Exploring the Potential of Nanoporous Materials for Advancing Ophthalmic Treatments
Abstract
:1. Introduction
2. Understanding Nanoporous Materials
2.1. Definition and Characteristics
2.2. The Potential as Ophthalmic Drug Carriers
3. Challenges with Current Drug Delivery Systems
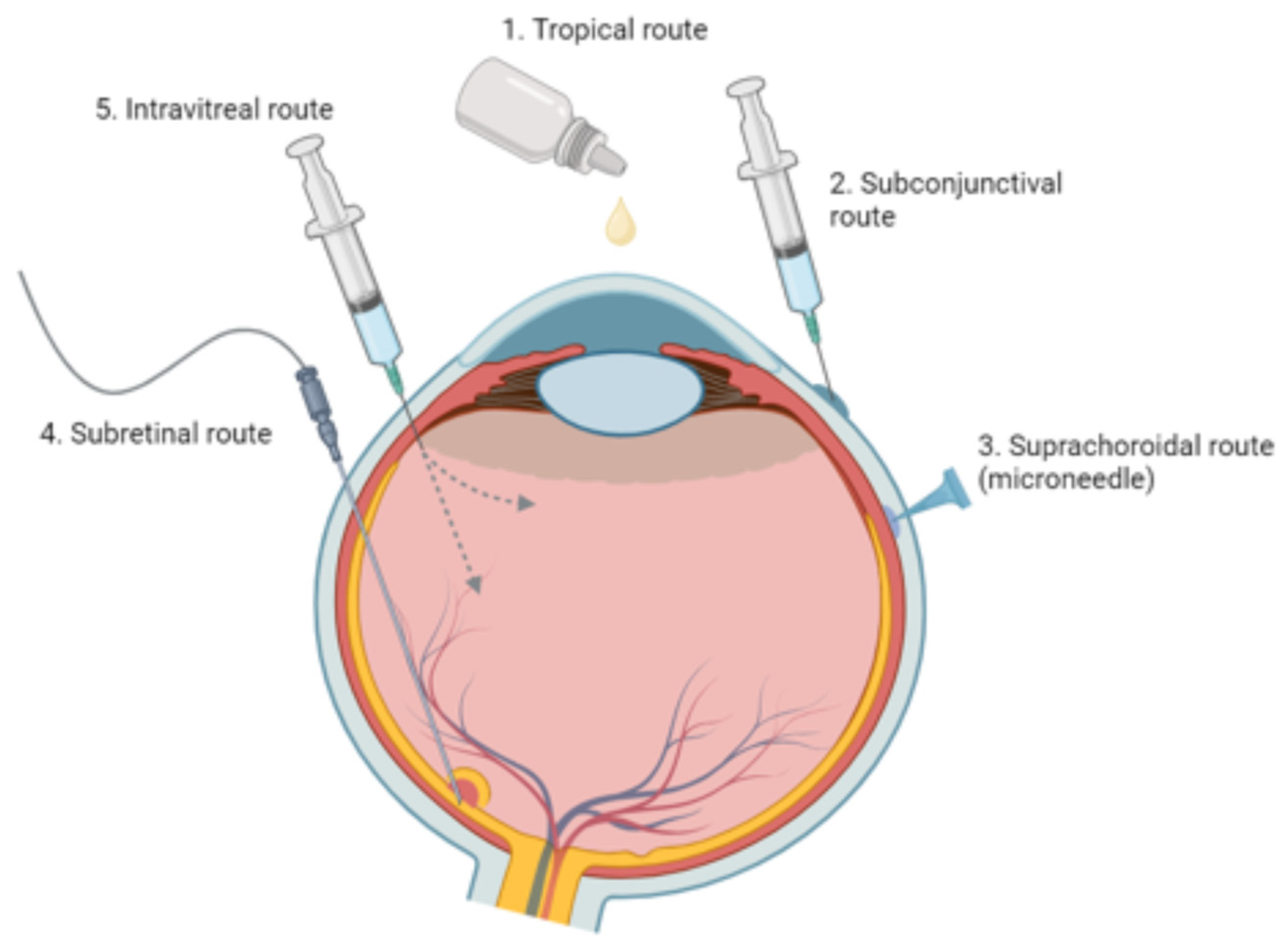
3.1. Principal Routes and Bioavailability
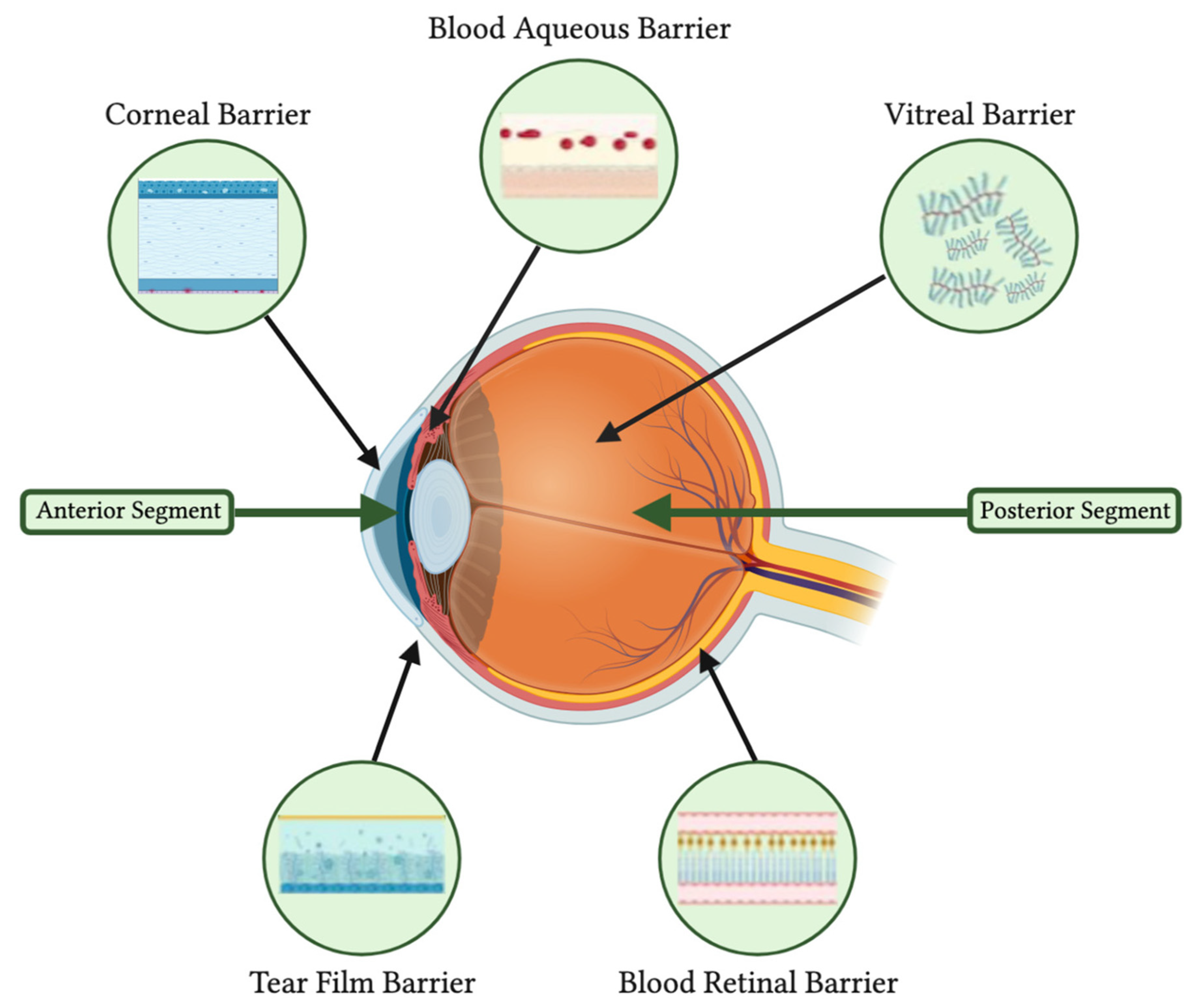
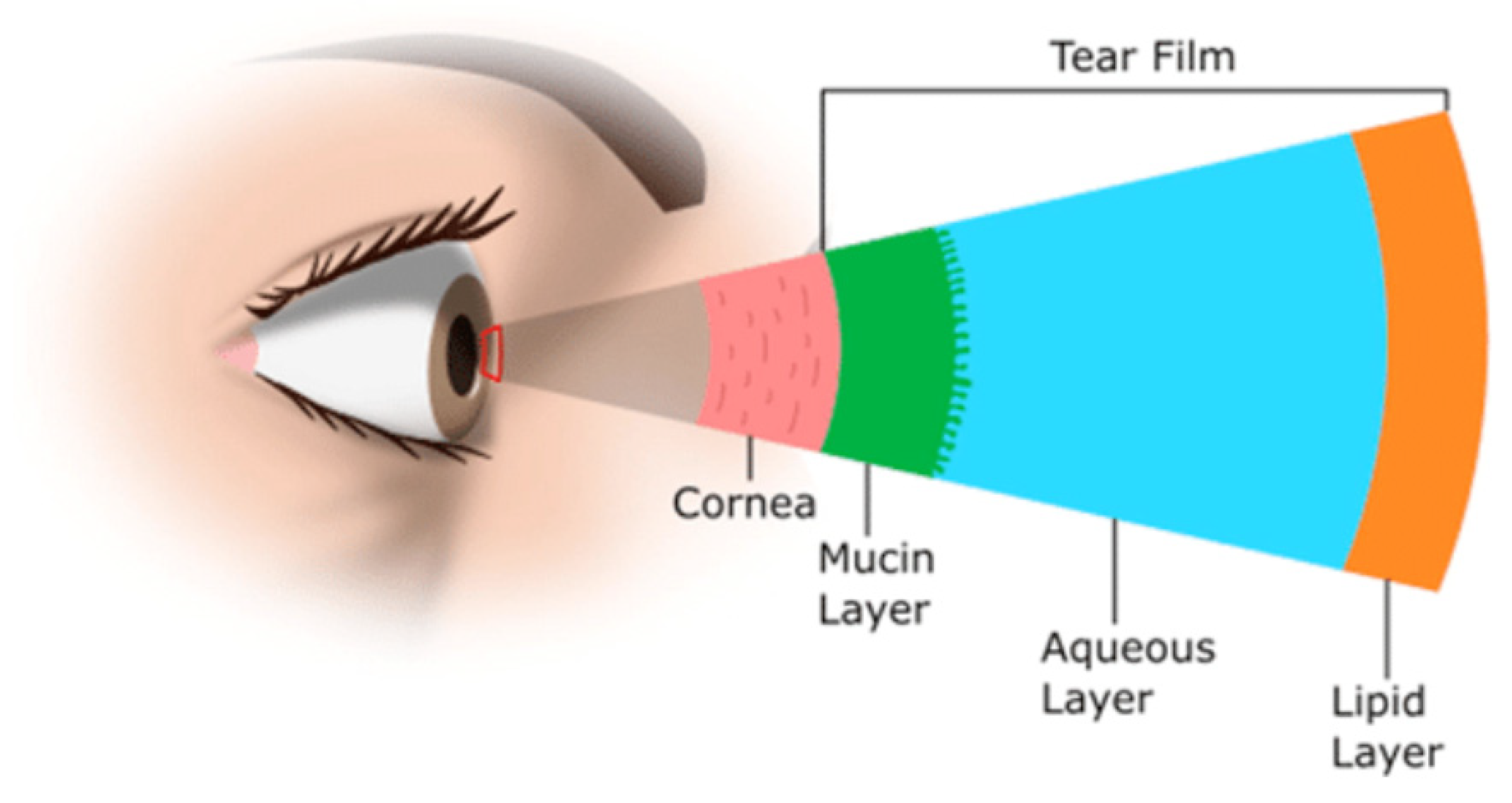
3.2. Challenges in Delivering Treatments to Ocular Tissues
4. Nanoporous Materials in Ocular Drug Delivery
4.1. Organic Nanoporous Materials for Ocular Drug Delivery
Nanoporous Hydrogels for Ocular Drug Delivery
4.2. Inorganic Nanoparticles for Ocular Drug Delivery
4.2.1. Mesoporous Silica for Ocular Drug Delivery
4.2.2. Nanoporous Polymers for Ocular Drug Delivery
4.2.3. Nanoporous Nanofibers for Ocular Drug Delivery
5. Nanoporous Materials in Intraocular Lenses
5.1. Structural and Functional Benefits of Nanoporous Intraocular Lenses
5.2. IOL Drug Delivery Potential
Drug Delivery Studies (Table 3)
| IOL Material Type | Key Features | Challenges | Current Usage Status | References |
|---|---|---|---|---|
| Crystalline films of sulfonated syndiotactic polystyrene (s-PS) |
| Theoretical drug depot capacity, not yet tested | Not currently in clinical use, research use only | [79] |
| poly(2-hydroxyethyl methacrylate) (pHEMA) hydrogel |
| Potential for porous pHEMA to calcify over long periods of time [13] | pHEMA hydrogel commonly used in IOLs pHEMA with nanopillar pores not currently in clinical use, research use only | [80] |
| Acrylate/methacrylate copolymer with Poly(methyl methacrylate) (PMMA) haptic part and nanoporous dexamethasone film | IOL optical properties largely unaffected by dexamethasone embedded film | Overall unstable drug release: three separate drug elution phases (burst, exponential, and steady release) | Not currently in clinical use, research use only | [35] |
5.3. Potential Drug Delivery NP Materials
5.4. Challenges and Future Considerations
6. Nanoporous Materials in Contact Lenses
6.1. Contact Lens Drug Delivery Potential
6.2. Development of Nanoporous CL Materials
6.3. Functional Benefits of Nanoporous Contact Lenses
6.4. Challenges and Future Considerations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wu, K.Y.; Joly-Chevrier, M.; Akbar, D.; Tran, S.D. Overcoming Treatment Challenges in Posterior Segment Diseases with Biodegradable Nano-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 1094. [Google Scholar] [CrossRef]
- Mabrouk, M.; Rajendran, R.; Soliman, I.E.; Ashour, M.M.; Beherei, H.H.; Tohamy, K.M.; Thomas, S.; Kalarikkal, N.; Arthanareeswaran, G.; Das, D.B. Nanoparticle- and Nanoporous-Membrane-Mediated Delivery of Therapeutics. Pharmaceutics 2019, 11, 294. [Google Scholar] [CrossRef]
- Fukumori, Y.; Takada, K.; Takeuchi, H. Nanoporous and Nanosize Materials for Drug Delivery Systems. In Nanotechnologies for the Life Sciences; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2007; ISBN 978-3-527-610419. [Google Scholar]
- Liu, D.; Wu, Q.; Chen, W.; Chen, K.; Lin, H.; Liu, F.; Xie, X.; Chen, H.; Chen, W. Nanoporous Gold Ring-Integrated Photothermal Intraocular Lens for Active Prevention of Posterior Capsular Opacification. Small 2022, 18, 2201098. [Google Scholar] [CrossRef]
- Wu, K.Y.; Ashkar, S.; Jain, S.; Marchand, M.; Tran, S.D. Breaking Barriers in Eye Treatment: Polymeric Nano-Based Drug-Delivery System for Anterior Segment Diseases and Glaucoma. Polymers 2023, 15, 1373. [Google Scholar] [CrossRef]
- Hadden, M.; Martinez-Martin, D.; Yong, K.-T.; Ramaswamy, Y.; Singh, G. Recent Advancements in the Fabrication of Functional Nanoporous Materials and Their Biomedical Applications. Materials 2022, 15, 2111. [Google Scholar] [CrossRef]
- Cho, E.C.; Au, L.; Zhang, Q.; Xia, Y. The Effects of Size, Shape, and Surface Functional Group of Gold Nanostructures on Their Adsorption and Internalization by Cells. Small 2010, 6, 517–522. [Google Scholar] [CrossRef]
- Engineering Precision Nanoparticles for Drug Delivery|Nature Reviews Drug Discovery. Available online: https://www.nature.com/articles/s41573-020-0090-8 (accessed on 19 September 2023).
- Afrand, M.; Ahmed, E.; Ahmed, W.; Ali, H.M.; Amjad, M.; Amli, H.; Awad, A.; Babar, H.; Behjani, M.A.; Booth, M.; et al. List of Contributors. In Emerging Nanotechnologies for Renewable Energy; Ahmed, W., Booth, M., Nourafkan, E., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. xi–xiii. ISBN 978-0-12-821346-9. [Google Scholar]
- Comprehensive Nanoscience and Nanotechnology. Available online: http://www.sciencedirect.com:5070/referencework/9780128122969/comprehensive-nanoscience-and-nanotechnology (accessed on 27 August 2023).
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular Drug Delivery Systems: An Overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Kim, E.-B.; Ameen, S.; Akhtar, M.S.; Shin, H.S. Iron-Nickel Co-Doped ZnO Nanoparticles as Scaffold for Field Effect Transistor Sensor: Application in Electrochemical Detection of Hexahydropyridine Chemical. Sens. Actuators B Chem. 2018, 275, 422–431. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Shin, H.S. ZnO Hollow Nano-Baskets for Mineralization of Cationic Dye. Mater. Lett. 2016, 183, 329–333. [Google Scholar] [CrossRef]
- Ameen, S.; Kim, E.-B.; Akhtar, M.S.; Shin, H.S. Electrochemical Detection of Resorcinol Chemical Using Unique Cabbage like ZnO Nanostructures. Mater. Lett. 2017, 209, 571–575. [Google Scholar] [CrossRef]
- Jang, G.-S.; Ameen, S.; Akhtar, M.S.; Shin, H.-S. Cobalt Oxide Nanocubes as Electrode Material for the Performance Evaluation of Electrochemical Supercapacitor. Ceram. Int. 2018, 44, 588–595. [Google Scholar] [CrossRef]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Nanoporous Organic Polymer Networks. Prog. Polym. Sci. 2012, 37, 530–563. [Google Scholar] [CrossRef]
- Nanoporous Materials—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/12908422/ (accessed on 19 September 2023).
- Hentze, H.-P.; Antonietti, M. Template Synthesis of Porous Organic Polymers. Curr. Opin. Solid. State Mater. Sci. 2001, 5, 343–353. [Google Scholar] [CrossRef]
- Delamo, E.; Urtti, A. Current and Future Ophthalmic Drug Delivery systems: A Shift to the Posterior Segment. Drug Discov. Today 2008, 13, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Langer, R.; Jia, X. Nanostructured Materials for Applications in Drug Delivery and Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 241–268. [Google Scholar] [CrossRef]
- Singh, G.; Ramadass, K.; Sooriyakumar, P.; Hettithanthri, O.; Vithange, M.; Bolan, N.; Tavakkoli, E.; Van Zwieten, L.; Vinu, A. Nanoporous Materials for Pesticide Formulation and Delivery in the Agricultural Sector. J. Control Release 2022, 343, 187–206. [Google Scholar] [CrossRef]
- Molecules|Free Full-Text|Controlled Drug Delivery Systems: Current Status and Future Directions. Available online: https://www.mdpi.com/1420-3049/26/19/5905 (accessed on 17 September 2023).
- Urtti, A. Challenges and Obstacles of Ocular Pharmacokinetics and Drug Delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- Akhter, M.H.; Ahmad, I.; Alshahrani, M.Y.; Al-Harbi, A.I.; Khalilullah, H.; Afzal, O.; Altamimi, A.S.A.; Najib Ullah, S.N.M.; Ojha, A.; Karim, S. Drug Delivery Challenges and Current Progress in Nanocarrier-Based Ocular Therapeutic System. Gels 2022, 8, 82. [Google Scholar] [CrossRef]
- Addo, R.T. Ocular Drug Delivery: Advances, Challenges and Applications, 1st ed.; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-47689-6. [Google Scholar]
- Chang, A.Y.; Purt, B. Biochemistry, Tear Film. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef]
- Wu, K.Y.; Kulbay, M.; Tanasescu, C.; Jiao, B.; Nguyen, B.H.; Tran, S.D. An Overview of the Dry Eye Disease in Sjögren’s Syndrome Using Our Current Molecular Understanding. Int. J. Mol. Sci. 2023, 24, 1580. [Google Scholar] [CrossRef]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous Humor Dynamics: A Review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-S.; Hou, P.-K.; Tai, T.-Y.; Lin, B.J. Blood-Ocular Barriers. Tzu Chi Med. J. 2008, 20, 25–34. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-Retinal Barrier. Eur. J. Ophthalmol. 2011, 21 (Suppl. 6), S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for Ocular Drug Delivery: Current Status and Translational Opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef]
- Wu, K.Y.; Fujioka, J.K.; Gholamian, T.; Zaharia, M.; Tran, S.D. Suprachoroidal Injection: A Novel Approach for Targeted Drug Delivery. Pharmaceuticals 2023, 16, 1241. [Google Scholar] [CrossRef]
- Fan, W.; Song, M.; Li, L.; Niu, L.; Chen, Y.; Han, B.; Sun, X.; Yang, Z.; Lei, Y.; Chen, X. Endogenous Dual Stimuli-Activated NO Generation in the Conventional Outflow Pathway for Precision Glaucoma Therapy. Biomaterials 2021, 277, 121074. [Google Scholar] [CrossRef]
- Karamitsos, A.; Lamprogiannis, L.; Karagkiozaki, V.; Laskarakis, A.; Papadopoulou, L.; Fatouros, D.; Ziakas, N.; Logothetidis, S.; Tsinopoulos, I. Design, Characterisation and Drug Release Study of Polymeric, Drug-eluting Single Layer Thin Films on the Surface of Intraocular Lenses. IET Nanobiotechnol. 2020, 14, 501–507. [Google Scholar] [CrossRef]
- Lai, C.-F.; Shiau, F.-J. Enhanced and Extended Ophthalmic Drug Delivery by pH-Triggered Drug-Eluting Contact Lenses with Large-Pore Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2023, 15, 18630–18638. [Google Scholar] [CrossRef]
- Luo, L.-J.; Nguyen, D.D.; Huang, C.-C.; Lai, J.-Y. Therapeutic Hydrogel Sheets Programmed with Multistage Drug Delivery for Effective Treatment of Corneal Abrasion. Chem. Eng. J. 2022, 429, 132409. [Google Scholar] [CrossRef]
- Sun, J.-G.; Jiang, Q.; Zhang, X.-P.; Shan, K.; Liu, B.-H.; Zhao, C.; Yan, B. Mesoporous Silica Nanoparticles as a Delivery System for Improving Antiangiogenic Therapy. Int. J. Nanomed. 2019, 14, 1489–1501. [Google Scholar] [CrossRef]
- Paiva, M.R.B.; Andrade, G.F.; Dourado, L.F.N.; Castro, B.F.M.; Fialho, S.L.; Sousa, E.M.B.; Silva-Cunha, A. Surface Functionalized Mesoporous Silica Nanoparticles for Intravitreal Application of Tacrolimus. J. Biomater. Appl. 2021, 35, 1019–1033. [Google Scholar] [CrossRef]
- Wu, M.; Wang, S.; Wang, Y.; Zhang, F.; Shao, T. Targeted Delivery of Mitomycin C-Loaded and LDL-Conjugated Mesoporous Silica Nanoparticles for Inhibiting the Proliferation of Pterygium Subconjunctival Fibroblasts. Exp. Eye Res. 2020, 197, 108124. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yuan, Z.; Gaeke, S.; Kao, W.W.-Y.; Li, S.K.; Miller, D.; Williams, B.; Park, Y.C. Laser-Activated Drug Implant for Controlled Release to the Posterior Segment of the Eye. ACS Appl. Bio Mater. 2021, 4, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Rohde, F.; Walther, M.; Wächter, J.; Knetzger, N.; Lotz, C.; Windbergs, M. In-Situ Tear Fluid Dissolving Nanofibers Enable Prolonged Viscosity-Enhanced Dual Drug Delivery to the Eye. Int. J. Pharm. 2022, 616, 121513. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, Z.; Chu, D.; Liu, R.; Zhu, L.; Shi, L.; Li, C.; Jin, L.; Zhang, X.; Li, J. Celastrol-Based Nanoporous Membranes Prevent Subconjunctival Fibrosis by Activating Autophagy. Mater. Today Adv. 2023, 18, 100356. [Google Scholar] [CrossRef]
- Pasqui, D.; De Cagna, M.; Barbucci, R. Polysaccharide-Based Hydrogels: The Key Role of Water in Affecting Mechanical Properties. Polymers 2012, 4, 1517–1534. [Google Scholar] [CrossRef]
- Jaspers, M.; Rowan, A.E.; Kouwer, P.H.J. Tuning Hydrogel Mechanics Using the Hofmeister Effect. Adv. Funct. Mater. 2015, 25, 6503–6510. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- pH-Sensitive Hydrogel for Coacervative Cloud Point Extraction and Spectrophotometric Determination of Cu (II): Optimization by Central Composite Design. Available online: https://www.infona.pl/resource/bwmeta1.element.springer-doi-10_1007-S13738-015-0653-5 (accessed on 23 August 2023).
- Management of Corneal Abrasions|AAFP. Available online: https://www.aafp.org/pubs/afp/issues/2004/0701/p123.html (accessed on 19 September 2023).
- Kumar, D.; Sailaja Chirravuri, S.V.; Shastri, N.R. Impact of Surface Area of Silica Particles on Dissolution Rate and Oral Bioavailability of Poorly Water Soluble Drugs: A Case Study with Aceclofenac. Int. J. Pharm. 2014, 461, 459–468. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-Crystal Template Mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Hurley, K.R.; Haynes, C.L. Critical Considerations in the Biomedical Use of Mesoporous Silica Nanoparticles. J. Phys. Chem. Lett. 2012, 3, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Sahlgren, C.; Linden, M. Multifunctional Mesoporous Silica Nanoparticles for Combined Therapeutic, Diagnostic and Targeted Action in Cancer Treatment. Curr. Drug Targets 2011, 12, 1166–1186. [Google Scholar] [CrossRef] [PubMed]
- Emerging Trends in Nanomedicine for Improving Ocular Drug Delivery: Light-Responsive Nanoparticles, Mesoporous Silica Nanoparticles, and Contact Lenses|ACS Biomaterials Science & Engineering. Available online: https://pubs.acs.org/doi/full/10.1021/acsbiomaterials.0c01347 (accessed on 19 September 2023).
- Aliancy, J.; Stamer, W.D.; Wirostko, B. A Review of Nitric Oxide for the Treatment of Glaucomatous Disease. Ophthalmol. Ther. 2017, 6, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, Y.; Song, M.; Deng, Y.; Sun, X.; Lei, Y. Prolonged Use of Nitric Oxide Donor Sodium Nitroprusside Induces Ocular Hypertension in Mice. Exp. Eye Res. 2021, 202, 108280. [Google Scholar] [CrossRef]
- Surface Functionalized Mesoporous Silica Nanoparticles for Intravitreal Application of Tacrolimus—Mayara Rodrigues Brandão Paiva, Gracielle Ferreira Andrade, Lays Fernanda Nunes Dourado, Brenda Fernanda Moreira Castro, Silvia Ligório Fialho, Edésia Martins Barros Sousa, Armando Silva-Cunha, 2021. Available online: https://journals.sagepub.com/doi/full/10.1177/0885328220977605 (accessed on 19 September 2023).
- Notario, B. Nanoporous Polymeric Materials: A New Class of Materials with Enhanced Properties. Prog. Mater. Sci. 2016. [Google Scholar] [CrossRef]
- Hughes, G.A. Nanostructure-Mediated Drug Delivery. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 22–30. [Google Scholar] [CrossRef]
- Peppas, N.A.; Langer, R. New Challenges in Biomaterials. Science 1994, 263, 1715–1720. [Google Scholar] [CrossRef]
- Small-Scale Systems for in Vivo Drug Delivery—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/14520404/ (accessed on 23 August 2023).
- Kim, S.W.; Petersen, R.V.; Feijen, J. Chapter 5—Polymeric Drug Delivery Systems. In Drug Design; Ariëns, E.J., Ed.; Medicinal Chemistry: A Series of Monographs; Academic Press: Cambridge, MA, USA, 1980; Volume 11, pp. 193–250. [Google Scholar]
- Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications|Chemical Reviews. Available online: https://pubs.acs.org/doi/10.1021/acs.chemrev.8b00593 (accessed on 27 August 2023).
- Langwald, S.V.; Ehrmann, A.; Sabantina, L. Measuring Physical Properties of Electrospun Nanofiber Mats for Different Biomedical Applications. Membranes 2023, 13, 488. [Google Scholar] [CrossRef]
- Divya, T.; Sureshkumar, A.; Sudhandiran, G. Autophagy Induction by Celastrol Augments Protection against Bleomycin-Induced Experimental Pulmonary Fibrosis in Rats: Role of Adaptor Protein P62/ SQSTM1. Pulm. Pharmacol. Ther. 2017, 45, 47–61. [Google Scholar] [CrossRef]
- Pharmaceutics|Free Full-Text|Nanofibers in Ocular Drug Targeting and Tissue Engineering: Their Importance, Advantages, Advances, and Future Perspectives. Available online: https://www.mdpi.com/1999-4923/15/4/1062 (accessed on 19 September 2023).
- Diebold, Y.; Calonge, M. Applications of Nanoparticles in Ophthalmology. Prog. Retin. Eye Res. 2010, 29, 596–609. [Google Scholar] [CrossRef]
- del Pozo-Rodríguez, A.; Delgado, D.; Gascón, A.R.; Solinís, M.Á. Lipid Nanoparticles as Drug/Gene Delivery Systems to the Retina. J. Ocul. Pharmacol. Ther. 2013, 29, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, S.S.; Lim, R.R.; Lakshminarayanan, R.; Mohan, R.R. Nanomedicine Approaches for Corneal Diseases. J. Funct. Biomater. 2015, 6, 277–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jones, L.; Gu, F.X. Nanomaterials for Ocular Drug Delivery. Macromol. Biosci. 2012, 12, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Liang, D.T.; Li, P. Fabrication and Analyses of Bionic Intraocular Lens with Meniscus Polymer Layer and Porous Structure. Opt. Commun. 2019, 430, 204–209. [Google Scholar] [CrossRef]
- Shin, M.-K.; Ji, Y.W.; Moon, C.-E.; Lee, H.; Kang, B.; Jinn, W.-S.; Ki, J.; Mun, B.; Kim, M.-H.; Lee, H.K.; et al. Matrix Metalloproteinase 9-Activatable Peptide-Conjugated Hydrogel-Based Fluorogenic Intraocular-Lens Sensor. Biosens. Bioelectron. 2020, 162, 112254. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, G.; Ji, Y.W.; Moon, C.-E.; Jung, Y.; Lee, H.K.; Lee, J.; Koh, W.-G. Real-Time and Label-Free Biosensing Using Moiré Pattern Generated by Bioresponsive Hydrogel. Bioact. Mater. 2023, 23, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.M.; Vasavada, A.R.; Johar, S.R.K.; Vasavada, V.A.; Vasavada, V.A. Post-Operative Capsular Opacification: A Review. Int. J. Biomed. Sci. 2007, 3, 237–250. [Google Scholar]
- Mazoteras, P.; Casaroli-Marano, R.P. In Vitro Biofilm Distribution on the Intraocular Lens Surface of Different Biomaterials. J. Cataract. Refract. Surg. 2015, 41, 1980. [Google Scholar] [CrossRef]
- Alvaro Toribio, H.M.-B.; Alvaro Toribio, H.M.-B. In Vitro Adherence of Conjunctival Bacteria to Different Oculoplastic Materials. Qwer 2018, 11, 1895–1901. [Google Scholar] [CrossRef]
- Sleath, B.; Blalock, S.; Covert, D.; Stone, J.L.; Skinner, A.C.; Muir, K.; Robin, A.L. The Relationship between Glaucoma Medication Adherence, Eye Drop Technique, and Visual Field Defect Severity. Ophthalmology 2011, 118, 2398–2402. [Google Scholar] [CrossRef]
- Cao, Y.; Samy, K.E.; Bernards, D.A.; Desai, T.A. Recent Advances in Intraocular Sustained-Release Drug Delivery Devices. Drug Discov. Today 2019, 24, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, A.R.; Googe, J.M., Jr.; Stockdale, C.R.; Bressler, N.M.; Brucker, A.J.; Elman, M.J.; Glassman, A.R. Diabetic Retinopathy Clinical Research Network Risk of Endophthalmitis After Intravitreal Drug Injection When Topical Antibiotics Are Not Required: The Diabetic Retinopathy Clinical Research Network Laser-Ranibizumab-Triamcinolone Clinical Trials. Arch. Ophthalmol. 2009, 127, 1581–1583. [Google Scholar] [CrossRef] [PubMed]
- Zuppolini, S.; Borriello, A.; Pellegrino, M.; Venditto, V.; Ambrosio, L.; Nicolais, L. Potential Contact and Intraocular Lenses Based on Hydrophilic/Hydrophobic Sulfonated Syndiotactic Polystyrene Membranes. J. King Saud. Univ. Sci. 2017, 29, 487–493. [Google Scholar] [CrossRef]
- Krajňák, T.; Černá, E.; Šuráňová, M.; Šamořil, T.; Zicha, D.; Vojtová, L.; Čechal, J. Replica-Mold Nanopatterned PHEMA Hydrogel Surfaces for Ophthalmic Applications. Sci. Rep. 2022, 12, 14497. [Google Scholar] [CrossRef] [PubMed]
- Vijayasekaran, S.; Chirila, T.V.; Robertson, T.A.; Lou, X.; Fitton, J.H.; Hicks, C.R.; Constable, I.J. Calcification of Poly(2-Hydroxyethyl Methacrylate) Hydrogel Sponges Implanted in the Rabbit Cornea: A 3-Month Study. J. Biomater. Sci. Polym. Ed. 2000, 11, 599–615. [Google Scholar] [CrossRef]
- MediPrint Ophthalmics, Inc. A Phase 2a Study of Safety, Tolerability, and Efficacy of Drug-Delivering Contact Lens LL-BMT1 in Patients With Primary Open-Angle Glaucoma or Ocular Hypertension; clinicaltrials.gov, 2022. Available online: https://classic.clinicaltrials.gov/ProvidedDocs/08/NCT04747808/Prot_000.pdf (accessed on 27 September 2023).
- Friedman, D.S. Latanoprost Eluting Contact Lens for Treating Glaucoma and Ocular Hypertension; clinicaltrials.gov, 2023. Available online: https://catalyst.harvard.edu/wp-content/uploads/2021/03/Friedman_Scientific_Abstract.pdf (accessed on 27 September 2023).
- Materials|Free Full-Text|Review of Potential Drug-Eluting Contact Lens Technologies. Available online: https://www.mdpi.com/1996-1944/16/10/3653 (accessed on 23 September 2023).
- Choi, J.H.; Li, Y.; Jin, R.; Shrestha, T.; Choi, J.S.; Lee, W.J.; Moon, M.J.; Ju, H.T.; Choi, W.; Yoon, K.C. The Efficiency of Cyclosporine A-Eluting Contact Lenses for the Treatment of Dry Eye. Curr. Eye Res. 2019, 44, 486–496. [Google Scholar] [CrossRef]
- Fabrication of Contact Lens Device with Integrated Microtubes for in Situ Extended Drug Delivery for Ocular Disease Treatment. Available online: https://ieeexplore.ieee.org/document/8808259 (accessed on 23 September 2023).
- Kaempferol Incorporated Bovine Serum Albumin Fibrous Films for Ocular Drug Delivery—Yin—2021—Macromolecular Bioscience—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/mabi.202100269 (accessed on 23 September 2023).
- Lai, C.-F.; Shiau, F.-J. Hydrogel Contact Lenses Embedded with Amine-Functionalized Large-Pore Mesoporous Silica Nanoparticles with Extended Hyaluronic Acid Release. Nanomaterials 2023, 13, 2441. [Google Scholar] [CrossRef]
- Oh, C.H.; Bae, J.-H.; Lee, H.M. Preparation of Porous Hydrogels Using Blowing Agents and Application to Contact Lenses. Polymer 2022, 46, 47–55. [Google Scholar] [CrossRef]
- A Multifunctional Smart Soft Contact Lens Device Enabled by Nanopore Thin Film for Glaucoma Diagnostics and In Situ Drug Delivery. Available online: https://ieeexplore.ieee.org/document/8768313/ (accessed on 23 September 2023).
- Lee, S.-H.; Shin, K.-S.; Kim, J.-W.; Kang, J.-Y.; Kim, J.-K. Stimulus-Responsive Contact Lens for IOP Measurement or Temperature-Triggered Drug Release. Transl. Vis. Sci. Technol. 2020, 9, 1. [Google Scholar] [CrossRef]

| Indication | Drug | DDS | Advantages and Considerations | Administration Route | Stage | Reference |
|---|---|---|---|---|---|---|
| Corneal abrasion | Epigallocatechin gallate (EGCG); 8-1,3-glucan; and SB431542 | Nanoporous hydrogels | Controlled and sequential release of multiple drugs tailored to address different stages of corneal tissue repair. | Topical | Preclinical—in vitro | [37] |
| Glaucoma | Nitric oxide (NO) | Mesoporous silica nanoparticles | Increased tissue permeability; tissue targeting; and drug penetration. Sustainable and stable delivery of NO to tissue site. | Topical | Preclinical—in vitro and in vivo | [34] |
| Ocular neovascularisation | Bevacizumab | Mesoporous silica nanoparticles | Effective preservation of bevacizumab concentration, without causing toxicity to tissues. | Injection (subconjunctival) | Preclinical—in vitro and in vivo | [38] |
| Posterior uveitis | Tacrolimus | Mesoporous silica nanoparticles | DDS achieved up to 7% TAC loading, without any damage to the retinal tissue or optic nerve tissue. | Injection (intravitreal) | Preclinical—in vitro and in vivo | [39] |
| Postoperative pterygium recurrence | Mitomycin C (MMC) | Mesoporous silica nanoparticles | Targeting and delivery of MMC was effective and the nanoparticles exhibited less toxicity compared to normal fibroblasts. | Injection (subconjunctival) | Preclinical—in vitro and in vivo | [40] |
| Posterior uveitis | Methotrexate (MTX) | Nanoporous polymers | DDS did not show any toxicity, immune or foreign body responses following implantation. | Implantation | Preclinical—in vitro and in vivo | [41] |
| Conjunctivitis | Gentamicin and dexamethasone | Nanoporous Naofibers | Prolonged resistance time, increased tear fluid viscosity and contact time. | Topical | Preclinical—in vitro and ex vivo | [42] |
| Subconjunctival fibrosis | Celastrol | Nanoporous nanofibers | DDS prevented burse release of celastrol and was still able to preserve the important PI3K/Akt/mTOR pathway-inhibiting effect of celastrol. | Implantation | Preclinical—in vitro and in vivo | [43] |
| IOL Material Type | Key Features | Challenges | Current Usage Status | References |
|---|---|---|---|---|
| Accommodating IOL: PDMS (Polydimethylsiloxane) polymer layers surrounding porous support ring and optical liquid |
| Clinical use not yet established | Not currently in clinical use, research use only | [70] |
| PEGDAAm hydrogel (Diacrylamide group-modified PEG diacrylamide) |
| Biomarker diffusion dependent on hydrogel density (nanopore size) | Not currently in clinical use, research use only | [71] |
| Acrylamide, acrylic acid, & methylenebisacrylamide hydrogel |
| Potentially negative effect on visual field (unknown) | Not currently in clinical use, research use only | [72] |
| Polymethyl methacrylate (PMMA) with nanoporous gold ring (NPG) |
|
| Not currently in clinical use, research use only | [4] |
| CL Material Type | Key Features | Challenges | Current Usage Status | References |
|---|---|---|---|---|
| 2-Hydroxylethylmethacrylate (2-HEMA) polymer base with nanoporous silica containing cyclosporine A (CsA) |
|
| Not currently in clinical use, research use only | [85] |
| PDMS (Polydimethylsiloxane) lens with embedded PDMS microtubes |
|
| Not currently in clinical use, research use only | [86] |
| HEMA (2- hydroxyethyl methacrylate) and MAA (methacrylic acid) lens base |
|
| Not currently in clinical use, research use only | [89] |
| Not a contact lens. Preliminary analysis of a BSA porous film to investigate its drug delivery capacity (Kaempferol). |
|
| Not currently in clinical use, research use only | [87] |
| CL Material Type | Key Features | Challenges | Current Usage Status | References |
|---|---|---|---|---|
| PDMS (Polydimethylsiloxane) silicone elastomer embedded with anodic aluminum oxide (AAO) thin film |
|
| Not currently in clinical use, research use only | [90] |
| HEMA (hydroxyethylmethacrylate) with timolol-loaded thermosensitive PNIPAM (poly(N-isopropylacrylamide)) |
|
| Not currently in clinical use, research use only | [91] |
| Timolol maleate salt (TMS) and brimonidine tartrate salt (BTS) loaded in mesoporous silica nanoparticles |
|
| Not currently in clinical use, research use only | [88] |
| Large-pore mesoporous silica nanoparticles (LPMSNs) functionalized with amine groups (LPMSN-amine) for the delivery of yaluronici acid (HA) |
|
| Not currently in clinical use, research use only | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.Y.; Brister, D.; Bélanger, P.; Tran, S.D. Exploring the Potential of Nanoporous Materials for Advancing Ophthalmic Treatments. Int. J. Mol. Sci. 2023, 24, 15599. https://doi.org/10.3390/ijms242115599
Wu KY, Brister D, Bélanger P, Tran SD. Exploring the Potential of Nanoporous Materials for Advancing Ophthalmic Treatments. International Journal of Molecular Sciences. 2023; 24(21):15599. https://doi.org/10.3390/ijms242115599
Chicago/Turabian StyleWu, Kevin Y., Danielle Brister, Paul Bélanger, and Simon D. Tran. 2023. "Exploring the Potential of Nanoporous Materials for Advancing Ophthalmic Treatments" International Journal of Molecular Sciences 24, no. 21: 15599. https://doi.org/10.3390/ijms242115599







