Novel Substituted Azoloazines with Anticoagulant Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biology
2.2.1. Anticoagulant Activity of the Target Compounds In Vitro
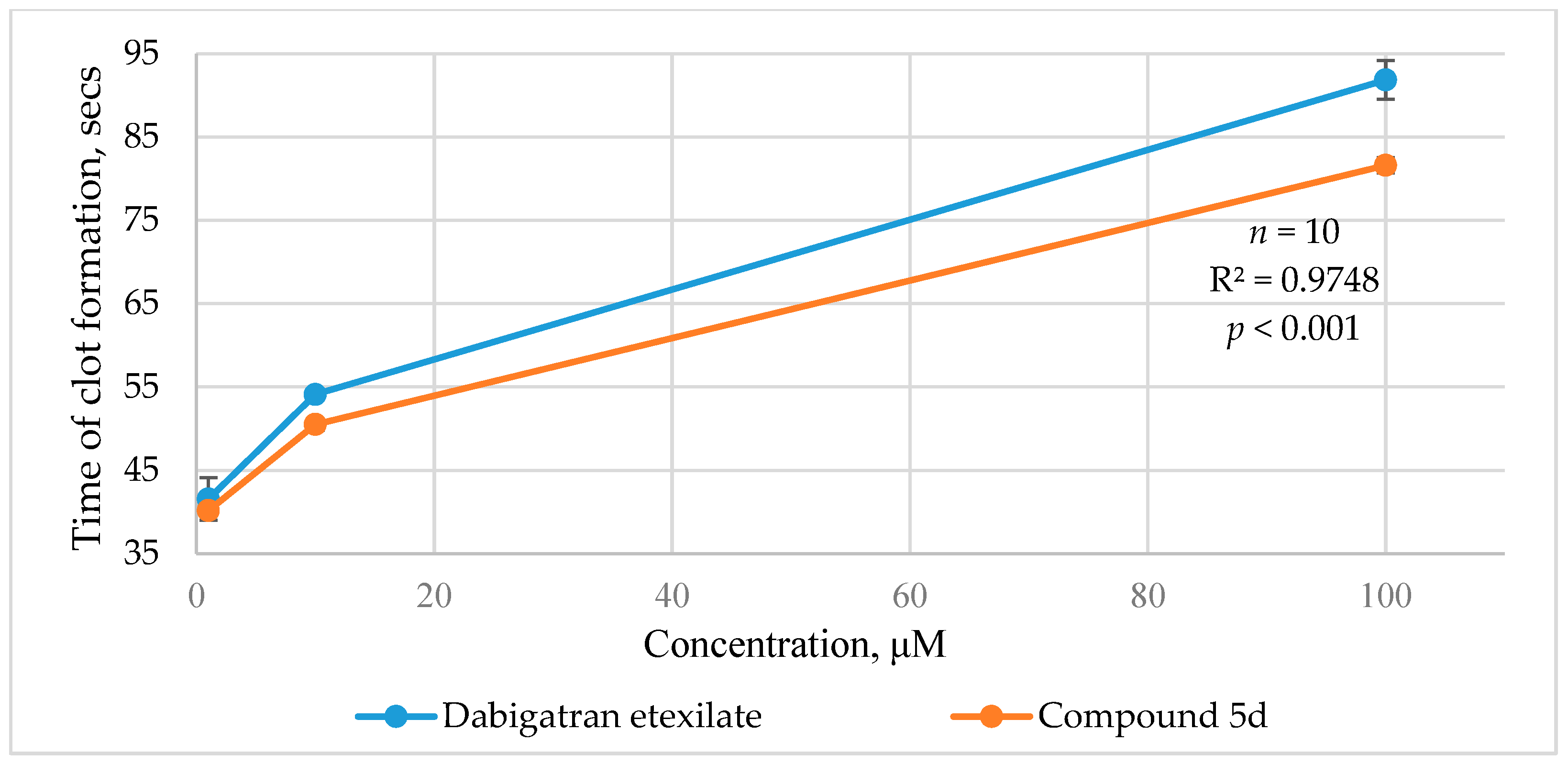
2.2.2. Ecarin Clotting Time
2.2.3. An Animal Study of Anticoagulant Activity
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Preparation of Nitro Derivatives of Azolo[1,5-a]pyrimidines 4c,d (Scheme 2)
3.1.2. General Procedure for the Preparation of Di(het)aryl-Substituted Azolo[1,5-a]pyrimidines 7a,b (Scheme 2)
3.1.3. General Procedure for the Preparation of 7-Aryl-5-methyl-4,7-dihydro-[1,2,4]triazolo[1,5-a]pyrimidine-6-carboxylates 10a,b (Scheme 2)
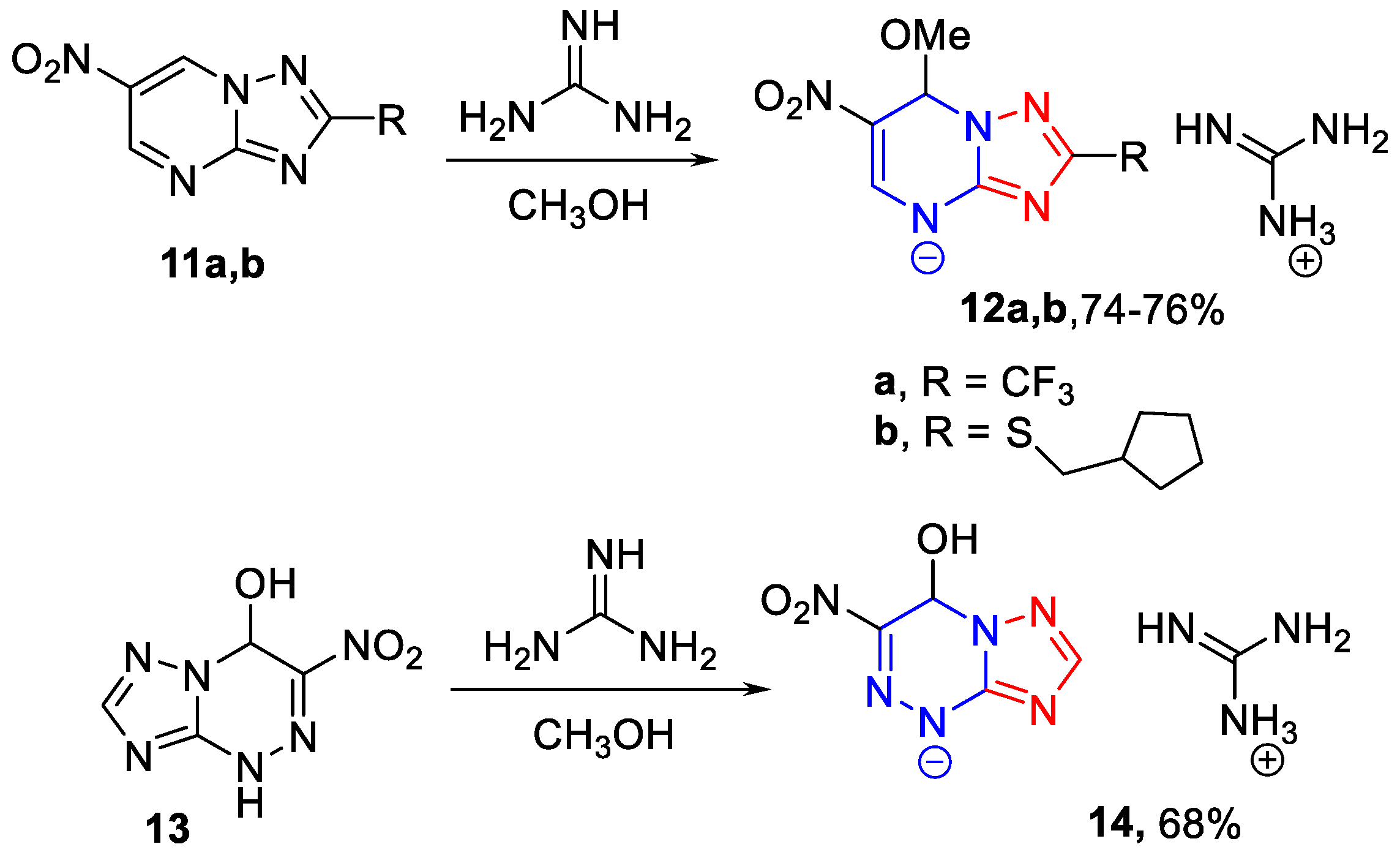
3.1.4. The General Synthesis of 6-Nitro-7-methoxy-4,7-dihydro-azolyl-4 guanidates 12a,b, 14 (Scheme 3)
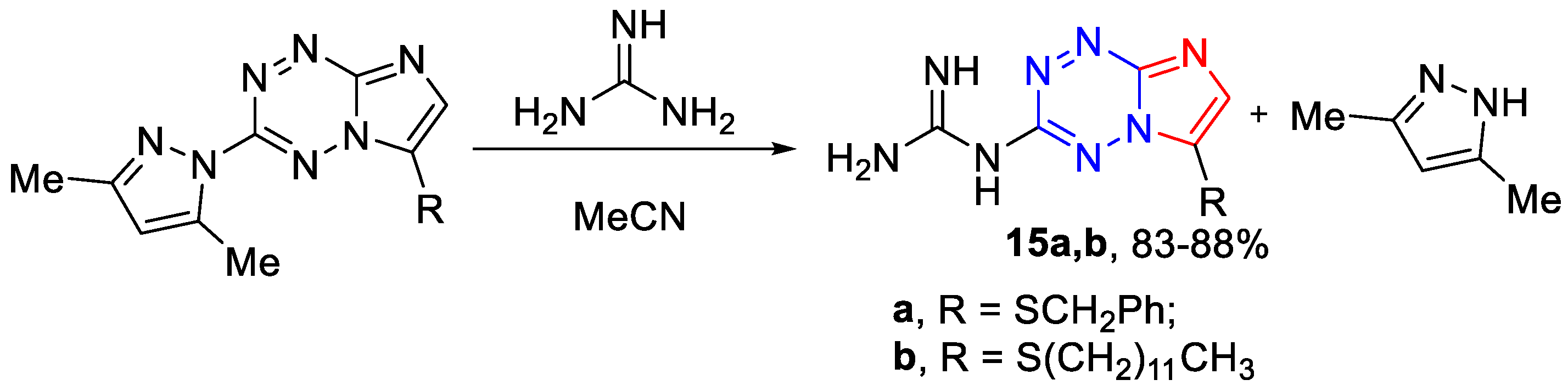
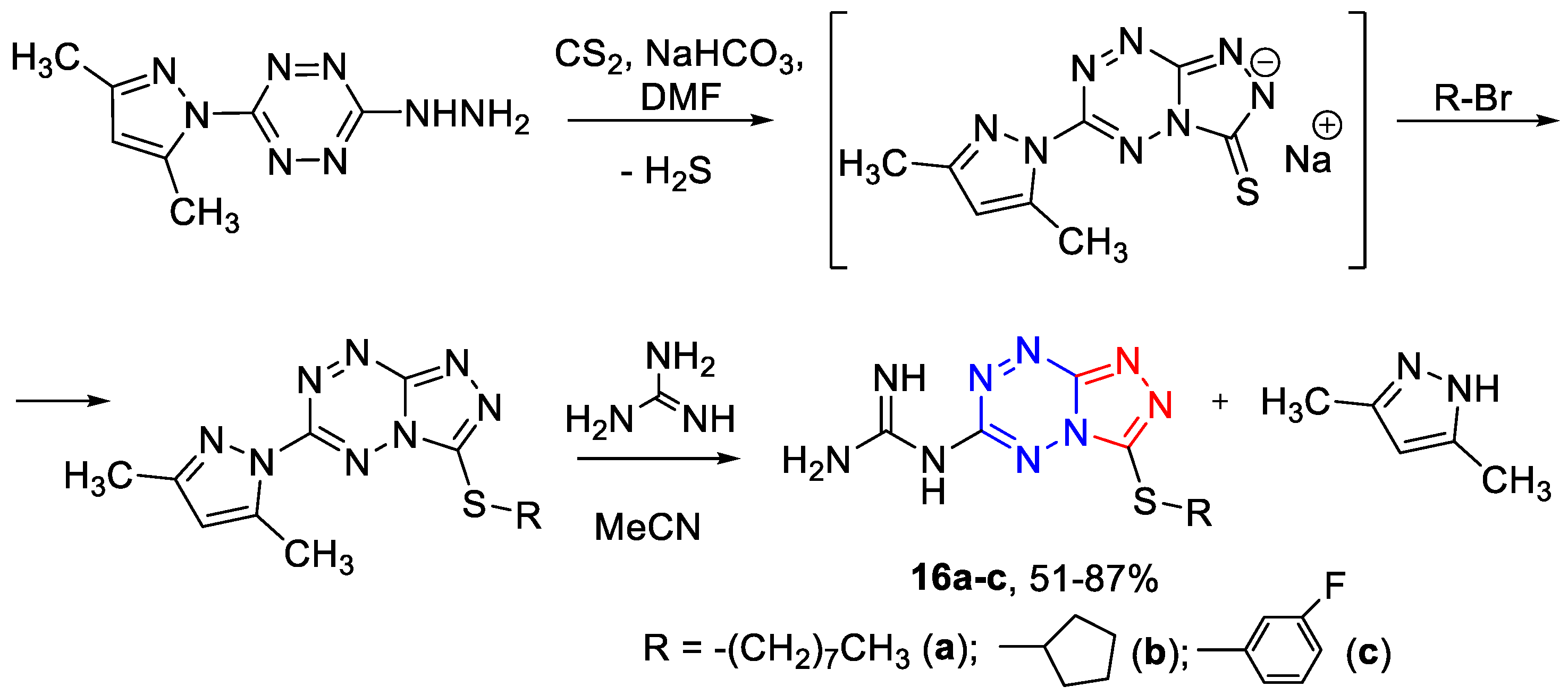
3.1.5. The General Synthetic Method of 6-Guanidino-3-alkylthio-[1,2,4]triazolo[4,3-b][1,2,4,5]tetrazines 16a–c (Scheme 5)
3.2. Biology
3.2.1. Animals
3.2.2. In Vitro Anticoagulant Assay
3.2.3. Ecarin Clotting Time
3.2.4. Anticoagulant Assay in Animals
3.2.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Testa, S.; Prandoni, P.; Paoletti, O.; Morandini, R.; Tala, M.; Dellanoce, C.; Giorgi-Pierfranceschi, M.; Betti, M.; Danzi, G.B.; Pan, A.; et al. Direct Oral Anticoagulant Plasma Levels’ Striking Increase in Severe COVID-19 Respiratory Syndrome Patients Treated with Antiviral Agents: The Cremona Experience. J. Thromb. Haemost. 2020, 18, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, S.H.; Thimmulappa, R.K. Antiviral, immunomodulatory, and anticoagulant effects of quercetin and its derivatives: Potential role in prevention and management of COVID-19. J. Pharm. Anal. 2022, 12, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Gupta, R.; Ghosh, A.; Misra, A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.; Franke, S.; Rüster, C.; Wolf, G. Advanced glycation end-products and the kidney. Eur. J. Clin. Investig. 2010, 40, 742–755. [Google Scholar] [CrossRef]
- Pinheiro, S.; Pinheiro, E.M.C.; Muri, E.M.F.; Pessôa, J.C.; Cadorini, M.A.; Greco, S.J. Biological Activities of [1,2,4]triazolo[1,5-a]pyrimidines and Analogs. Med. Chem. Res. 2020, 29, 1751–1776. [Google Scholar] [CrossRef]
- Gurevich, K.G.; Urakov, A.L.; Rozit, G.A.; Klen, E.É.; Samorodov, A.V.; Khaliullin, F.A. Synthesis and Antiplatelet and Anticoagulant Activity of Thietane-Containing 2-(5-Bromo-2,4-Dihydro-3-Oxo-1,2,4-Triazolyl-4)Acetate Salts. Pharm. Chem. J. 2021, 55, 417–422. [Google Scholar] [CrossRef]
- Muhammad, Z.A.; Farghaly, T.A.; Althagafi, I.; Al-Hussain, S.A.; Zaki, M.E.A.; Harras, M.F. Synthesis of antimicrobial azoloazines and molecular docking for inhibiting COVID-19. J. Heterocycl. Chem. 2021, 58, 1286–1301. [Google Scholar] [CrossRef]
- Rusinov, V.L.; Sapozhnikova, I.M.; Spasov, A.A.; Chupakhin, O.N. Fused azoloazines with antidiabetic activity. Russ. Chem. Bull. 2022, 71, 2561–2594. [Google Scholar] [CrossRef]
- Ilin, I.; Lipets, E.; Sulimov, A.; Kutov, D.; Shikhaliev, K.; Potapov, A.; Krysin, M.; Zubkov, F.; Sapronova, L.; Ataullakhanov, F.; et al. New factor Xa inhibitors based on 1,2,3,4-tetrahydroquinoline developed by molecular modelling. J. Mol. Graph. Model. 2019, 89, 215–224. [Google Scholar] [CrossRef]
- Savateev, K.V.; Ulomsky, E.N.; Fedotov, V.V.; Rusinov, V.L.; Sivak, K.V.; Lyubishin, M.M.; Kuzmich, N.N.; Aleksandrov, A.G. 6-Nitrotriazolo[1,5-a]pyrimidines as Promising Structures for Pharmacotherapy of Septic Conditions. Russ. J. Bioorg. Chem. 2017, 43, 421–428. [Google Scholar] [CrossRef]
- Esteban-Parra, G.M.; Sebastián, E.S.; Cepeda, J.; Sánchez-González, C.; Rivas-García, L.; Llopis, J.; Aranda, P.; Sánchez-Moreno, M.; Quirós, M.; Rodríguez-Diéguez, A. Anti-diabetic and anti-parasitic properties of a family of luminescent zinc coordination compounds based on the 7-amino-5-methyl-1,2,4-triazolo[1,5-a]pyrimidine ligand. J. Inorg. Biochem. 2020, 212, 111235. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.S.; Huang, X.; Chen, L.Z.; Liu, M.M.; Shi, J.B. Design and synthesis of novel pyrazolo[4,3-d]pyrimidines as potential therapeutic agents for acute lung injury. J. Enzym. Inhib. Med. Chem. 2019, 34, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Ishmetova, R.I.; Ignatenko, N.K.; Korotina, A.V.; Ganebnykh, I.N.; Slepukhin, P.A.; Babkova, V.A.; Gerasimova, N.A.; Evstigneeva, N.P.; Zilberberg, N.V.; Kungurov, N.V.; et al. Synthesis and biological activity of 3-guanidino-6-R-imidazo[1,2-b]- and 6-guanidino-3-R-[1,2,4]triazolo[4,3-b][1,2,4,5]tetrazines. Russ. Chem. Bull. 2018, 67, 2079–2087. [Google Scholar] [CrossRef]
- Klimenko, K.; Lyakhov, S.; Shibinskaya, M.; Karpenko, A.; Marcou, G.; Horvath, D.; Zenkova, M.; Goncharova, E.; Amirkhanov, R.; Krysko, A.; et al. Virtual screening, synthesis and biological evaluation of DNA intercalating antiviral agents. Bioorg. Med. Chem. Lett. 2017, 27, 3915–3919. [Google Scholar] [CrossRef]
- Kunjiappan, S.; Theivendren, S.; Pavadai, P.; Govindaraj, S.; Sankaranarayanan, M.; Somasundaram, B.; Arunachalam, S.; Pandian, S.R.K.; Damodar, N.A. Design and in silico modeling of Indoloquinoxaline incorporated keratin nanoparticles for modulation of glucose metabolism in 3T3-L1 adipocytes. Biotechnol. Prog. 2020, 36, e2904. [Google Scholar] [CrossRef]
- Sadykhov, G.A.; Belyaev, D.V.; Vakhrusheva, D.V.; Eremeeva, N.I.; Khramtsova, E.E.; Pervova, M.G.; Rusinov, G.L.; Verbitskiy, E.V.; Chupakhin, O.N.; Charushin, V.N. New Approach to Biologically Active Indolo[2,3-b]quinoxaline Derivatives through Intramolecular Oxidative Cyclodehydrogenation. ChemistrySelect 2022, 7, e202200497. [Google Scholar] [CrossRef]
- Oukoloff, K.; Lucero, B.; Francisco, K.R.; Brunden, K.R.; Ballatore, C. 1,2,4-Triazolo[1,5-a]pyrimidines in drug design. Eur. J. Med. Chem. 2019, 165, 332–346. [Google Scholar] [CrossRef]
- Yu, W.; Goddard, C.; Clearfield, E.; Mills, C.; Xiao, T.; Guo, H.; Morrey, J.D.; Motter, N.E.; Zhao, K.; Block, T.M.; et al. Design, synthesis, and biological evaluation of triazolo-pyrimidine derivatives as novel inhibitors of hepatitis B virus surface antigen (HBsAg) secretion. J. Med. Chem. 2011, 54, 5660–5670. [Google Scholar] [CrossRef]
- Girasolo, M.A.; Canfora, P.; Sabatino, D.; Schillaci, E.; Foresti, S.; Rubino, G.; Ruisi, G.S. Synthesis, characterization, crystal structures and in vitro antistaphylococcal activity of organotin(IV) derivatives with 5,7-disubstituted-1,2,4-triazolo[1,5-a]pyrimidine. J. Inorg. Biochem. 2012, 106, 156–163. [Google Scholar] [CrossRef]
- Bhatt, J.D.; Chudasama, C.J.; Patel, K.D. Pyrazole clubbed triazolo[1,5-a]pyrimidine hybrids as an anti-tubercular agents: Synthesis, in vitro screening and molecular docking study. Bioorg. Med. Chem. 2015, 23, 7711–7716. [Google Scholar] [CrossRef]
- Gamal-Eldeen, A.M.; Hamdy, N.A.; Abdel-Aziz, H.A.; El-Hussieny, E.A.; Fakhr, I.M.I. Induction of intrinsic apoptosis pathway in colon cancer HCT-116 cells by novel 2-substituted-5,6,7,8-tetrahydronaphthalene derivatives. Eur. J. Med. Chem. 2014, 77, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Y.; Li, C.S.; Li, P.; Bai, X.Q.; Guo, S.Y.; Jin, Y.; Piao, S.J. Synthesis and evaluation of ursolic acid-based 1,2,4-triazolo[1,5-a]pyrimidines derivatives as anti-inflammatory agents. Mol. Divers. 2022, 26, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Mąkosza, M.; Wojciechowski, K. Nucleophilic Substitution of Hydrogen in Heterocyclic Chemistry. Chem. Rev. 2004, 104, 2631–2666. [Google Scholar] [CrossRef]
- Mąkosza, M. Nucleophilic substitution of hydrogen in electron-deficient arenes, a general process of great practical value. Chem. Soc. Rev. 2010, 39, 2855–2868. [Google Scholar] [CrossRef] [PubMed]
- Verbitskiy, E.V.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Recent Advances in Direct C–H Functionalization of Pyrimidines. Synthesis 2018, 50, 193–210. [Google Scholar] [CrossRef]
- Rasputin, N.A.; Demina, N.S.; Irgashev, R.A.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Direct (het)arylation of [1,2,4]triazolo[1,5-a]pyrimidines: Both eliminative and oxidative pathways. Tetrahedron 2017, 73, 5500–5508. [Google Scholar] [CrossRef]
- Bhagat, S.; Sharma, R.; Sawant, D.M.; Sharma, L.; Chakraborti, A.K. LiOH·H2O as a Novel Dual Activation Catalyst for Highly Efficient and Easy Synthesis of 1,3-Diaryl-2-Propenones by Claisen–Schmidt Condensation under Mild Conditions. J. Mol. Catal. A Chem. 2006, 244, 20–24. [Google Scholar] [CrossRef]
- Wei, W.; Qunrong, W.; Liqin, D.; Aiqing, Z.; Duoyuan, W. Synthesis of Dinitrochalcones by Using Ultrasonic Irradiation in the Presence of Potassium Carbonate. Ultrason. Sonochem. 2005, 12, 411–414. [Google Scholar] [CrossRef]
- Fedorova, O.V.; Zhidovinova, M.S.; Rusinov, G.L.; Ovchinnikova, I.G. Aminoazoles in the three component synthesis of 7substituted 6ethoxycarbonyl5methyl4,7dihydroazolo[1,5a]pyrimidines. Russ. Chem. Bull. Int. Ed. 2003, 52, 1768–1769. [Google Scholar] [CrossRef]
- Ishmetova, R.I.; Babkov, D.A.; Kucheryavenko, A.F.; Babkova, V.A.; Sirotenko, V.S.; Ignatenko, N.K.; Tolschina, S.G.; Vassiliev, P.M.; Rusinov, G.L.; Spasov, A.A. In silico consensus activity prediction, rational synthesis, and evaluation of antiglycation and antiplatelet activities of 3,6-disubstituted 1,2,4,5-tetrazines. Russ. Chem. Bull. 2020, 69, 768–773. [Google Scholar] [CrossRef]
- Quan, M.L.; Pinto, D.J.P.; Smallheer, J.M.; Ewing, W.R.; Rossi, K.A.; Luettgen, J.M.; Seiffert, D.A.; Wexler, R.R. Factor XIa Inhibitors as New Anticoagulants. J. Med. Chem. 2018, 61, 7425–7447. [Google Scholar] [CrossRef]
- Abd El-Sattar, N.E.A.; Badawy, E.H.K.; Abdel-Mottaleb, M.S.A. Synthesis of Some Pyrimidine, Pyrazole, and Pyridine Derivatives and Their Reactivity Descriptors. J. Chem. 2018, 2018, 8795061. [Google Scholar] [CrossRef]
- Du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting Animal Research: Explanation and Elaboration for the ARRIVE Guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Spasov, A.A.; Kucheryavenko, A.F.; Gaidukova, K.A.; Kosolapov, V.A.; Zhukovskaya, O.N. Antiplatelet Activity of New Derivatives of Benzimidazole Containing Sterically Hindered Phenolic Group in Their Structure. Res. Result. Pharmacol. 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Nowak, G. The ecarin clotting time, a universal method to quantify direct thrombin inhibitors. Pathophysiol. Haemost. Thromb. 2003, 33, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.Q.; Yang, T.; Xiao, W.; Fan, L.; Wu, Y.; Terrando, N.; Wang, T.L. Prolonged Neuroinflammation after Lipopolysaccharide Exposure in Aged Rats. PLoS ONE 2014, 9, e106331. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Mannucci, P.M. Direct oral anticoagulants and venous thromboembolism. Eur. Respir. Rev. 2016, 141, 295–302. [Google Scholar] [CrossRef]
- Jackson, S.P.; Darbousset, R.; Schoenwaelder, S.M. Thromboinflammation: Challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood 2019, 133, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.P.; Viswanathan, S.; Wang, M.; Sun, L.Q.; Clark, G.C.; D’Elia, R.V. Current and future developments in the treatment of virus-induced hypercytokinemia. Future Med. Chem. 2017, 9, 169–178. [Google Scholar] [CrossRef]
- Pawlinski, R.; Pedersen, B.; Schabbauer, G.; Tencati, M.; Holscher, T.; Boisvert, W.; Andrade-Gordon, P.; Frank, R.D.; Mackman, N. Role of Tissue Factor and Protease-Activated Receptors in a Mouse Model of Endotoxemia. Blood 2004, 103, 1342–1347. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, L.; Xie, M.; Liu, J.; Zhou, B.; Wu, R.; Cao, L.; Kroemer, G.; Wang, H.; Billiar, T.R.; et al. TMEM173 Drives Lethal coagulation in Sepsis. Cell Host Microbe 2020, 27, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Behrens, E.M.; Koretzky, G.A. Review: Cytokine Storm Syndrome: Looking Toward the Precision Medicine Era. Arthritis Rheumatol. 2017, 69, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-G.; Liu, Y.; Zhang, J.-M.; Cui, S.-C.; Zhang, Z.-G.; Zhu, H.-L. The Research Progress of Direct Thrombin Inhibitors. Mini-Rev. Med. Chem. 2020, 20, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Alouidor, B.; Sweeney, R.E.; Tat, T.; Wong, R.K.; Yoon, J.-Y. Microfluidic Point-of-Care Ecarin-Based Clotting and Chromogenic Assays for Monitoring Direct Thrombin Inhibitors. J. Extracorpor. Technol. 2019, 51, 29–37. [Google Scholar] [CrossRef]

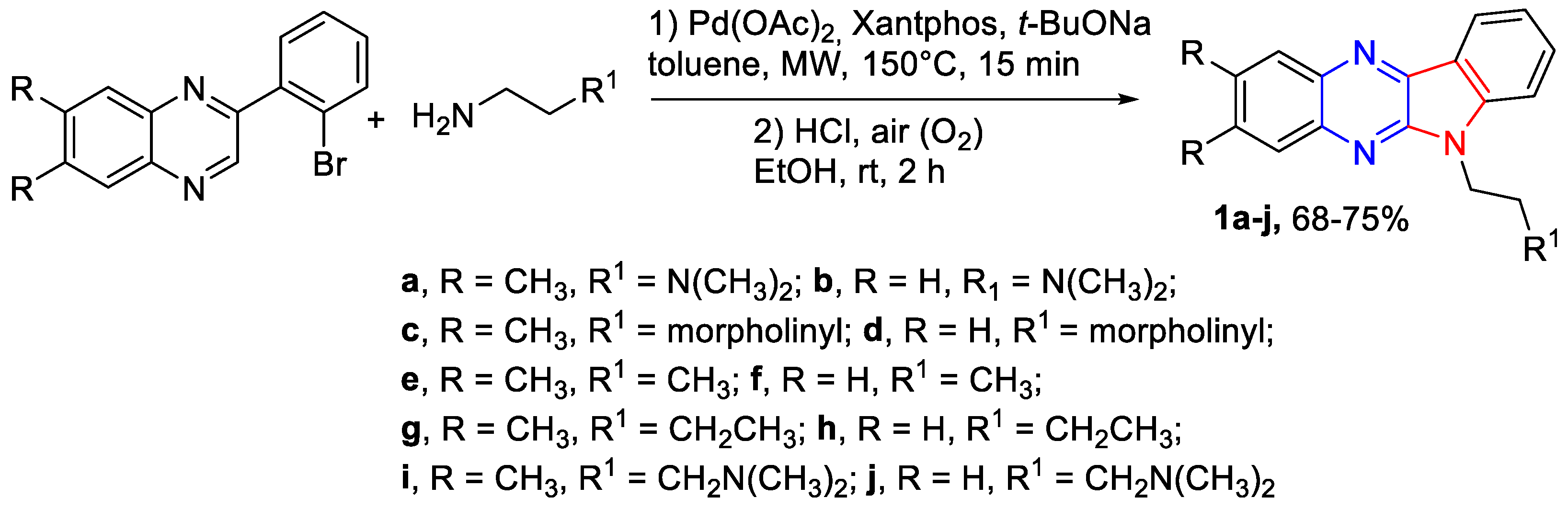

| No. | Compound | Structure | Coagulogram Parameters | ||
|---|---|---|---|---|---|
| APTT, s | TT, s | PT, s | |||
| 1 | Control | 46.23 ± 0.22 | 11.65 ± 0.06 | 14.60 ± 0.10 | |
| 2 | Dabigatran etexilate | 75.26 ± 2.61 * | 70.23 ± 3.15 * | 14.20 ± 0.38 * | |
| 3 | 1a | 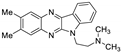 | 22.1 ± 5.9 | 22.7 ± 2.0 * | 14.5 ± 0.0 |
| 4 | 1b | 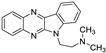 | 26.8 ± 2.4 | 23.9 ± 0.9 * | 13.3 ± 0.2 |
| 5 | 1c | 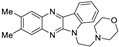 | 27.1 ± 2.0 | 26.2 ± 1.0 * | 13.8 ± 0.4 |
| 6 | 1d | 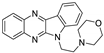 | 18.2 ± 0.5 | 26.7 ± 1.3 * | 13.4 ± 0.3 |
| 7 | 1e |  | 29.6 ± 0.2 | 38.0 ± 0.5 * | 13.4 ± 0.1 |
| 8 | 1f | 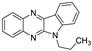 | 24.8 ± 4.2 | 39.3 ± 1.8 * | 13.1 ± 0.3 |
| 9 | 1g |  | 30.6 ± 1.6 | 34.1 ± 0.9 * | 11.7 ± 0.4 |
| 10 | 1h |  | 25.7 ± 3.1 | 35.6 ± 1.1 * | 13.9 ± 0.1 |
| 11 | 1i |  | 32.1 ± 4.8 | 21.9 ± 1.2 * | 13.8 ± 0.3 |
| 12 | 1j |  | 32.5 ± 0.1 | 22.0 ± 2.7 * | 14.2 ± 0.4 |
| 13 | 5a |  | 26.3 ± 2.9 | 43.8 ± 4.3 * | 13.4 ± 0.1 |
| 14 | 5b | 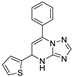 | 25.9 ± 2.2 | 21.0 ± 0.0 * | 15.8 ± 0.3 |
| 15 | 5c | 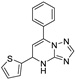 | 27.6 ± 1.9 | 41.4 ± 3.1 * | 13.9 ± 0.2 |
| 16 | 5d |  | 61.78 ± 4.58 * | 154.43 ± 11.14 * | 14.08 ± 0.4 |
| 17 | 7a | 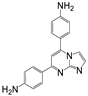 | 56.23 ± 5.05 | 66.58 ± 4.64 * | 11.62 ± 1.00 |
| 18 | 7b | 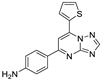 | 35.0 ± 0.2 | 32.3 ± 0.9 * | 13.6 ± 0.5 |
| 19 | 8c | 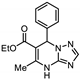 | 34.6 ± 0.7 | 34.7 ± 0.7 * | 14.2 ± 0.1 |
| 20 | 10a |  | 30.9 ± 0.6 | 48.6 ± 22.4 * | 13.2 ± 1.0 |
| 21 | 10b |  | 30.6 ± 2.6 | 27.1 ± 1.4 * | 12.7 ± 0.2 |
| 22 | 12a | 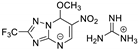 | 25.1 ± 0.8 | 24.3 ± 3.5 * | 14.2 ± 0.1 |
| 23 | 12b |  | 26.2 ± 1.5 | 25.6 ± 3.9 * | 14.8 ± 0.4 |
| 24 | 14 |  | 24.5 ± 0.8 | 23.8 ± 3.5 * | 14.5 ± 0.5 |
| 25 | 15a | 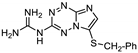 | 34.0 ± 0.6 | 32.8 ± 0.8 * | 14.5 ± 0.3 |
| 26 | 15b | 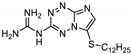 | 34.1 ± 0.5 | 33.7 ± 0.6 * | 14.0 ± 0.2 |
| 27 | 16a | 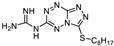 | 29.1 ± 1.4 | 34.3 ± 4.7 * | 12.2 ± 0.2 |
| 28 | 16b | 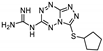 | 24.7 ± 1.2 | 22.7 ± 1.7 * | 13.9 ± 0.5 |
| 29 | 16 |  | 26.3 ± 1.1 | 25.9 ± 1.6 * | 14.1 ± 0.3 |
| No. | Compound | Coagulogram Parameters | ||
|---|---|---|---|---|
| APTT, s | TT, s | PT, s | ||
| 1 | Control | 46.23 ± 0.22 | 11.65 ± 0.06 | 14.60 ± 0.10 |
| 2 | LPS control | 56.28 ± 1.60 * | 9.01 ± 0.37 * | 15.10 ± 0.07 |
| 3 | Dabigatran etexilate | 135.13 ± 15.17 *# | 125.45 ± 1.54 *# | 15.73 ± 0.20 |
| 4 | 5d | 61.78 ± 4.58 * | 154.43 ± 7.14 *# | 14.08 ± 0.4 |
| Compound | Δ% of Thrombin Time Prolongation Relative to Control | EC50, μM | |||
| 10 μM | 5 μM | 1 μM | 0.1 μM | ||
| Dabigatran etexilate | 302.0 ± 34.4 * | 176.1 ± 15.3 * | 42.3 ± 5.6 * | 1.4 ± 0.4 * | 1.4 |
| 5d | 467.1 ± 14.4 *# | 194.1 ± 10.0 *# | 46.5 ± 6.7 * | 4.3 ± 1.0 * | 1.3 |
| Compound | Δ % of Thrombin Time Prolongation Relative To Control + LPS | IC50, μM | |||
| 10 μM | 5 μM | 1 μM | 0.1 μM | ||
| Dabigatran etexilate | 292.0 ± 0.6 # | 292.0 ± 0.6 # | 51.7 ± 0.5 | 16.3 ± 0.2 # | 0.76 |
| 5d | 354.1 ± 6.5 #$ | 234.1 ± 4.2 #$ | 56.3 ± 1.1 * | 8.4 ± 1.6 | 0.78 |
| Compound | Coagulogram Parameters of Animals without Hypercytokinemia | ||
| APTT, s | TT, s | PT, s | |
| Control group | 38.3 ± 1.4 | 57.7 ± 1.8 | 28.1 ± 1.4 |
| Dabigatran etexilate | 137.5 ± 2.2 * | 603.9 ± 14.2 * | 31.2 ± 1.3 |
| 5d | 59.2 ± 8.3 * | 325.3 ± 5.1 * | 24.5 ± 1.1 |
| Compound | Coagulogram Parameters of Animals with Hypercytokinemia | ||
| APTT, s | TT, s | PT, s | |
| LPS group | 18.4 ± 0.8 | 44.1 ± 1.7 | 21.2 ± 1.3 |
| Dabigatran etexilate | 41.0 ± 2.0 # | 566.1 ± 11.5 # | 28.1 ± 1.0 |
| 5d | 28.7 ± 1.0 # | 640.3 ± 7.4 #$ | 26.4 ± 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spasov, A.A.; Fedorova, O.V.; Rasputin, N.A.; Ovchinnikova, I.G.; Ishmetova, R.I.; Ignatenko, N.K.; Gorbunov, E.B.; Sadykhov, G.A.o.; Kucheryavenko, A.F.; Gaidukova, K.A.; et al. Novel Substituted Azoloazines with Anticoagulant Activity. Int. J. Mol. Sci. 2023, 24, 15581. https://doi.org/10.3390/ijms242115581
Spasov AA, Fedorova OV, Rasputin NA, Ovchinnikova IG, Ishmetova RI, Ignatenko NK, Gorbunov EB, Sadykhov GAo, Kucheryavenko AF, Gaidukova KA, et al. Novel Substituted Azoloazines with Anticoagulant Activity. International Journal of Molecular Sciences. 2023; 24(21):15581. https://doi.org/10.3390/ijms242115581
Chicago/Turabian StyleSpasov, Alexander A., Olga V. Fedorova, Nikolay A. Rasputin, Irina G. Ovchinnikova, Rashida I. Ishmetova, Nina K. Ignatenko, Evgeny B. Gorbunov, Gusein A. o. Sadykhov, Aida F. Kucheryavenko, Kseniia A. Gaidukova, and et al. 2023. "Novel Substituted Azoloazines with Anticoagulant Activity" International Journal of Molecular Sciences 24, no. 21: 15581. https://doi.org/10.3390/ijms242115581






