A Novel Quinazoline Derivative Prevents and Treats Arsenic-Induced Liver Injury by Regulating the Expression of RecQ Family Helicase
Abstract
:1. Introduction
2. Results
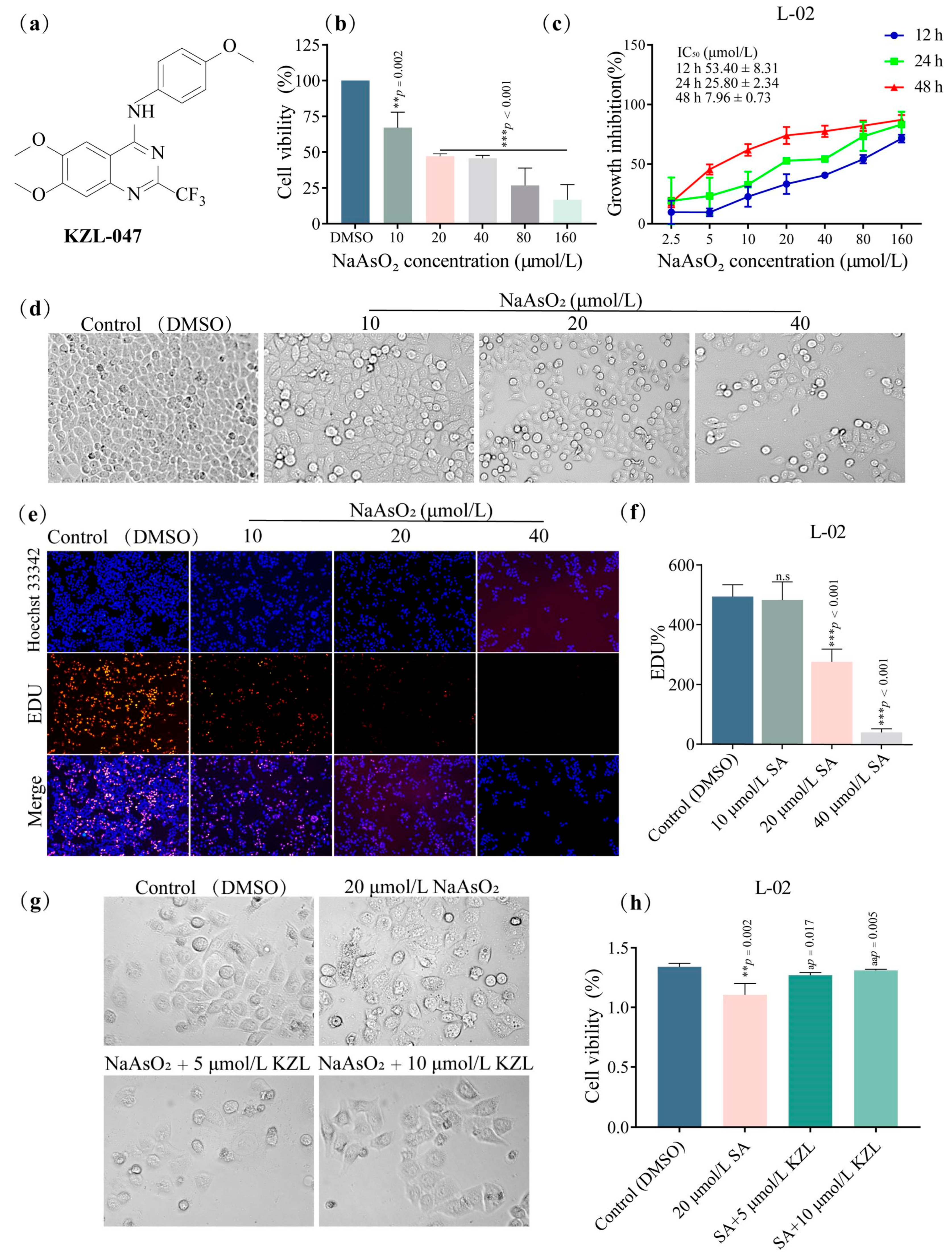
2.1. Effect of KZL-047 on the Growth of L-02 Cells
2.1.1. Effect of Different Concentrations of KZL-047 on the Proliferation of L-02 Cells
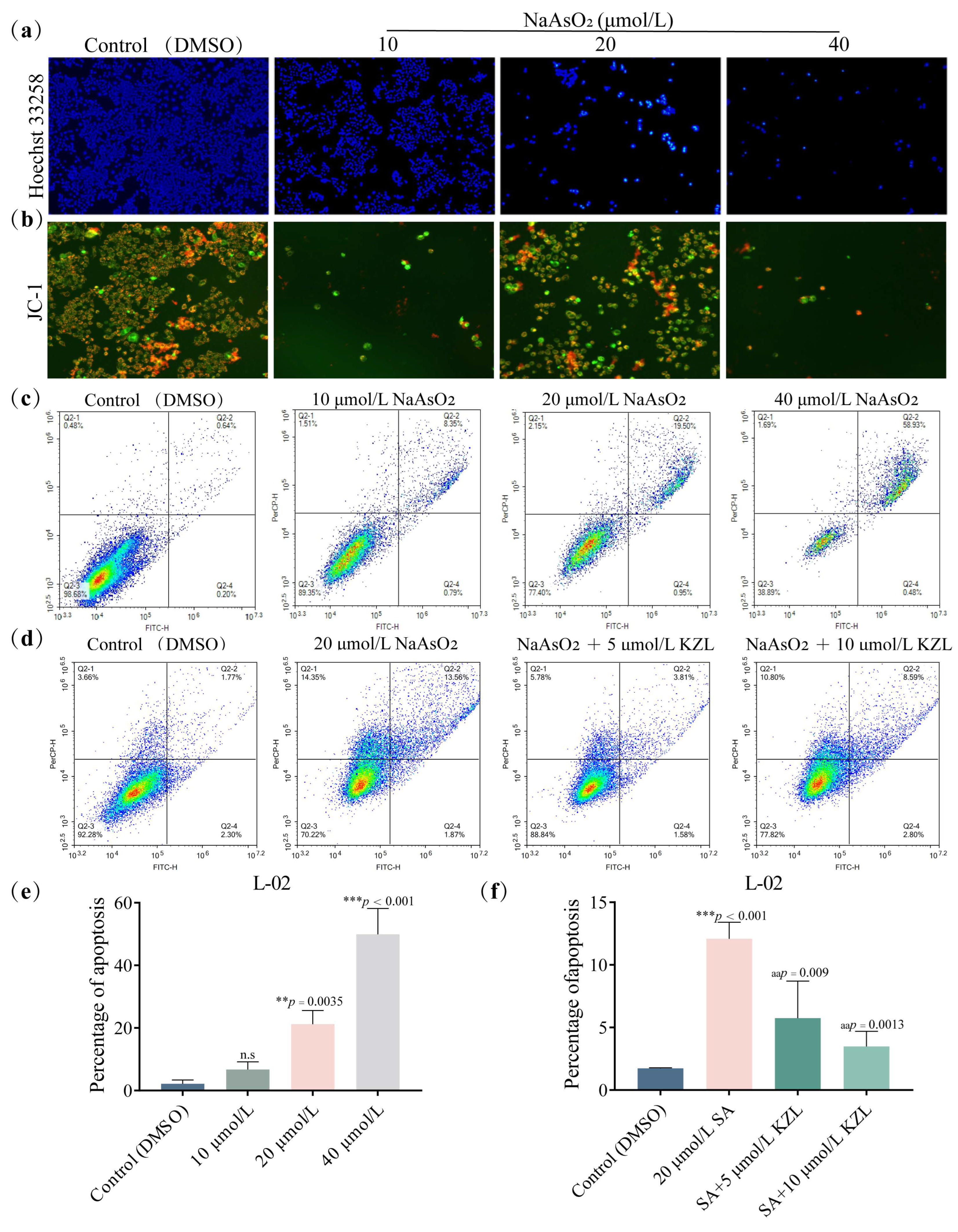
2.1.2. Effect of Different Concentrations of KZL-047 on Apoptosis of L-02 Cells
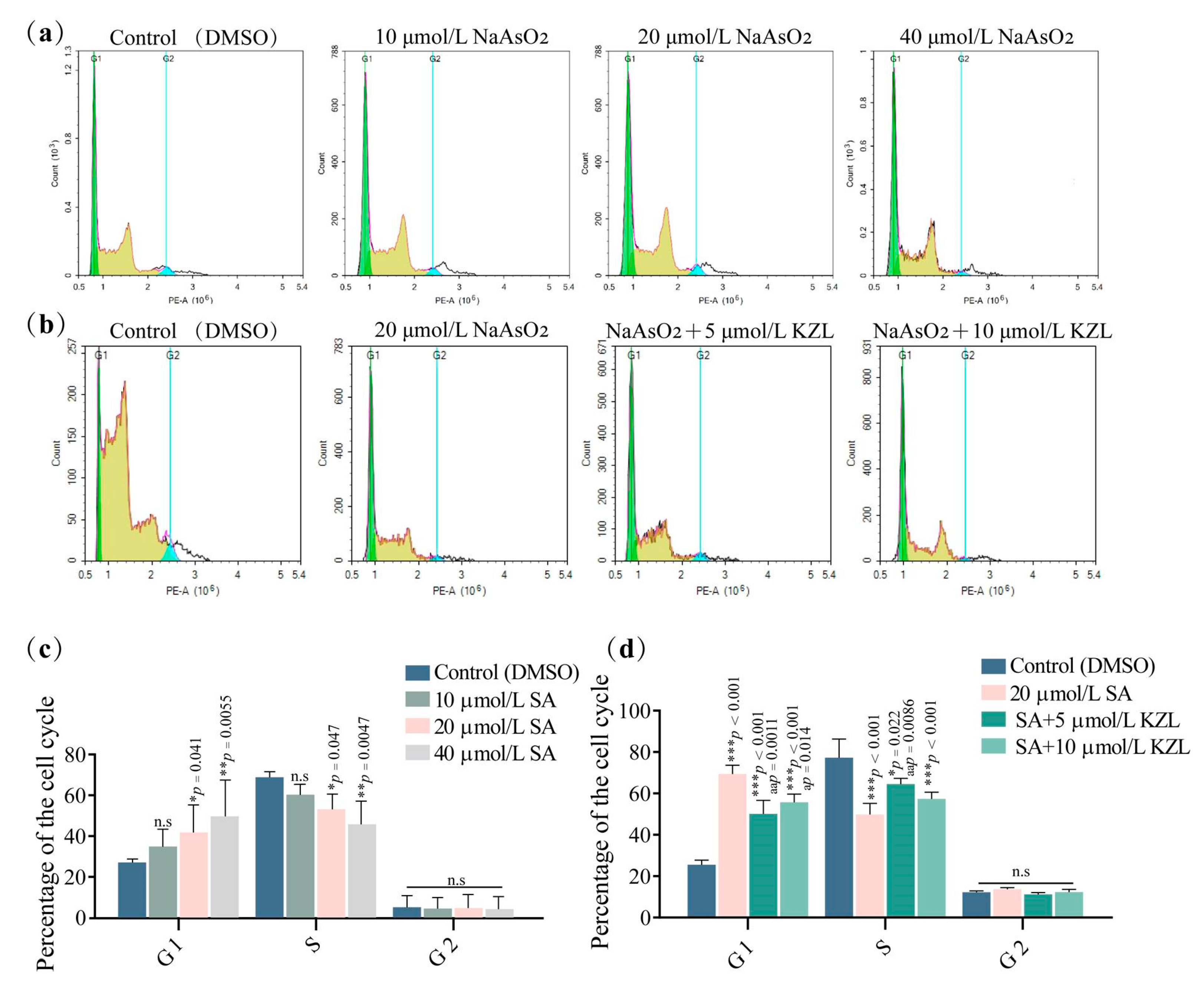
2.1.3. Effect of Different Concentrations of KZL-047 on the Cycle of L-02 Cells
2.2. Effect of KZL-047 on the RecQ Helicase Expression of L-02 Cells
2.3. Effect of KZL-047 on the RecQ Helicase Proteins Expression of L-02 Cells Analyzed by Western Blotting
2.4. Effect of KZL-047 on SA-induced Liver Injury in Mice
2.4.1. Effects of KZL-047 on Body Weight, Liver Weight, and Liver Coefficient in Mice
2.4.2. Effect of KZL-047 on Serum ALT, AST, and TBIL in Mice
2.4.3. Effects of KZL-047 on Malondialdehyde (MDA), Glutathione (GSH), and Superoxide Dismutase (SOD) in Mouse Liver Tissue
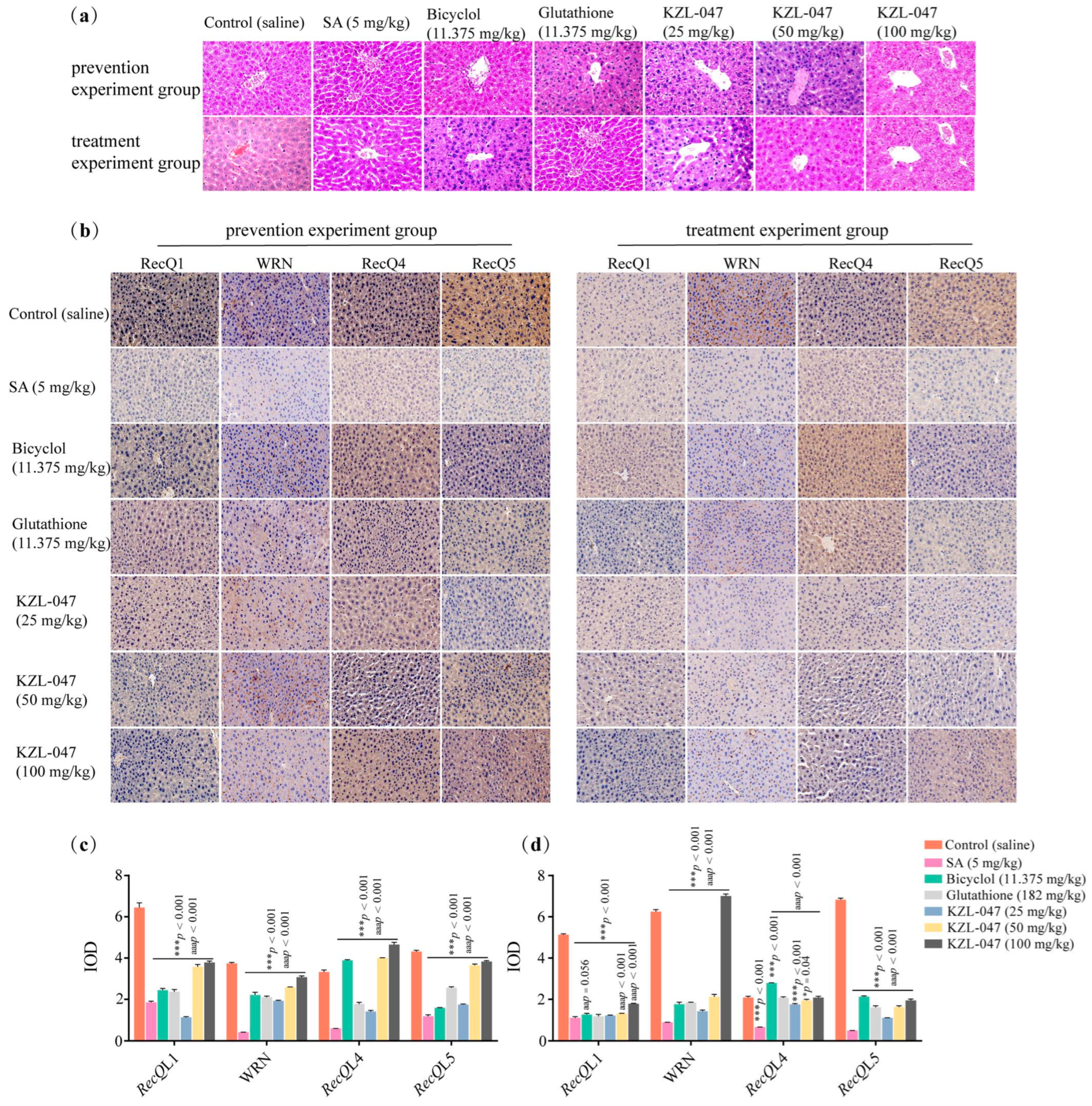
2.4.4. Effect of KZL-047 on Pathological Changes in Liver Tissues of Mice
3. Discussion
4. Materials and Methods
4.1. Materials, Cells, and Experimental Animals
4.2. Cell Culture and Compound Treatment
4.3. Cell Growth Assay
4.3.1. Cell Proliferation Assay
4.3.2. EdU (5-ethynyl-2’-deoxyuridine) Assay
4.3.3. Hoechst 33258 Staining
4.3.4. Mitochondrial Membrane Potential (MMP) Assay
4.3.5. Cell Apoptosis Assay
4.3.6. Cell Cycle Assay
4.4. Gene and Protein Expression Assay
4.4.1. Molecular Docking Assay
4.4.2. RNA Extraction and Reverse Transcription Assay
4.4.3. Real-Time Fluorescence Quantitative PCR Assay
4.4.4. Western Blotting
4.5. Animal Experiment
4.5.1. Grouping and Processing
4.5.2. Liver Coefficient Assay
4.5.3. Biochemical Indicators in Serum Assay
4.5.4. Indicators of Oxidative Stress in Liver Tissue Assay
4.5.5. Pathological Changes in Liver Tissue Assay
4.5.6. Immunohistochemical Assay
4.6. Statistical Analysis
4.7. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, L.; Liu, Y.; Wang, D.; Zhu, K.; Zou, Z.; Zhang, A. Imbalanced inflammatory response in subchronic arsenic-induced liver injury and the protective effects of Ginkgo biloba extract in rats: Potential role of cytokines mediated cell–cell interactions. Environ. Toxicol. 2021, 36, 2073–2092. [Google Scholar] [CrossRef] [PubMed]
- Amal, H.; Gong, G.; Yang, H.; Joughin, B.A.; Wang, X.; Knutson, C.G.; Kartawy, M.; Khaliulin, I.; Wishnok, J.S.; Tannenbaum, S.R. Low doses of arsenic in a mouse model of human exposure and in neuronal culture lead to S-nitrosylation of synaptic proteins and apoptosis via nitric oxide. Int. J. Mol. Sci. 2020, 21, 3948. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xiao, T.; Zhang, A. Associations between and risks of trace elements related to skin and liver damage induced by arsenic from coal burning. Ecotoxicol. Environ. Saf. 2021, 208, 111719. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shi, L.; Xiao, T.; Xue, J.; Li, J.; Wang, P.; Wu, L.; Dai, X.; Ni, X.; Liu, Q. microRNA-21, via the HIF-1α/VEGF signaling pathway, is involved in arsenite-induced hepatic fibrosis through aberrant cross-talk of hepatocytes and hepatic stellate cells. Chemosphere 2021, 266, 129177. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Sun, J.; Wang, L.; Wang, P.; Xiao, T.; Wang, S.; Liu, Q. The lncRNA HOTAIR via miR-17-5p is involved in arsenite-induced hepatic fibrosis through regulation of Th17 cell differentiation. J. Hazard. Mater. 2023, 443 Pt B, 130276. [Google Scholar] [CrossRef]
- Fatoki, J.O.; Badmus, J.A. Arsenic as an environmental and human health antagonist: A review of its toxicity and disease initiation. J. Hazard. Mater. Adv. 2022, 5, 100052. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Goyer, R.A.; Achanzar, W.; Waalkes, M.P. Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenicals. Toxicol. Sci. 2000, 55, 460–467. [Google Scholar] [CrossRef]
- Zhou, Q.; Xi, S. A review on arsenic carcinogenesis: Epidemiology, metabolism, genotoxicity and epigenetic changes. Regul. Toxicol. Pharmacol. 2018, 99, 78–88. [Google Scholar] [CrossRef]
- Medda, N.; De, S.K.; Maiti, S. Different mechanisms of arsenic related signaling in cellular proliferation, apoptosis and neo-plastic transformation. Ecotoxicol. Environ. Saf. 2021, 208, 111752. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, C.; Zheng, P.; Dan, Q.; Luo, H.; Ma, X.; Lu, C. Arsenic suppresses GDF1 expression via ROS-dependent downregulation of specificity protein 1. Environ. Pollut. 2021, 271, 116302. [Google Scholar] [CrossRef]
- Zhou, X.; Speer, R.M.; Volk, L.; Hudson, L.G.; Liu, K.J. Arsenic co-carcinogenesis: Inhibition of DNA repair and interaction with zinc finger proteins. Semin. Cancer Biol. 2021, 76, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.A.; Gileadi, O. RecQ helicases in DNA repair and cancer targets. Essays Biochem. 2020, 64, 819–830. [Google Scholar] [PubMed]
- Das, T.; Pal, S.; Ganguly, A. Human RecQ helicases in transcription-associated stress management: Bridging the gap between DNA and RNA metabolism. Biol. Chem. 2021, 402, 617–636. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Fan, H.; Zheng, K.; Qin, X.; Yang, L.; Yang, Y.; Duan, Y.; Zhang, Q.; Zeng, C.; Hu, L. Synthesis and anti-tumor activity of [1,4] dioxino [2,3-f] quinazoline derivatives as dual inhibitors of c-Met and VEGFR-2. Bioorganic Chem. 2019, 88, 102916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Potgieter, T.I.; Kosche, E.; Rückert, J.; Ostermann, E.; Schulz, T.; Empting, M.; Brune, W. Thioxothiazolo [3,4-a]quinazoline derivatives inhibit the human cytomegalovirus alkaline nuclease. Antivir. Res. 2023, 217, 105696. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.S.; Li, P.; Yu, J.; Cheng, S.; Yu, G.; Ahmad, M.; Meng, X.L.; Luo, H.; Xu, B.X. Discovery of novel N-aryl-2-trifluoromethyl-quinazoline-4-amine derivatives as the inhibitors of tubulin polymerization in leukemia cells. Eur. J. Med. Chem. 2023, 256, 115470. [Google Scholar] [CrossRef]
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Ali, S.A.; Arshia; Ishtiaq, M.; Khan, K.M. Quinazoline and quinazolinone as important medicinal scaffolds: A comparative patent review (2011–2016). Expert Opin. Ther. Pat. 2018, 28, 281–297. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Arsenic contamination, consequences and remediation techniques: A review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270. [Google Scholar] [CrossRef]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula-ScienceDirect. Geosci. Front. 2020, 12, 101079. [Google Scholar] [CrossRef]
- Flora, S. Preventive and therapeutic strategies for acute and chronic human arsenic exposure. In Arsenic in Drinking Water and Food; Springer: Berlin/Heidelberg, Germany, 2020; pp. 341–370. [Google Scholar]
- Sanchez, T.R.; Powers, M.; Perzanowski, M.; George, C.M.; Graziano, J.H.; Navas-Acien, A. A Meta-analysis of Arsenic Exposure and Lung Function: Is There Evidence of Restrictive or Obstructive Lung Disease? Curr. Environ. Health Rep. 2018, 5, 244–254. [Google Scholar] [CrossRef]
- Niño, S.A.; Morales-Martínez, A.; Chi-Ahumada, E.; Carrizales, L.; Salgado-Delgado, R.; Pérez-Severiano, F.; Díaz-Cintra, S.; Jiménez-Capdeville, M.E.; Zarazúa, S. Arsenic exposure contributes to the bioenergetic damage in an Alzheimer’s disease model. ACS Chem. Neurosci. 2018, 10, 323–336. [Google Scholar] [PubMed]
- Guo, P.; Chen, S.; Li, D.; Zhang, J.; Luo, J.; Zhang, A.; Yu, D.; Bloom, M.S.; Chen, L.; Chen, W. SFPQ is involved in regulating arsenic-induced oxidative stress by interacting with the miRNA-induced silencing complexes. Environ. Pollut. 2020, 261, 114160. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wei, B.K.; Wang, J.; Dong, G.; Wang, X. Taurine Supplementation Ameliorates Arsenic-Induced Hepatotoxicity and Oxidative Stress in Mouse. Adv. Exp. Med. Biol. 2019, 1155, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Adedara, I.A.; Fabunmi, A.T.; Ayenitaju, F.C.; Atanda, O.E.; Adebowale, A.A.; Ajayi, B.O.; Owoeye, O.; Rocha, J.B.T.; Farombi, E.O. Neuroprotective mechanisms of selenium against arsenic-induced behavioral impairments in rats. Neurotoxicology 2020, 76, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xiao, T.; Wang, Q.; Liang, B.; Zhang, A. Effects of Essential Trace Elements and Oxidative Stress on Endemic Arsenism Caused by Coal Burning in PR China. Biol. Trace Elem. Res. 2020, 198, 25–36. [Google Scholar] [CrossRef]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Cmaj 2005, 172, 367–379. [Google Scholar] [CrossRef]
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. Jama 2015, 313, 2263–2273. [Google Scholar] [CrossRef]
- Li, H.; Li, J.R.; Huang, M.H.; Chen, J.H.; Lv, X.Q.; Zou, L.L.; Tan, J.L.; Dong, B.; Peng, Z.G.; Jiang, J.D. Bicyclol Attenuates Liver Inflammation Induced by Infection of Hepatitis C Virus via Repressing ROS-Mediated Activation of MAPK/NF-κB Signaling Pathway. Front. Pharmacol. 2018, 9, 1438. [Google Scholar] [CrossRef]
- Qiu, M.; Chen, L.; Tan, G.; Ke, L.; Zhang, S.; Chen, H.; Liu, J. A reactive oxygen species activation mechanism contributes to JS-K-induced apoptosis in human bladder cancer cells. Sci. Rep. 2015, 5, 15104. [Google Scholar] [CrossRef]
- Tao, Y.; Qiu, T.; Yao, X.; Jiang, L.; Wang, N.; Jia, X.; Wei, S.; Wang, Z.; Pei, P.; Zhang, J.; et al. Autophagic-CTSB-inflammasome axis modulates hepatic stellate cells activation in arsenic-induced liver fibrosis. Chemosphere 2020, 242, 124959. [Google Scholar] [CrossRef]
- Ozturk, M.; Metin, M.; Altay, V.; Bhat, R.A.; Ejaz, M.; Gul, A.; Unal, B.T.; Hasanuzzaman, M.; Nibir, L.; Nahar, K.; et al. Arsenic and Human Health: Genotoxicity, Epigenomic Effects, and Cancer Signaling. Biol. Trace Elem. Res. 2022, 200, 988–1001. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.L.; Sun, B.F.; Huang, L.R.; Yuan, H.B.; Zhang, S.; Chen, J.; Yu, Z.J.; Luo, H. Potent Inhibition of miR-34b on Migration and Invasion in Metastatic Prostate Cancer Cells by Regulating the TGF-β Pathway. Int. J. Mol. Sci. 2017, 18, 2762. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, J.; Fu, W.; Zheng, X.; Ren, L.; Liu, S.; Wang, J.; Ji, T.; Du, G. 3-O-acetyl-11-keto-β-boswellic acid exerts anti-tumor effects in glioblastoma by arresting cell cycle at G2/M phase. J. Exp. Clin. Cancer Res. 2018, 37, 132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Wen, Z.H.; Wan, K.; Yuan, D.; Zeng, X.; Liang, G.; Zhu, J.; Xu, B.; Luo, H. A novel synthesized 3′, 5′-diprenylated chalcone mediates the proliferation of human leukemia cells by regulating apoptosis and autophagy pathways. Biomed. Pharmacother. 2018, 106, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zeng, X.; Li, S.; Sun, Z.; Yu, J.; Chen, C.; Shen, X.; Pan, W.; Luo, H. A novel tanshinone analog exerts anti-cancer effects in prostate cancer by inducing cell apoptosis, arresting cell cycle at G2 phase and blocking metastatic ability. Int. J. Mol. Sci. 2019, 20, 4459. [Google Scholar]
- Mehrzadi, S.; Fatemi, I.; Malayeri, A.R.; Khodadadi, A.; Mohammadi, F.; Mansouri, E.; Rashno, M.; Goudarzi, M. Ellagic acid mitigates sodium arsenite-induced renal and hepatic toxicity in male Wistar rats. Pharmacol. Rep. 2018, 70, 712–719. [Google Scholar] [CrossRef]
- Mahalanobish, S.; Saha, S.; Dutta, S.; Sil, P.C. Mangiferin alleviates arsenic induced oxidative lung injury via upregulation of the Nrf2-HO1 axis. Food Chem. Toxicol. 2019, 126, 41–55. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Abdel Moneim, A.E.; Bauomy, A.A.; Khalil, M.; Al-Shaebi, E.M.; Al-Quraishy, S. Chlorogenic acid prevents hepatotoxicity in arsenic-treated mice: Role of oxidative stress and apoptosis. Mol. Biol. Rep. 2020, 47, 1161–1171. [Google Scholar] [CrossRef]
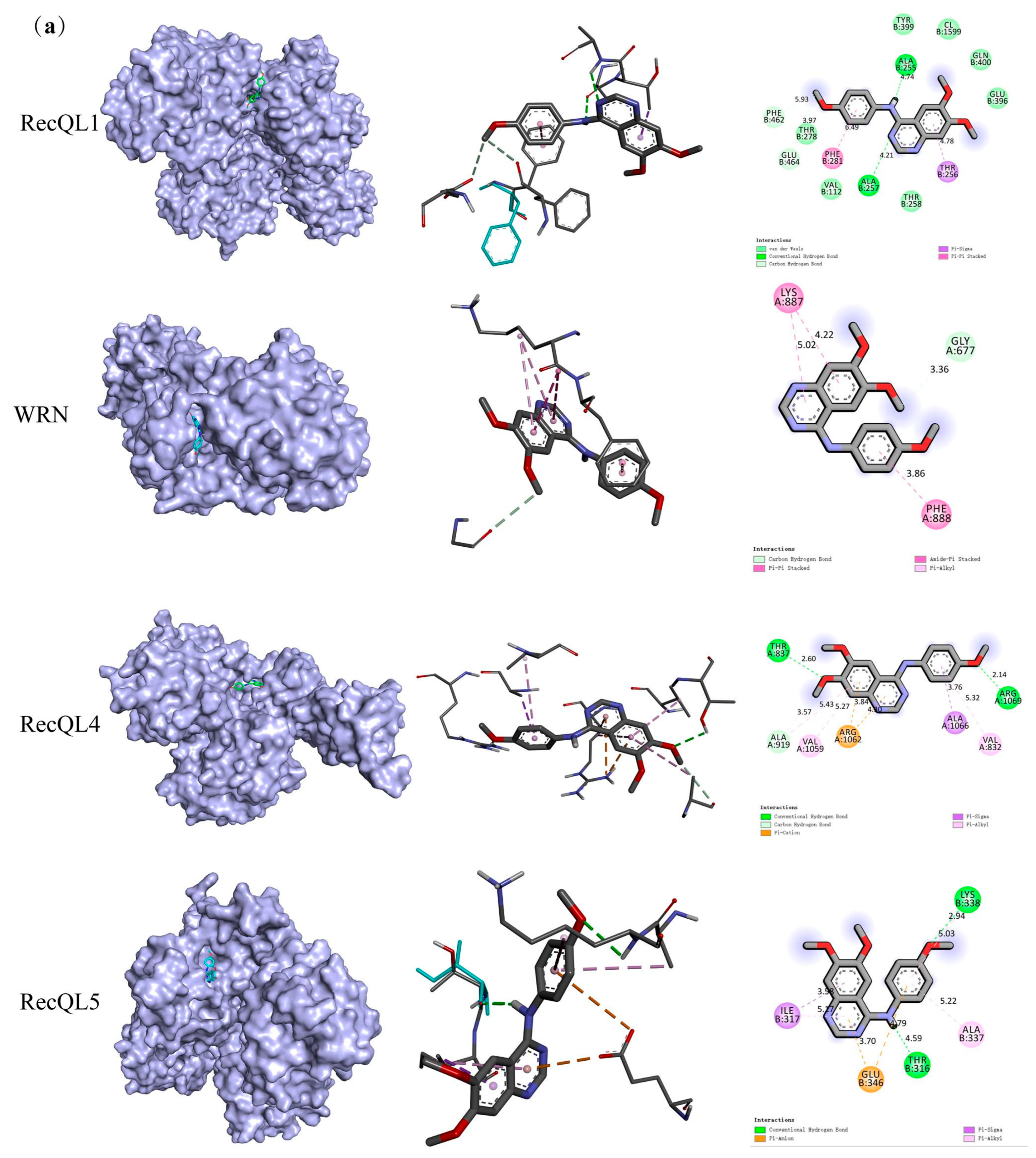

| Target Symbol | Entry | PDB ID | Binding Affinity (kcal/mol) |
|---|---|---|---|
| RecQL1 | P46063 | 2V1X | −7.9 |
| WRN | Q14191 | 6YHR | −8.2 |
| RecQL4 | O94761 | 5LST | −6.6 |
| RecQL5 | O94762 | 5LB3 | −7.5 |
| Group (mg/kg) | Prevention Experiment Group | Treatment Experiment Group | ||||
|---|---|---|---|---|---|---|
| Body Weight, g | Liver Weight, g | Liver Coefficient, % | Body Weight, g | Liver Weight, g | Liver Coefficient, % | |
| Control (saline) | 47.40 ± 0.93 | 1.78 ± 0.08 | 3.75 ± 0.22 | 51.6 ± 1.11 | 1.91 ± 0.06 | 3.68 ± 0.12 |
| SA (5.0) | 35.07 ± 1.77 ** | 2.20 ± 0.06 ** | 6.27 ± 0.35 aa | 43.83 ± 1.78 ** | 2.31 ± 0.05 ** | 5.27 ± 0.30 ** |
| Bicyclol (11.375) | 46.13 ± 1.16 aa | 1.89 ± 0.12 aa | 4.10 ± 0.23 aa | 50.05 ± 1.34 aa | 1.90 ± 0.06 aa | 3.79 ±0.20 aa |
| glutathione (182) | 47.43 ± 1.29 aa | 1.87 ± 0.17 aa | 3.94 ± 0.40 aa | 50.92 ± 0.92 aa | 1.95 ± 0.04 aa | 3.83 ± 0.07 aa |
| KZL-047 (25) | 45.50 ± 1.90 aa | 1.85 ± 0.08 aa | 4.08 ± 0.18 aa | 50.07 ± 1.47 aa | 1.99 ± 0.10 aa | 3.97 ± 0.10 aa |
| KZL-047 (50) | 46.18 ± 1.68 aa | 1.86 ± 0.05 aa | 4.03 ± 0.17 aa | 50.98 ± 1.24 aa | 1.90 ± 0.05 aa | 3.74 ± 0.12 aa |
| KZL-047 (100) | 47.35 ± 1.54 aa | 1.78 ± 0.11 aa | 3.76 ± 0.18 aa | 51.17 ± 1.27 aa | 1.89 ± 0.05 aa | 3.69 ± 0.06 aa |
| Group (mg/kg) | Prevention Experiment Group | Treatment Experiment Group | ||||
|---|---|---|---|---|---|---|
| ALT, U/mL | AST, U/mL | TBIL, μmol/L | ALT, U/mL | AST, U/mL | TBIL, μmol/L | |
| Control (saline) | 35.01 ± 6.37 | 99.08 ± 5.45 | 19.20 ± 4.99 | 45.54 ± 4.10 | 78.95 ± 5.36 | 15.36 ± 1.26 |
| SA (5.0) | 71.59 ± 11.75 ** | 174.24 ± 26.66 ** | 37.56 ± 7.90 ** | 86.60 ± 10.62 ** | 154.30 ± 14.26 ** | 32.04 ± 4.73 ** |
| Bicyclol (11.375) | 36.98 ± 4.74 aa | 100.12 ± 2.67 aa | 18.25 ± 4.52 aa | 52.48 ± 9.67 aa | 77.40 ± 2.91 aa | 16.39 ± 2.30 aa |
| glutathione (182) | 34.81 ± 3.91 aa | 97.42 ± 2.44 aa | 17.22 ± 4.37 aa | 43.84 ± 4.10 aa | 76.00 ± 3.38 aa | 15.09 ± 1.83 aa |
| KZL-047 (25) | 35.03 ± 0.96 aa | 96.93 ± 5.65 aa | 18.23 ± 4.24 aa | 52.36 ± 8.68 aa | 79.54 ± 5.98 aa | 14.98 ± 5.94 aa |
| KZL-047 (50) | 33.98 ± 0.93 aa | 94.78 ± 5.34 aa | 16.50 ±3.38 aa | 49.38 ± 5.23 aa | 78.02 ± 4.14 aa | 14.80 ± 2.82 aa |
| KZL-047 (100) | 32.76 ± 1.03 aa | 91.89 ± 8.68 aa | 15.12 ± 2.27 aa | 44.28 ± 3.05 aa | 75.23 ± 3.88 aa | 14.28 ± 3.36 aa |
| Group (mg/kg) | Prevention Experiment Group | Treatment Experiment Group | ||||
|---|---|---|---|---|---|---|
| MDA nmol/mg | GSH, μmol/g | SOD, U/g | MDA, nmol/mg | GSH, μmol/g | SOD, U/g | |
| Control (saline) | 25.69 ± 2.67 | 39.60 ± 1.89 | 151.12 ± 3.19 | 15.19 ± 0.59 | 37.16 ± 5.93 | 96.09 ± 8.34 |
| SA (5.0) | 36.71 ± 3.65 ** | 21.06 ± 1.42 ** | 95.97 ± 3.33 ** | 26.14 ± 4.14 ** | 21.41 ± 1.40 ** | 50.60 ± 7.76 ** |
| Bicyclol (11.375 | 28.53 ± 3.15 aa | 37.79 ± 0.73 aa | 147.17 ± 3.39 aa | 16.83 ± 1.70 aa | 34.25 ± 0.51 aa | 87.24 ± 5.86 aa |
| glutathione (182) | 27.87 ± 4.10 aa | 38.19 ± 0.87 aa | 147.72 ± 3.54 aa | 16.07 ± 1.55 aa | 35.22 ± 1.04 aa | 88.84 ±3.16 aa |
| KZL-047 (25) | 26.05 ± 4.10 aa | 37.89 ± 0.79 aa | 145.62 ± 5.19 aa | 16.84 ± 2.53 aa | 35.07 ± 0.84 aa | 88.55 ± 1.66 aa |
| KZL-047 (50) | 25.80 ± 1.68 aa | 38.26 ± 1.75 aa | 147.75 ± 5.01 aa | 15.07 ± 1.14 aa | 39.02 ± 3.84 aa | 90.13 ±1.21 aa |
| KZL-047 (100) | 25.72 ± 0.43 aa | 38.56 ± 1.50 aa | 148.41 ± 6.09 aa | 14.85 ± 1.22 aa | 39.42 ± 3.62 aa | 74.13 ± 5.68 aa |
| Gene | Primer | Primer Sequence |
|---|---|---|
| RECQL1 | Forward Reverse | 5′-AAGAATGGCGTCCGTTTCAG-3′ 5′-AGCTCTTGTTGCCTTTCCGT-3′ |
| WRN | Forward Reverse | 5′-GGCACGGTAGAACCAACTCA-3′ 5′-TGCTCTTCATTGGGTGCTGG-3′ |
| RECQL4 | Forward Reverse | 5′-ATCCTGTCTGGCATCTCCAC-3′ 5′-TGCAGGACAGATTCCCGTTG-3′ |
| RECQL5 | Forward Reverse | 5′-AGCCACCATACCACCTTTCC-3′ 5′-GGGCATGCACACAAAGACG-3′ |
| GAPDH | Forward Reverse | 5′-GGAGTCCACTGGCTCTTCA-3′ 5′-GTCATGAGTCCTTCACGATACC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Mo, M.; Yang, L.; Yu, J.; Li, J.; Cheng, S.; Sun, B.; Xu, B.; Zhang, A.; Luo, H. A Novel Quinazoline Derivative Prevents and Treats Arsenic-Induced Liver Injury by Regulating the Expression of RecQ Family Helicase. Int. J. Mol. Sci. 2023, 24, 15521. https://doi.org/10.3390/ijms242115521
Yang H, Mo M, Yang L, Yu J, Li J, Cheng S, Sun B, Xu B, Zhang A, Luo H. A Novel Quinazoline Derivative Prevents and Treats Arsenic-Induced Liver Injury by Regulating the Expression of RecQ Family Helicase. International Journal of Molecular Sciences. 2023; 24(21):15521. https://doi.org/10.3390/ijms242115521
Chicago/Turabian StyleYang, Heping, Min Mo, Langlang Yang, Jia Yu, Jiao Li, Sha Cheng, Baofei Sun, Bixue Xu, Aihua Zhang, and Heng Luo. 2023. "A Novel Quinazoline Derivative Prevents and Treats Arsenic-Induced Liver Injury by Regulating the Expression of RecQ Family Helicase" International Journal of Molecular Sciences 24, no. 21: 15521. https://doi.org/10.3390/ijms242115521
APA StyleYang, H., Mo, M., Yang, L., Yu, J., Li, J., Cheng, S., Sun, B., Xu, B., Zhang, A., & Luo, H. (2023). A Novel Quinazoline Derivative Prevents and Treats Arsenic-Induced Liver Injury by Regulating the Expression of RecQ Family Helicase. International Journal of Molecular Sciences, 24(21), 15521. https://doi.org/10.3390/ijms242115521





