Insertion of Transposable Elements in AVR-Pib of Magnaporthe oryzae Leading to LOSS of the Avirulent Function
Abstract
:1. Introduction
2. Results
2.1. Effectiveness of the Pib Gene and Frequency of AVR-Pib Alleles
2.2. Virulence Function of AVR-Pib Variations against the Pib Gene
2.3. Distribution of Haplotypes of AVR-Pib of M. oryzae
2.4. Selection Pressure on AVR-Pib in M. oryzae
2.5. Adaption of TE Insertion in AVR-Pib
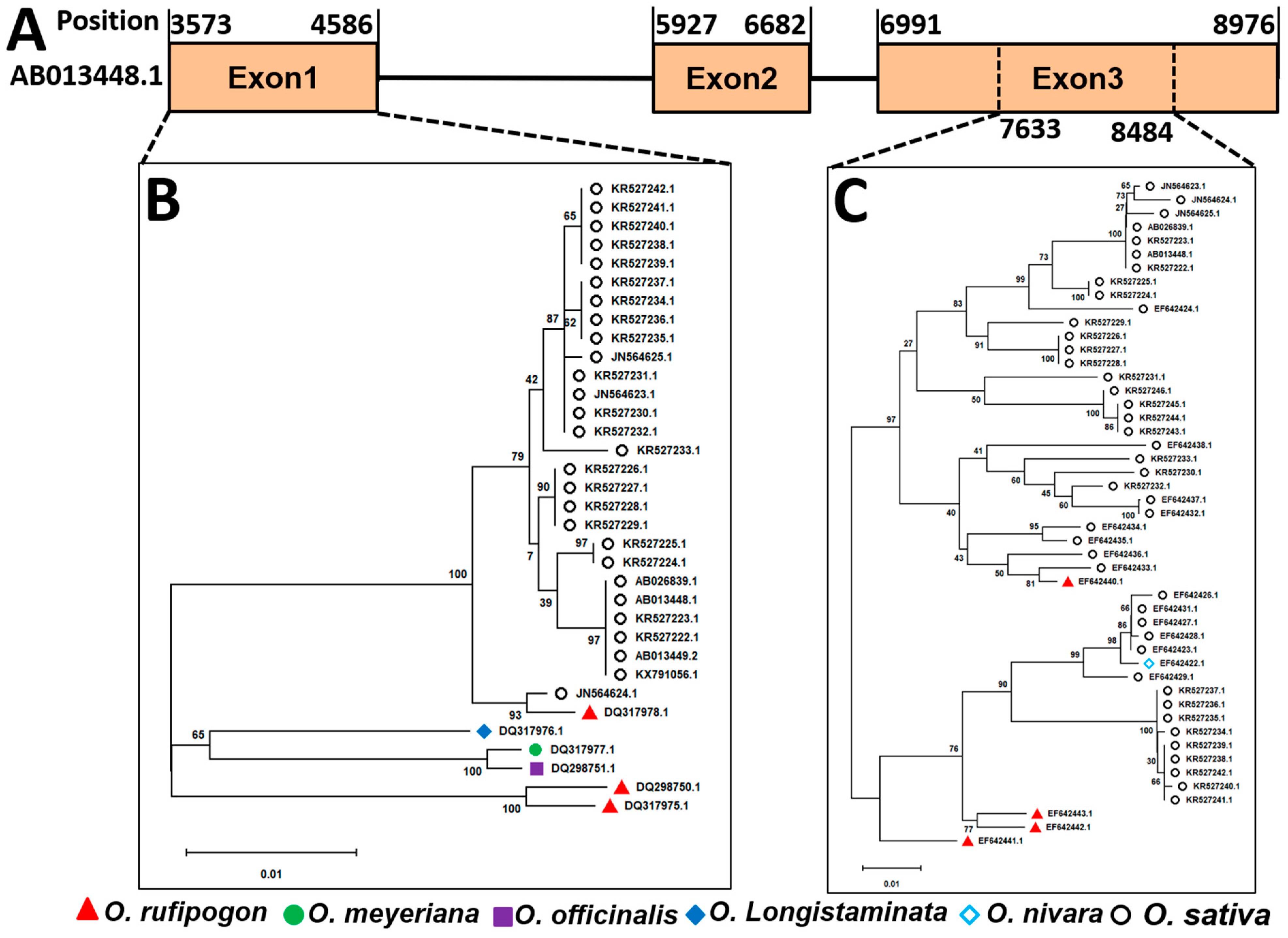
2.6. Phylogeny of Pib Allele Partial to CDS Regions
3. Discussion
4. Materials and Methods
4.1. Blast Isolates, Rice Accessions, Culture and Pathogenicity Identification
4.2. DNA Extraction, PCR Amplification and DNA Sequencing
4.3. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Woolhouse, M.; Webster, J.; Domingo, E.; Charlesworth, B.; Levin, B. Biological and biomedical implications of the co-evolution of pathogens and their hosts. Nat. Genet. 2002, 32, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xiao, G.; Telebanco-Yanoria, M.J.; Siazon, P.M.; Padilla, J.; Opulencia, R.; Bigirimana, J.; Habarugira, G.; Wu, J.; Li, M.; et al. The broad-spectrum rice blast resistance (R) gene Pita2 encodes a novel R protein unique from Pita. Rice 2022, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Daniel, J.; Wang, Z. The arms race between Magnaporthe oryzae and rice: Diversity and interaction of Avr and R genes. J. Integr. Agric. 2017, 16, 2746–2760. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, G.; Zhang, L.; Deng, L.; Wang, Y.; Wang, Y. Analysis of blast-resistance of rice germplasm and pathogenicity of rice blast fungus Magnaporthe oryzae in Heilongjiang Province. J. Plant Prot. 2017, 44, 209–216. (In Chinese) [Google Scholar]
- Du, Y.; Ruan, H.; Shi, N.; Gan, L.; Yang, X.; Chen, F. Pathogenicity analysis of Magnaporthe grisea against major Pi-genes and main rice varieties in Fujian Province. J. Plant Prot. 2016, 43, 442–451. (In Chinese) [Google Scholar]
- Zhang, S.; Zhong, X.; Qiao, G.; Shen, L.; Zhou, T.; Peng, Y. Difference in virulence of Magnaporthe oryzae from Sichuan, Chongqing and Guizhou. Southwest China J. Agric. Sci. 2017, 30, 359–365. (In Chinese) [Google Scholar]
- Yang, J.; Chen, S.; Zeng, L.; Li, Y.; Chen, Z.; Zhu, X. Evaluation on resistance of major rice blast resistance genes to Magnaporthe grisea isolates collected from indica rice in Guangdong Province, China. Chin. J. Rice Sci. 2008, 22, 190–196. (In Chinese) [Google Scholar]
- Li, J.; Li, C.; Chen, Y.; Lei, C.; Ling, Z. Evaluation of twenty-two blast resistance genes in Yunnan using monogenetic rice lines. Acta Phytophylacica Sin. 2005, 32, 113–119. (In Chinese) [Google Scholar]
- Xiao, G.; Yang, J.; Zhu, X.; Wu, J.; Zhou, B. Prevalence of ineffective haplotypes at the rice blast resistance (R) gene loci in Chinese elite hybrid rice varieties revealed by sequence-based molecular diagnosis. Rice 2020, 13, 6. [Google Scholar] [CrossRef]
- Miyamoto, M.; Ando, I.; Rybka, K.; Kodama, O.; Kawasaki, S. High resolution mapping of the indica-derived rice blast resistance genes. I. Pi-b. Mol. Plant-Microbe Interact. 1996, 9, 6–13. [Google Scholar] [CrossRef]
- Monna, L.; Miyao, A.; Zhong, H.S.; Yano, M.; Iwamoto, M.; Umehara, Y.; Umehara, Y.; Kurata, N.; Hayasaka, H.; Sasaki, T. Saturation mapping with subclones of YACs: DNA marker production targeting the rice blast disease resistance gene, Pi-b. Theor. Appl. Genet. 1997, 94, 170–176. [Google Scholar] [CrossRef]
- Wang, Z.; Yano, M.; Yamanouchi, U.; Iwamoto, M.; Monna, L.; Hayasaka, H.; Katayose, Y.; Sasaki, T. The Pib gene for rice blast resistance belongs to the nucleotide binding and leucine-rich repeat class of plant disease resistance genes. Plant J. 1999, 19, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Xu, X.; Ma, J.; Wang, S.; Tian, P.; Meng, L. Distribution of blast resistance genes Pib, Pita, Pi5, Pi25 and Pi54 in mini-core collection of Chinese rice germplasm. J. Plant Genet. Resour. 2019, 20, 1240–1246, 1254. (In Chinese) [Google Scholar]
- Shi, K.; Lei, C.; Cheng, Z.; Xu, X.; Wang, J.; Wan, J. Distribution of two blast resistance genes Pita and Pib in major rice cultivars in China. J. Plant Genet. Resour. 2009, 10, 21–26. (In Chinese) [Google Scholar]
- Li, J.; Wang, T.; Xu, M. Identification of Pi-ta and Pi-b genes for rice blast resistance of rice landraces from Yunnan Province. Chin. J. Rice Sci. 2012, 26, 593–599. (In Chinese) [Google Scholar] [CrossRef]
- Yang, M.; Cheng, Z.; Cheng, S.; Qian, J.; Yin, M.; Wu, C. Cloning and analysis of Pi-ta and Pib gene homologues from Yuannan Yuanjiang type of common wild rice (Oryza rufipogon Griff). Plant Physiol. Commun. 2007, 43, 483–486. (In Chinese) [Google Scholar]
- Olukayode, T.; Quime, B.; Shen, Y.C.; Yanoria, M.J.; Zhang, S.; Yang, J.; Zhu, X.; Shen, W.C.; von Tiedemann, A.; Zhou, B. Dynamic insertion of Pot3 in AvrPib prevailing in a field rice blast population in the Philippines led to the high virulence frequency against the resistance gene Pib in rice. Phytopathology 2019, 109, 870–877. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Wu, W.; He, L.; Yang, X.; Pan, Q. Function and evolution of Magnaporthe oryzae avirulence gene AvrPib responding to the rice blast resistance gene Pib. Sci. Rep. 2015, 5, 11642. [Google Scholar] [CrossRef]
- Ray, S.; Singh, P.K.; Gupta, D.K.; Mahato, A.K.; Sarkar, C.; Rathour, R.; Singh, N.K.; Sharma, T.R. Analysis of Magnaporthe oryzae genome reveals a fungal effector, which is able to induce resistance response in transgenic rice line containing resistance gene, Pi54. Front. Plant Sci. 2016, 7, 1140. [Google Scholar] [CrossRef]
- Wu, J.; Kou, Y.; Bao, J.; Li, Y.; Tang, M.; Zhu, X.; Ponaya, A.; Xiao, G.; Li, J.; Li, C.; et al. Comparative genomics identifies the Magnaporthe oryzae avirulence effector AvrPi9 that triggers Pi9-mediated blast resistance in rice. New Phytol. 2015, 206, 1463–1475. [Google Scholar] [CrossRef]
- Yoshida, K.; Saitoh, H.; Fujisawa, S.; Kanzaki, H.; Matsumura, H.; Yoshida, K.; Tosa, Y.; Chuma, I.; Takano, Y.; Win, J.; et al. Association genetics reveals three novel avirulence genes from the rice blast fungal pathogen Magnaporthe oryzae. Plant Cell. 2009, 21, 1573–1591. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, B.; Wu, J.; Lu, G.; Hu, Y.; Zhang, X.; Zhang, Z.; Zhao, Q.; Feng, Q.; Zhang, H.; et al. The Magnaporthe oryzae avirulence gene AVR-Pizt encodes a predicted secreted protein that triggers the immunity in rice mediated by the blast resistance gene Piz-t. Mol. Plant-Microbe Interact. 2009, 22, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Fudal, I.; Bohnert, H.U.; Tharreau, D.; Lebrun, M.H. Transposition of MINE, a composite retrotransposon, in the avirulence gene ACE1 of the rice blast fungus Magnaporthe grisea. Fungal Genet. Biol. 2005, 42, 761–772. [Google Scholar] [CrossRef]
- Farman, M.L.; Leong, S.A. Chromosome walking to the AVR1-CO39 avirulence gene of Magnaporthe grisea: Discrepancy between the physical and genetic maps. Genetics 1998, 150, 1049–1058. [Google Scholar] [CrossRef]
- Orbach, M.J.; Farrall, L.; Sweigard, J.A.; Chumley, F.G.; Valent, B. A telomeric avirulence gene determines efficacy for the rice blast resistance gene Pi-ta. Plant Cell 2000, 12, 2019–2032. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Sweigard, J.A.; Valent, B. The PWL host specificity gene family in the blast fungus Magnaporthe grisea. Mol. Plant-Microbe Interact. 1995, 8, 939–948. [Google Scholar] [CrossRef]
- Sweigard, J.A.; Carroll, A.M.; Kang, S.; Farrall, L.; Chumley, F.G.; Valent, B. Identification, cloning, and characterization of PWL2, a gene for host species specificity in the rice blast fungus. Plant Cell 1995, 7, 1221–1233. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Xing, J.; Jia, Y.; Peng, Z.; Shi, Y.; He, Q.; Shu, F.; Zhang, W.; Zhang, Z.; Deng, H. Characterization of molecular identity and pathogenicity of rice blast fungus in Hunan Province of China. Plant Dis. 2017, 101, 557–561. [Google Scholar] [CrossRef]
- Chuma, I.; Isobe, C.; Hotta, Y.; Ibaragi, L.; Futamata, N.; Kusaba, M.; Yoshida, K.; Terauchi, R.; Fujita, Y.; Nakayashiki, H.; et al. Multiple translocation of the AVR-Pita effector gene among chromosomes of the rice blast fungus Magnaporthe oryzae and related species. PLoS Pathog. 2011, 7, e1002147. [Google Scholar] [CrossRef]
- Dai, Y.; Jia, Y.; Correll, J.; Wang, X.; Wang, Y. Diversification evolution of the avirulence gene AVR-Pita1 in field isolates of Magnaporthe oryzae. Fungal Genet. Biol. 2010, 47, 973–980. [Google Scholar] [CrossRef]
- Kang, S.; Lebrun, M.H.; Farrall, L.; Valent, B. Gain of virulence caused by insertion of a Pot3 transposon in a Magnaporthe grisea avirulence gene. Mol. Plant-Microbe Interact. 2001, 14, 671–674. [Google Scholar] [CrossRef]
- Zhou, E.; Jia, Y.; Singh, P.; Correll, J.; Lee, F.N. Instability of the Magnaporthe oryzae avirulence gene AVR-Pita alters virulence. Fungal Genet. Biol. 2007, 44, 1024–1034. [Google Scholar] [CrossRef]
- Li, J. Breeding of Yunnan rice; identification of rice blast resistance genes in japonica rice in Yunnan. In Yunnan Rice; Jiang, Z., Ed.; Yunnan Science and Technology Press: Kunming, China, 1995; pp. 178–189. (In Chinese) [Google Scholar]
- Wang, C.A.; Guncar, G.; Forwood, J.K.; Teh, T.; Catanzariti, A.; Lawrence, G.J.; Loughlin, F.E.; Mackay, J.P.; Schirra, H.J.; Anderson, P.A.; et al. Crystal structures of flax rust avirulence proteins AvrL567-A and -D reveal details of the structural basis for flax disease resistance specificity. Plant Cell 2007, 19, 2898–2912. [Google Scholar] [CrossRef]
- Kanzaki, H.; Yoshida, K.; Saitoh, H.; Fujisaki, K.; Hirabuchi, A.; Alaux, L.; Fournier, E.; Tharreau, D.; Terauchi, R. Arms race co-evolution of Magnaporthe oryzae AVR-Pik and rice Pik genes driven by their phyical interactions. Plant J. 2012, 72, 894–907. [Google Scholar] [CrossRef]
- Wu, W.; Wang, L.; Zhang, S.; Li, Z.; Zhang, Y.; Lin, F.; Pan, Q. Stepwise arms race between AvrPik and Pik alleles in the rice blast pathosystem. Mol. Plant-Microbe Interact. 2014, 27, 759–769. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Li, C.; Bi, Y.; Fu, X.; Wang, R. Novel haplotypes and networks of AVR-Pik alleles in Magnaporthe oryzae. BMC Plant Biol. 2019, 19, 204. [Google Scholar] [CrossRef]
- Jia, Y.; Valent, B.; Lee, F.N. Determination of host responses to Magnaporthe grisea on detached rice leaves using a spot inoculation method. Plant Dis. 2003, 87, 129–133. [Google Scholar] [CrossRef]
- Tai, T.; Tanksley, S.D. A rapid and inexpensive method for isolation of total DNA from dehydrated plant tissue. Plant Mol. Biol. Rep. 1990, 8, 297–303. [Google Scholar] [CrossRef]
- Rozas, J.; Sánchez-Del, B.J.; Messeguer, X.; Rozas, R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 2003, 19, 2496–2497. [Google Scholar] [CrossRef]
- Clement, M.; Posada, D.; Crandall, K. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef]
- Fontaine, C.; Lovett, P.N.; Sanou, H.; Maley, J.; Bouvet, J.-M. Genetic diversity of the shea tree (Vitellaria paradoxa C.F. Gaertn), detected by RAPD and chloroplast microsatellite markers. Heredity 2004, 93, 639–648. [Google Scholar] [CrossRef]
- Rzhetsky, A.; Nei, M.A. Simple method for estimating and testing minimum evolution trees. Mol. Biol. Evol. 1992, 9, 945–967. [Google Scholar]

| Locations | No. of Isolates | PCR Detection a | Pathogenicity Assay b | |||
|---|---|---|---|---|---|---|
| Genotype and No. of Isolates and Frequency (%) | No. of Avirulence Isolates and Frequency (%) | |||||
| L1 | L2 | L3 | Total Isolates and Frequency (%) | |||
| Central | 54 | 22 (40.7) | 3 (5.6)) | 0 | 25 (46.3) B | 41 (75.9) AB |
| Northeastern | 72 | 23 (31.9) | 3 (4.2) | 0 | 26 (36.1) B | 54 (75.0) B |
| Northwestern | 15 | 11 (73.3) | 0 | 0 | 11 (73.3) A | 15 (100) A |
| Southeastern | 33 | 10 (30.3) | 6 (18.2) | 1 (3.0) | 17 (51.5) B | 19 (57.6) C |
| Southwestern | 28 | 9 (32.1) | 8 (28.6) | 2 (7.1) | 19 (67.9) A | 15 (53.6) C |
| Western | 164 | 29 (17.7) | 33 (20.1) | 2 (1.2) | 64 (39.0) B | 79 (48.2) C |
| Total | 366 | 104 (28.4) | 53 (14.5) | 5 (1.4) | 162 (44.3) | 223 (60.9) |
| XI | 149 | 31 (20.8) | 34 (22.8) | 5 (3.4) | 70 (47.0) * | 69 (46.3) ** |
| GJ | 217 | 73 (33.6) | 19 (8.8) | 0 | 92 (42.4) * | 154 (71.0) ** |
| Total | 366 | 104 (28.4) | 53 (14.5) | 5 (1.4) | 162 (44.3) | 223 (60.9) |
| Haplotype | No. of Isolates | % of Total | Variant Locus a | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5′ UTR | CDS Regions | 3′ UTR | ||||||||||||||||||
| −338 | Between −325 and −326 | Between −239 and −240 | Between −216 and −217 | −192 | −175 | Between −210 and −211 | −93 | 137 | 141 | 146 | 148 | 158 | 160 | +70 | +154 | +218 | +232 | |||
| KM887844 | T | - | - | - | C | C | - | T | A | T | T | C | A | T | C | G | A | C | ||
| H01 | 33 | 26.2 | . | . | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| H02 | 4 | 3.2 | . | . | . | C | . | . | . | A | . | . | . | . | . | . | . | T | . | . |
| H03 | 4 | 3.2 | . | . | . | C | T | . | . | . | . | . | . | . | . | . | . | . | . | . |
| H04 | 1 | 0.8 | . | ACTTA | . | C | . | . | . | . | . | . | . | . | . | . | T | . | . | . |
| H05 | 8 | 6.3 | C | . | . | C | . | . | . | . | . | . | . | . | . | C | . | . | . | . |
| H06 | 1 | 0.8 | C | . | ACGTTA | C | . | . | . | . | . | . | . | . | . | C | . | . | . | . |
| H07 | 3 | 2.4 | . | . | . | C | . | T | . | . | T | . | . | . | C | G | . | . | . | A |
| H08 | 1 | 0.8 | . | . | . | C | . | . | ACA | . | . | A | C | G | . | . | . | . | . | . |
| H09 | 13 | 10.3 | . | ACTTA | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| H10 | 10 | 7.9 | . | AGTTA | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| H11 | 2 | 1.6 | . | ATTA | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| H12 | 10 | 7.9 | . | . | . | C | . | . | ACA | . | . | . | . | . | . | . | . | . | . | . |
| Pot2 | 6 | 4.8 | −275 insert Pot2 | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . | |
| Pot3 rev-A | 1 | 0.8 | −240 insert Pot3 | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . | |
| Pot3 rev-B | 2 | 1.6 | −240 insert Pot3 | . | C | . | . | . | . | . | . | . | . | . | C | . | . | . | . | |
| Pot3-A | 24 | 19.1 | −240 insert Pot3 | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . | |
| Pot3-B | 2 | 1.6 | −240 insert Pot3 | . | C | . | . | . | . | . | . | . | . | . | C | . | . | . | . | |
| Pot3-C | 1 | 0.8 | −240 insert Pot3 | . | C | . | . | . | . | . | . | . | . | . | . | . | . | C | . | |
| Haplotype | Variant Locus a | Disease Reaction b | |||||
|---|---|---|---|---|---|---|---|
| 46 | 47 | 49 | 50 | 53 | 54 | ||
| KM887844 | E | F | I | R | Y | F | |
| H01 | . | . | . | . | . | . | 24R + 5M + 4? |
| H02 | . | . | . | . | . | . | 3R + 1M |
| H03 | . | . | . | . | . | . | 4R |
| H04 | . | . | . | . | . | . | 1R |
| H05 | . | . | . | . | . | L | 7R + 1? |
| H06 | . | . | . | . | . | L | 1R |
| H07 | V | . | . | . | S | V | 3R |
| H08 | . | L | T | G | . | . | 1S |
| H09 | . | . | . | . | . | . | 11R + 2M |
| H10 | . | . | . | . | . | . | 9R + 1M |
| H11 | . | . | . | . | . | . | 2S |
| H12 | . | . | . | . | . | . | 9R + 1M |
| Pot2 | . | . | . | . | . | . | 7S |
| Pot3 rev-A c | . | . | . | . | . | . | 1S |
| Pot3 rev-B | . | . | . | . | . | L | 2S |
| Pot3-A | . | . | . | . | . | . | 22S + 2M |
| Pot3-B | . | . | . | . | . | . | 2S |
| Pot3-C | . | . | . | . | . | L | 1S |
| Haplotype | Regions | Production c | ||||||
|---|---|---|---|---|---|---|---|---|
| Central | Northeastern | Northwestern | Southwestern | Southeastern | Western | XI | GJ | |
| H01 | 11(47.8) a | 9(60.0) | 2(15.4) | 10(52.6) | 0 | 1(2.0) | 10(20.8) | 23(29.5) |
| H02 | 0 | 0 | 0 | 4(21.1) | 0 | 0 | 4(8.3) | 0 |
| H03 | 4(17.4) | 0 | 0 | 0 | 0 | 0 | 0 | 4(5.1) |
| H04 | 0 | 0 | 0 | 0 | 0 | 1(2.0) | 0 | 1(1.3) |
| H05 | 1(4.3) | 1(6.7) | 3(23.1) | 0 | 2(33.3) | 1(2.0) | 2(4.2) | 6(7.7) |
| H06 | 0 | 0 | 0 | 0 | 1(16.7) | 0 | 1(2.1) | 0 |
| H07 | 0 | 0 | 0 | 0 | 0 | 3(6.0) | 0 | 3(3.8) |
| H08 | 0 | 0 | 0 | 0 | 0 | 1(2.0) | 1(2.1) | 0 |
| H09 | 2(8.7) | 0 | 0 | 0 | 0 | 11(22.0) | 0 | 13(16.7) |
| H10 | 2(8.7) | 0 | 8(61.5) | 0 | 0 | 0 | 0 | 10(12.8) |
| H11 | 0 | 0 | 0 | 0 | 1(16.7) | 1(2.0) | 1(2.1) | 1(1.3) |
| H12 | 0 | 3(20.0) | 0 | 1(5.3) | 0 | 6(12.0) | 6(12.5) | 4(5.1) |
| Pot2 | 0 | 1(6.7) | 0 | 4(21.1) | 0 | 1(2.0) | 6(12.5) | 0 |
| Pot3 rev | 2(8.7) | 1(6.7) | 0 | 0 | 0 | 0 | 0 | 3(3.8) |
| Pot3 | 1(4.3) | 0 | 0 | 0 | 2(33.3) | 24(48.0) | 17 | 10(12.8) |
| Total | 23 | 15 | 13 | 19 | 6 | 50 | 48 | 78 |
| No. of haplotypes | 7 | 5 | 3 | 4 | 4 | 10 | 9 | 11 |
| Index of diversity b | 0.71 | 0.59 | 0.54 | 0.63 | 0.72 | 0.70 | 0.79 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Lu, L.; Li, C.; Wang, Q.; Shi, Z. Insertion of Transposable Elements in AVR-Pib of Magnaporthe oryzae Leading to LOSS of the Avirulent Function. Int. J. Mol. Sci. 2023, 24, 15542. https://doi.org/10.3390/ijms242115542
Li J, Lu L, Li C, Wang Q, Shi Z. Insertion of Transposable Elements in AVR-Pib of Magnaporthe oryzae Leading to LOSS of the Avirulent Function. International Journal of Molecular Sciences. 2023; 24(21):15542. https://doi.org/10.3390/ijms242115542
Chicago/Turabian StyleLi, Jinbin, Lin Lu, Chengyun Li, Qun Wang, and Zhufeng Shi. 2023. "Insertion of Transposable Elements in AVR-Pib of Magnaporthe oryzae Leading to LOSS of the Avirulent Function" International Journal of Molecular Sciences 24, no. 21: 15542. https://doi.org/10.3390/ijms242115542
APA StyleLi, J., Lu, L., Li, C., Wang, Q., & Shi, Z. (2023). Insertion of Transposable Elements in AVR-Pib of Magnaporthe oryzae Leading to LOSS of the Avirulent Function. International Journal of Molecular Sciences, 24(21), 15542. https://doi.org/10.3390/ijms242115542






