Artemisia annua Extract Attenuate Doxorubicin-Induced Hepatic Injury via PI-3K/Akt/Nrf-2-Mediated Signaling Pathway in Rats
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Content of Artemisia annua Leaves
2.2. GC-MS Analysis of Artemisia annua Leaf Extract
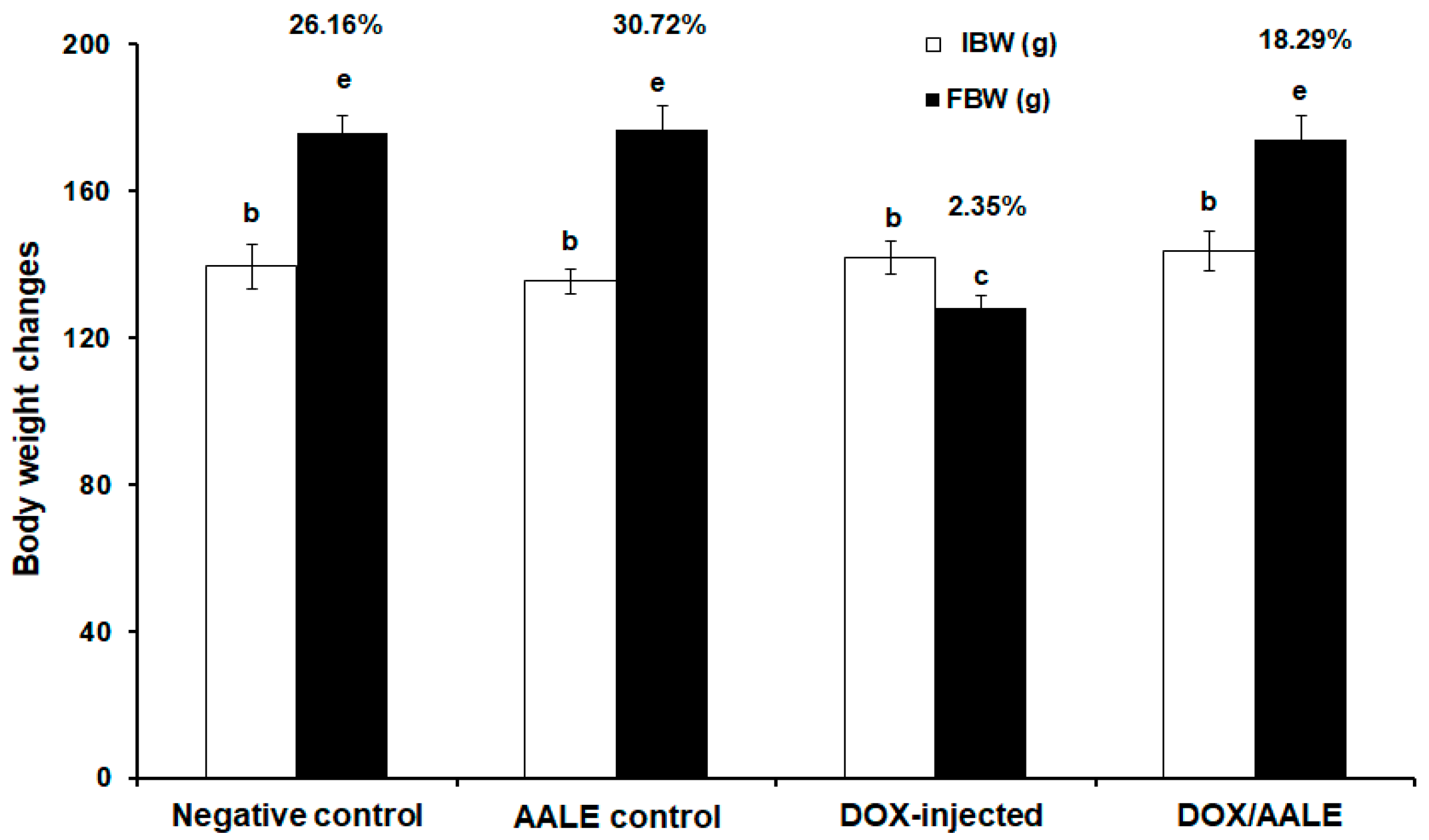
2.3. Effect of AALE Treatment on the Percentages of Body Weight Changes
2.4. Effect of AALE Treatment on the Alterations of Serum Biochemical Parameters Induced by DOX
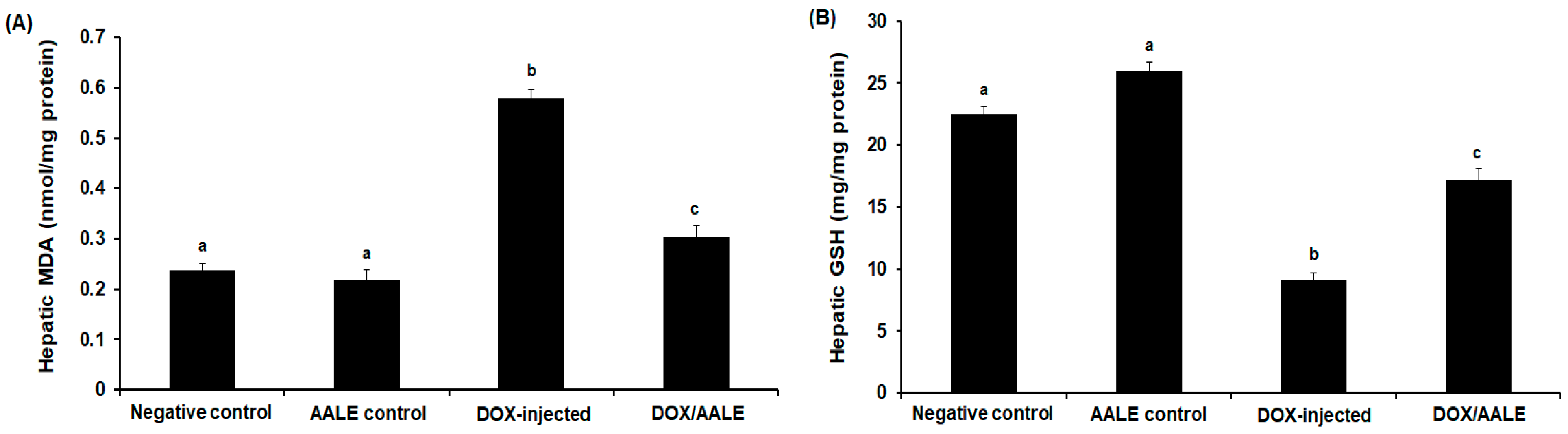
2.5. Treatment with AALE Modulated the Alterations of Hepatic Biochemical Parameters Induced by DOX
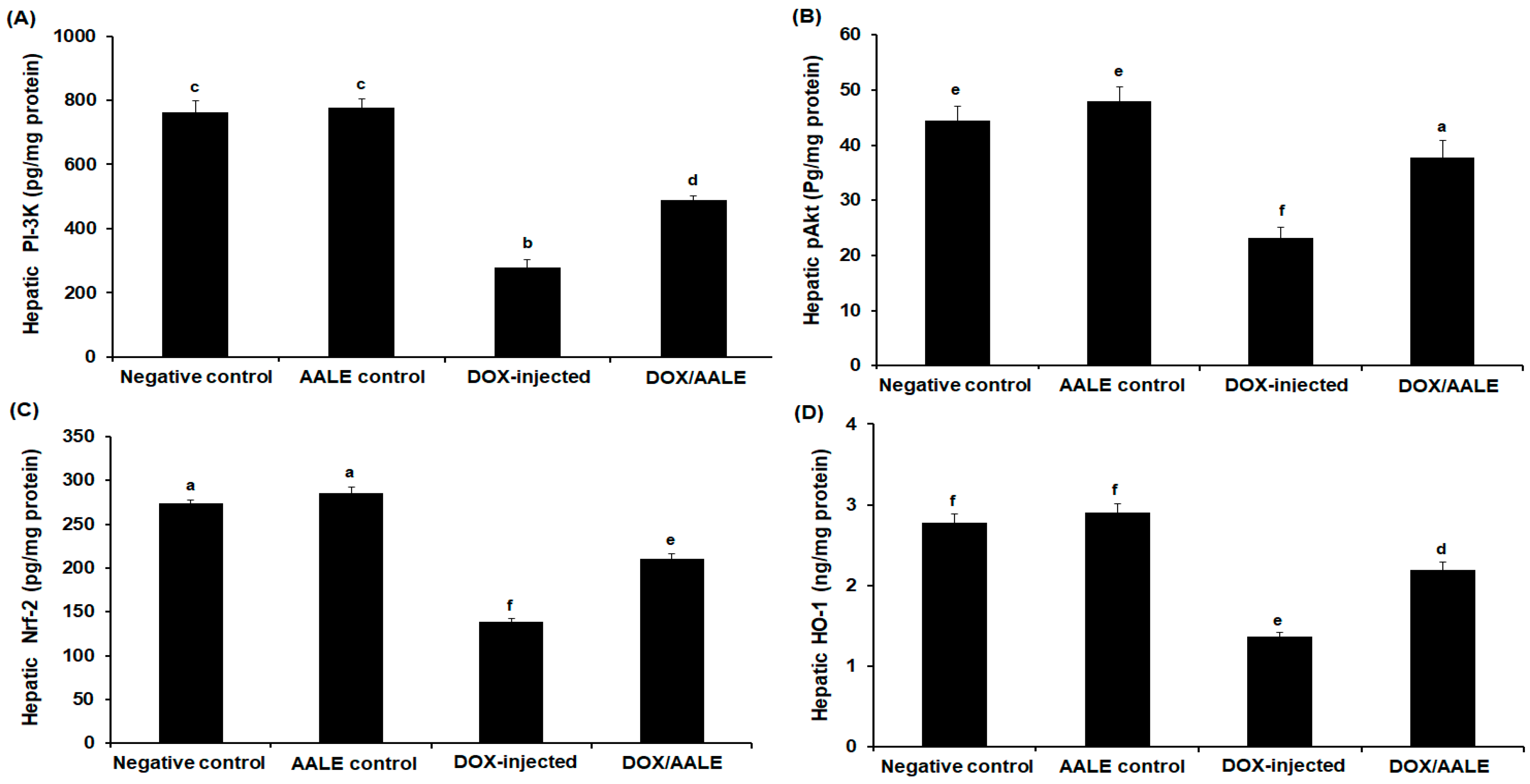
2.6. Effect of AALE Treatment on Antioxidant Gene Expression
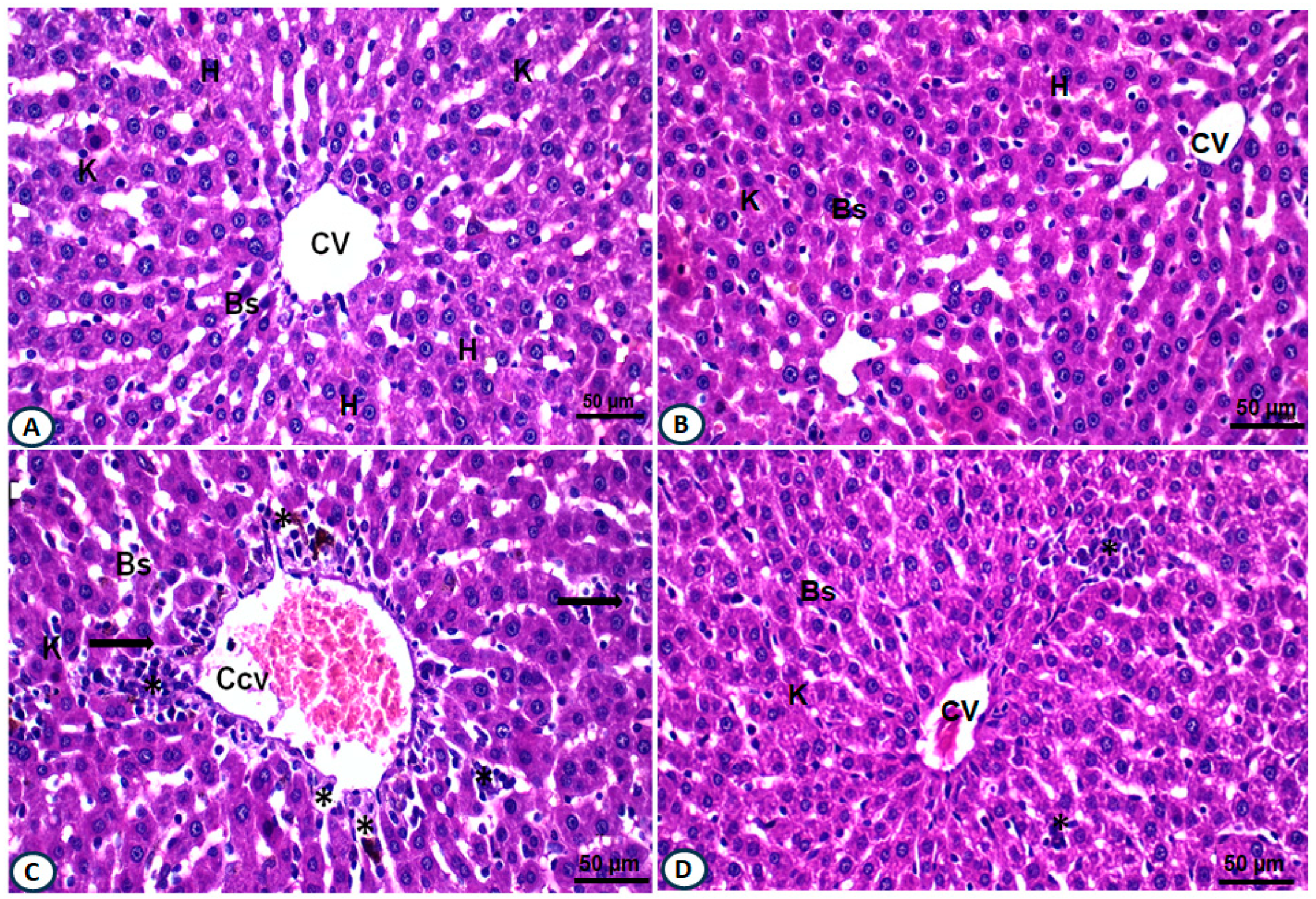
2.7. Treatment with AALE Restored Hepatic Histopathological Alterations Induced by DOX
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Collection of Plant and Extract Preparation
4.3. Determination of Phytochemical Content of A. annua Leaves
4.4. Gas Chromatography and Mass Spectrum (GC-MS) Profiling of AALE
4.5. Rats and Experimental Design
4.6. Biochemical Analysis
4.7. Molecular Analysis
4.8. Histopathological Investigations
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Naggar, S.A.; El-Said, K.S. Antitumor efficacy of EDTA co-treatment with cisplatin in tumor-bearing mice. Braz. J. Pharm. Sci. 2020, 56, e18536. [Google Scholar] [CrossRef]
- Argenziano, M.; Gigliotti, C.L.; Clemente, N.; Boggio, E.; Ferrara, B.; Trotta, F.; Pizzimenti, S.; Barrera, G.; Boldorini, R.; Bessone, F.; et al. Improvement in the anti-tumor efficacy of doxorubicin nanosponges in in vitro and in mice bearing breast tumor models. Cancers 2020, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yuan, S.; Zhao, Q.; Wang, B.O.; Wang, X.; Li, K. Quercetin enhances chemotherapeutic effect of doxorubicin against human breast cancer cells while reducing toxic side effects of it. Biomed. Pharmacother. 2018, 100, 441–447. [Google Scholar] [CrossRef]
- Tan, Q.; Yan, X.; Song, L.; Yi, H.; Li, P.; Sun, G.; Yu, D.; Li, L.; Zeng, Z.; Guo, Z. Induction of mitochondrial dysfunction and oxidative damage by antibiotic drug doxycycline enhances the responsiveness of glioblastoma to chemotherapy. Med. Sci. Monit. 2017, 23, 4117–4125. [Google Scholar] [CrossRef]
- Timm, K.N.; Ball, V.; Miller, J.J.; Savic, D.; West, J.A.; Griffin, J.L.; Tyler, D.J. Metabolic effects of doxorubicin on the rat liver assessed with hyperpolarized MRI and metabolomics. Front. Physiol. 2022, 12, 782745. [Google Scholar] [CrossRef]
- Shaw, P.; Chattopadhyay, A. Nrf2-ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. J. Cell. Physiol. 2020, 235, 3119–3130. [Google Scholar] [CrossRef]
- Wang, R.; Deng, D.; Shao, N.; Xu, Y.; Xue, L.; Peng, Y.; Liu, Y.; Zhi, F. Evodiamine activates cellular apoptosis through suppressing PI3K/AKT and activating MAPK in glioma. OncoTargets Ther. 2018, 11, 1183–1192. [Google Scholar] [CrossRef]
- Sabnam, S.; Pal, A. Relevance of Erk1/2-PI3K/Akt signaling pathway in CEES-induced oxidative stress regulates inflammation and apoptosis in keratinocytes. Cell Biol. Toxicol. 2019, 35, 541–564. [Google Scholar] [CrossRef]
- Wali, A.F.; Rashid, S.; Rashid, S.M.; Ansari, M.A.; Khan, M.R.; Haq, N.; Alhareth, D.Y.; Ahmad, A.; Rehman, M.U. Naringenin regulates doxorubicin-induced liver dysfunction: Impact on oxidative stress and inflammation. Plants 2020, 9, 550. [Google Scholar] [CrossRef]
- Al Asmari, A.F.; Alharbi, M.; Alqahtani, F.; Alasmari, F.; AlSwayyed, M.; Alzarea, S.I.; Al-Alallah, I.A.; Alghamdi, A.; Hakami, H.M.; Alyousef, M.K.; et al. Diosmin alleviates doxorubicin-induced liver injury via modulation of oxidative stress-mediated hepatic inflammation and apoptosis via Nf-kB and MAPK pathway: A preclinical study. Antioxidants 2021, 10, 1998. [Google Scholar] [CrossRef]
- Xing, Y.Y.; Xu, Y.Q.; Jin, X.; Shi, L.L.; Guo, S.W.; Yan, S.M.; Shi, B.L. Optimization extraction and characterization of Artemisia ordosica polysaccharide and its beneficial effects on antioxidant function and gut microbiota in rats. RSC Adv. 2020, 10, 26151–26164. [Google Scholar] [CrossRef]
- Nurlybekova, A.; Kudaibergen, A.; Kazymbetova, A.; Amangeldi, M.; Baiseitova, A.; Ospanov, M. Traditional use, phytochemical profiles, and pharmacological properties of Artemisia genus from central Asia. Molecules 2022, 27, 5128. [Google Scholar] [CrossRef]
- Mohanty, B.; Puri, S.; Kesavan, V. A review on therapeutic potential of Artemisia nilagirica. J. Plant Biochem. Physiol. 2018, 6, 205. [Google Scholar] [CrossRef]
- Lang, S.J.; Schmiech, M.; Hafner, S.; Paetz, C.; Steinborn, C.; Huber, R.; El Gaafary, M.; Werner, K.; Schmidt, C.Q.; Syrovets, T.; et al. Antitumor activity of an Artemisia annua herbal preparation and identification of active ingredients. Phytomedicine 2019, 62, 152962. [Google Scholar] [CrossRef]
- Yang, X.L.; Deng, S.Q.; De Philippis, R.; Chen, L.Z.; Hu, C.Z.; Zhang, W.H. Chemical composition of volatile oil from Artemisia ordosica and its allelopathic effects on desert soil microalgae, Palmellococcus miniatus. Plant Physiol. Biochem. 2012, 51, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.Y.; Zheng, Y.K.; Yang, S.; Zhang, L.H.; Guo, S.W.; Shi, L.L.; Xu, Y.Q.; Jin, X.; Yan, S.M.; Shi, B.L. Artemisia ordosica polysaccharide alleviated lipopolysaccharide-induced oxidative stress of broilers via Nrf2/Keap1 and TLR4/NF-κB pathway. Ecotoxicol. Environ. Saf. 2021, 223, 112566. [Google Scholar] [CrossRef]
- Wang, Q.H.; Pa, B.; Gong, J.H.; Bao, W.Q.; Hao, J.S.; Xu, Y.H. Phenylpropanoids, flavonoids, and terpenoids from Artemisia ordosica Krasch. Magn. Reason. Chem. 2019, 57, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, I.; Özmen, Ö.; Tutun, H.; Yalçın, A.; Türk, A. Artemisinin attenuates doxorubicin induced cardiotoxicity and hepatotoxicity in rats. Biotech. Histochem. 2020, 95, 121–128. [Google Scholar] [CrossRef]
- Mokhamer, E.M.; Zidan, A.A.; El.Ghayesh, N.K.; Abdel-Aziz, K.K. Attenuation of trichloroacetic acid-induced hepatocellular carcinoma by Artemisia judaica ethanolic extract in male rats. J. Basic Appl. Zool. 2022, 83, 2426. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Peter, S.; Alven, S.; Maseko, R.B.; Aderibigbe, B.A. Doxorubicin-based hybrid compounds as potential anticancer agents: A review. Molecules 2022, 27, 4478. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dong, Z.; Lan, X.; Liao, Z.; Chen, M. Sweroside alleviated LPS-induced inflammation via SIRT1 mediating NF-kappaB and FoxO1 signaling pathways in RAW264.7 Cells. Molecules 2019, 24, 872. [Google Scholar] [CrossRef] [PubMed]
- Septembre-Malaterre, A.; Lalarizo Rakoto, M.; Marodon, C.; Bedoui, Y.; Nakab, J.; Simon, E.; Hoarau, L.; Savriama, S.; Strasberg, D.; Guiraud, P.; et al. Artemisia annua, a Traditional plant brought to light. Int. J. Mol. Sci. 2020, 21, 4986. [Google Scholar] [CrossRef] [PubMed]
- Chukwurah, P.N.; Brisibe, E.A.; Osuagwu, A.N.; Okoko, T. Protective capacity of Artemisia annua as a potent antioxidant remedy against free radical damage. Asian Pac. J. Trop. Biomed. 2014, 4, 92–98. [Google Scholar] [CrossRef]
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Chellappan, D.K. Phytochemistry and pharmacological activity of the genus artemisia. Arch. Pharm. Res. 2021, 44, 439. [Google Scholar] [CrossRef] [PubMed]
- Bordean, M.E.; Ungur, R.A.; Toc, D.A.; Borda, I.M.; Marțiș, G.S.; Pop, C.R.; Filip, M.; Vlassa, M.; Nasui, B.A.; Pop, A.; et al. Antibacterial and phytochemical screening of Artemisia species. Antioxidants 2023, 12, 596. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Sinan, K.I.; Sieniawska, E.; Sawicki, R.; Maciejewska-Turska, M.; Skalikca-Woźniak, K.; Luca, S.V. Unveiling the phytochemical profile and biological potential of five Artemisia species. Antioxidants 2022, 11, 1017. [Google Scholar] [CrossRef]
- Mazandarani, M.; Majidi, Z.; Zarghami, M.; Parastoo, M.; Hemati, H.; Fatemeh, F. Total phenol, flavonoid, anthocyanin, and antioxidant activities in different parts of Artemisia annua L. in two localities (North of Iran). J. Med. Plants Prod. 2012, 1, 13–21. [Google Scholar]
- Wan, X.L.; Niu, Y.; Zheng, X.C.; Huang, Q.; Su, W.P.; Zhang, J.F.; Zhang, L.L.; Wang, T. Antioxidant capacities of Artemisia annua L. leaves and enzymatically treated Artemisia annua L. in vitro and in broilers. Anim. Feed Sci. Technol. 2016, 221, 27–34. [Google Scholar] [CrossRef]
- Hameed, I.H.; Altameme, H.J.; Idan, S.A. Artemisia annua: Biochemical products analysis of methanolic aerial parts extract and anti-microbial capacity. Res. J. Pharmaceut. Biol. Chem. Sci. 2016, 7, 1843–1868. [Google Scholar]
- Liu, M.; Su, Y.; Guo, Y. Headspace-low water absorption trap technique: Analysis of low-abundance volatile compounds from fresh Artemisia Annua L. with GC–MS. J. Chromatogr. Sci. 2022, 60, 907–915. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A sesquiterpene with countless biological properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Dangroo, N.A.; Singh, J.; Gupta, N.; Singh, S.; Kaul, A.; Khuroo, M.A.; Sangwan, P.L. T- and B-cell immunosuppressive activity of novel α-santonin analogs with humoral and cellular immune response in Balb/c mice. MedChemComm 2016, 8, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Ziaei, A.; Ramezani, M.; Wright, L.; Paetz, C.; Schneider, B.; Amirghofran, Z. Identification of spathulenol in Salvia mirzayanii and the immunomodulatory effects. Phytother. Res. 2011, 25, 557–562. [Google Scholar] [CrossRef]
- Dundar, H.A.; Kiray, M.; Kir, M.; Kolatan, E.; Bagriyanik, A.; Altun, Z.; Aktas, S.; Ellidokuz, H.; Yilmaz, O.; Mutafoglu, K.; et al. Protective effect of acetyl-L-carnitine against doxorubicin-induced cardiotoxicity in wistar albino rats. Arch. Med. Res. 2016, 47, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Shaker, R.A.; Abboud, S.H.; Assad, H.C.; Hadi, N. Enoxaparin attenuates doxorubicin induced cardiotoxicity in rats via interfering with oxidative stress, inflammation, and apoptosis. BMC Pharmacol. Toxicol. 2018, 19, 3. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Todorovic, V.; Sobajic, S.; Mahajna, J.; Gerić, M.; Tur, J.A.; Bartoszek, A. Natural products counteracting cardiotoxicity during cancer chemotherapy: The special case of doxorubicin, a comprehensive review. Int. J. Mol. Sci. 2021, 22, 10037. [Google Scholar] [CrossRef]
- Yi, X.; Wang, F.; Feng, Y.; Zhu, J.; Wu, Y. Danhong injection attenuates doxorubicin-induced cardiotoxicity in rats via suppression of apoptosis: Network pharmacology analysis and experimental validation. Front. Pharmacol. 2022, 13, 929302. [Google Scholar] [CrossRef]
- Hozayen, W.G.; Abou Seif, H.S.; Amin, S. Protective effects of ruitn and/or hesperidin against doxorubicin induced hepatotoxicity. Int. J. Clin. Nutr. 2014, 2, 11–17. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Elkomy, M.H.; Fahim, H.I.; Ashour, M.B.; Naguib, I.A.; Alghamdi, B.S.; Mahmoud, H.U.R.; Ahmed, N.A. Rutin and quercetin counter doxorubicin-induced liver toxicity in wistar rats via their modulatory effects on inflammation, oxidative stress, apoptosis, and Nrf2. Oxid. Med. Cell Longev. 2022, 2022, 2710607. [Google Scholar] [CrossRef]
- Bradic, J.; Andjic, M.; Novakovic, J.; Kocovic, A.; Tomovic, M.; Petrovic, A.; Nikolic, M.; Mitrovic, S.; Jakovljevic, V.; Pecarski, D. Lady’s Bedstraw as a powerful antioxidant for attenuation of doxorubicin-induced cardiotoxicity. Antioxidants 2023, 12, 1277. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Lalrinengi, C. Treatment of mice with naringin alleviates the doxorubicin-induced oxidative stress in the liver of Swiss albino mice. MOJ Anat. Physiol. 2017, 4, 267–278. [Google Scholar] [CrossRef]
- Kebiechc, M.; Lakroun, Z.; Lahouel, M.; Bouayed, J.; Meraihi, A. Evaluation of epirubicin-induced acute oxidative stress toxicity in rat liver cells and mitochondria, and the prevention of toxicity through quercetin administration. Exp. Toxicol. Pathol. 2009, 61, 161–167. [Google Scholar] [CrossRef]
- El-Said, K.S.; Atta, A.; Mobasher, M.A.; Germoush, M.O.; Mohamed, T.M.; Salem, M.M. Quercetin mitigates rheumatoid arthritis by inhibiting adenosine deaminase in rats. Mol. Med. 2022, 28, 24. [Google Scholar] [CrossRef]
- El-Said, K.S.; Hussein, S.; Alrashdi, B.M.; Mahmoud, H.A.; Ibrahim, M.A.; Elbakry, M.; El-Tantawy, H.; Kabil, D.I.; El-Naggar, S.A. Musa sp. leaves extract ameliorates the hepato-renal toxicities induced by cadmium in mice. Molecules 2022, 27, 559. [Google Scholar] [CrossRef]
- Barakat, B.M.; Ahmed, H.I.; Bahr, H.I.; Elbahaie, A.M. Protective effect of boswellic acids against doxorubicin-induced hepatotoxicity: Impact on Nrf2/HO-1 defense pathway. Oxid. Med. Cell Longev. 2018, 2018, 8296451. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef]
- Prasanna, P.L.; Renu, K.; Valsala Gopalakrishnan, A. New molecular and biochemical insights of doxorubicin-induced hepatotoxicity. Life Sci. 2020, 250, 117599. [Google Scholar] [CrossRef]
- Alshabanah, O.A.; Hafez, M.M.; Al-Harbi, M.M.; Hassan, Z.K.; Al Rejaie, S.S.; Asiri, Y.A.; Sayed-Ahmed, M.M. Doxorubicin toxicity can be ameliorated during antioxidant L-carnitine supplementation. Oxid. Med. Cell Longev. 2010, 3, 428–433. [Google Scholar] [CrossRef]
- Mete, R.; Oran, M.; Topcu, B.; Oznur, M.; Seber, E.S.; Gedikbasi, A.; Yetisyigit, T. Protective effects of onion (Allium cepa) extract against doxorubicin-induced hepatotoxicity in rats. Toxicol. Ind. Health 2016, 32, 551–557. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Y.; Wang, L.; Zhang, G.; Su, S.; Zhang, R.; Zhang, J.; Li, A.; Shang, C.; Bi, B.; et al. Alleviation of doxorubicin–induced hepatorenal toxicities with sesamin via the suppression of oxidative stress. Hum. Exp. Toxicol. 2016, 35, 1183–1193. [Google Scholar] [CrossRef]
- Espírito Santo, S.G.; Monte, M.G.; Polegato, B.F.; Barbisan, L.F.; Romualdo, G.R. Protective effects of omega-3 supplementation against doxorubicin-induced deleterious effects on the liver and kidneys of rats. Molecules 2023, 28, 3004. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of ûavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Hiai, S.; Oura, H.; Odaka, Y.; Nakajima, T. A colorimetric estimation of Ginseng saponins. Planta Med. 1975, 28, 363–369. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Ekiert, H.; Świątkowska, J.; Klin, P.; Rzepiela, A.; Szopa, A. Artemisia annua–importance in traditional medicine and current state of knowledge on chemistry, biological activity, and possible applications. Planta Med. 2021, 87, 584–599. [Google Scholar] [CrossRef]
- Warpe, V.S.; Mali, V.R.; Arulmozhi, S.; Bodhankar, S.L.; Mahadik, K.R. Cardioprotective effect of ellagic acid on doxorubicin induced cardiotoxicity in wistar rats. J. Acute Med. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 6th ed.; Churchill Livingstone: London, UK, 2008; pp. 352–360. [Google Scholar]

| Phytochemical Analysis | AAL |
|---|---|
| Total phenolic (mg GAE/g DW) | 21.36 ± 0.85 |
| Total flavonoids (mg QE/g DW) | 69.86 ± 2.37 |
| TAC (mg AAE/g DW) | 287.65 ± 5.54 |
| Saponin (mg/g DW) | 415.28 ± 3.95 |
| Anthocyanin (mg ECG/g DW) | 3.98 ± 0.45 |
| DPPH scavenging % | 81% ± 1.57 |
| IC50 of DPPH (mg/mL) | 4.55 ± 0.34 |
| No. | RT (min) | Name | M.F. | P.A% |
|---|---|---|---|---|
| 1 | 3.65 | 1,2-15,16-Diepoxyhexadecane | C16H30O2 | 3.65 |
| 2 | 4.25 | 3,5-Hexadien-2-ol2-methyl | C7H12O | 3.93 |
| 3 | 5.14 | Cholestan -3-ol,2 methylene, (3ß,5α) | C28H48O | 1.78 |
| 4 | 8.75 | 3,5-Heptadienal,2- ethylidene-6-methyl | C10H14O | 3.87 |
| 5 | 9.27 | Exo-2,7,7-trimethylbicyclo[2.2.1] eptan-2-ol | C10H18O | 2.30 |
| 6 | 12.02 | 2-Cyclohexen-1-one, 3-methyl-6-(1-methylethyl) | C10H16O | 2.65 |
| 7 | 13.19 | 3,5-Heptadien-2-ol, 2,6-dimethyl | C9H16O | 6.57 |
| 8 | 13.47 | 2,4,6-Trimethyl-1,3,6-heptatriene | C10H16 | 11.37 |
| 9 | 15.63 | β-Caryophyllenea | C10H24 | 8.95 |
| 10 | 16.21 | 3-Butenoic acid, 2-oxo-4-phenyl | C10H8O3 | 9.81 |
| 11 | 17.59 | 1,3,3-Trimethyl-2-oxabicyclo[2.2.2]octane | C10H18O | 2.78 |
| 12 | 22.37 | Spathulenol | C15H24O | 6.83 |
| 13 | 25.00 | Phytol | C20H40O | 3.54 |
| 14 | 25.39 | α-Santonin | C15H18O3 | 7.79 |
| 15 | 26.04 | Naphtho[1,2-b]furan-2,6(3H,4H)-dione,3a,5,5a,9, 9a,9b-Hexahydro-9-hydroxy-3,5a,9-trimethyl | C15H20O4 | 3.25 |
| 16 | 27.33 | 1-Naphthalenecarboxylic acid, 5,6,7,8-tetrahydro | C11H12O2 | 1.32 |
| 17 | 29.72 | Propanedioic acid, (phenylmethyl)-, diethyl ester | C14H18O4 | 2.74 |
| Groups | AST (U/L) | ALT (U/L) | ALP (U/L) | T.B. (mg/dL) | D.B. (mg/dL) |
|---|---|---|---|---|---|
| Normal control | 34.17 ± 1.15 f | 23.43 ± 0.59 a | 287.6 ± 4.04 e | 0.68 ± 0.014 c | 0.136 ± 0.005 a |
| AALE control | 30.64 ± 0.83 f | 20.21 ± 0.73 a | 281.2 ± 2.95 e | 0.60 ± 0.011 c | 0.129 ± 0.008 a |
| DOX-treated | 97.58 ± 1.46 b | 67.78 ± 1.44 e | 511.8 ± 5.86 b | 1.57 ± 0.056 b | 0.417 ± 0.009 d |
| DOX/AALE | 56.39 ± 1.68 c | 34.89 ± 0.96 a,d | 347.2 ± 6.49 f | 0.92 ± 0.015 c,f | 0.251 ± 0.007 e |
| Groups | SOD | CAT | GPX | GST | GR |
|---|---|---|---|---|---|
| Normal control | 1.01 ± 0.05 e | 1.00 ± 0.06 a | 1.02 ± 0.05 d | 1.00 ± 0.07 f | 1.00 ± 0.06 c |
| AALE control | 1.12 ± 0.03 e | 1.25 ± 0.04 a | 1.30 ± 0.06 d | 1.28 ± 0.05 f | 1.31 ± 0.04 c |
| DOX-treated | 0.46 ± 0.08 b | 0.53 ± 0.05 f | 0.37 ± 0.04 e | 0.41 ± 0.06 b | 0.50 ± 0.009 a |
| DOX/AALE | 0.85 ± 0.06 e | 0.91 ± 0.06 a | 0.78 ± 0.09 d | 0.92 ± 0.05 f | 0.74 ± 0.007 a,c |
| Gene | Accession Number | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|---|
| SOD | NM_017050 | CGAGCATGGGTTCCATGTC | CTGGACCGCCATGTTTCTTAG |
| CAT | NM_012520.2 | ACAACTCCCAGAAGCCTAAGAATG | GCTTTTCCCTTGGCAGCTATG |
| GPX | NM_030826.4 | GGAGAATGGCAAGAATGAAGA | CCGCAGGAAGGTAAAGAG |
| GST | XM_343545.8 | GCTGGAGTGGAGTTTGAAGAA | GTCCTGACCACGTCAACATAG |
| GR | NM_053906.2 | TTCTGGAACTCGTCCACTAGG | CCATGTGGTTACTGCACTACTTCC |
| β-actin | NM_031144.3 | ATCGCTGACAGGATGCAGAAG | AGAGCCACCAATCCACACAGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Said, K.S.; Haidyrah, A.S.; Mobasher, M.A.; Khayyat, A.I.A.; Shakoori, A.; Al-Sowayan, N.S.; Barnawi, I.O.; Mariah, R.A. Artemisia annua Extract Attenuate Doxorubicin-Induced Hepatic Injury via PI-3K/Akt/Nrf-2-Mediated Signaling Pathway in Rats. Int. J. Mol. Sci. 2023, 24, 15525. https://doi.org/10.3390/ijms242115525
El-Said KS, Haidyrah AS, Mobasher MA, Khayyat AIA, Shakoori A, Al-Sowayan NS, Barnawi IO, Mariah RA. Artemisia annua Extract Attenuate Doxorubicin-Induced Hepatic Injury via PI-3K/Akt/Nrf-2-Mediated Signaling Pathway in Rats. International Journal of Molecular Sciences. 2023; 24(21):15525. https://doi.org/10.3390/ijms242115525
Chicago/Turabian StyleEl-Said, Karim Samy, Ahmed S. Haidyrah, Maysa A. Mobasher, Arwa Ishaq A. Khayyat, Afnan Shakoori, Noorah Saleh Al-Sowayan, Ibrahim Omar Barnawi, and Reham A. Mariah. 2023. "Artemisia annua Extract Attenuate Doxorubicin-Induced Hepatic Injury via PI-3K/Akt/Nrf-2-Mediated Signaling Pathway in Rats" International Journal of Molecular Sciences 24, no. 21: 15525. https://doi.org/10.3390/ijms242115525
APA StyleEl-Said, K. S., Haidyrah, A. S., Mobasher, M. A., Khayyat, A. I. A., Shakoori, A., Al-Sowayan, N. S., Barnawi, I. O., & Mariah, R. A. (2023). Artemisia annua Extract Attenuate Doxorubicin-Induced Hepatic Injury via PI-3K/Akt/Nrf-2-Mediated Signaling Pathway in Rats. International Journal of Molecular Sciences, 24(21), 15525. https://doi.org/10.3390/ijms242115525






