Hyaluronic Acid Conjugated with 17β-Estradiol Effectively Alleviates Estropause-Induced Cognitive Deficits in Rats
Abstract
1. Introduction
2. Results
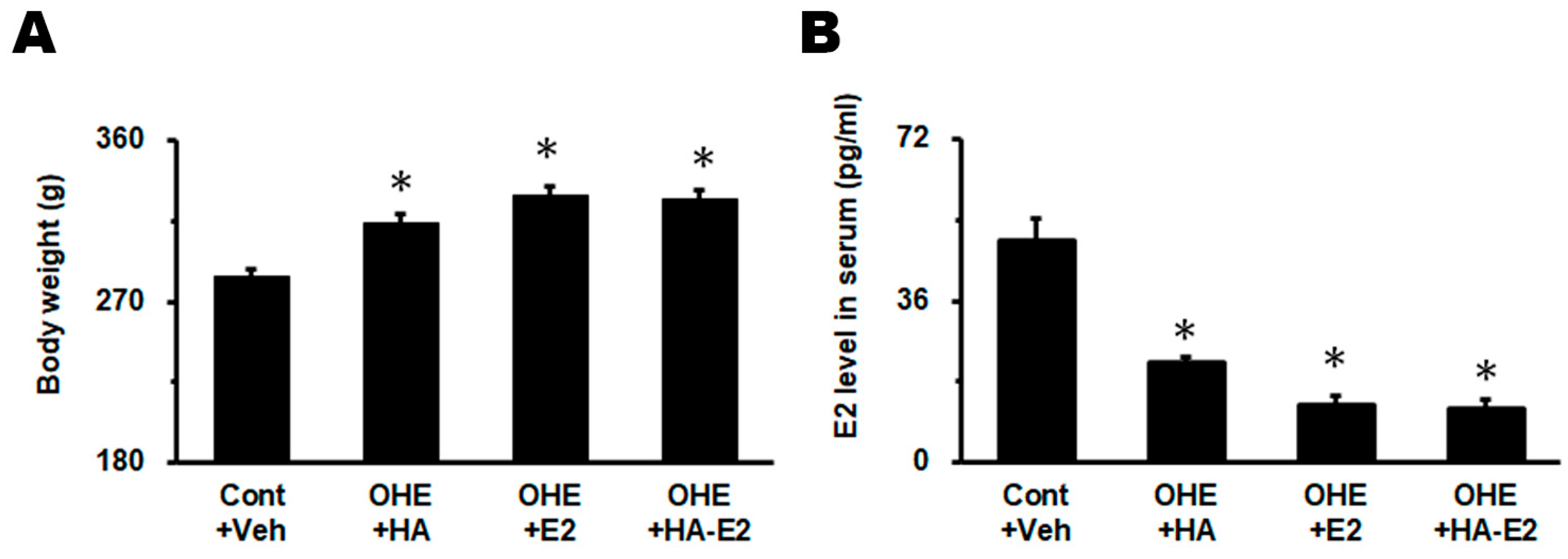
2.1. Effect of Exogenous HA-E2 Treatment on Animal Body Weights and Serum E2 Levels in OHE Rats
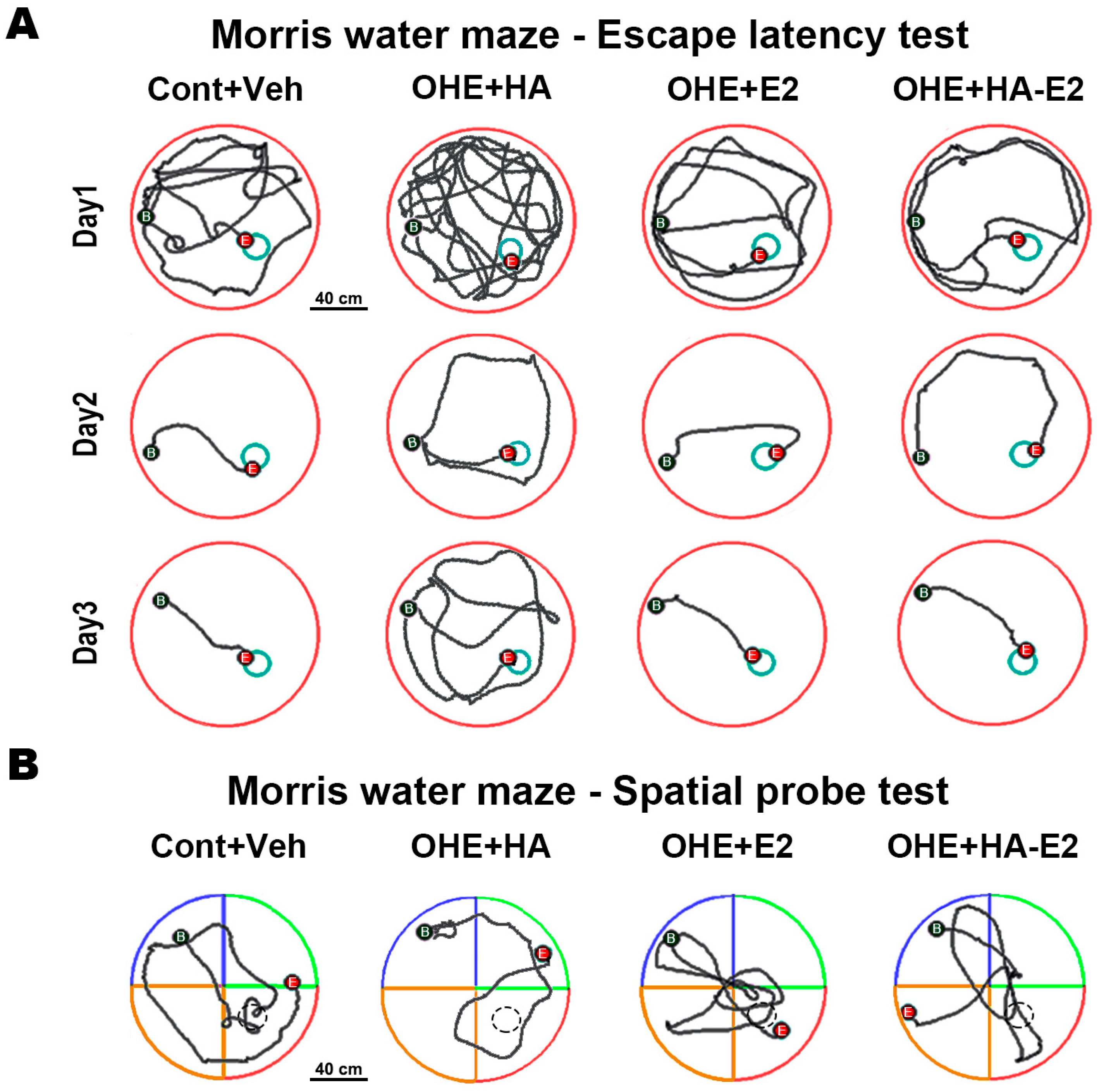
2.2. Exogenous HA-E2 Treatment for Spatial Learning and Memory in OHE Rats
2.2.1. Escape Latency Test
2.2.2. Spatial Probe Task
2.3. Exogenous HA-E2 Treatment on Cholinergic Septo-Hippocampal Innervation in OHE Rats
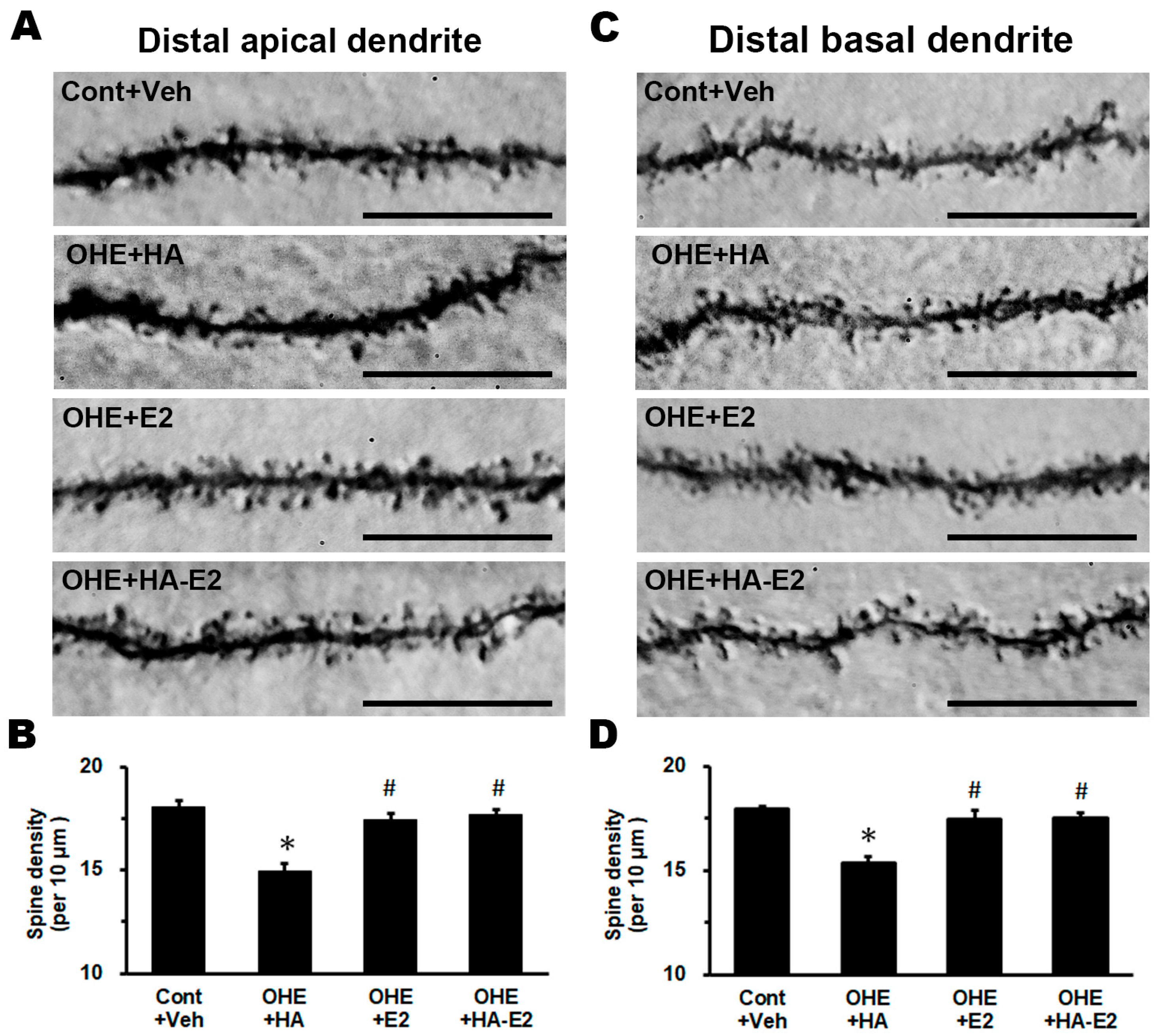
2.4. Exogenous HA-E2 Effect on Dendritic Spine of Hippocampal CA1 Pyramidal Neurons
3. Discussion
3.1. Exogenous E2 or HA-E2 Treatment Did Not Alter Serum E2 Level or Body Weight
3.2. Exogenous E2 or HA-E2 Treatment Modulated Cholinergic Innervation
3.3. Exogenous E2 or HA-E2 Treatment Repopulated the Spine Density of CA1 Pyramidal Neuron
3.4. Limitations of the Present Study
4. Materials and Methods
4.1. Animals
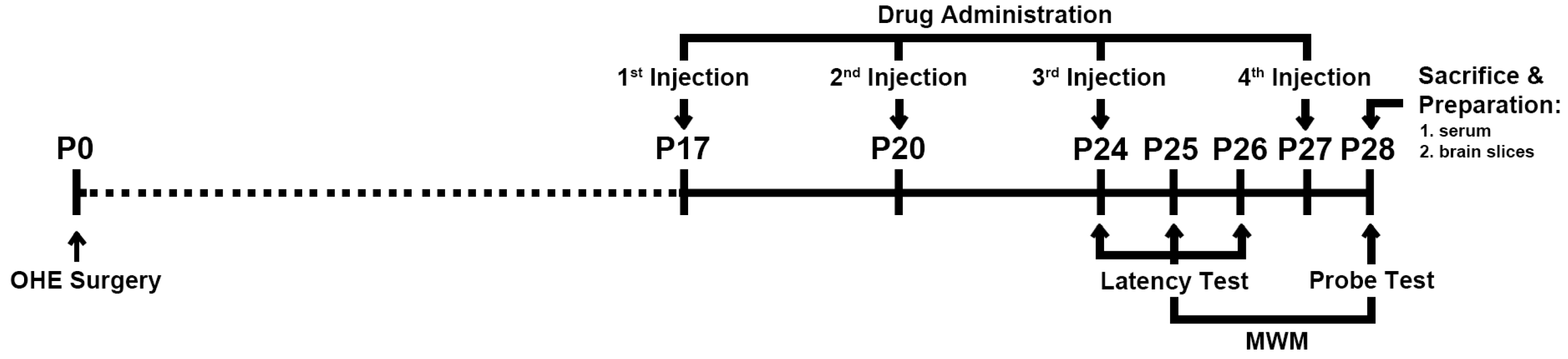
4.2. Experiment Schedule
4.3. Drug Administration
4.4. MWM Task
4.4.1. Escape Latency Test
4.4.2. Spatial Probe Test
4.5. Sacrifice and Preparation of Serum and Brain Tissues
4.6. IHC Staining
4.7. Intracellular Dye Injection and Immunoconversion
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Choi, H.J.; Lee, A.J.; Kang, K.S.; Song, J.H.; Zhu, B.T. 4-hydroxyestrone, an endogenous estrogen metabolite, can strongly protect neuronal cells against oxidative damage. Sci. Rep. 2020, 10, 7283. [Google Scholar] [CrossRef]
- Chen, J.R.; Lim, S.H.; Chung, S.C.; Lee, Y.F.; Wang, Y.J.; Tseng, G.F.; Wang, T.J. Reproductive experience modified dendritic spines on cortical pyramidal neurons to enhance sensory perception and spatial learning in rats. Exp. Anim. 2017, 66, 61–74. [Google Scholar] [CrossRef]
- Wang, T.J.; Chen, J.R.; Wang, W.J.; Wang, Y.J.; Tseng, G.F. Genistein partly eases aging and estropause-induced primary cortical neuronal changes in rats. PLoS ONE 2014, 9, e89819. [Google Scholar] [CrossRef]
- Chen, J.R.; Yan, Y.T.; Wang, T.J.; Chen, L.J.; Wang, Y.J.; Tseng, G.F. Gonadal hormones modulate the dendritic spine densities of primary cortical pyramidal neurons in adult female rat. Cereb. Cortex 2009, 19, 2719–2727. [Google Scholar] [CrossRef]
- Jett, S.; Malviya, N.; Schelbaum, E.; Jang, G.; Jahan, E.; Clancy, K.; Hristov, H.; Pahlajani, S.; Niotis, K.; Loeb-Zeitlin, S.; et al. Endogenous and exogenous estrogen exposures: How women’s reproductive health can drive brain aging and inform alzheimer’s prevention. Front. Aging Neurosci. 2022, 14, 831807. [Google Scholar] [CrossRef]
- Viña, J.; Lloret, A. Why women have more Alzheimer’s disease than men: Gender and mitochondrial toxicity of amyloid-beta peptide. J. Alzheimers Dis. 2010, 20 (Suppl. S2), S527–S533. [Google Scholar] [CrossRef]
- Casadesus, G.; Rolston, R.K.; Webber, K.M.; Atwood, C.S.; Bowen, R.L.; Perry, G.; Smith, M.A. Menopause, estrogen, and gonadotropins in Alzheimer’s disease. Adv. Clin. Chem. 2008, 45, 139–153. [Google Scholar] [CrossRef]
- Bove, R.; Secor, E.; Chibnik, L.B.; Barnes, L.L.; Schneider, J.A.; Bennett, D.A.; De Jager, P.L. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology 2014, 82, 222–229. [Google Scholar] [CrossRef]
- Henderson, V.W.; Benke, K.S.; Green, R.C.; Cupples, L.A.; Farrer, L.A. Postmenopausal hormone therapy and Alzheimer’s disease risk: Interaction with age. J. Neurol. Neurosurg. Psychiatry 2005, 76, 103–105. [Google Scholar] [CrossRef]
- Shao, H.; Breitner, J.C.; Whitmer, R.A.; Wang, J.; Hayden, K.; Wengreen, H.; Corcoran, C.; Tschanz, J.; Norton, M.; Munger, R.; et al. Hormone therapy and Alzheimer disease dementia: New findings from the Cache County Study. Neurology 2012, 79, 1846–1852. [Google Scholar] [CrossRef]
- Kim, Y.J.; Soto, M.; Branigan, G.L.; Rodgers, K.; Brinton, R.D. Association between menopausal hormone therapy and risk of neurodegenerative diseases: Implications for precision hormone therapy. Alzheimers Dement. 2021, 7, e12174. [Google Scholar] [CrossRef]
- Dalal, P.K.; Agarwal, M. Postmenopausal syndrome. Indian J. Psychiatry 2015, 57, S222–S232. [Google Scholar] [CrossRef]
- Lisman, J.; Buzsáki, G.; Eichenbaum, H.; Nadel, L.; Ranganath, C.; Redish, A.D. Viewpoints: How the hippocampus contributes to memory, navigation and cognition. Nat. Neurosci. 2017, 20, 1434–1447. [Google Scholar] [CrossRef]
- Bekkers, J.M. Pyramidal neurons. Curr. Biol. 2011, 21, R975. [Google Scholar] [CrossRef]
- Moser, M.B.; Trommald, M.; Andersen, P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial-learning in adult-rats suggests the formation of new synapses. Proc. Natl. Acad. Sci. USA 1994, 91, 12673–12675. [Google Scholar] [CrossRef]
- Frankfurt, M.; Luine, V. The evolving role of dendritic spines and memory: Interaction(s) with estradiol. Horm. Behav. 2015, 74, 28–36. [Google Scholar] [CrossRef]
- Chen, M.H.; Hong, C.L.; Wang, Y.T.; Wang, T.J.; Chen, J.R. The effect of astaxanthin treatment on the rat model of fetal alcohol spectrum disorders (FASD). Brain Res. Bull. 2022, 183, 57–72. [Google Scholar] [CrossRef]
- Chen, M.H.; Wang, T.J.; Chen, L.J.; Jiang, M.Y.; Wang, Y.J.; Tseng, G.F.; Chen, J.R. The effects of astaxanthin treatment on a rat model of Alzheimer’s disease. Brain Res. Bull. 2021, 172, 151–163. [Google Scholar] [CrossRef]
- Chen, J.R.; Tseng, G.F.; Wang, Y.J.; Wang, T.J. Exogenous dehydroisoandrosterone sulfate reverses the dendritic changes of the central neurons in aging male rats. Exp. Gerontol. 2014, 57, 191–202. [Google Scholar] [CrossRef]
- Chen, J.R.; Wang, T.J.; Lim, S.H.; Wang, Y.J.; Tseng, G.F. Testosterone modulation of dendritic spines of somatosensory cortical pyramidal neurons. Brain Struct. Funct. 2013, 218, 1407–1417. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef]
- Haam, J.; Yakel, J.L. Cholinergic modulation of the hippocampal region and memory function. J. Neurochem. 2017, 142 (Suppl. S2), 111–121. [Google Scholar] [CrossRef] [PubMed]
- Teles-Grilo Ruivo, L.M.; Mellor, J.R. Cholinergic modulation of hippocampal network function. Front. Synaptic Neurosci. 2013, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, P.; Dumas, J. Estrogen-cholinergic interactions: Implications for cognitive aging. Horm. Behav. 2015, 74, 173–185. [Google Scholar] [CrossRef]
- Luine, V.N.; Renner, K.J.; McEwen, B.S. Sex-dependent differences in estrogen regulation of choline acetyltransferase are altered by neonatal treatments. Endocrinology 1986, 119, 874–878. [Google Scholar] [CrossRef]
- Vincze, B.; Kapuvári, B.; Udvarhelyi, N.; Horváth, Z.; Mátrai, Z.; Czeyda-Pommersheim, F.; Kőhalmy, K.; Kovács, J.; Boldizsár, M.; Láng, I.; et al. Serum estrone concentration, estrone sulfate/estrone ratio and BMI are associated with human epidermal growth factor receptor 2 and progesterone receptor status in postmenopausal primary breast cancer patients suffering invasive ductal carcinoma. Springerplus 2015, 4, 387. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Halake, K.; Lee, J. Functional hyaluronic acid conjugates based on natural polyphenols exhibit antioxidant, adhesive, gelation, and self-healing properties. J. Ind. Eng. Chem. 2017, 54, 44–51. [Google Scholar] [CrossRef]
- Schmidt, M.; Kimmig, R. The benefits and risks of hormonal replacement therapy—An update. Gynakol. Geburtshilfliche Rundsch. 2006, 46, 166–173. [Google Scholar] [CrossRef]
- Dubey, R.K.; Imthurn, B.; Zacharia, L.C.; Jackson, E.K. Hormone replacement therapy and cardiovascular disease. Hypertension 2004, 44, 789–795. [Google Scholar] [CrossRef]
- Peters, A.; Sherman, L.S. Diverse roles for hyaluronan and hyaluronan receptors in the developing and adult nervous system. Int. J. Mol. Sci. 2020, 21, 5988. [Google Scholar] [CrossRef]
- Curcio, M.; Cirillo, G.; Rouaen, J.R.C.; Saletta, F.; Nicoletta, F.P.; Vittorio, O.; Iemma, F. Natural polysaccharide carriers in brain delivery: Challenge and perspective. Pharmaceutics 2020, 12, 1183. [Google Scholar] [CrossRef] [PubMed]
- Patriarca, M.T.; Barbosa de Moraes, A.R.; Nader, H.B.; Petri, V.; Martins, J.R.; Gomes, R.C.; Soares, J.M., Jr. Hyaluronic acid concentration in postmenopausal facial skin after topical estradiol and genistein treatment: A double-blind, randomized clinical trial of efficacy. Menopause 2013, 20, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Wronski, T.J.; Schenck, P.A.; Cintrón, M.; Walsh, C.C. Effect of body weight on osteopenia in ovariectomized rats. Calcif. Tissue Int. 1987, 40, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.J.; Choi, Y.; Park, D. Improvement of cognitive function in ovariectomized rats by human neural stem cells overexpressing choline acetyltransferase via secretion of NGF and BDNF. Int. J. Mol. Sci. 2022, 23, 5560. [Google Scholar] [CrossRef]
- Singh, M.; Meyer, E.M.; Millard, W.J.; Simpkins, J.W. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994, 644, 305–312. [Google Scholar] [CrossRef]
- Singh, M.; Meyer, E.M.; Simpkins, J.W. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology 1995, 136, 2320–2324. [Google Scholar] [CrossRef]
- Shughrue, P.J.; Scrimo, P.J.; Merchenthaler, I. Estrogen binding and estrogen receptor characterization (ERalpha and ERbeta) in the cholinergic neurons of the rat basal forebrain. Neuroscience 2000, 96, 41–49. [Google Scholar] [CrossRef]
- Solum, D.T.; Handa, R.J. Localization of estrogen receptor alpha (ER alpha) in pyramidal neurons of the developing rat hippocampus. Brain Res. Dev. Brain Res. 2001, 128, 165–175. [Google Scholar] [CrossRef]
- Gibbs, R.B.; Nelson, D.; Hammond, R. Role of GPR30 in mediating estradiol effects on acetylcholine release in the hippocampus. Horm. Behav. 2014, 66, 339–345. [Google Scholar] [CrossRef]
- Hammond, R.; Nelson, D.; Gibbs, R.B. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology 2011, 36, 182–192. [Google Scholar] [CrossRef]
- Kanju, P.M.; Parameshwaran, K.; Sims-Robinson, C.; Uthayathas, S.; Josephson, E.M.; Rajakumar, N.; Dhanasekaran, M.; Suppiramaniam, V. Selective cholinergic depletion in medial septum leads to impaired long term potentiation and glutamatergic synaptic currents in the hippocampus. PLoS ONE 2012, 7, e31073. [Google Scholar] [CrossRef]
- Robertson, R.T.; Gallardo, K.A.; Claytor, K.J.; Ha, D.H.; Ku, K.H.; Yu, B.P.; Lauterborn, J.C.; Wiley, R.G.; Yu, J.; Gall, C.M.; et al. Neonatal treatment with 192 IgG-saporin produces long-term forebrain cholinergic deficits and reduces dendritic branching and spine density of neocortical pyramidal neurons. Cereb. Cortex 1998, 8, 142–155. [Google Scholar] [CrossRef]
- Fréchette, M.; Rennie, K.; Pappas, B.A. Developmental forebrain cholinergic lesion and environmental enrichment: Behaviour, CA1 cytoarchitecture and neurogenesis. Brain Res. 2009, 1252, 172–182. [Google Scholar] [CrossRef]
- Bora, S.H.; Liu, Z.; Kecojevic, A.; Merchenthaler, I.; Koliatsos, V.E. Direct, complex effects of estrogens on basal forebrain cholinergic neurons. Exp. Neurol. 2005, 194, 506–522. [Google Scholar] [CrossRef] [PubMed]
- Abrahám, I.M.; Koszegi, Z.; Tolod-Kemp, E.; Szego, E.M. Action of estrogen on survival of basal forebrain cholinergic neurons: Promoting amelioration. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S104–S112. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.; Akama, K.; Alves, S.; Brake, W.G.; Bulloch, K.; Lee, S.; Li, C.; Yuen, G.; Milner, T.A. Tracking the estrogen receptor in neurons: Implications for estrogen-induced synapse formation. Proc. Natl. Acad. Sci. USA 2001, 98, 7093–7100. [Google Scholar] [CrossRef]
- Matsuda, K.; Sakamoto, H.; Mori, H.; Hosokawa, K.; Kawamura, A.; Itose, M.; Nishi, M.; Prossnitz, E.R.; Kawata, M. Expression and intracellular distribution of the G protein-coupled receptor 30 in rat hippocampal formation. Neurosci. Lett. 2008, 441, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.D.; Sharma, K.; Nyakas, C.; Vij, U. Estrogen receptor alpha and beta immunoreactive neurons in normal adult and aged female rat hippocampus: A qualitative and quantitative study. Brain Res. 2005, 1056, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.M.; Fink, S.E.; Shah, R.A.; Janssen, W.G.; Hayashi, S.; Milner, T.A.; McEwen, B.S.; Morrison, J.H. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J. Neurosci. 2002, 22, 3608–3614. [Google Scholar] [CrossRef]
- Bohacek, J.; Daniel, J.M. The ability of oestradiol administration to regulate protein levels of oestrogen receptor alpha in the hippocampus and prefrontal cortex of middle-aged rats is altered following long-term ovarian hormone deprivation. J. Neuroendocrinol. 2009, 21, 640–647. [Google Scholar] [CrossRef]
- Cersosimo, M.G.; Benarroch, E.E. Estrogen actions in the nervous system: Complexity and clinical implications. Neurology 2015, 85, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Sargin, D.; Mercaldo, V.; Yiu, A.; Higgs, G.; Han, J.-H.; Frankland, P.; Josselyn, S. CREB regulates spine density of lateral amygdala neurons: Iimplications for memory allocation. Front. Behav. Neurosci. 2013, 7, 209. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Cohen, R.S.; Pandey, S.C. Effects of estrogen treatment on expression of brain-derived neurotrophic factor and cAMP response element-binding protein expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendocrinology 2005, 81, 294–310. [Google Scholar] [CrossRef]
- Galasso, C.; Orefice, I.; Pellone, P.; Cirino, P.; Miele, R.; Ianora, A.; Brunet, C.; Sansone, C. On the Neuroprotective role of astaxanthin: New perspectives? Mar. Drugs 2018, 16, 247. [Google Scholar] [CrossRef]
- Lian, W.W.; Zhou, W.; Zhang, B.Y.; Jia, H.; Xu, L.-j.; Liu, A.-l.; Du, G.-h. DL0410 ameliorates cognitive disorder in SAMP8 mice by promoting mitochondrial dynamics and the NMDAR-CREB-BDNF pathway. Acta. Pharmacol. Sin. 2021, 42, 1055–1068. [Google Scholar] [CrossRef]
- Kochlamazashvili, G.; Henneberger, C.; Bukalo, O.; Dvoretskova, E.; Senkov, O.; Lievens, P.M.; Westenbroek, R.; Engel, A.K.; Catterall, W.A.; Rusakov, D.A.; et al. The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic L-type Ca2+ channels. Neuron 2010, 67, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, D.; Ren, Y.; Guo, S.; Li, J.; Ma, S.; Yao, M.; Guan, F. Injectable hyaluronic acid hydrogel loaded with BMSC and NGF for traumatic brain injury treatment. Mater. Today Bio. 2022, 13, 100201. [Google Scholar] [CrossRef]
- Djoudi, A.; Molina-Peña, R.; Ferreira, N.; Ottonelli, I.; Tosi, G.; Garcion, E.; Boury, F. Hyaluronic acid scaffolds for loco-regional therapy in nervous system related disorders. Int. J. Mol. Sci. 2022, 23, 12174. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.J.; Nguyen, C.; Chun, H.N.; Llorente, I.L.; Chiu, A.S.; Machnicki, M.; Zarembinski, T.I.; Carmichael, S.T. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J. Cereb. Blood Flow Metab. 2017, 37, 1030–1045. [Google Scholar] [CrossRef]
- Sherren, N.; Pappas, B.A. Selective acetylcholine and dopamine lesions in neonatal rats produce distinct patterns of cortical dendritic atrophy in adulthood. Neuroscience 2005, 136, 445–456. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Begum, T.; Reza, F. Hormonal Influences on Cognitive Function. Malays. J. Med. Sci. 2018, 25, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Siiteri, P.K.; Murai, J.T.; Hammond, G.L.; Nisker, J.A.; Raymoure, W.J.; Kuhn, R.W. The serum transport of steroid hormones. Recent Prog. Horm. Res. 1982, 38, 457–510. [Google Scholar] [CrossRef] [PubMed]



| Groups | ||||
|---|---|---|---|---|
| Con + Veh | OHE + HA | OHE + E2 | OHE + HA-E2 | |
| Animal numbers | 17 | 11 | 23 | 24 |
| Escape latency (seconds) | ||||
| Day1 (p = 0.172) | 62.9 (IQR = 41.4–77.5) | 60.1 (IQR = 25.3–78.6) | 40.5 (IQR = 22.3–73.9) | 34.0 (IQR = 20.2–58.6) |
| Day2 (p = 0.210) | 8.7 (IQR = 5.2–15.3) | 9.4 (IQR = 7.2–22.9) | 16.5 (IQR = 6.0–22.2) | 9.6 (IQR = 6.2–27.1) |
| Day3 (p = 0.007) | 6.6 (IQR = 5.0–10.1) | 11.6 (IQR = 10.9–21.9) * | 7.7 (IQR = 5.1–10.8) | 6.0 (IQR = 4.4–9.4) # |
| Swimming distance (meters) | ||||
| Day1 (p = 0.170) | 11.6 (IQR = 8.5–14.0) | 11.5 (IQR = 4.9–16.2) | 8.2 (IQR = 4.4–13.4) | 6.8 (IQR = 4.4–11.7) |
| Day2 (p = 0.257) | 1.6 (IQR = 1.0–2.5) | 2.4 (IQR = 1.4–5.5) | 3.7 (IQR = 1.2–4.9) | 2.0 (IQR = 1.3–5.4) |
| Day3 (p = 0.004) | 1.2 (IQR = 0.9–1.7) | 2.9 (IQR = 2.0–4.1) * | 1.5 (IQR = 1.0–2.4) # | 1.2 (IQR = 0.9–1.7) # |
| Swimming speed (cm/s) | ||||
| Day1 (p = 0.811) | 19.2 (IQR = 16.8–21.2) | 19.6 (IQR = 18.9–21.7) | 20.0 (IQR = 17.9–21.1) | 19.4 (IQR = 17.7–21.9) |
| Day2 (p = 0.711) | 21.9 (IQR = 18.5–23.9) | 22.2 (IQR = 19.3–25.4) | 20.0 (IQR = 18.2–24.3) | 21.0 (IQR = 21.0–22.9) |
| Day3 (p = 0.728) | 19.2 (IQR = 16.8–23.2) | 20.4 (IQR = 18.5–23.0) | 20.9 (IQR = 18.3–24.5) | 19.3 (IQR = 17.4–23.6) |
| Groups | ||||
|---|---|---|---|---|
| Con + Veh | OHE + HA | OHE + E2 | OHE + HA-E2 | |
| Animal numbers | 17 | 11 | 23 | 24 |
| Swimming distance in the target quadrant | ||||
| Value (meter) (p = 0.011) | 1.8 (IQR = 1.6–2.3) | 1.3 (IQR = 0.8–1.4) * | 1.9 (IQR = 1.3–2.4) # | 1.9 (IQR = 1.4–2.3) # |
| Ratio (%) (p = 0.006) | 31.1 (IQR = 28.3–41.4) | 23.8 (IQR = 17.0–25.9) * | 31.3 (IQR = 21.1–39.8) # | 32.3 (IQR = 24.0–39.4) # |
| Swimming time in the target quadrant | ||||
| Value (second) (p = 0.002) | 9.4 (IQR = 8.0–13.1) | 5.6 (IQR = 3.9–8.0) * | 9.6 (IQR = 6.4–13.5) # | 10.1 (IQR = 7.2–12.9) # |
| Ratio (%) (p = 0.002) | 31.2 (IQR = 26.7–43.6) | 18.7 (IQR = 13.1–26.8) * | 31.9 (IQR = 21.3–45.0) # | 33.7 (IQR = 24.1–43.1) # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.-H.; Lin, H.-C.; Chao, T.; Lee, V.S.-Y.; Hou, C.-L.; Wang, T.-J.; Chen, J.-R. Hyaluronic Acid Conjugated with 17β-Estradiol Effectively Alleviates Estropause-Induced Cognitive Deficits in Rats. Int. J. Mol. Sci. 2023, 24, 15569. https://doi.org/10.3390/ijms242115569
Chen M-H, Lin H-C, Chao T, Lee VS-Y, Hou C-L, Wang T-J, Chen J-R. Hyaluronic Acid Conjugated with 17β-Estradiol Effectively Alleviates Estropause-Induced Cognitive Deficits in Rats. International Journal of Molecular Sciences. 2023; 24(21):15569. https://doi.org/10.3390/ijms242115569
Chicago/Turabian StyleChen, Mu-Hsuan, Hsiao-Chun Lin, Tzu Chao, Viola Szu-Yuan Lee, Chia-Lung Hou, Tsyr-Jiuan Wang, and Jeng-Rung Chen. 2023. "Hyaluronic Acid Conjugated with 17β-Estradiol Effectively Alleviates Estropause-Induced Cognitive Deficits in Rats" International Journal of Molecular Sciences 24, no. 21: 15569. https://doi.org/10.3390/ijms242115569
APA StyleChen, M.-H., Lin, H.-C., Chao, T., Lee, V. S.-Y., Hou, C.-L., Wang, T.-J., & Chen, J.-R. (2023). Hyaluronic Acid Conjugated with 17β-Estradiol Effectively Alleviates Estropause-Induced Cognitive Deficits in Rats. International Journal of Molecular Sciences, 24(21), 15569. https://doi.org/10.3390/ijms242115569






