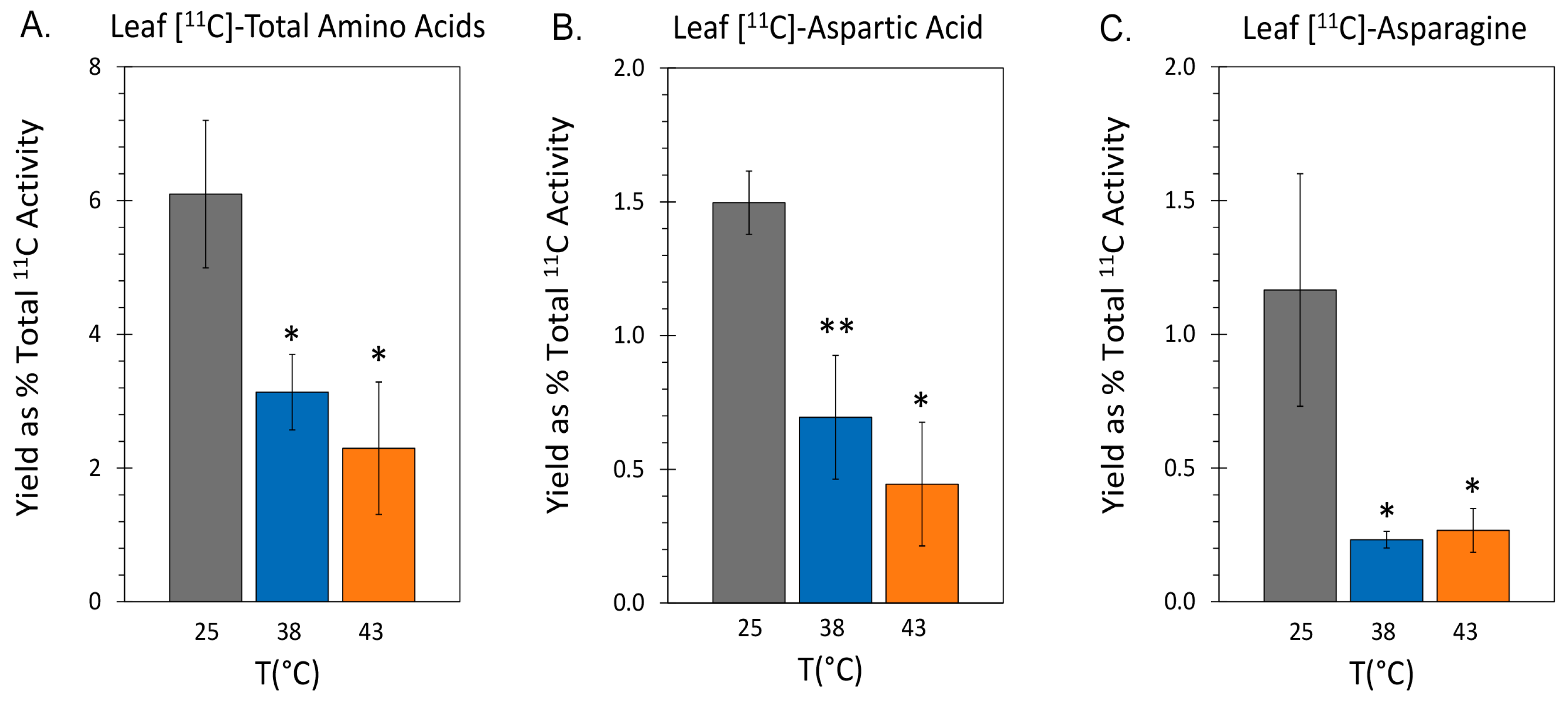
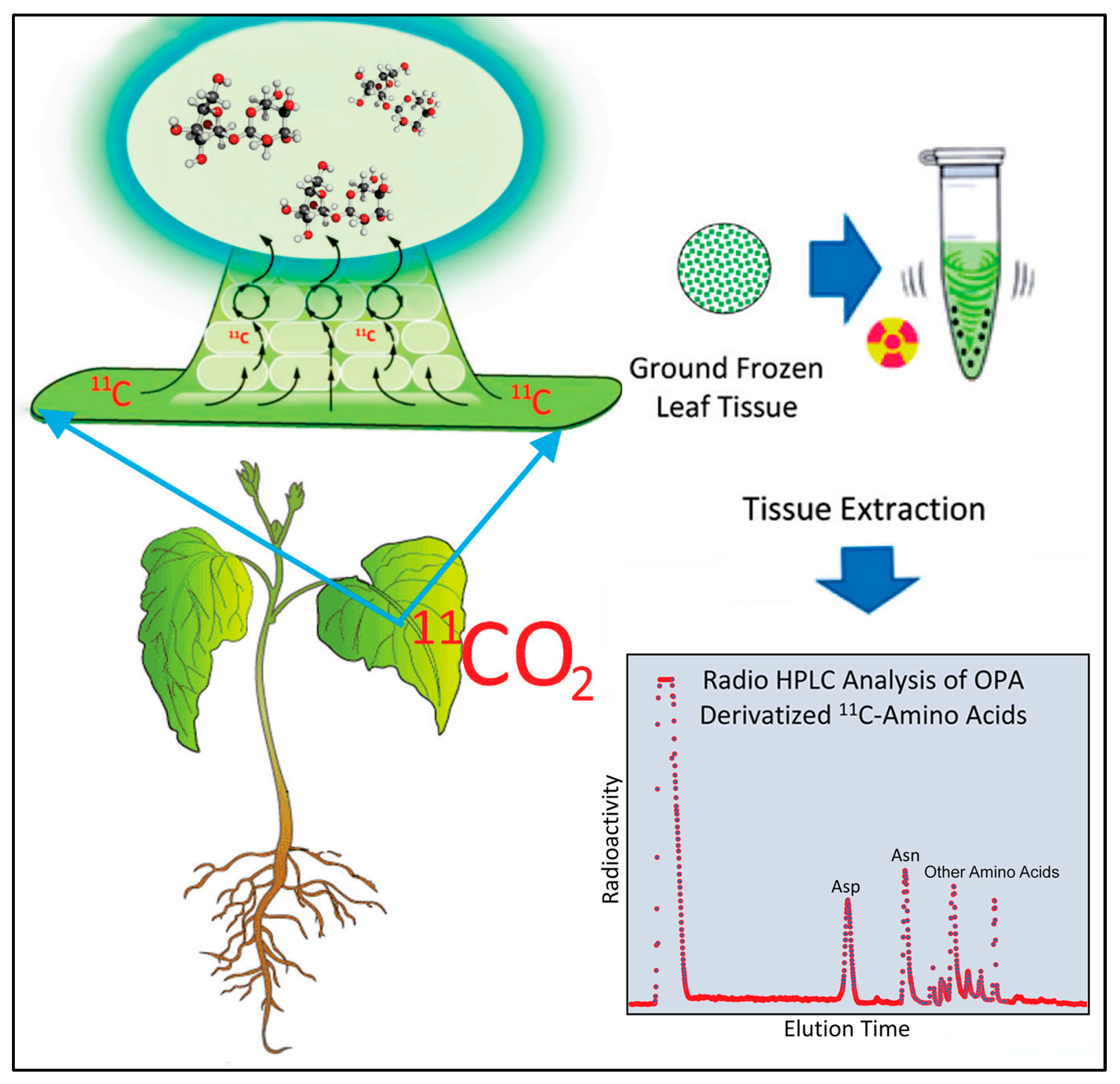
2.3. Effect of Heat Stress on ‘New’ Carbon Partitioning (as 11C) into Amino Acids
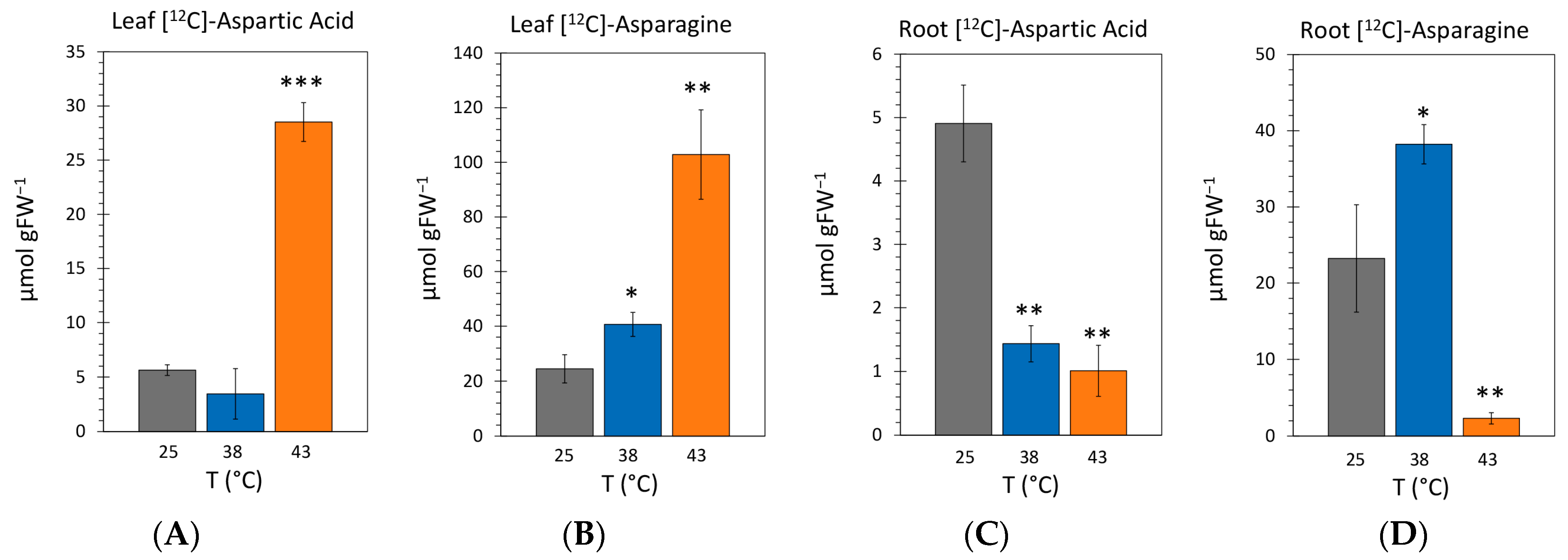
2.4. Effect of Heat Stress on the Endogenous Concentrations of [12C]-Aspartic Acid and [12C]-Asparagine
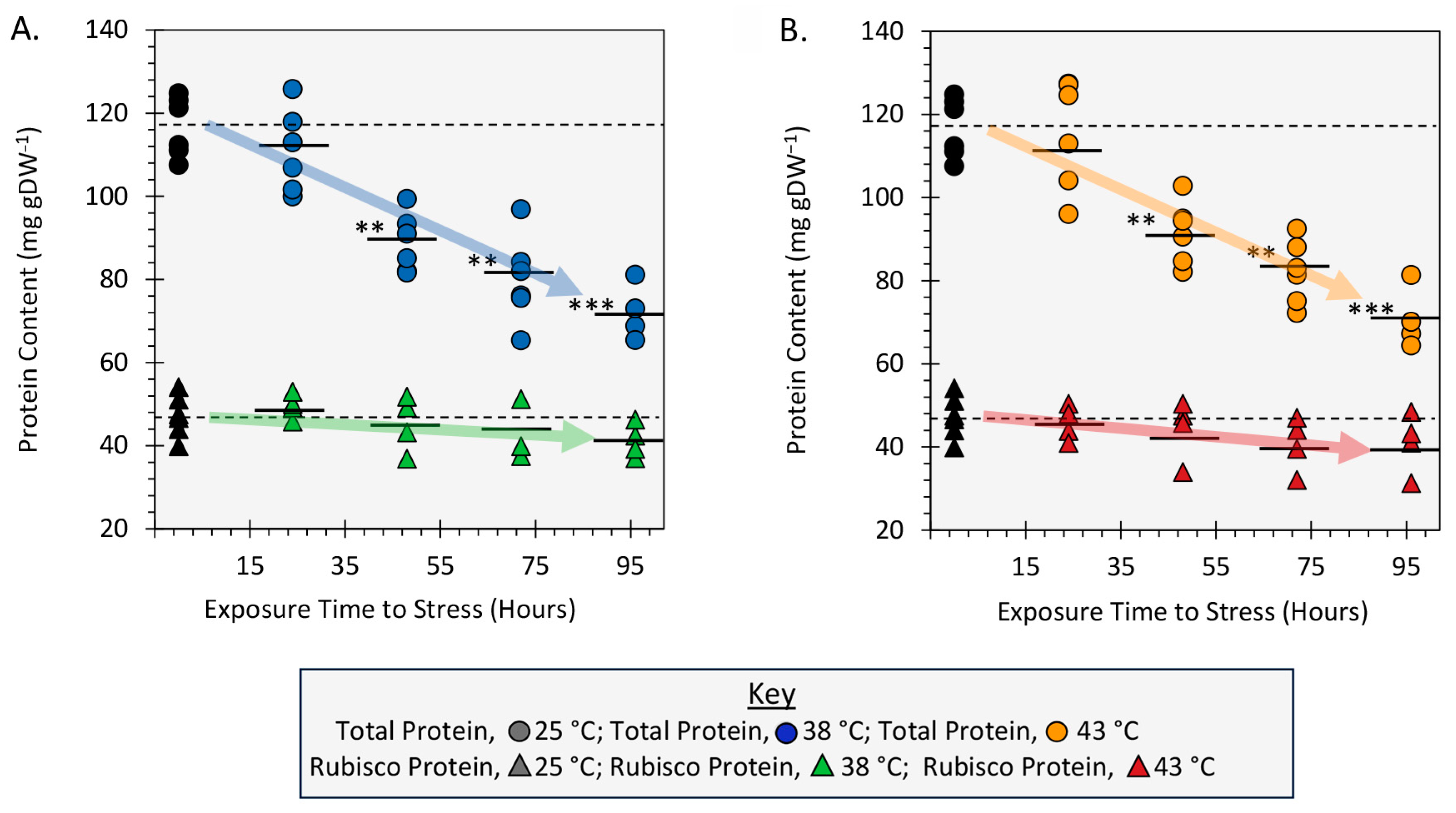
2.5. Effect of Heat Stress on Leaf Total Protein and Rubisco Protein Content
3. Discussion
4. Materials and Methods
4.1. Plant Growth
4.2. Nicotine Analysis
4.3. Production and Administration of Radioactive 11CO2 and 13NO3−
4.4. [11/12C]-Aspartic Acid and [11/12C]-Asparagine Analyses
4.5. Protein Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keller, C.F. Global warming 2007: An update to global warming: The balance of evidence and its policy implications. Sci. World J. 2007, 7, 381–399. [Google Scholar] [CrossRef][Green Version]
- Schnoor, J.L. The IPCC fourth assessment. Environ. Sci. Technol. 2007, 41, 1503. [Google Scholar] [CrossRef][Green Version]
- De Costa, W.A.J.M. A review of the possible impacts of climate change on forests in the humid tropics. J. Natl. Sci. Found. Sri 2011, 39, 281–302. [Google Scholar] [CrossRef]
- Jones, N. Climate assessments: 25 years of the IPCC. Nature 2013, 501, 298–299. [Google Scholar] [CrossRef]
- Kintisch, E. Climate science. For researchers, IPCC leaves a deep impression. Science 2013, 342, 24. [Google Scholar] [CrossRef]
- Deryng, D.; Conway, D.; Ramankutty, N.; Price, J.; Warren, R. Global crop yield response to extreme heat stress under multiple climate change futures. Environ. Res. Lett. 2014, 9, 034011. [Google Scholar] [CrossRef]
- Horton, D.; Johnson, N.C.; Singh, D.; Swain, D.; Rajaratnam, B.; Diffenbaugh, N. Contribution of changes in atmospheric circulation patterns to extreme temperature trends. Nature 2015, 522, 465–469. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef]
- Delmotte, M.V.; Zhai, P.; Pörtner, H.O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Oki, W.; Péan, C.; Pidcock, R.; et al. (Eds.) IPCC Global Warming of 1.5 °C—An IPCC Special Report on the Impacts of Global Warming of 1.5 C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways; IPPC: Geneva, Switzerland, 2018; Volume 2. [Google Scholar]
- Akter, N.; Islam, M.R. Heat stress effects and management in wheat: A review. Agron. Sustain. Dev. 2017, 37, 37–54. [Google Scholar] [CrossRef]
- Baldwin, I.T. The alkaloidal responses of wild tobacco to real and simulated herbivory. Oecologia 1988, 77, 378–381. [Google Scholar] [CrossRef]
- Baldwin, I.T. Mechanism of damage-induced alkaloid production in wild tobacco. J. Chem. Ecol. 1989, 15, 1661–1680. [Google Scholar] [CrossRef]
- Baldwin, I.T. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc. Natl. Acad. Sci. USA 1998, 95, 8113–8118. [Google Scholar] [CrossRef]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural Products (Secondary Metabolites). In Biochemistry & Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 1250–1318. [Google Scholar]
- Bedewitz, M.A.; Góngora-Castillo, E.; Uebler, J.B.; Gonzales-Vigil, E.; Wiegert-Rininger, K.E.; Childs, K.L.; Hamilton, J.P.; Vaillancourt, B.; Yeo, Y.S.; Chappell, J.; et al. A root-expressed L-phenylalanine: 4-hydroxyphenylpyruvate aminotransferase is required for tropane alkaloid biosynthesis in Atropa belladonna. Plant Cell 2014, 26, 3745–3762. [Google Scholar] [CrossRef]
- Biastoff, S.; Brandt, W.; Dräger, B. Putrescine N-methyltransferase—The start for alkaloids. Phytochemistry 2009, 70, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, C.; Cordeiro, A.; Alcázar, R.; Borrell, A.; Culiañez-Macià, F.A.; Tiburcio, A.F.; Altabella, T. Localization of arginine decarboxylase in tobacco plants. Physiol. Plant. 2004, 120, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Katoh, A.; Ohki, H.; Inai, K.; Hashimoto, T. Molecular regulation of nicotine biosynthesis. Plant Biotechnol. 2005, 22, 389–392. [Google Scholar] [CrossRef]
- Shitan, N.; Minami, S.; Morita, M.; Hayashida, M.; Ito, S.; Takanashi, K.; Omote, H.; Moriyama, Y.; Sugiyama, A.; Goossens, A.; et al. Involvement of the leaf-specific multidrug and toxic compound extrusion (MATE) transporter Nt-JAT2 in vacuolar sequestration of nicotine in Nicotiana tabacum. PLoS ONE 2014, 9, e108789. [Google Scholar] [CrossRef]
- Shitan, N.; Hayashida, M.; Yazaki, K. Translocation and accumulation of nicotine via distinct spatio-temporal regulation of nicotine transporters in Nicotiana tabacum. Plant Signal. Behav. 2015, 10, e1035852. [Google Scholar] [CrossRef][Green Version]
- Leete, E.; Siegfried, K.J. The biogenesis of nicotine. III. Further observations on the incorporation of ornithine into the pyrrolidine ring. J. Am. Chem. Soc. 1957, 19, 4529–4531. [Google Scholar] [CrossRef]
- Tso, T.C. Organic metabolism—Alkaloids. In Production, Physiology and Biochemistry of the Tobacco Plant; Ideals Inc.: Beltsville, MD, USA, 1993. [Google Scholar]
- Voipio, I.; Autio, J. Responses of red-leaved lettuce to light intensity, UV-A radiation, and root zone temperature. Acta Hortic. 1995, 399, 183–190. [Google Scholar] [CrossRef]
- Anttonen, M.J.; Hoppula, K.I.; Nestby, R.; Verheul, M.J.; Kar-Jalainen, R.O. Influence of fertilization, mulch color, early forcing, fruit order, planting date, shading, growing environment, and genotype on the contents of selected phenolics in strawberry (Fragaria ananassa Duch.) fruits. J. Agric. Food Chem. 2006, 54, 2614–2620. [Google Scholar] [CrossRef] [PubMed]
- Coolong, T.W.; Randle, W.M. The influence of root zone temperature on growth and flavour precursors in Allium cepa L. J. Hortic. Sci. Biotech. 2006, 81, 199–204. [Google Scholar] [CrossRef]
- Ziegler, J.; Facchini, P.J. Alkaloid biosynthesis: Metabolism and trafficking. Annu. Rev. Plant Biol. 2008, 59, 735–769. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, L.; Laakso, S.; Rosenqvist, H. The effect of temperature on growth, indole alkaloid accumulation and lipid composition of Catharanthus roseus cell suspension cultures. Plant Cell Rep. 1992, 11, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Q.; Zhang, X.; Li, R.; Jia, Y.; Ef, A.-A.; Jia, A.; Hu, L.; Hu, X. Hydrogen sulfide mediates nicotine biosynthesis in tobacco (Nicotiana tabacum) under high temperature conditions. Plant Physiol. Biochem. 2016, 104, 174–179. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Ji, J.; Li, P.; Yu, L.; Abd-Allah, E.F.; Luo, Y.; Hu, L.; Hu, X. High Temperature Induces expression of tobacco transcription factor NtMYC2a to regulate nicotine and JA biosynthesis. Front. Physiol. 2016, 7, 465. [Google Scholar] [CrossRef]
- Cheng, T.; Hu, L.; Wang, P.; Yang, X.; Peng, Y.; Lu, Y.; Chen, J.; Shi, J. Carbon monoxide potentiates high temperature-induced nicotine biosynthesis in tobacco. Int. J. Mol. Sci. 2018, 19, 188. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Mechanism for deactivation of Rubisco under moderate heat stress. Physiol. Plant. 2004, 122, 513–519. [Google Scholar] [CrossRef]
- Schrader, S.M.; Wise, R.R.; Wacholtz, W.F.; Ort, D.R.; Sharkey, T.D. Thylakoid membrane responses to moderately high leaf temperature in Pima cotton. Plant Cell Environ. 2004, 27, 725–735. [Google Scholar] [CrossRef]
- Perdomo, J.A.; Capó-Bauçà, S.; Carmo-Silva, E.; Galmés, J. Rubisco and Rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit. Front. Plant Sci. 2017, 8, 490. [Google Scholar] [CrossRef]
- Degen, G.E.; Orr, D.J.; Carmo-Silva, E. Heat-induced changes in the abundance of wheat Rubisco activase isoforms. New Phytol. 2021, 229, 1298–1311. [Google Scholar] [CrossRef]
- Zheng, Z.-L. Carbon and nitrogen nutrient balance signaling in plants. Plant Signal. Behav. 2009, 4, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Ferrieri, R.A.; Herman, E.; Babst, B.; Schueller, M.J. Managing the Soil Nitrogen Cycle in Agroecosystem. In Soil Nitrogen Uses and Environmental Impacts; Lai, R., Stewart, B.A., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2018; Chapter 14; pp. 343–360. [Google Scholar]
- Lohaus, G.; Burba, M.; Heldt, H.W. Comparison of the contents of sucrose and amino acids in the leaves, phloem sap and taproots of high and low sugar-producing hybrids of sugar beet (Beta vulgaris L.). J. Exp. Bot. 1994, 45, 1097–1101. [Google Scholar] [CrossRef]
- Rentsch, D.; Schmidt, S.; Tegeder, M. Transporters for uptake and allocation of organic nitrogen compounds in plants. FEBS Lett. 2007, 581, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef]
- Tegeder, M.; Hammes, U.Z. The way out and in: Phloem loading and unloading of amino acids. Curr. Opin. Plant Biol. 2018, 43, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Nie, J.; Bai, R.; Sui, X. Amino acid transporters in plants: Identification and function. Plants 2020, 9, 972. [Google Scholar] [CrossRef]
- Pratelli, R.; Pilot, G. Regulation of amino acid metabolic enzymes and transporters in plants. J. Exp. Bot. 2014, 65, 5535–5556. [Google Scholar] [CrossRef]
- Zhai, Y.; Guo, M.; Wang, H.; Lu, J.; Liu, J.; Zhang, C.; Gong, Z.; Lu, M. Autophagy, a conserved mechanism for protein degradation, responds to heat, and other abiotic stresses in Capsicum annuum L. Front. Plant Sci. 2016, 7, 131. [Google Scholar] [CrossRef]
- Su, T.; Yang, M.; Wang, P.; Zhao, Y.; Ma, C. Interplay between the ubiquitin proteasome system and ubiquitin-mediated autophagy in plants. Cells 2020, 9, 2219. [Google Scholar] [CrossRef]
- Zhang, Y.; Min, H.; Shi, C.; Xia, G.; Lai, Z. Transcriptome analysis of the role of autophagy in plant response to heat stress. PLoS ONE 2021, 16, e0247783. [Google Scholar] [CrossRef] [PubMed]
- Lea, P.J.; Sodek, L.; Parry, M.A.J.; Shewry, P.R.; Halford, N.G. Asparagine in plants. Ann. Appl. Biol. 2006, 150, 1–26. [Google Scholar] [CrossRef]
- Batool, T.; Makky, E.A.; Jalal, M.; Yusoff, M.M. A comprehensive review on L-Asparaginase and its applications. Appl. Biochem. Biotech. 2016, 178, 900–923. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Xu, S.; Zhang, H.; Xu, M.; Yang, T.; Wang, L.; Qian, H.; Zhang, H.; Fang, H.; et al. Simultaneous cell disruption and semi-quantitative activity assays for high-throughput screening of thermostable L-Asparaginases. Sci. Rep. 2018, 8, 7915. [Google Scholar] [CrossRef]
- Ferrieri, R.A.; Wolf, A.P. The chemistry of positron-emitting nucleogenic (hot) atoms with regard to preparation of labeled compounds of practical utility. Radiochim. Acta 1983, 34, 69–83. [Google Scholar] [CrossRef]
- Ferrieri, R.A. Production and Application of Synthetic Precursors Labeled with Carbon-11 and Fluorine-18. In Handbook of Radiopharmaceuticals: Radiochemistry and Applications; Welch, M.J., Redvanly, C.S., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2003. [Google Scholar]
- Ferrieri, R.A.; Gray, D.W.; Babst, B.A.; Schueller, M.J.; Schlyer, D.J.; Thorpe, M.R.; Orians, C.M.; Lerdau, M. Use of carbon-11 in Populus shows that exogenous jasmonic acid increases biosynthesis of isoprene from recently fixed carbon. Plant Cell Environ. 2005, 25, 591–602. [Google Scholar] [CrossRef]
- Wu, X.; Xiong, E.; Wang, W.; Scali, M.; Cresti, M. Universal sample preparation method integrating trichloroacetic acid/acetone precipitation with phenol extraction for crop proteomic analysis. Nat. Protoc. 2014, 9, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.B.; Natarajan, S.S. A rapid method for depletion of Rubisco from soybean (Glycine max) leaf for proteomic analysis of lower abundance proteins. Phytochemistry 2009, 70, 1958–1964. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kessler, D.; Gase, K.; Baldwin, I.T. Field experiments with transformed plants reveal the sense of floral scents. Science 2008, 321, 1200–1202. [Google Scholar] [CrossRef]
- Baracchi, D.; Marples, A.; Jenkins, A.J.; Leitch, A.R.; Chittka, L. Nicotine in floral nectar pharmacologically influences bumblebee learning of floral features. Sci. Rep. 2017, 7, 1951. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waller, S.; Powell, A.; Noel, R.; Schueller, M.J.; Ferrieri, R.A. Radiocarbon Flux Measurements Reveal Mechanistic Insight into Heat-Stress Induction of Nicotine Biosynthesis in Nicotiana attenuata. Int. J. Mol. Sci. 2023, 24, 15509. https://doi.org/10.3390/ijms242115509
Waller S, Powell A, Noel R, Schueller MJ, Ferrieri RA. Radiocarbon Flux Measurements Reveal Mechanistic Insight into Heat-Stress Induction of Nicotine Biosynthesis in Nicotiana attenuata. International Journal of Molecular Sciences. 2023; 24(21):15509. https://doi.org/10.3390/ijms242115509
Chicago/Turabian StyleWaller, Spenser, Avery Powell, Randi Noel, Michael J. Schueller, and Richard A. Ferrieri. 2023. "Radiocarbon Flux Measurements Reveal Mechanistic Insight into Heat-Stress Induction of Nicotine Biosynthesis in Nicotiana attenuata" International Journal of Molecular Sciences 24, no. 21: 15509. https://doi.org/10.3390/ijms242115509
APA StyleWaller, S., Powell, A., Noel, R., Schueller, M. J., & Ferrieri, R. A. (2023). Radiocarbon Flux Measurements Reveal Mechanistic Insight into Heat-Stress Induction of Nicotine Biosynthesis in Nicotiana attenuata. International Journal of Molecular Sciences, 24(21), 15509. https://doi.org/10.3390/ijms242115509





