New Therapeutic Strategies for Obesity and Its Metabolic Sequelae: Brazilian Cerrado as a Unique Biome
Abstract
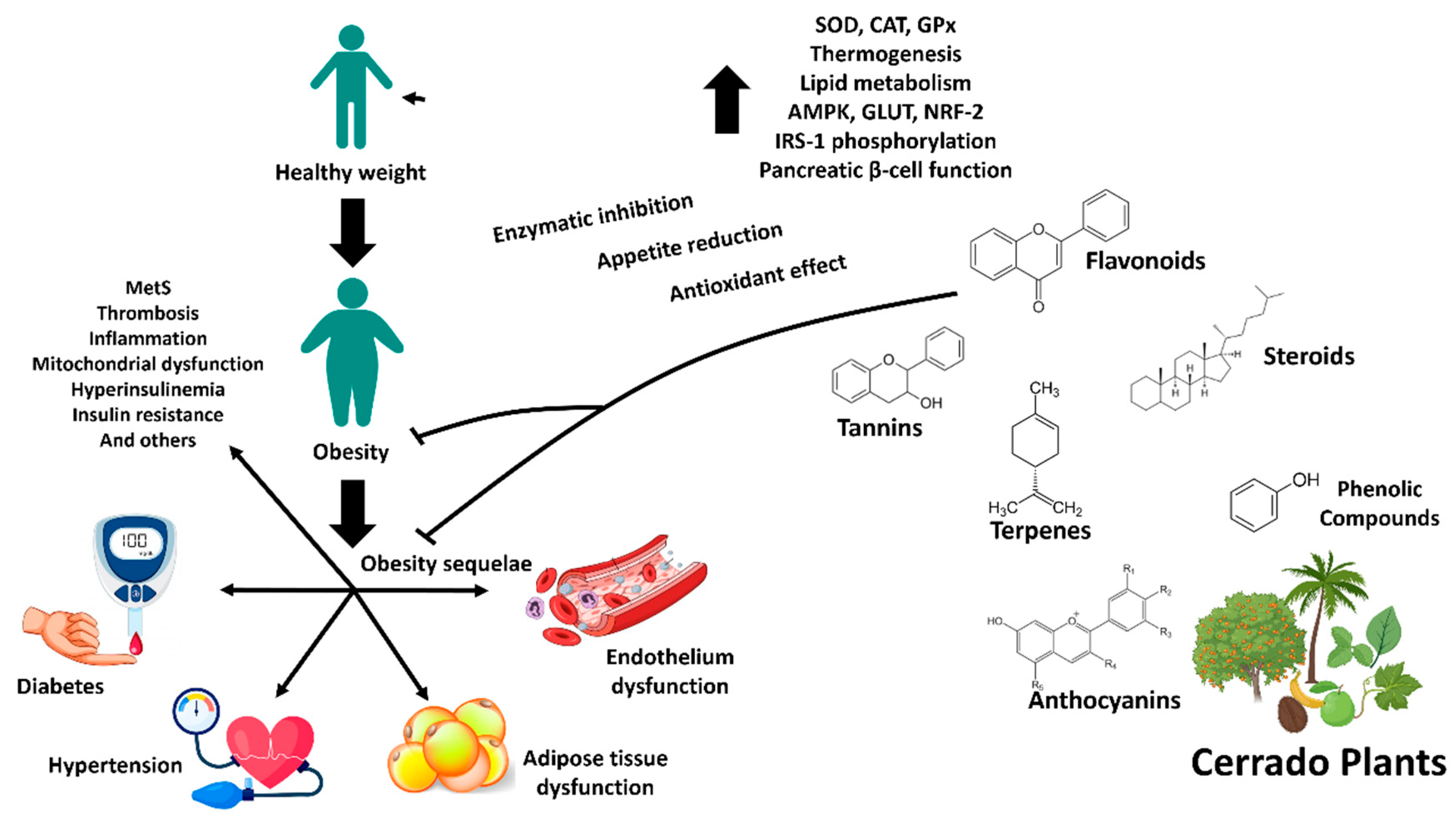
1. Introduction
2. Chemical Constitution of Native Plants of the Brazilian Cerrado
2.1. Polyphenols
2.2. Terpenes and Sterols
| Medicinal Plant | Phytochemical Constituents | Biological Properties | References |
|---|---|---|---|
| Acrocomia aculeata | Gallic, vanillic, caffeic, and ferulic acid, rutin, quercetin, campesterol, stigmasterol, β-sitosterol, lupeol, and lupeol acetate | In vitro and in vivo antioxidant activity, hypoglycemic, hypotriglyceridemic anticancer and cardioprotective effect | [2,12,54] |
| Alibertia edulis | Caffeic acid, quercetin 3-rhamnosyl-(1 → 6)-galactoside and iridois ioxide | Hypoglycemiant effect, protection against hemolysis and oxidative stress | [55] |
| Alibertia verrucosa | Phenolic compounds and tocopherols | Antioxidant activity | [56] |
| Anacardium humile | Phenolic compounds, anthocyanins and tocopherols | Antioxidant activity | [56] |
| Annona crassiflora | Epicatechin and quercetin | Antioxidant, antiproliferative and wound healing | [57] |
| Alkaloids, specially the isolated one, stevagallin | Anti-obesity capacity, inhibition against pancreatic lipase with low cytotoxicity | [58] | |
| Annona muricata | Total phenolic compounds, flavonoids and proanthocyanidins (total quantification) | Antioxidant activity, in vitro antidiabetic and inhibition of α-amylase, lipase, α-glucosidase, non-enzymatic glycation, and lipid peroxidation | [59] |
| Phenols, flavonoids, saponins, tannins, steroids, and alkaloids | Antidiabetic and antiglycation | [60] | |
| Bactris setosa | Phenolic compounds (anthocyanins and non-anthocyanin phenolic compounds) and carotenoids | Oxidative and nitrosative protection | [61,62] |
| Banisteriopsis argyrophylla | Catechin, flavonoids, glycosylated kaempferol, procyanidins, and megastigmane glucosides | α-amylase, α-glucosidase, lipase, and glycation inhibitors, antidiabetic and antioxidant | [63] |
| Byrsonima verbascifolia | Resveratrol and ferulic acid | Antimutagenic, antigenotoxic and antioxidant activity | [64] |
| Buchenavia tomentosa | Phenols, carotenoids and tocopherols | Antioxidant activity | [56,65] |
| Campomanesia cambessedeana | Catechin, ethyl gallate and propyl gallate | Antimutagenic, antigenotoxic and antioxidant activity | [64] |
| Caryocar brasiliense | Gallic acid, quinic acid, quercetin, and quercetin 3-O-arabinos | Antioxidant activity | [66] |
| Phenolic acids and tannins, including corilagin and geraniin | Antidiabetic effect | [67] | |
| Cedrela odorata | Gallic acid, catechin and gallocatechin | Hyperglycemia reduction and antioxidant activity in vivo | [68] |
| Dipteryx alata | Gallic acid and its derivatives, such as gallic acid esters and gallotannins | Antioxidant and antiproliferative activity | [69] |
| p-Coumaric, ellagic, caffeic, ferulic, and gallic acid and hydroxybenzoic, catechin and epicatechin | Antioxidant activity | [70] | |
| Phenolic compounds (total quantification) | In vivo antioxidant activity | [71] | |
| Phenols and terpenes | Antioxidant activity and Caenorhabditis elegans life expectancy increase | [72] | |
| Eschweilera nanat | Rutin and hyperoside | Antioxidant activity | [73] |
| Eugenia dysenterica | Proanthocyanidins, flavonoids, phenolic acids, quercetin, kaempferol derivatives, free and total ellagic acid | Antioxidant activity, pancreatic lipase inhibition, lower body weight and fat mass, improved hyperglycemia, dyslipidemia and fecal triglycerides excretion | [74] |
| Quercetin and gallic acid | Inhibitory effects on hydrolases, antioxidant and antiglycation properties | [75] | |
| Phenolic compounds (total quantification), myricetin, quercetin and kaempferol | Antioxidant, antiproliferative and antimutagenic potential | [76] | |
| Eugenia klotzschiana | More than 35 compounds (see ref.) | Antioxidant and antibacterial effect | [77] |
| Guazuma ulmifolia | Flavan-3-ol-derived flavonoids, including monomers and dimers, condensed tannins, and glycosylated flavonoids | In vitro and in vivo antioxidant activity | [10] |
| Hancornia speciosa | Phenolic compounds (total quantification) | Antioxidant activity | [78] |
| Hymenaea stignocarpa | Caffeic acid, quercetin-3-rutinoside, kaempferol; quercetin-3-rhamnoside | α-amylase and α-glucosidase inhibition, glycemic profile improved | [79] |
| Hyptis Jacq. | Phenolic acids, flavonoids, cinnamic acid derivatives, chlorogenic acid and rosmarinic acid | Antioxidant activity | [80] |
| Kielmeyera coriacea | Protocatechuic acid, procyanidins A, B, and C and epicatechin | Antioxidant and antiglycation activity, and LPL inhibition. | [81]. |
| Mauritia flexuosa | Total phenolic compounds and β-carotene (total quantification) | Antioxidant activity | [82] |
| Carotenoids and polyphenols (catechin, quercetin and gallic acid) | Antioxidant activity, antimutagenic, antimicrobial | [83,84,84,85,86,87,88,89] | |
| Mauritiella armata | Palmitic, estearic, oleic, linoleic, linolenic acid, tocopherol, and α-tocopherol | Antioxidant activity | [90] |
| Myrcia bella | Flavonoids and phenolic acids derivatives | Antimutagenic and antioxidant activity | [91] |
| Passiflora setacea | Polyphenols | Antidiabetic and anti-inflammatory effect, with insulin, HOMA IR, PPAR-γ and IL-6 levels improvement | [92] |
| Pouteria ramiflora | Friedelin, epifriedelanol, taraxerol, triterpens and FFA | Antioxidant and α-amylase inhibition | [93] |
| Pouteria torta | Phenolic compounds, flavonoids, catechin and epicatechin | Antioxidant and α-amylase inhibition | [94] |
| Psidium cattleianum | Epicatechin, gallic, coumaric, and ferulic acid, myricetin and quercetin | Antioxidant and antimicrobial activity and antiproliferative effect on human cancer cells | [95] |
| Rollinia mucosa | Phenolic compounds and tocopherols | Antioxidant activity | [56] |
| Schinus terebinthifolius Raddi | O-glycosylated flavonols, gallotannins and gallic acid along with its derivatives | Antioxidant, antidiabetic and antiproliferative activities | [96,97] |
| Sterculia striata | Oleic acid, phytosterols β-sitosterol, stigmasteroland, campesterol γ-, δ-, α- and β-tocopherol, ellagic, ferulic, methoxyphenylacetic and protocatechuic acids | Antioxidant activity | [98] |
| Solanum lycocarpu | 24 phenolic compounds (see ref.) | Antioxidant activity | [99] |
| Vochysiaceae species | Polyphenols, such as flavonoids and condensed tannins | Antioxidant and inhibitory potential against human α-amylase and protein glycation | [100] |
| Senna velutina | 21 compounds (see ref.) | Antioxidant, in vitro and in vivo antitumor effects | [101,102] |
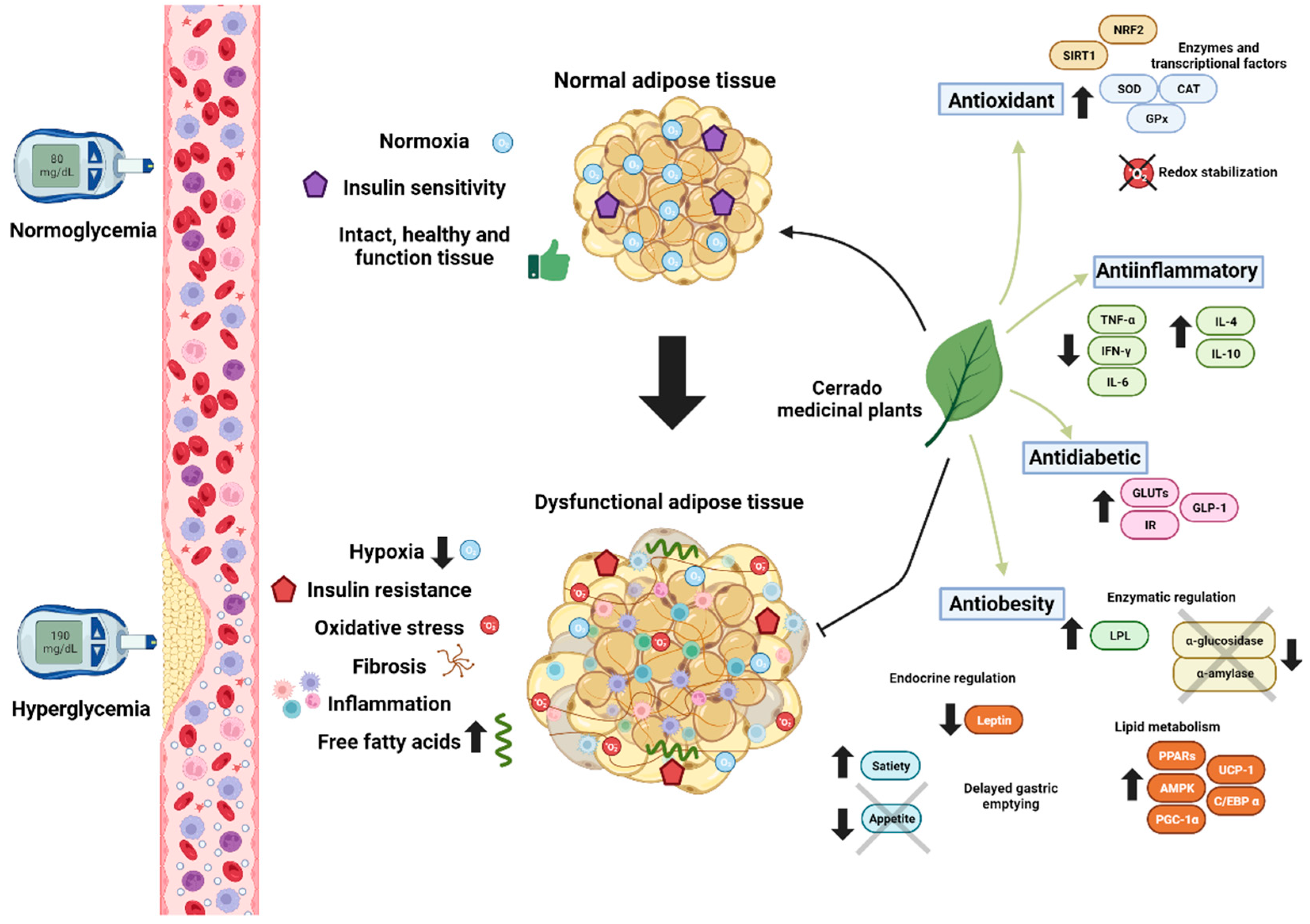
3. Obesity, Dyslipidemia and Inflammation
3.1. Antilipidemic Effects of Cerrado Plants through Enzymatic Inhibition
3.2. Anti-Inflammatory Effect
3.3. Adipocyte Differentiation, Adipogenesis, and Browning
3.4. Modulation of Neuroendocrine Mechanisms
| Therapeutic Properties | Medicinal Plant | Experimental Condition | Refs. | Medicinal Plant | ExperimentalCondition | Model | Treatment/Dose | Refs. |
|---|---|---|---|---|---|---|---|---|
| In Vitro | In Vivo | |||||||
| Antilipidemic effect | - | - | - | Casearia sylvestris | High fat diet | Swiss and C57BL/6 LDLr-null mice | 250 and 500 mg/kg extract | [124] |
| - | - | - | Eugenia dysenterica | High-fat high-sucrose diet | C57BL/6J mice | 7 and 14 mg gallic acid equivalent of extract/kg | [74] | |
| Enzymatic inhibition | Kielmeyera coriacea | α-amylase, α-glucosidase, and pancreatic lipase inhibition, antioxidant and antiglycation assays | [81] | - | - | - | - | - |
| Banisteriopsis argyrophylla | α-amylase, α-glucosidase, and pancreatic lipase inhibition, antioxidant and antiglycation assays | [63] | - | - | - | - | - | |
| Annona muricata | α-amylase, α-glucosidase, and pancreatic lipase inhibition, antioxidant, antiglycation assays and cytotoxic assays | [59] | - | - | - | - | - | |
| Annona crassiflora | Lipase inhibition and cytotoxic assay | [58] | - | - | - | - | - | |
| Anti-inflammatory effect | Serjania lethalis, Cupania vernalis, Casearia sylvestris | Determination of nitric oxide production and cytotoxicity assay | [131] | Xylopia aromatica | High carbohydrate | BALB/c mice | 50, 100 and 200 mg/kg extract | [123] |
| - | - | - | Pyrostegia venusta | High-carbohydrate-refined diet | BALB/c | 300 mg/kg extract | [129] | |
| - | - | - | Eugenia dysenterica | High-fat high-sucrose diet | C57BL/6J mice | 7 and 14 mg gallic acid equivalent of extract/kg | [74,130] | |
| Adipocyte differentiation, adipogenesis, and browning | Hippeastrum stapfianum | Activation of PPAR-α, PPAR-γ and antioxidant assays | [135] | Davilla elliptica | High-lard/high-sugar diet | Swiss mice | 0.26 mg/kg extract | [125] |
| - | - | - | Passiflora setacea | Clinical trial | Overweight male volunteers and BV-2 microglial cells | 50 g, 150 g of pulp in two phases (humans), phenolic mebolites (cells) | [92] | |
| Modulation of neuroendocrine mechanisms | - | - | - | Anacardium occidentale | Clinical trial | Women at cardiometabolic risk | 15 g of Brazil nuts + 30 g of cashew nuts | [144] |
| - | - | - | Caryocar. brasiliense | Liver injury induction with carbon tetrachloride | Wistar rats | 3 or 6 mL/kg of almond oil | [145] | |
4. Type 2 Diabetes, Oxidative Stress and Glycation
4.1. Antidiabetic Effects of Cerrado Plants
| Therapeutic Properties | Medicinal Plant | Experimental Condition | Refs. | Medicinal Plant | Experimental Condition | Model | Treatment/ Dose | Refs. |
|---|---|---|---|---|---|---|---|---|
| In Vitro | In Vivo/Clinical Studies | |||||||
| Enzymatic inhibition and antidiabetic effects | Annona muricata | α-amylase, α-glucosidase, and pancreatic lipase, associated with antioxidant and antiglycation assays | [59] | Annona muricata | Mice—streptozotocin (STZ)-induced T1D | Male BALB/c mice and in erythrocytes of diabetic patients | 1.0 mg/kg seed oil | [152] |
| Annona muricata | antidiabetic, and antiglycation potentials | [60] | Annona muricata | STZ-induced T2D | Male wistar rats | 100 mg/kg or 200 mg/kg extract | [153] | |
| Acrocomia aculeata | Antioxidant and cytotoxic assays | [2] | Acrocomia aculeata | Normal and non-obese T2D rats | Male wistar and Goto-Kakizaki rats | 200 mg/kg extract | [12] | |
| - | - | - | Acrocomia aculeata | STZ- and low HFD induction | Male wistar rats | 40 or 160 g of kernel oil | [154] | |
| Banisteriopsis argyrophylla | Inhibition of α-amylase, α-glucosidase, and lipase and antioxidant assays | [63] | Acrocomia aculeata | Normal, STZ and frutose-induced diet | Male wistar rats | 3, 30 or 300 mg/kg pulp oil | [155] | |
| Eugenia dysenterica | Inhibitory effects on hydrolases, antioxidant and antiglycation properties | [75] | Hymenaea stignocarpa | Normal and healthy | Women | Replacement of normal flour with pulp flour at 10, 20 and 30% | [79] | |
| - | - | - | Caryocar brasiliense | Normal rats | Male swiss mice and THP-1, CCL-13 and CR-1458 cell lines | 100 mg/kg extract and fractions | [67] | |
| - | - | - | Terminalia phaeocarpa | Normal rats | Male swiss mice and THP-1 cell line | 100 mg/kg extract and fractions | [156] | |
| - | - | - | Eugenia florida | Normal and Alloxan-induced | Male Wistar rats | 200 mg/kg extract | [43] | |
| - | - | - | Alibertia edulis | Normal and HDF-induced | Male swiss mice | 200 and 400 mg/kg extract | [53] | |
| - | - | - | Anacardium othonianum | Normal and healthy | Women | 400 mL juice | [159] | |
| - | - | - | Siolmatra brasiliensis | Normal and HDF-induce | Male C57Bl/6J mice | 125 or 250 mg/kg extract | [160] | |
| - | - | - | Eugenia dysenterica | Metabolic syndrome | Woman | 300 mL juice | [161] | |
| Redox imbalance and antioxidant potential | Mauritia flexuosa | Free radical scavenging | [85] | Acrocomia aculeata | Oxidative stress induction | C. elegans | 500–1000 μg/mL | [2] |
| Mauritia flexuosa | Free radical and hydroxyl scavenging, and reducing iron | [82] | Acrocomia aculeata | Normal and non-obese T2D rats | Wistar and Goto-Kakizaki rats | 200 mg/kg | [12] | |
| Annona crassiflora | ABTS free radicals capture | [162] | - | - | - | - | - | |
| Solanum lycocarpum | DPPH, FRAP and ORAC techniques | [99,163] | - | - | - | - | - | |
| Dipteryx alata | Antioxidant profile with antiproliferative activity | [69] | - | - | - | - | - | |
4.2. The Redox Imbalance and Antioxidant Potential of Phytochemical Compounds from the Cerrado Plants
4.3. Limitations of the Study
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Life Expectancy Increases by 5 Years, but Inequalities Persist. Available online: https://www.who.int/news/item/19-05-2016-life-expectancy-increased-by-5-years-since-2000-but-health-inequalities-persist (accessed on 8 May 2023).
- Monteiro-Alfredo, T.; Matafome, P.; Iacia, B.P.; Antunes, K.Á.; dos Santos, J.M.; da Silva Melo da Cunha, J.; Oliveira, S.; Oliveira, A.S.; Campos, J.F.; Magalhães, M.; et al. Acrocomia aculeata (Jacq.) Lodd. Ex Mart. Leaves Increase SIRT1 Levels and Improve Stress Resistance. Oxidative Med. Cell. Longev. 2020, 2020, e5238650. [Google Scholar] [CrossRef] [PubMed]
- Vatner, S.F.; Zhang, J.; Oydanich, M.; Berkman, T.; Naftalovich, R.; Vatner, D.E. Healthful Aging Mediated by Inhibition of Oxidative Stress. Ageing Res. Rev. 2020, 64, 101194. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization—Background—The Global Burden of Chronic. Available online: https://www.who.int/nutrition/topics/2_background/en/ (accessed on 26 July 2019).
- Monteiro-Alfredo, T.; Caramelo, B.; Arbeláez, D.; Amaro, A.; Barra, C.; Silva, D.; Oliveira, S.; Seiça, R.; Matafome, P. Distinct Impact of Natural Sugars from Fruit Juices and Added Sugars on Caloric Intake, Body Weight, Glycaemia, Oxidative Stress and Glycation in Diabetic Rats. Nutrients 2021, 13, 2956. [Google Scholar] [CrossRef] [PubMed]
- Borghi-Silva, A.; Back, G.D.; Garcia de Araújo, A.S.; Oliveira, M.R.; da Luz Goulart, C.; Silva, R.N.; Bassi, D.; Mendes, R.G.; Arena, R. COVID-19 Seen from a Syndemic Perspective: Impact of Unhealthy Habits and Future Perspectives to Combat These Negative Interactions in Latin America. Prog. Cardiovasc. Dis. 2022, 71, 72–78. [Google Scholar] [CrossRef]
- Chu, D.-T.; Minh Nguyet, N.T.; Nga, V.T.; Thai Lien, N.V.; Vo, D.D.; Lien, N.; Nhu Ngoc, V.T.; Son, L.H.; Le, D.-H.; Nga, V.B.; et al. An Update on Obesity: Mental Consequences and Psychological Interventions. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 155–160. [Google Scholar] [CrossRef]
- Safaei, M.; Sundararajan, E.A.; Driss, M.; Boulila, W.; Shapi’i, A. A Systematic Literature Review on Obesity: Understanding the Causes & Consequences of Obesity and Reviewing Various Machine Learning Approaches Used to Predict Obesity. Comput. Biol. Med. 2021, 136, 104754. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA Damage Response in Cancer. Redox Biol. 2018, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.M.; Alfredo, T.M.; Antunes, K.Á.; da Cunha, J.d.S.M.; Costa, E.M.A.; Lima, E.S.; Silva, D.B.; Carollo, C.A.; Schmitz, W.O.; Boleti, A.P.d.A.; et al. Guazuma ulmifolia Lam. Decreases Oxidative Stress in Blood Cells and Prevents Doxorubicin-Induced Cardiotoxicity. Oxidative Med. Cell. Longev. 2018, 2018, e2935051. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Alfredo, T.; Oliveira, S.; Amaro, A.; Rosendo-Silva, D.; Antunes, K.; Pires, A.S.; Teixo, R.; Abrantes, A.M.; Botelho, M.F.; Castelo-Branco, M.; et al. Hypoglycaemic and Antioxidant Properties of Acrocomia aculeata (Jacq.) Lodd Ex Mart. Extract Are Associated with Better Vascular Function of Type 2 Diabetic Rats. Nutrients 2021, 13, 2856. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity. Available online: https://www.who.int/health-topics/obesity (accessed on 13 April 2023).
- Nevill, A.M.; Duncan, M.J.; Myers, T. BMI Is Dead; Long Live Waist-Circumference Indices: But Which Index Should We Choose to Predict Cardio-Metabolic Risk? Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1642–1650. [Google Scholar] [CrossRef]
- Elffers, T.W.; de Mutsert, R.; Lamb, H.J.; de Roos, A.; van Dijk, K.W.; Rosendaal, F.R.; Jukema, J.W.; Trompet, S. Body Fat Distribution, in Particular Visceral Fat, Is Associated with Cardiometabolic Risk Factors in Obese Women. PLoS ONE 2017, 12, e0185403. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.P.; de Souza Santos, R.; Palmer, B.F.; Clegg, D.J. Determinants of Body Fat Distribution in Humans May Provide Insight about Obesity-Related Health Risks. J. Lipid Res. 2019, 60, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D. Role of Body Fat Distribution and the Metabolic Complications of Obesity. J. Clin. Endocrinol. Metab. 2008, 93, S57–S63. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes. Facts 2017, 10, 207–215. [Google Scholar] [CrossRef]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic Syndrome, Mediterranean Diet, and Polyphenols: Evidence and Perspectives. J. Cell. Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization—2011 the World Traditional Medicines Situation. In Traditional Medicines: Global Situation, Issues and Challenges; WHO: Geneva, Switzerland, 2011; Volume 3, pp. 1–14. [Google Scholar]
- Teng, Z.; Shen, Y. Research progress of genetic engineering on medicinal plants. Zhongguo Zhong Yao Za Zhi 2015, 40, 594–601. [Google Scholar]
- De Luca, V.; Salim, V.; Atsumi, S.M.; Yu, F. Mining the Biodiversity of Plants: A Revolution in the Making. Science 2012, 336, 1658–1661. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, J.T.G.; Da Silva Baldivia, D.; de Castro, D.T.H.; dos Santos, H.F.; dos Santos, C.M.; Oliveira, A.S.; Alfredo, T.M.; Vilharva, K.N.; de Picoli Souza, K.; dos Santos, E.L. The Immunoregulatory Function of Polyphenols: Implications in Cancer Immunity. J. Nutr. Biochem. 2020, 85, 108428. [Google Scholar] [CrossRef]
- Fonseca, C.R.; Venticinque, E.M. Biodiversity Conservation Gaps in Brazil: A Role for Systematic Conservation Planning. Nat. Conserv. 2018, 16, 61–67. [Google Scholar] [CrossRef]
- de Carvalho, A.P.A.; Conte-Junior, C.A. Health Benefits of Phytochemicals from Brazilian Native Foods and Plants: Antioxidant, Antimicrobial, Anti-Cancer, and Risk Factors of Metabolic/Endocrine Disorders Control. Trends Food Sci. Technol. 2021, 111, 534–548. [Google Scholar] [CrossRef]
- Dos Reis, S.O.; da Luz, T.C.; da Silva Couto, C.V.M.; Dalbó, J.; Nunes, L.d.C.; Martins, M.C.; Silva, P.I.; da Silva, A.M.A.; Trivilin, L.O. Juçara (Euterpe edulis Mart.) Supplementation Reduces Aberrant Crypt Foci and Increases SOD1 Expression in the Colorectal Mucosa of Carcinogenesis-Induced Rats. Nutr. Cancer 2020, 72, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Nehring, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Effect of in Vitro Gastrointestinal Digestion on the Bioaccessibility of Phenolic Compounds, Minerals, and Antioxidant Capacity of Mimosa Scabrella Bentham Honeydew Honeys. Food Res. Int. 2017, 99, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zeng, L.; He, Y.; Liu, X.; Zhang, T.; Wang, Q. Effects of Dietary Tannic Acid on Obesity and Gut Microbiota in C57BL/6J Mice Fed with High-Fat Diet. Foods 2022, 11, 3325. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, F.; Nisa, M.U.; Hussain, H.A.; Khan, M.K.; Ahmad, R.S.; Ahmad, N.; Imran, M.; Umbreen, H. Effect of Hydrolysable Tannin on Nutrient Intake Obesity and Other Associated Metabolic Risk Factors in Polycystic Rats. Transl. Med. Commun. 2021, 6, 10. [Google Scholar] [CrossRef]
- Khalil, H.E.; Abdelwahab, M.F.; Ibrahim, H.-I.M.; AlYahya, K.A.; Altaweel, A.A.; Alasoom, A.J.; Burshed, H.A.; Alshawush, M.M.; Waz, S. Cichoriin, a Biocoumarin, Mitigates Oxidative Stress and Associated Adverse Dysfunctions on High-Fat Diet-Induced Obesity in Rats. Life 2022, 12, 1731. [Google Scholar] [CrossRef]
- Singh, L.R.; Kumar, A.; Upadhyay, A.; Gupta, S.; Palanati, G.R.; Sikka, K.; Siddiqi, M.I.; Yadav, P.N.; Sashidhara, K.V. Discovery of Coumarin-Dihydroquinazolinone Analogs as Niacin Receptor 1 Agonist with in-Vivo Anti-Obesity Efficacy. Eur. J. Med. Chem. 2018, 152, 208–222. [Google Scholar] [CrossRef]
- Goto, T.; Takahashi, N.; Hirai, S.; Kawada, T. Various Terpenoids Derived from Herbal and Dietary Plants Function as PPAR Modulators and Regulate Carbohydrate and Lipid Metabolism. PPAR Res. 2010, 2010, 483958. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Shen, X.; Deng, R.; Zhang, Y.; Zheng, X. Dietary Anthocyanin-Rich Extract of Açai Protects from Diet-Induced Obesity, Liver Steatosis, and Insulin Resistance with Modulation of Gut Microbiota in Mice. Nutrition 2021, 86, 111176. [Google Scholar] [CrossRef]
- Xu, H.; Liu, M.; Liu, H.; Zhao, B.; Zheng, M.; Liu, J. Anthocyanins from Purple Corn Ameliorated Obesity in High Fat Diet-Induced Obese Mice through Activating Hepatic AMPK. J. Funct. Foods 2021, 84, 104582. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, Y.; Ali, H.S.; El-Kherbetawy, M.K.; Elkazzaz, A.Y.; ElSayed, M.H.; Elshormilisy, A.; Eltrawy, A.H.; Abed, S.Y.; Alshahrani, A.M.; Hashish, A.A.; et al. Saponin-Rich Extract of Tribulus Terrestris Alleviates Systemic Inflammation and Insulin Resistance in Dietary Obese Female Rats: Impact on Adipokine/Hormonal Disturbances. Biomed. Pharmacother. 2022, 147, 112639. [Google Scholar] [CrossRef]
- Tafolla-Arellano, J.C.; González-León, A.; Tiznado-Hernández, M.E.; Zacarías García, L.; Báez-Sañudo, R. Composición, Fisiología y Biosíntesis de La Cutícula En Plantas. Rev. Fitotec. Mex. 2013, 36, 3–12. [Google Scholar] [CrossRef][Green Version]
- Saad, B.; Ghareeb, B.; Kmail, A. Metabolic and Epigenetics Action Mechanisms of Antiobesity Medicinal Plants and Phytochemicals. Evid. Based Complement. Altern. Med. 2021, 2021, 9995903. [Google Scholar] [CrossRef]
- Francini-Pesenti, F.; Spinella, P.; Calò, L.A. Potential Role of Phytochemicals in Metabolic Syndrome Prevention and Therapy. Diabetes Metab. Syndr. Obes. 2019, 12, 1987–2002. [Google Scholar] [CrossRef]
- Speer, H.; D’Cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and Human Health-A Focus on Oxidative Stress, Inflammation and Disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Verpoorte, R. Exploration of Nature’s Chemodiversity: The Role of Secondary Metabolites as Leads in Drug Development. Drug Discov. Today 1998, 5, 232–238. [Google Scholar] [CrossRef]
- Jucá, M.M.; Cysne Filho, F.M.S.; de Almeida, J.C.; Mesquita, D.d.S.; Barriga, J.R.d.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E.; et al. Flavonoids: Biological Activities and Therapeutic Potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef]
- Araújo, J. Química de Alimentos—Teoria e Prática—7a Edição. Available online: https://www.agrolivros.com.br/nutricao-e-tecnologia-de-alimentos/livro-quimica-de-alimentos-teoria-e-pratica--p (accessed on 9 September 2019).
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Nunes, Â.A.; Buccini, D.F.; Jaques, J.A.S.; Portugal, L.C.; Guimarães, R.C.A.; Favaro, S.P.; Caldas, R.A.; Carvalho, C.M.E. Effect of Acrocomia aculeata Kernel Oil on Adiposity in Type 2 Diabetic Rats. Plant Foods Hum. Nutr. 2018, 73, 61–67. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Ganai, S.A.; Ahmad, T.; Gani, M. Understanding the Role of Active Components from Plant Sources in Obesity Management. J. Saudi Soc. Agric. Sci. 2019, 18, 168–176. [Google Scholar] [CrossRef]
- Patel, M.D.; Thompson, P.D. Phytosterols and Vascular Disease. Atherosclerosis 2006, 186, 12–19. [Google Scholar] [CrossRef]
- Tang, S.; Fang, C.; Liu, Y.; Tang, L.; Xu, Y. Anti-Obesity and Anti-Diabetic Effect of Ursolic Acid against Streptozotocin/High Fat Induced Obese in Diabetic Rats. J. Oleo Sci. 2022, 71, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Chen, B.; Bai, Y.; Miao, T.; Rui, L.; Zhang, H.; Xia, B.; Li, Y.; Gao, S.; Wang, X.-D.; et al. Lycopene in Protection against Obesity and Diabetes: A Mechanistic Review. Pharmacol. Res. 2020, 159, 104966. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Kumar, P.; Deshmukh, R.R.; Bishayee, A.; Kumar, S. Pentacyclic Triterpenes: New Tools to Fight Metabolic Syndrome. Phytomedicine 2018, 50, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lee, J.; Salazar Hernandez, M.A.; Mazitschek, R.; Ozcan, U. Treatment of Obesity with Celastrol. Cell 2015, 161, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Oliveira, P.; Fraga-Corral, M.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Scientific Basis for the Industrialization of Traditionally Used Plants of the Rosaceae Family. Food Chem. 2020, 330, 127197. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Alfredo, T.; Maurino dos Santos, J.; Ávila Antunes, K.; Melo, J.; Botelho, W.; Boleti, A.P.; Pires, A.S.; Marques, I.; Abrantes, A.M.; Botelho, M.F.; et al. Acrocomia aculeata Associated with Doxorubicin: Cardioprotection and Anticancer Activity. Front. Pharmacol. 2023, 14, 1223933. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo de Santana Aquino, D.; Monteiro, T.A.; Lima Cardoso, C.A.; Heredia Vieira, S.C.; Vieira, M.d.C.; de Picoli Souza, K.; Amaya-Farfan, J.; Borges Castro Carvalho, G.C.; Moura, C.S.; Morato, P.N. Investigation of the Antioxidant and Hypoglycemiant Properties of Alibertia edulis (L.C. Rich.) A.C. Rich. Leaves. J. Ethnopharmacol. 2020, 253, 112648. [Google Scholar] [CrossRef] [PubMed]
- Borges, P.R.S.; Edelenbos, M.; Larsen, E.; Hernandes, T.; Nunes, E.E.; de Barros Vilas Boas, E.V.; Pires, C.R.F. The Bioactive Constituents and Antioxidant Activities of Ten Selected Brazilian Cerrado Fruits. Food Chem. X 2022, 14, 100268. [Google Scholar] [CrossRef] [PubMed]
- Prado, L.G.; Arruda, H.S.; Peixoto Araujo, N.M.; de Oliveira Braga, L.E.; Banzato, T.P.; Pereira, G.A.; Figueiredo, M.C.; Ruiz, A.L.T.G.; Eberlin, M.N.; de Carvalho, J.E.; et al. Antioxidant, Antiproliferative and Healing Properties of Araticum (Annona crassiflora Mart.) Peel and Seed. Food Res. Int. 2020, 133, 109168. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.N.; Justino, A.B.; Martins, M.M.; Peixoto, L.G.; Vilela, D.D.; Santos, P.S.; Teixeira, T.L.; da Silva, C.V.; Goulart, L.R.; Pivatto, M.; et al. Stephalagine, an Alkaloid with Pancreatic Lipase Inhibitory Activity Isolated from the Fruit Peel of Annona crassiflora Mart. Ind. Crops Prod. 2017, 97, 324–329. [Google Scholar] [CrossRef]
- Justino, A.B.; Miranda, N.C.; Franco, R.R.; Martins, M.M.; da Silva, N.M.; Espindola, F.S. Annona muricata Linn. Leaf as a Source of Antioxidant Compounds with in Vitro Antidiabetic and Inhibitory Potential against α-Amylase, α-Glucosidase, Lipase, Non-Enzymatic Glycation and Lipid Peroxidation. Biomed. Pharmacother. 2018, 100, 83–92. [Google Scholar] [CrossRef]
- Olasehinde, O.R.; Afolabi, O.B.; Owolabi, O.V.; Akawa, A.B.; Omiyale, O.B. GC–MS Analysis of Phytochemical Constituents of Methanolic Fraction of Annona muricata Leaf and Its Inhibition against Two Key Enzymes Linked to Type II Diabetes. Sci. Afr. 2022, 16, e01178. [Google Scholar] [CrossRef]
- Boeing, J.S.; Ribeiro, D.; Chisté, R.C.; Visentainer, J.V.; Costa, V.M.; Freitas, M.; Fernandes, E. Chemical Characterization and Protective Effect of the Bactris setosa Mart. Fruit against Oxidative/Nitrosative Stress. Food Chem. 2017, 220, 427–437. [Google Scholar] [CrossRef]
- Dantas, M.B.V.C.; Júnior, O.R.P.; Campos, L.T.d.P.; Campos, É.G. Compounds of Tucum-Do-Cerrado (Bactris setosa) Fruit with Antioxidant Activity. Nat. Prod. Res. 2023, 37, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, D.M.O.; Justino, A.B.; Sousa, R.M.F.; Munoz, R.A.A.; de Aquino, F.J.T.; Martins, M.M.; Goulart, L.R.; Pivatto, M.; Espindola, F.S.; de Oliveira, A. Antioxidant Compounds from Banisteriopsis argyrophylla Leaves as α-Amylase, α-Glucosidase, Lipase, and Glycation Inhibitors. Bioorg. Chem. 2020, 105, 104335. [Google Scholar] [CrossRef] [PubMed]
- Malta, L.G.; Ghiraldini, F.G.; Reis, R.; Oliveira, M.d.V.; Silva, L.B.; Pastore, G.M. In Vivo Analysis of Antigenotoxic and Antimutagenic Properties of Two Brazilian Cerrado Fruits and the Identification of Phenolic Phytochemicals. Food Res. Int. 2012, 49, 604–611. [Google Scholar] [CrossRef]
- Ferreira, B.A.; da Silva, A.R.A.; Filbido, G.S.; Narita, I.M.P.; Pinheiro, A.P.d.O.; da Cruz e Silva, D.; Nascimento, E.; Villa, R.D.; de Oliveira, A.P. In Vitro Bioaccessibility of the Bioactive Compounds and Minerals in the Pulp and Peel of Buchenavia Tomentosa Eichler Fruits and Their Antioxidant Capacities. Meas. Food 2022, 8, 100064. [Google Scholar] [CrossRef]
- Roesler, R.; Catharino, R.R.; Malta, L.G.; Eberlin, M.N.; Pastore, G. Antioxidant Activity of Caryocar brasiliense (Pequi) and Characterization of Components by Electrospray Ionization Mass Spectrometry. Food Chem. 2008, 110, 711–717. [Google Scholar] [CrossRef]
- Caldeira, A.S.P.; Mbiakop, U.C.; Pádua, R.M.; van de Venter, M.; Matsabisa, M.G.; Campana, P.R.V.; Cortes, S.F.; Braga, F.C. Bioguided Chemical Characterization of Pequi (Caryocar brasiliense) Fruit Peels towards an Anti-Diabetic Activity. Food Chem. 2021, 345, 128734. [Google Scholar] [CrossRef] [PubMed]
- Giordani, M.A.; Collicchio, T.C.M.; Ascêncio, S.D.; de Oliveira Martins, D.T.; Balogun, S.O.; Bieski, I.G.C.; da Silva, L.A.; Colodel, E.M.; de Souza, R.L.; de Souza, D.L.P.; et al. Hydroethanolic Extract of the Inner Stem Bark of Cedrela Odorata Has Low Toxicity and Reduces Hyperglycemia Induced by an Overload of Sucrose and Glucose. J. Ethnopharmacol. 2015, 162, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Alves, S.C.; Pereira, R.S.; Pereira, A.B.; Ferreira, A.; Mecha, E.; Silva, A.B.; Serra, A.T.; Bronze, M.R. Identification of Functional Compounds in Baru (Dipteryx alata Vog.) Nuts: Nutritional Value, Volatile and Phenolic Composition, Antioxidant Activity and Antiproliferative Effect. Food Res. Int. 2020, 131, 109026. [Google Scholar] [CrossRef]
- Lemos, M.R.B.; Siqueira, E.M.d.A.; Arruda, S.F.; Zambiazi, R.C. The Effect of Roasting on the Phenolic Compounds and Antioxidant Potential of Baru Nuts [Dipteryx alata Vog.]. Food Res. Int. 2012, 48, 592–597. [Google Scholar] [CrossRef]
- Siqueira, E.M.d.A.; Marin, A.M.F.; da Cunha, M.d.S.B.; Fustinoni, A.M.; de Sant’Ana, L.P.; Arruda, S.F. Consumption of Baru Seeds [Dipteryx alata Vog.], a Brazilian Savanna Nut, Prevents Iron-Induced Oxidative Stress in Rats. Food Res. Int. 2012, 45, 427–433. [Google Scholar] [CrossRef]
- Leite, N.R.; de Araújo, L.C.A.; Dos Santos da Rocha, P.; Agarrayua, D.A.; Ávila, D.S.; Carollo, C.A.; Silva, D.B.; Estevinho, L.M.; de Picoli Souza, K.; Dos Santos, E.L. Baru Pulp (Dipteryx alata Vogel): Fruit from the Brazilian Savanna Protects against Oxidative Stress and Increases the Life Expectancy of Caenorhabditis Elegans via SOD-3 and DAF-16. Biomolecules 2020, 10, 1106. [Google Scholar] [CrossRef]
- Outuki, P.M.; de Francisco, L.M.B.; Hoscheid, J.; Bonifácio, K.L.; Barbosa, D.S.; Cardoso, M.L.C. Development of Arabic and Xanthan Gum Microparticles Loaded with an Extract of Eschweilera nana Miers Leaves with Antioxidant Capacity. Colloids Surf. A Physicochem. Eng. Asp. 2016, 499, 103–112. [Google Scholar] [CrossRef]
- Donado-Pestana, C.M.; Belchior, T.; Genovese, M.I. Phenolic Compounds from Cagaita (Eugenia dysenterica DC.) Fruit Prevent Body Weight and Fat Mass Gain Induced by a High-Fat, High-Sucrose Diet. Food Res. Int. 2015, 77, 177–185. [Google Scholar] [CrossRef]
- Justino, A.B.; Guerra Silva, H.C.; Franco, R.R.; de Oliveira Cavalcante Pimentel, I.; Silva, N.F.; Saraiva, A.L.; Espindola, F.S. Flavonoids and Proanthocyanidins-Rich Fractions from Eugenia dysenterica Fruits and Leaves Inhibit the Formation of Advanced Glycation End-Products and the Activities of α-Amylase and α-Glucosidase. J. Ethnopharmacol. 2022, 285, 114902. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Carvalho-Silva, L.B.; Morales, J.P.; Malta, L.G.; Muramoto, M.T.; Ferreira, J.E.M.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Maróstica Junior, M.R.; Pastore, G.M. Evaluation of the Antioxidant, Antiproliferative and Antimutagenic Potential of Araçá-Boi Fruit (Eugenia stipitata Mc Vaugh—Myrtaceae) of the Brazilian Amazon Forest. Food Res. Int. 2013, 50, 70–76. [Google Scholar] [CrossRef]
- Carneiro, N.S.; Alves, C.C.F.; Alves, J.M.; Egea, M.B.; Martins, C.H.G.; Silva, T.S.; Bretanha, L.C.; Balleste, M.P.; Micke, G.A.; Silveira, E.V.; et al. Chemical Composition, Antioxidant and Antibacterial Activities of Essential Oils from Leaves and Flowers of Eugenia Klotzschiana Berg (Myrtaceae). An. Acad. Bras. Ciênc. 2017, 89, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- de Lima, J.P.; Fante, C.A.; Freitas Pires, C.R.; Nunes, E.E.; Alves, R.R.; de Siqueira Elias, H.H.; Nunes, C.A.; de Barros Vilas Boas, E.V. The Antioxidative Potential and Volatile Constituents of Mangaba Fruit over the Storage Period. Sci. Hortic. 2015, 194, 1–6. [Google Scholar] [CrossRef]
- da Silva, C.P.; Soares-Freitas, R.A.M.; Sampaio, G.R.; Santos, M.C.B.; do Nascimento, T.P.; Cameron, L.C.; Ferreira, M.S.L.; Arêas, J.A.G. Identification and Action of Phenolic Compounds of Jatobá-Do-Cerrado (Hymenaea stignocarpa Mart.) on α-Amylase and α-Glucosidase Activities and Flour Effect on Glycemic Response and Nutritional Quality of Breads. Food Res. Int. 2019, 116, 1076–1083. [Google Scholar] [CrossRef]
- dos Santos, K.P.; Sedano-Partida, M.D.; Sala-Carvalho, W.R.; Loureiro, B.O.S.J.; da Silva-Luz, C.L.; Furlan, C.M. Biological Activity of Hyptis Jacq. (Lamiaceae) Is Determined by the Environment. Ind. Crops Prod. 2018, 112, 705–715. [Google Scholar] [CrossRef]
- Justino, A.B.; Santana, E.C.; Franco, R.R.; Queiroz, J.S.; Silva, H.C.G.; de Lima, J.P.; Saraiva, A.L.; Martins, M.M.; Lemos de Morais, S.A.; de Oliveira, A.; et al. Antioxidant Compounds of Kielmeyera coriacea Mart. with α-Amylase, Lipase and Advanced Glycation End-Product Inhibitory Activities. J. Pharm. Biomed. Anal. 2021, 206, 114387. [Google Scholar] [CrossRef]
- Cândido, T.L.N.; Silva, M.R.; Agostini-Costa, T.S. Bioactive Compounds and Antioxidant Capacity of Buriti (Mauritia flexuosa L.f.) from the Cerrado and Amazon Biomes. Food Chem. 2015, 177, 313–319. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.M.; Siqueira, E.P.; Nunes, Y.R.F.; Cota, B.B. Flavonoids from Leaves of Mauritia flexuosa. Rev. Bras. Farmacogn. 2013, 23, 614–620. [Google Scholar] [CrossRef]
- Koolen, H.H.F.; da Silva, F.M.A.; Gozzo, F.C.; de Souza, A.Q.L.; de Souza, A.D.L. Antioxidant, Antimicrobial Activities and Characterization of Phenolic Compounds from Buriti (Mauritia flexuosa L. f.) by UPLC–ESI-MS/MS. Food Res. Int. 2013, 51, 467–473. [Google Scholar] [CrossRef]
- Leite, P.I.P.; Barreto, S.M.A.G.; Freitas, P.R.; de Araújo, A.C.J.; Paulo, C.L.R.; de Almeida, R.S.; de Assis, C.F.; Padilha, C.E.A.; Ferrari, M.; de Sousa Junior, F.C. Extraction of Bioactive Compounds from Buriti (Mauritia flexuosa L.) Fruit by Eco-Friendly Solvents: Chemical and Functional Characterization. Sustain. Chem. Pharm. 2021, 22, 100489. [Google Scholar] [CrossRef]
- Nobre, C.B.; Sousa, E.O.; Camilo, C.J.; Machado, J.F.; Silva, J.M.F.L.; Filho, J.R.; Coutinho, H.D.M.; Costa, J.G.M. Antioxidative Effect and Phytochemical Profile of Natural Products from the Fruits of “Babaçu” (Orbignia speciose) and “Buriti” (Mauritia flexuosa). Food Chem. Toxicol. 2018, 121, 423–429. [Google Scholar] [CrossRef]
- Resende, L.M.; Franca, A.S.; Oliveira, L.S. Buriti (Mauritia flexuosa L. f.) Fruit by-Products Flours: Evaluation as Source of Dietary Fibers and Natural Antioxidants. Food Chem. 2019, 270, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.; Antunes, L.; Darin, J.; Mercadante, A.; Bianchi, M.D.L. Buriti (Mauritia flexuosa) Oil: Evaluation of the Mutagenic and Antimutagenic Potential by the Micronucleus Test in Vivo. Toxicol. Lett. 2010, 196, S163–S164. [Google Scholar] [CrossRef]
- Rudke, A.R.; Andrade, K.S.; Mazzutti, S.; Zielinski, A.A.F.; Alves, V.R.; Vitali, L.; Ferreira, S.R.S. A Comparative Study of Phenolic Compounds Profile and in Vitro Antioxidant Activity from Buriti (Mauritia flexuosa) by-Products Extracts. LWT 2021, 150, 111941. [Google Scholar] [CrossRef]
- de Souza, F.G.; Náthia-Neves, G.; de Araújo, F.F.; Dias Audibert, F.L.; Delafiori, J.; Neri-Numa, I.A.; Catharino, R.R.; de Alencar, S.M.; de Almeida Meireles, M.A.; Pastore, G.M. Evaluation of Antioxidant Capacity, Fatty Acid Profile, and Bioactive Compounds from Buritirana (Mauritiella armata Mart.) Oil: A Little-Explored Native Brazilian Fruit. Food Res. Int. 2021, 142, 110260. [Google Scholar] [CrossRef]
- Serpeloni, J.M.; Leal Specian, A.F.; Ribeiro, D.L.; Tuttis, K.; Vilegas, W.; Martínez-López, W.; Dokkedal, A.L.; Saldanha, L.L.; de Syllos Cólus, I.M.; Varanda, E.A. Antimutagenicity and Induction of Antioxidant Defense by Flavonoid Rich Extract of Myrcia bella Cambess. in Normal and Tumor Gastric Cells. J. Ethnopharmacol. 2015, 176, 345–355. [Google Scholar] [CrossRef]
- Duarte, I.; de Souza, M.C.M.; Curinga, R.M.; Mendonça, H.M.; de Oliveira, L.d.L.; Milenkovic, D.; Hassimotto, N.M.A.; Costa, A.M.; Malaquias, J.V.; Borges, T.K.d.S. Effect of Passiflora Setacea Juice and Its Phenolic Metabolites on Insulin Resistance Markers in Overweight Individuals and on Microglial Cell Activity. Food Funct. 2022, 13, 6498–6509. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.M.; Dutra Gomes, J.V.; Jamal, C.M.; Cunha Neto, Á.; Santos, M.L.; Fagg, C.W.; Fonseca-Bazzo, Y.M.; Magalhães, P.d.O.; de Sales, P.M.; Silveira, D. Triterpenes from Pouteria ramiflora (Mart.) Radlk. Leaves (Sapotaceae). Food Chem. Toxicol. 2017, 109, 1063–1068. [Google Scholar] [CrossRef]
- de Sales, P.M.; de Souza, P.M.; Dartora, M.; Resck, I.S.; Simeoni, L.A.; Fonseca-Bazzo, Y.M.; de Oliveira Magalhães, P.; Silveira, D. Pouteria Torta Epicarp as a Useful Source of α-Amylase Inhibitor in the Control of Type 2 Diabetes. Food Chem. Toxicol. 2017, 109, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.L.; Haas, L.I.R.; Chaves, F.C.; Salvador, M.; Zambiazi, R.C.; da Silva, W.P.; Nora, L.; Rombaldi, C.V. Araçá (Psidium cattleianum Sabine) Fruit Extracts with Antioxidant and Antimicrobial Activities and Antiproliferative Effect on Human Cancer Cells. Food Chem. 2011, 128, 916–922. [Google Scholar] [CrossRef]
- dos Santos da Rocha, P.; de Araújo Boleti, A.P.; do Carmo Vieira, M.; Carollo, C.A.; da Silva, D.B.; Estevinho, L.M.; dos Santos, E.L.; de Picoli Souza, K. Microbiological Quality, Chemical Profile as Well as Antioxidant and Antidiabetic Activities of Schinus Terebinthifolius Raddi. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 220, 36–46. [Google Scholar] [CrossRef]
- Olinto, S.C.F.; Calil-Silveira, J.; Dias, R.B.A.; Valle, M.M.R.; Serrano-Nascimento, C.; Rocha, P.S.; Monteiro-Alfredo, T.; dos Santos, E.L.; de Picoli Souza, K.; Nunes, M.T. Antiproliferative and Cytotoxic Effects of Schinus Terebinthifolia Leaf Extract on Thyroid Follicular Cells. Rev. Bras. Farmacogn. 2020, 30, 693–700. [Google Scholar] [CrossRef]
- de Britto Policarpi, P.; Turcatto, L.; Demoliner, F.; Ferrari, R.A.; Bascuñan, V.L.A.F.; Ramos, J.C.; Jachmanián, I.; Vitali, L.; Micke, G.A.; Block, J.M. Nutritional Potential, Chemical Profile and Antioxidant Activity of Chichá (Sterculia striata) Nuts and Its by-Products. Food Res. Int. 2018, 106, 736–744. [Google Scholar] [CrossRef]
- Pereira, A.P.A.; Angolini, C.F.F.; Paulino, B.N.; Lauretti, L.B.C.; Orlando, E.A.; Silva, J.G.S.; Neri-Numa, I.A.; Souza, J.D.R.P.; Pallone, J.A.L.; Eberlin, M.N.; et al. A Comprehensive Characterization of Solanum lycocarpum St. Hill and Solanum oocarpum Sendtn: Chemical Composition and Antioxidant Properties. Food Res. Int. 2019, 124, 61–69. [Google Scholar] [CrossRef]
- Franco, R.R.; Justino, A.B.; Martins, M.M.; Silva, C.G.; Campana, P.R.V.; Lopes, J.C.D.; De Almeida, V.L.; Espindola, F.S. Phytoscreening of Vochysiaceae Species: Molecular Identification by HPLC-ESI-MS/MS and Evaluating of Their Antioxidant Activity and Inhibitory Potential against Human α-Amylase and Protein Glycation. Bioorg. Chem. 2019, 91, 103122. [Google Scholar] [CrossRef]
- Campos, J.F.; de Castro, D.T.H.; Damião, M.J.; Vieira Torquato, H.F.; Paredes-Gamero, E.J.; Carollo, C.A.; Estevinho, L.M.; de Picoli Souza, K.; Dos Santos, E.L. The Chemical Profile of Senna velutina Leaves and Their Antioxidant and Cytotoxic Effects. Oxidative Med. Cell. Longev. 2016, 2016, 8405957. [Google Scholar] [CrossRef]
- Castro, D.T.H.; Campos, J.F.; Damião, M.J.; Torquato, H.F.V.; Paredes-Gamero, E.J.; Carollo, C.A.; Rodrigues, E.G.; de Picoli Souza, K.; dos Santos, E.L. Ethanolic Extract of Senna velutina Roots: Chemical Composition, In Vitro and In Vivo Antitumor Effects, and B16F10-Nex2 Melanoma Cell Death Mechanisms. Oxidative Med. Cell. Longev. 2019, 2019, e5719483. [Google Scholar] [CrossRef]
- Luna, E.M.; Freitas, T.S.; Campina, F.F.; Costa, M.S.; Rocha, J.E.; Cruz, R.P.; Sena Júnior, D.L.; Silveira, Z.S.; Macedo, N.S.; Pinheiro, J.C.A.; et al. Evaluation of Phytochemical Composition, Toxicity in Drosophila Melanogaster and Effects on Antibiotics Modulation of Plathymenia Reticulata Benth Extract. Toxicol. Rep. 2021, 8, 732–739. [Google Scholar] [CrossRef]
- Marinho, B.M.; Guimarães, V.H.D.; Sousa, J.N.; Moraes, D.S.; Gomes, E.S.B.; Vieira, C.R.; dos Reis, S.T.; Costa, T.d.O.; Farias, L.C.; Guimarães, A.L.S.; et al. Brazilian Cerrado Plant (Arnica) Lychnophora ericoides Mart. (Asteraceae) Toxicity Characterization in Mice. Phytomed. Plus 2022, 2, 100154. [Google Scholar] [CrossRef]
- Mendonça, L.a.B.M.; Matias, R.; Zanella, D.F.P.; Porto, K.R.A.; Guilhermino, J.F.; Moreira, D.L.; Roel, A.R.; Pott, A.; Carvalho, C.M.E. Toxicity and Phytochemistry of Eight Species Used in the Traditional Medicine of Sul-Mato-Grossense, Brazil. Braz. J. Biol. 2020, 80, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Palozi, R.A.C.; Guarnier, L.P.; Romão, P.V.M.; Nocchi, S.R.; dos Santos, C.C.; Lourenço, E.L.B.; Silva, D.B.; Gasparotto, F.M.; Gasparotto Junior, A. Pharmacological Safety of Plinia cauliflora (Mart.) Kausel in Rabbits. Toxicol. Rep. 2019, 6, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Traesel, G.K.; de Souza, J.C.; de Barros, A.L.; Souza, M.A.; Schmitz, W.O.; Muzzi, R.M.; Oesterreich, S.A.; Arena, A.C. Acute and Subacute (28 Days) Oral Toxicity Assessment of the Oil Extracted from Acrocomia aculeata Pulp in Rats. Food Chem. Toxicol. 2014, 74, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, C.; Teschke, R. Herbal Hepatotoxicity: Clinical Characteristics and Listing Compilation. Int. J. Mol. Sci. 2016, 17, 588. [Google Scholar] [CrossRef]
- Murphy, R.; Carroll, R.W.; Krebs, J.D. Pathogenesis of the Metabolic Syndrome: Insights from Monogenic Disorders. Mediat. Inflamm. 2013, 2013, e920214. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic Syndrome: Pathophysiology, Management, and Modulation by Natural Compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Rodrigues, T.; Matafome, P.; Santos-Silva, D.; Sena, C.; Seiça, R. Reduction of Methylglyoxal-Induced Glycation by Pyridoxamine Improves Adipose Tissue Microvascular Lesions. Available online: https://www.hindawi.com/journals/jdr/2013/690650/ (accessed on 8 February 2021).
- Teixeira, G.P.; Faria, R.X. Influence of Purinergic Signaling on Glucose Transporters: A Possible Mechanism against Insulin Resistance? Eur. J. Pharmacol. 2021, 892, 173743. [Google Scholar] [CrossRef]
- Saadeldeen, F.S.A.; Niu, Y.; Wang, H.; Zhou, L.; Meng, L.; Chen, S.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Liu, Z.; Kang, W. Natural Products: Regulating Glucose Metabolism and Improving Insulin Resistance. Food Sci. Hum. Wellness 2020, 9, 214–228. [Google Scholar] [CrossRef]
- Rodrigues, T.; Matafome, P.; Sereno, J.; Almeida, J.; Castelhano, J.; Gamas, L.; Neves, C.; Gonçalves, S.; Carvalho, C.; Arslanagic, A.; et al. Methylglyoxal-Induced Glycation Changes Adipose Tissue Vascular Architecture, Flow and Expansion, Leading to Insulin Resistance. Sci. Rep. 2017, 7, 1698. [Google Scholar] [CrossRef] [PubMed]
- Saad, B. Prevention and Treatment of Obesity-Related Inflammatory Diseases by Edible and Medicinal Plants and Their Active Compounds. Immuno 2022, 2, 609–629. [Google Scholar] [CrossRef]
- Boonchaya-anant, P.; Apovian, C.M. Metabolically Healthy Obesity—Does It Exist? Curr. Atheroscler. Rep. 2014, 16, 441. [Google Scholar] [CrossRef]
- Rutkowski, J.M.; Davis, K.E.; Scherer, P.E. Mechanisms of Obesity and Related Pathologies: The Macro- and Microcirculation of Adipose Tissue. FEBS J. 2009, 276, 5738–5746. [Google Scholar] [CrossRef] [PubMed]
- Bakker, S.J.; IJzerman, R.G.; Teerlink, T.; Westerhoff, H.V.; Gans, R.O.; Heine, R.J. Cytosolic Triglycerides and Oxidative Stress in Central Obesity: The Missing Link between Excessive Atherosclerosis, Endothelial Dysfunction, and Beta-Cell Failure? Atherosclerosis 2000, 148, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.P.; Gastaldi, G.; Bobbioni-Harsch, E.; Arboit, P.; Gobelet, C.; Dériaz, O.; Golay, A.; Witztum, J.L.; Giacobino, J.-P. Lipid Peroxidation in Skeletal Muscle of Obese as Compared to Endurance-Trained Humans: A Case of Good vs. Bad Lipids? FEBS Lett. 2003, 551, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.A. Mitochondrial Oxidative Stress and Inflammation: An Slalom to Obesity and Insulin Resistance. J. Physiol. Biochem. 2006, 62, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Mastrototaro, L.; Roden, M. Insulin Resistance and Insulin Sensitizing Agents. Metabolism 2021, 125, 154892. [Google Scholar] [CrossRef] [PubMed]
- Gooda Sahib, N.; Saari, N.; Ismail, A.; Khatib, A.; Mahomoodally, F.; Abdul Hamid, A. Plants’ Metabolites as Potential Antiobesity Agents. Sci. World J. 2012, 2012, e436039. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Ferreira, A.V.M.; Oliveira, M.C.; Teixeira, M.M.; Brandão, M.G.L. Effects of Xylopia aromatica (Lam.) Mart. Fruit on Metabolic and Inflammatory Dysfunction Induced by High Refined Carbohydrate-Containing-Diet in Mice. Food Res. Int. 2014, 62, 541–550. [Google Scholar] [CrossRef][Green Version]
- Frediani Brant, N.M.; Mourão Gasparotto, F.; de Oliveira Araújo, V.; Christian Maraschin, J.; Lima Ribeiro, R.d.C.; Botelho Lourenço, E.L.; Cardozo Junior, E.L.; Gasparotto Junior, A. Cardiovascular Protective Effects of Casearia Sylvestris Swartz in Swiss and C57BL/6 LDLr-Null Mice Undergoing High Fat Diet. J. Ethnopharmacol. 2014, 154, 419–427. [Google Scholar] [CrossRef]
- Sousa, J.N.; Mafra, V.; Marinho, B.M.; Guimarães, V.H.D.; Oliveira, L.P.; dos Reis, S.T.; Costa, T.; Vieira, C.R.; de Paula, A.M.B.; Guimarães, A.L.S.; et al. Oral Treatment with Davilla elliptica A. St,-Hil. Leaves Improves Liver Steatosis and Lipid Metabolism on a Diet-Induced Obese Mice Model. Phytomed. Plus 2021, 1, 100130. [Google Scholar] [CrossRef]
- Brisson, D.; Larouche, M.; Chebli, J.; Khoury, E.; Gaudet, D. Correlation between Chylomicronemia Diagnosis Scores and Post-Heparin Lipoprotein Lipase Activity. Clin. Biochem. 2023, 114, 67–72. [Google Scholar] [CrossRef]
- Holmbäck, U.; Forslund, A.; Grudén, S.; Alderborn, G.; Söderhäll, A.; Hellström, P.M.; Lennernäs, H. Effects of a Novel Combination of Orlistat and Acarbose on Tolerability, Appetite, and Glucose Metabolism in Persons with Obesity. Obes. Sci. Pract. 2020, 6, 313–323. [Google Scholar] [CrossRef]
- Pu, Y.; Chen, L.; He, X.; Cao, J.; Jiang, W. Soluble Polysaccharides Decrease Inhibitory Activity of Banana Condensed Tannins against Porcine Pancreatic Lipase. Food Chem. 2023, 418, 136013. [Google Scholar] [CrossRef]
- Veloso, C.d.C.; de Oliveira, M.C.; Oliveira, C.d.C.; Rodrigues, V.G.; Giusti-Paiva, A.; Teixeira, M.M.; Duarte, I.D.; Ferreira, A.V.M.; Perez, A.d.C. Hydroethanolic Extract of Pyrostegia venusta (Ker Gawl.) Miers Flowers Improves Inflammatory and Metabolic Dysfunction Induced by High-Refined Carbohydrate Diet. J. Ethnopharmacol. 2014, 151, 722–728. [Google Scholar] [CrossRef]
- Donado-Pestana, C.M.; dos Santos-Donado, P.R.; Daza, L.D.; Belchior, T.; Festuccia, W.T.; Genovese, M.I. Cagaita Fruit (Eugenia dysenterica DC.) and Obesity: Role of Polyphenols on Already Established Obesity. Food Res. Int. 2018, 103, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, D.R.; Mineo, J.R.; de Souza, M.A.; de Paula, J.E.; Espindola, L.S.; Espindola, F.S. Down-Modulation of Nitric Oxide Production in Murine Macrophages Treated with Crude Plant Extracts from the Brazilian Cerrado. J. Ethnopharmacol. 2005, 99, 37–41. [Google Scholar] [CrossRef]
- Semple, R.K.; Chatterjee, V.K.K.; O’Rahilly, S. PPAR Gamma and Human Metabolic Disease. J. Clin. Investig. 2006, 116, 581–589. [Google Scholar] [CrossRef]
- Vanden Berghe, W.; Vermeulen, L.; Delerive, P.; De Bosscher, K.; Staels, B.; Haegeman, G. A Paradigm for Gene Regulation: Inflammation, NF-κB and PPAR. Adv. Exp. Med. Biol. 2003, 544, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Lee, C.-H.; Tiep, S.; Yu, R.T.; Ham, J.; Kang, H.; Evans, R.M. Peroxisome-Proliferator-Activated Receptor Delta Activates Fat Metabolism to Prevent Obesity. Cell 2003, 113, 159–170. [Google Scholar] [CrossRef]
- Gomes-Copeland, K.K.P.; Meireles, C.G.; Gomes, J.V.D.; Torres, A.G.; Sinoti, S.B.P.; Fonseca-Bazzo, Y.M.; Magalhães, P.d.O.; Fagg, C.W.; Simeoni, L.A.; Silveira, D. Hippeastrum stapfianum (Kraenzl.) R.S.Oliveira & Dutilh (Amaryllidaceae) Ethanol Extract Activity on Acetylcholinesterase and PPAR-α/γ Receptors. Plants 2022, 11, 3179. [Google Scholar] [CrossRef] [PubMed]
- Hithamani, G.; Ganesan, P. Polyphenols from Indian Cereal Grains Inhibit 3T3-L1 Adipogenesis through Modulating Early and Late Phase Adipogenic Markers. Food Biosci. 2022, 50, 102075. [Google Scholar] [CrossRef]
- Li, K.K.; Peng, J.M.; Zhu, W.; Cheng, B.H.; Li, C.M. Gallocatechin Gallate (GCG) Inhibits 3T3-L1 Differentiation and Lipopolysaccharide Induced Inflammation through MAPK and NF-κB Signaling. J. Funct. Foods 2017, 30, 159–167. [Google Scholar] [CrossRef]
- Bartelt, A.; Heeren, J. Adipose Tissue Browning and Metabolic Health. Nat. Rev. Endocrinol. 2014, 10, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.L.; Molica, F.; Kwak, B.R. Browning of White Adipose Tissue as a Therapeutic Tool in the Fight against Atherosclerosis. Metabolites 2021, 11, 319. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.-Y.; Li, X.-Y.; Wang, Y.-L.; Lang, D.-Q.; Liu, L.; Yi, Y.-K.; Liu, Q.; Shen, C.-Y. Natural Bioactive Constituents from Herbs and Nutraceuticals Promote Browning of White Adipose Tissue. Pharmacol. Res. 2022, 178, 106175. [Google Scholar] [CrossRef] [PubMed]
- Alves-Santos, A.M.; Fernandes, D.C.; Naves, M.M.V. Baru (Dipteryx alata Vog.) Fruit as an Option of Nut and Pulp with Advantageous Nutritional and Functional Properties: A Comprehensive Review. NFS J. 2021, 24, 26–36. [Google Scholar] [CrossRef]
- Mattes, R.D.; Kris-Etherton, P.M.; Foster, G.D. Impact of Peanuts and Tree Nuts on Body Weight and Healthy Weight Loss in Adults12. J. Nutr. 2008, 138, 1741S–1745S. [Google Scholar] [CrossRef]
- Dall’Agnol, R.; Lino von Poser, G. The Use of Complex Polysaccharides in the Management of Metabolic Diseases: The Case of Solanum lycocarpum Fruits. J. Ethnopharmacol. 2000, 71, 337–341. [Google Scholar] [CrossRef]
- Mayumi Usuda Prado Rocha, D.; Paula Silva Caldas, A.; Simões e Silva, A.C.; Bressan, J.; Hermana Miranda Hermsdorff, H. Nut-Enriched Energy Restricted Diet Has Potential to Decrease Hunger in Women at Cardiometabolic Risk: A Randomized Controlled Trial (Brazilian Nuts Study). Nutr. Res. 2023, 109, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.R.d.O.; de Santana, F.C.; Torres-Leal, F.L.; de Melo, I.L.P.; Yoshime, L.T.; Matos-Neto, E.M.; Seelaender, M.C.L.; Araújo, C.M.M.; Cogliati, B.; Mancini-Filho, J. Pequi (Caryocar brasiliense Camb.) Almond Oil Attenuates Carbon Tetrachloride-Induced Acute Hepatic Injury in Rats: Antioxidant and Anti-Inflammatory Effects. Food Chem. Toxicol. 2016, 97, 205–216. [Google Scholar] [CrossRef]
- Fowler, M.J. Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- WHO. Cardiovascular Diseases. Available online: https://www.who.int/westernpacific/health-topics/cardiovascular-diseases (accessed on 11 October 2021).
- Viigimaa, M.; Sachinidis, A.; Toumpourleka, M.; Koutsampasopoulos, K.; Alliksoo, S.; Titma, T. Macrovascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 110–116. [Google Scholar] [CrossRef]
- Mohamed, S.M.; Shalaby, M.A.; El-Shiekh, R.A.; El-Banna, H.A.; Emam, S.R.; Bakr, A.F. Metabolic Syndrome: Risk Factors, Diagnosis, Pathogenesis, and Management with Natural Approaches. Food Chem. Adv. 2023, 3, 100335. [Google Scholar] [CrossRef]
- Olasehinde, O.R.; Afolabi, O.B. Identification of Bioactive Constituents of Chloroform Fraction from Annona muricata Leaf, Its Antioxidant Activity and Inhibitory Potential against Carbohydrate-Hydrolyzing α-Amylase and α-Glucosidase Activities Linked to Type II Diabetes Mellitus: In Vitro Study. J. Herbmed. Pharmacol. 2022, 12, 100–108. [Google Scholar] [CrossRef]
- Ali Asgar, M. Anti-Diabetic Potential of Phenolic Compounds: A Review. Int. J. Food Prop. 2013, 16, 91–103. [Google Scholar] [CrossRef]
- Pinto, L.C.; Cerqueira-Lima, A.T.; Suzarth, S.d.S.; de Souza, R.; Tosta, B.R.; da Silva, H.B.; Pires, A.d.O.; Queiroz, G.d.A.; Teixeira, T.O.; Dourado, K.M.C.; et al. Anonna muricata L. (Soursop) Seed Oil Improves Type 1 Diabetes Parameters in Vivo and in Vitro. PharmaNutrition 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Florence, N.T.; Benoit, M.Z.; Jonas, K.; Alexandra, T.; Désiré, D.D.P.; Pierre, K.; Théophile, D. Antidiabetic and Antioxidant Effects of Annona muricata (Annonaceae), Aqueous Extract on Streptozotocin-Induced Diabetic Rats. J. Ethnopharmacol. 2014, 151, 784–790. [Google Scholar] [CrossRef]
- Nunes, Â.A.; Buccini, D.F.; dos Santos Jaques, J.A.; Portugal, L.C.; Guimarães, R.C.A.; Favaro, S.P.; de Araújo Caldas, R.; Carvalho, C.M.E. Effect of Dietary Acrocomia aculeata Kernel Oil Rich in Medium Chain Fatty Acids on Type 2 Diabetic Rats. J. Funct. Foods 2020, 75, 104295. [Google Scholar] [CrossRef]
- da Silva, P.V.B.; Ramiro, M.M.; Iriguchi, E.K.K.; Corrêa, W.A.; Lowe, J.; Cardoso, C.A.L.; Arena, A.C.; Kassuya, C.A.L.; Muzzi, R.M. Antidiabetic, Cytotoxic and Antioxidant Activities of Oil Extracted from Acrocomia aculeata Pulp. Nat. Prod. Res. 2019, 33, 2413–2416. [Google Scholar] [CrossRef]
- Gomes, J.H.d.S.; Mbiakop, U.C.; Oliveira, R.L.; Stehmann, J.R.; de Pádua, R.M.; Cortes, S.F.; Braga, F.C. Polyphenol-Rich Extract and Fractions of Terminalia phaeocarpa Eichler Possess Hypoglycemic Effect, Reduce the Release of Cytokines, and Inhibit Lipase, α-Glucosidase, and α-Amilase Enzymes. J. Ethnopharmacol. 2021, 271, 113847. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.G.; Salles, B.C.C.; Bini, I.F.; Castaldini, L.P.; Silva, L.C.D.; Vilela, A.A.; Micheloni, A.L.C.; da Silva, G.M.; da Silva, P.H.C.; Maure, A.K.; et al. Phytochemical Composition, Antioxidant and in Vivo Antidiabetic Activities of the Hydroethanolic Extract of Eugenia florida DC. (Myrtaceae) Leaves. South Afr. J. Bot. 2019, 123, 317–332. [Google Scholar] [CrossRef]
- Mbiakop, U.C.; Gomes, J.H.S.; Pádua, R.M.; Lemos, V.S.; Braga, F.C.; Cortes, S.F. Oral Sub-Chronic Treatment with Terminalia phaeocarpa Eichler (Combretaceae) Reduces Liver PTP1B Activity in a Murine Model of Diabetes. J. Ethnopharmacol. 2023, 306, 116164. [Google Scholar] [CrossRef]
- e Silva, A.L.L.; dos Santos, D.C.; de Sousa, T.L.; Silva, F.G.; Egea, M.B. “Cerrado” Cashew (Anacardium othonianum Rizz.) Juice Improves Metabolic Parameters in Women: A Pilot Study. J. Funct. Foods 2020, 69, 103950. [Google Scholar] [CrossRef]
- Talpo, T.C.; Motta, B.P.; de Oliveira, J.O.; Figueiredo, I.D.; Pinheiro, C.G.; dos Santos, C.H.C.; de Carvalho, M.G.; Brunetti, I.L.; Baviera, A.M. Siolmatra Brasiliensis Stem Extract Ameliorates Antioxidant Defenses and Mitigates Glycoxidative Stress in Mice with High-Fat Diet-Induced Obesity. Obes. Res. Clin. Pract. 2022, 16, 130–137. [Google Scholar] [CrossRef]
- de Araujo, R.L.; Tomás-Barberán, F.A.; dos Santos, R.F.; Martinez-Blazquez, J.A.; Genovese, M.I. Postprandial Glucose-Lowering Effect of Cagaita (Eugenia dysenterica DC) Fruit Juice in Dysglycemic Subjects with Metabolic Syndrome: An Exploratory Study. Food Res. Int. 2021, 142, 110209. [Google Scholar] [CrossRef] [PubMed]
- de Souza, V.R.; Pereira, P.A.P.; Queiroz, F.; Borges, S.V.; de Deus Souza Carneiro, J. Determination of Bioactive Compounds, Antioxidant Activity and Chemical Composition of Cerrado Brazilian Fruits. Food Chem. 2012, 134, 381–386. [Google Scholar] [CrossRef]
- Morais, M.G.; Saldanha, A.A.; Azevedo, L.S.; Mendes, I.C.; Rodrigues, J.P.C.; Amado, P.A.; Farias, K.d.S.; Zanuncio, V.S.S.; Cassemiro, N.S.; da Silva, D.B.; et al. Antioxidant and Anti-Inflammatory Effects of Fractions from Ripe Fruits of Solanum lycocarpum St. Hil. (Solanaceae) and Putative Identification of Bioactive Compounds by GC–MS and LC-DAD-MS. Food Res. Int. 2022, 156, 111145. [Google Scholar] [CrossRef] [PubMed]
- Neves, C.; Rodrigues, T.; Sereno, J.; Simões, C.; Castelhano, J.; Gonçalves, J.; Bento, G.; Gonçalves, S.; Seiça, R.; Domingues, M.R.; et al. Dietary Glycotoxins Impair Hepatic Lipidemic Profile in Diet-Induced Obese Rats Causing Hepatic Oxidative Stress and Insulin Resistance. Oxidative Med. Cell. Longev. 2019, 2019, e6362910. [Google Scholar] [CrossRef]
- Al-Aubaidy, H.A.; Jelinek, H.F. Oxidative DNA Damage and Obesity in Type 2 Diabetes Mellitus. Eur. J. Endocrinol. 2011, 164, 899–904. [Google Scholar] [CrossRef]
- Bao, W.; Song, F.; Li, X.; Rong, S.; Yang, W.; Zhang, M.; Yao, P.; Hao, L.; Yang, N.; Hu, F.B.; et al. Plasma Heme Oxygenase-1 Concentration Is Elevated in Individuals with Type 2 Diabetes Mellitus. PLoS ONE 2010, 5, e12371. [Google Scholar] [CrossRef]
- Chandrakumar, L.; Bagyánszki, M.; Szalai, Z.; Mezei, D.; Bódi, N. Diabetes-Related Induction of the Heme Oxygenase System and Enhanced Colocalization of Heme Oxygenase 1 and 2 with Neuronal Nitric Oxide Synthase in Myenteric Neurons of Different Intestinal Segments. Oxidative Med. Cell. Longev. 2017, 2017, e1890512. [Google Scholar] [CrossRef]
- Dong, Q.Y.; Cui, Y.; Chen, L.; Song, J.; Sun, L. Urinary 8-Hydroxydeoxyguanosine Levels in Diabetic Retinopathy Patients. Eur. J. Ophthalmol. 2008, 18, 94–98. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Agarwal, E.; Bajaj, P.; Rao, N.S. 4-Hydroxy-2-Nonenal, an Oxidative Stress Marker in Crevicular Fluid and Serum in Type 2 Diabetes with Chronic Periodontitis. Contemp. Clin. Dent. 2013, 4, 281–285. [Google Scholar] [CrossRef]
- Toyokuni, S.; Yamada, S.; Kashima, M.; Ihara, Y.; Yamada, Y.; Tanaka, T.; Hiai, H.; Seino, Y.; Uchida, K. Serum 4-Hydroxy-2-Nonenal-Modified Albumin Is Elevated in Patients with Type 2 Diabetes Mellitus. Antioxid. Redox Signal. 2000, 2, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ Res 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Okeleji, L.O.; Ajayi, A.F.; Adebayo-Gege, G.; Aremu, V.O.; Adebayo, O.I.; Adebayo, E.T. Epidemiologic Evidence Linking Oxidative Stress and Pulmonary Function in Healthy Populations. Chronic Dis. Transl. Med. 2021, 7, 88–99. [Google Scholar] [CrossRef]
- Barbosa, K.B.F.; Costa, N.M.B.; Alfenas, R.d.C.G.; De Paula, S.O.; Minim, V.P.R.; Bressan, J. Estresse oxidativo: Conceito, implicações e fatores modulatórios. Rev. Nutr. 2010, 23, 629–643. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal Prospects of Antioxidants: A Review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant Activity in Meat Treated with Oregano and Sage Essential Oils. Food Chem. 2008, 106, 1188–1194. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity and Its Impact on Metabolic Syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J.-W. Reactive Oxygen Species Facilitate Adipocyte Differentiation by Accelerating Mitotic Clonal Expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef]
- Kaneto, H.; Matsuoka, T. Involvement of Oxidative Stress in Suppression of Insulin Biosynthesis under Diabetic Conditions. Int. J. Mol. Sci. 2012, 13, 13680–13690. [Google Scholar] [CrossRef]
- Hang, Y.; Yamamoto, T.; Benninger, R.K.P.; Brissova, M.; Guo, M.; Bush, W.; Piston, D.W.; Powers, A.C.; Magnuson, M.; Thurmond, D.C.; et al. The MafA Transcription Factor Becomes Essential to Islet β-Cells Soon After Birth. Diabetes 2014, 63, 1994–2005. [Google Scholar] [CrossRef]
- Zhang, C.; Moriguchi, T.; Kajihara, M.; Esaki, R.; Harada, A.; Shimohata, H.; Oishi, H.; Hamada, M.; Morito, N.; Hasegawa, K.; et al. MafA Is a Key Regulator of Glucose-Stimulated Insulin Secretion. Mol. Cell. Biol. 2005, 25, 4969–4976. [Google Scholar] [CrossRef] [PubMed]
- Matafome, P.; Sena, C.; Seiça, R. Methylglyoxal, Obesity, and Diabetes. Endocrine 2013, 43, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Alfredo, T.; Matafome, P. Gut Metabolism of Sugars: Formation of Glycotoxins and Their Intestinal Absorption. Diabetology 2022, 3, 596–605. [Google Scholar] [CrossRef]
- Bianchi, M.d.L.P.; Antunes, L.M.G. Radicais livres e os principais antioxidantes da dieta. Rev. Nutr. 1999, 12, 123–130. [Google Scholar] [CrossRef]
- de Giffoni de Carvalho, J.T.; da Silva Baldivia, D.; Leite, D.F.; de Araújo, L.C.A.; de Toledo Espindola, P.P.; Antunes, K.A.; Rocha, P.S.; de Picoli Souza, K.; dos Santos, E.L. Medicinal Plants from Brazilian Cerrado: Antioxidant and Anticancer Potential and Protection against Chemotherapy Toxicity. Oxidative Med. Cell. Longev. 2019, 2019, e3685264. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hu, M. Bioavailability Challenges Associated with Development of Anti-Cancer Phenolics. Mini Rev. Med. Chem. 2010, 10, 550–567. [Google Scholar] [CrossRef]
- Souza, J.E.D.; Casanova, L.M.; Costa, S.S. Bioavailability of Phenolic Compounds: A Major Challenge for Drug Development? Rev. Fitos 2015, 9, 55–67. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro-Alfredo, T.; Macedo, M.L.R.; de Picoli Souza, K.; Matafome, P. New Therapeutic Strategies for Obesity and Its Metabolic Sequelae: Brazilian Cerrado as a Unique Biome. Int. J. Mol. Sci. 2023, 24, 15588. https://doi.org/10.3390/ijms242115588
Monteiro-Alfredo T, Macedo MLR, de Picoli Souza K, Matafome P. New Therapeutic Strategies for Obesity and Its Metabolic Sequelae: Brazilian Cerrado as a Unique Biome. International Journal of Molecular Sciences. 2023; 24(21):15588. https://doi.org/10.3390/ijms242115588
Chicago/Turabian StyleMonteiro-Alfredo, Tamaeh, Maria Lígia Rodrigues Macedo, Kely de Picoli Souza, and Paulo Matafome. 2023. "New Therapeutic Strategies for Obesity and Its Metabolic Sequelae: Brazilian Cerrado as a Unique Biome" International Journal of Molecular Sciences 24, no. 21: 15588. https://doi.org/10.3390/ijms242115588
APA StyleMonteiro-Alfredo, T., Macedo, M. L. R., de Picoli Souza, K., & Matafome, P. (2023). New Therapeutic Strategies for Obesity and Its Metabolic Sequelae: Brazilian Cerrado as a Unique Biome. International Journal of Molecular Sciences, 24(21), 15588. https://doi.org/10.3390/ijms242115588






