From Fruit Waste to Medical Insight: The Comprehensive Role of Watermelon Rind Extract on Renal Adenocarcinoma Cellular and Transcriptomic Dynamics
Abstract
:1. Introduction
Objectives
2. Results
2.1. Metabolite Analysis of Phytoconstituents of Watermelon Rind Extraction
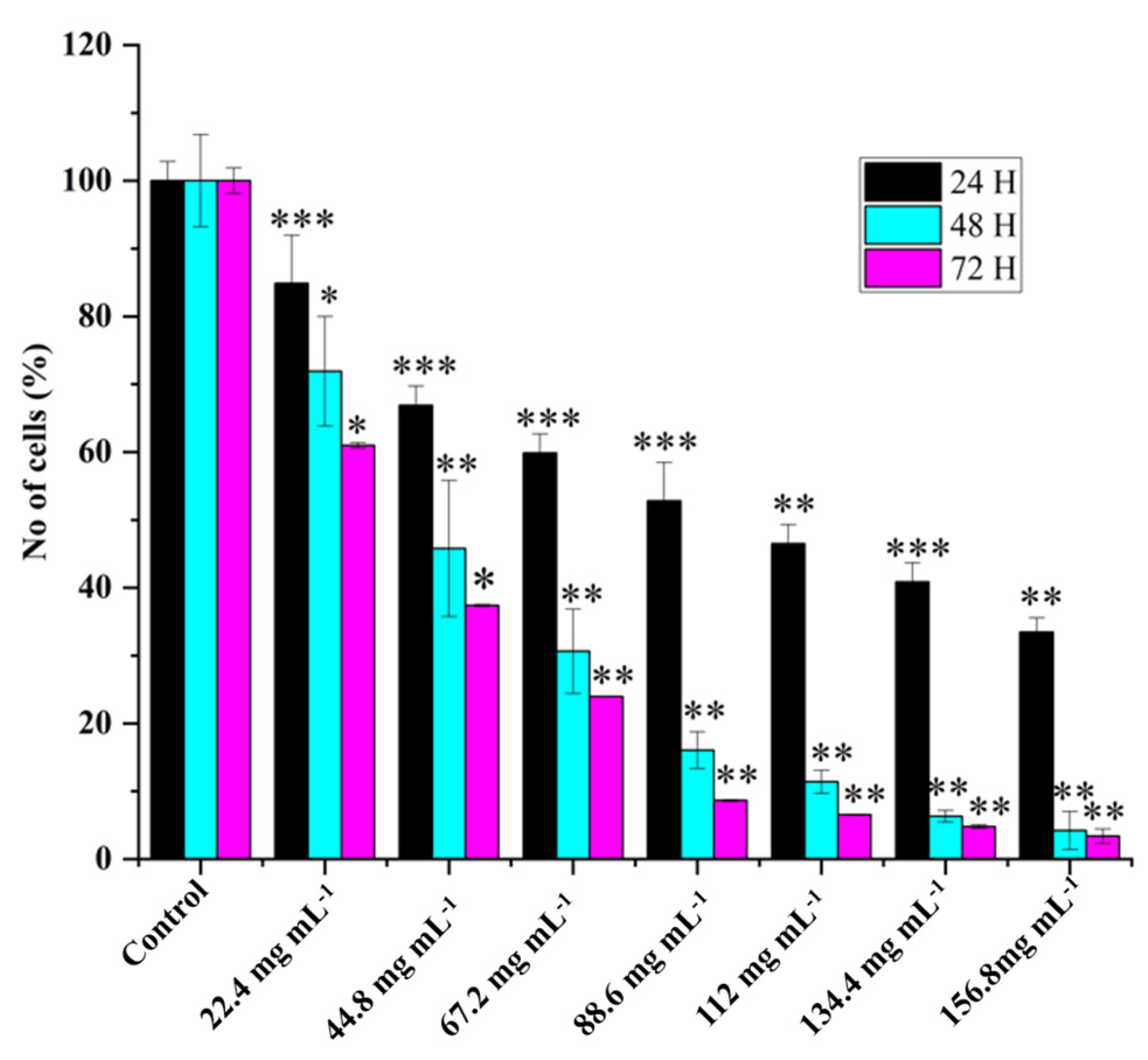
2.2. HRAC-769-P Cell Proliferation Was Affected by Concentration and Duration of Treatment
2.3. Apoptosis versus Senescence
2.4. Assessment of Cell Migration Inhibition and Metastasis Using Wound Healing Assay
2.5. RNAseq Assessment of Differentially Expressed Genes between WRE-Treated and Control Cells
2.6. Pathway Enrichment Analysis
3. Discussion
3.1. Role of Nutraceutical Compounds
3.2. Differential Impact on Apoptosis and Senescence
3.3. The Intricacies of Nitric Oxide Signaling
3.4. Watermelon Rind Extract Modulates a Complex Network of Genes
3.5. Intrinsic and Extrinsic Apoptotic Pathways
3.6. Promising Effects of Upregulated Transcripts on Tumor Growth Inhibition
3.7. Promising Effects of Downregulated Transcripts on Tumor Growth Inhibition
3.8. Multiple Gene Targets and Potential Therapeutic Avenues
3.9. NF-kappa B and TNF Signaling Pathway
4. Material and Methods
4.1. Plant Material and Extraction Process
4.2. Chemical Characterization of Watermelon Rind Extract Using LC-MS
4.3. Cell Culture
4.4. Treatments
4.5. Cell Proliferation Assay
4.6. Poly Caspase Assay
4.7. SA-Beta-Gal Assay
4.8. Wound Healing Assay
4.9. RNA Isolation and RNA-Seq Library Preparation
4.10. RNA-Seq Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Perla, V.; Nadimi, M.; Reddy, R.; Hankins, G.R.; Nimmakayala, P.; Harris, R.T.; Valluri, J.; Sirbu, C.; Reddy, U.K. Effect of ghost pepper on cell proliferation, apoptosis, senescence and global proteomic profile in human renal adenocarcinoma cells. PLoS ONE 2018, 13, e0206183. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharm. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, M.S. Nutrition and cancer: A review of the evidence for an anti-cancer diet. Nutr. J. 2004, 3, 19. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospecting 2021, 11, 5–13. [Google Scholar] [CrossRef] [PubMed]
- El Gizawy, H.A.; El-Haddad, A.E.; Attia, Y.M.; Fahim, S.A.; Zafer, M.M.; Saadeldeen, A.M. In Vitro Cytotoxic Activity and Phytochemical Characterization (UPLC/T-TOF-MS/MS) of the Watermelon (Citrullus lanatus) Rind Extract. Molecules 2022, 27, 2480. [Google Scholar] [CrossRef]
- Manivannan, A.; Lee, E.-S.; Han, K.; Lee, H.-E.; Kim, D.-S. Versatile Nutraceutical Potentials of Watermelon—A Modest Fruit Loaded with Pharmaceutically Valuable Phytochemicals. Molecules 2020, 25, 5258. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anticancer agents. Chemical reviews 2009, 109, 3012–3043. [Google Scholar] [CrossRef]
- Alsayed, H.; Ahmed, A.R.; Al-Sayed, H.M.A. Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. Ann. Agric. Sci. 2013, 58, 83–95. [Google Scholar] [CrossRef]
- Mushtaq, M.; Sultana, B.; Bhatti, H.N.; Asghar, M. RSM based optimized enzyme-assisted extraction of antioxidant phenolics from underutilized watermelon (Citrullus lanatus Thunb.) rind. J. Food Sci. Technol. 2014, 52, 5048–5056. [Google Scholar] [CrossRef]
- Zamuz, S.; Munekata, P.E.; Gullón, B.; Rocchetti, G.; Montesano, D.; Lorenzo, J.M. Citrullus lanatus as source of bioactive components: An up-to-date review. Trends Food Sci. Technol. 2021, 111, 208–222. [Google Scholar] [CrossRef]
- Rimando, A.M.; Perkins-Veazie, P.M. Determination of citrulline in watermelon rind. J. Chromatogr. A 2005, 1078, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019, 8, 5574. [Google Scholar] [CrossRef]
- Fesseha, M.; Hong, M.Y. Effects of Watermelon Consumption on Cellular Proliferation, and Apoptosis in Rat Colon (P05-019-19). Curr. Dev. Nutr. 2019, 3, nzz030.P05-019-19. [Google Scholar] [CrossRef]
- Itahana, K.; Campisi, J.; Dimri, G.P. Methods to detect biomarkers of cellular senescence: The senescence-associated β-galactosidase assay. Biol. Aging Methods Protocols. 2007, 371, 21–31. [Google Scholar]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Mintz, J.; Anastasia, V.; Omar, R.; Khushi, S.; Gabriella, G.; Joshua, M.; Hare, R.R.; Himanshu, A. Current advances of nitric oxide in cancer and anticancer therapeutics. Vaccines 2021, 9, 94. [Google Scholar] [CrossRef]
- Kong, H.; Chandel, N.S. Reactive oxygen species and cancer. In Oxidative Stress; Sies, H., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 619–637. [Google Scholar]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Vogler, M.; Dinsdale, D.; Dyer, M.J.S.; Cohen, G.M. Bcl-2 inhibitors: Small molecules with a big impact on cancer therapy. Cell Death Differ. 2008, 16, 360–367. [Google Scholar] [CrossRef]
- Grespi, F.; Soratroi, C.; Krumschnabel, G.; Sohm, B.; Ploner, C.; Geley, S.; Hengst, L.; Häcker, G.; Villunger, A. BH3-only protein Bmf mediates apoptosis upon inhibition of CAP-dependent protein synthesis. Cell Death. Differ. 2010, 17, 1672–1683. [Google Scholar] [CrossRef]
- Yan, W.; Huang, J.; Zhang, Q. Role of Metastasis Suppressor KAI1/CD82 in Different Cancers. J. Oncol. 2021, 2021, 9924473. [Google Scholar] [CrossRef]
- Peng, X.; Pan, K.; Zhao, W.; Zhang, J.; Yuan, S.; Wen, X.; Zhou, W.; Yu, Z. NPTX1 inhibits colon cancer cell proliferation through down-regulating cyclin A2 and CDK2 expression. Cell Biol. Int. 2018, 42, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, Y.; Zhao, W.; You, S.; Feng, M.; Xie, C.; Chi, X.; Zhang, Y.; Wang, X. As a downstream target of the AKT pathway, NPTX1 inhibits proliferation and promotes apoptosis in hepatocellular carcinoma. Biosci. Rep. 2019, 39, BSR20181662. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Su, J.; He, H.; Zhan, Y.; Liu, H. Hsa_circ_0070269 inhibits hepatocellular carcinoma progression through modulating miR-182/NPTX1 axis. Biomed. Pharm. 2019, 120, 109497. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, G.; An, K.; Shi, L. NPTX1 inhibits pancreatic cancer cell proliferation and migration and enhances chemotherapy sensitivity by targeting RBM10. Oncol. Lett. 2022, 23, 154. [Google Scholar] [CrossRef]
- Huo, L.; Wang, B.; Zheng, M.; Zhang, Y.; Xu, J.; Yang, G.; Guan, Q. miR-128-3p inhibits glioma cell proliferation and differentiation by targeting NPTX1 through IRS-1/PI3K/AKT signaling pathway. Exp. Med. 2019, 17, 2921–2930. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Q.; Zhao, G.; Zhang, J.; Yuan, H.; Feng, T.; Ou, D.; Gu, R.; Li, S.; Li, K.; et al. Loss of TRIM31 promotes breast cancer progression through regulating K48- and K63-linked ubiquitination of p53. Cell Death Dis. 2021, 12, 945. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Y.; Wang, Y.; Zhang, Y.; Luo, Q.; Man, X.; Wei, F.; Yu, X. Downregulation of Foxo3 and TRIM31 by miR-551b in side population promotes cell proliferation, invasion, and drug resistance of ovarian cancer. Med. Oncol. 2016, 33, 126. [Google Scholar] [CrossRef]
- Viera, M.; Yip, G.W.C.; Shen, H.-M.; Baeg, G.H.; Bay, B.H. Targeting CD82/KAI1 for Precision Therapeutics in Surmounting Metastatic Potential in Breast Cancer. Cancers 2021, 13, 4486. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liang, C.; Hua, Y.; Miao, C.; Zhang, J.; Xu, A.; Zhao, K.; Liu, S.; Tian, Y.; Dong, H.; et al. The metastasis suppressor CD82/KAI1 regulates cell migration and invasion via inhibiting TGF-β 1/Smad signaling in renal cell carcinoma. Oncotarget 2017, 8, 51559–51568. [Google Scholar] [CrossRef]
- Chen, T.; Du, D.; Chen, J.; Zhou, P.; Weinstein, J.N.; Yao, L.; Liu, Y. ZC3H12A Expression in Different Stages of Colorectal Cancer. Oncoscience 2019, 6, 301–311. [Google Scholar] [CrossRef]
- Miekus, K.; Kotlinowski, J.; Lichawska-Cieslar, A.; Rys, J.; Jura, J. Activity of MCPIP1 RNase in tumor associated processes. J. Exp. Clin. Cancer Res. 2019, 38, 421. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, S.; Han, Y.; Sun, J.; Xv, L.; Wu, L.; Wang, Y.; Ming, L. Linc00472 suppresses proliferation and promotes apoptosis through elevating PDCD4 expression by sponging miR-196a in colorectal cancer. Aging 2018, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Wang, G. LINC00472 suppressed by ZEB1 regulates the miR-23a-3p/FOXO3/BID axis to inhibit the progression of pancreatic cancer. J. Cell. Mol. Med. 2021, 25, 8312–8328. [Google Scholar] [CrossRef]
- Ma, S.; Rubin, B.P. Apoptosis-associated tyrosine kinase 1 inhibits growth and migration and promotes apoptosis in melanoma. Lab. Investig. 2014, 94, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Zhang, Y.; Li, D.D.; Zhang, F.L.; Liu, H.Y.; Liao, X.H.; Xie, H.Y.; Lu, Q.; Zhang, L.; Hong, Q.; et al. RNF144A functions as a tumor suppressor in breast cancer through ubiquitin ligase activity-dependent regulation of stability and oncogenic functions of HSPA2. Cell Death Differ. 2020, 27, 1105–1118. [Google Scholar] [CrossRef]
- Bostanabad, S.Y.; Noyan, S.; Dedeoglu, B.G.; Gurdal, H. Overexpression of β-Arrestins inhibits proliferation and motility in triple negative breast cancer cells. Sci. Rep. 2021, 11, 1539. [Google Scholar] [CrossRef]
- Peng, Y.; Li, H.; Fu, Y.; Guo, S.; Qu, C.; Zhang, Y.; Zong, B.; Liu, S. JAM2 predicts a good prognosis and inhibits invasion and migration by suppressing EMT pathway in breast cancer. Int. Immunopharmacol. 2022, 103, 108430. [Google Scholar] [CrossRef]
- Zheng, J.M.; Gan, M.F.; Yu, H.Y.; Ye, L.X.; Yu, Q.X.; Xia, Y.H.; Zhou, H.X.; Bao, J.Q.; Guo, Y.Q. KDF1, a Novel Tumor Suppressor in Clear Cell Renal Cell Carcinoma. Front. Oncol. 2021, 11, 686678. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yang, J.; Bai, Y.; Li, Q.; Yao, Y.; Liu, C.; Wu, F.; Zhang, J.; Zhang, Y. Corrigendum: ENC1 Facilitates Colorectal Carcinoma Tumorigenesis and Metastasis via JAK2/STAT5/AKT Axis-Mediated Epithelial Mesenchymal Transition and Stemness. Front. Cell Dev. Biol. 2021, 9, 758671. [Google Scholar] [CrossRef]
- Liu, H.; Liu, D.; Li, Z. Expression and clinical significance of ENC1 in gastrointestinal tumors: Bioinformatics analysis based on a public gene database. J. Gastrointest. Oncol. 2023, 14, 824. [Google Scholar] [CrossRef] [PubMed]
- Malaer, J.D.; Marrufo, A.M.; Mathew, P.A. 2B4 (CD244, SLAMF4) and CS1 (CD319, SLAMF7) in systemic lupus erythematosus and cancer. Clin. Immunol. 2018, 204, 50–56. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, P.; Hyslop, S.; Blake, M.K.; Godbehere, S.; Amalfitano, A.; Aldhamen, Y.A. SLAMF7 Signaling Reprograms T Cells toward Exhaustion in the Tumor Microenvironment. J. Immunol. 2021, 206, 193–205. [Google Scholar] [CrossRef]
- Cheng, M.; Michalski, S.; Kommagani, R. Role for Growth Regulation by Estrogen in Breast Cancer 1 (GREB1) in Hormone-Dependent Cancers. Int. J. Mol. Sci. 2018, 19, 2543. [Google Scholar] [CrossRef]
- Rae, J.M.; Michael, D.J.; Kevin, E.C.; Joshua, O.; José, M.L.; Marco, M.G.; Kenneth, J.P.; Marc, E.L. GREB1 is a novel androgen-regulated gene required for prostate cancer growth. Prostate 2006, 66, 886–894. [Google Scholar] [CrossRef]
- Guo, Y.; Shi, W.; Fang, R. miR-18a-5p promotes melanoma cell proliferation and inhibits apoptosis and autophagy by targeting EPHA7 signaling. Mol. Med. Rep. 2020, 23, 1. [Google Scholar] [CrossRef]
- Tsuboi, M.; Mori, H.; Bunai, T.; Kageyama, S.; Suzuki, M.; Okudela, K.; Takamochi, K.; Ogawa, H.; Shinmura, K.; Niwa, H.; et al. Secreted form of EphA7 in lung cancer. Int. J. Oncol. 2010, 36, 635–640. [Google Scholar]
- Bartolomé, R.A.; Robles, J.; Martin-Regalado, Á.; Pintado-Berninches, L.; Burdiel, M.; Jaén, M.; Aizpurúa, C.; Imbaud, J.I.; Casal, J.I. CDH6-activated αIIbβ3 crosstalks with α2β1 to trigger cellular adhesion and invasion in metastatic ovarian and renal cancers. Mol. Oncol. 2021, 15, 1849–1865. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, S.; Li, S.; Wang, J.; Lin, H.; Fu, W. High expression of oncogene cadherin-6 correlates with tumor progression and a poor prognosis in gastric cancer. Cancer Cell Int. 2021, 21, 493. [Google Scholar] [CrossRef]
- Gugnoni, M.; Sancisi, V.; Gandolfi, G.; Manzotti, G.; Ragazzi, M.; Giordano, D.; Tamagnini, I.; Tigano, M.; Frasoldati, A.; Piana, S.; et al. Cadherin-6 promotes EMT and cancer metastasis by restraining autophagy. Oncogene 2016, 36, 667–677. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, W.; Kayed, H.; Zheng, P.; Giese, N.A.; Friess, H.; Kleeff, J. Loss of ONECUT1 expression in human pancreatic cancer cells. Oncol. Rep. 2008, 19, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.J.; Wu, Y.; Johnson, R.A.; Woollard, J.R.; Bergstralh, E.J.; Cicek, M.S.; Bakeberg, J.; Rossetti, S.; Heyer, C.M.; Petersen, G.M.; et al. Germline PKHD1 mutations are protective against colorectal cancer. Hum. Genet. 2011, 129, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk, B.J.; Simpson-Haidaris, P.J. Fibrinogen assembly, secretion, and deposition into extracellular matrix by MCF-7 human breast carcinoma cells. Cancer Res. 2000, 60, 2033–2039. [Google Scholar] [PubMed]
- Knight, T.E.; Edwards, H.; Meshinchi, S.; Taub, J.W.; Ge, Y. “FLipping” the Story: FLT3-Mutated Acute Myeloid Leukemia and the Evolving Role of FLT3 Inhibitors. Cancers 2022, 14, 3398. [Google Scholar] [CrossRef]
- Stirewalt, D.L.; Radich, J.P. The role of FLT3 in haematopoietic malignancies. Nat. Rev. Cancer 2003, 3, 650–665. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, F.; Wang, B.; He, L.; Liu, Y.; Liu, Z. Dock2 in the development of inflammation and cancer. Eur. J. Immunol. 2018, 48, 915–922. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Cui, Y.; Zhang, X. Suppression of RNA editing by miR-17 inhibits the stemness of melanoma stem cells. Mol. Nucleic Acids 2022, 27, 439–455. [Google Scholar] [CrossRef]
- Lei, Y.; Henderson, B.R.; Emmanuel, C.; Harnett, P.R.; Defazio, A. Inhibition of ANKRD1 sensitizes human ovarian cancer cells to endoplasmic reticulum stress-induced apoptosis. Oncogene 2014, 34, 485–495. [Google Scholar] [CrossRef]
- Wang, L.; Yue, Y.; Zhang, L.; Jing, M.; Ma, M.; Liu, C.; Li, Y.; Xu, S.; Wang, K.; Wang, X.; et al. PAQR5 inhibits the growth and metastasis of clear cell renal cell carcinoma by suppressing the JAK/STAT3 signaling pathway. Cell Oncol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Liu, W.; Yan, X.; Yang, M.; Yao, S.; Shu, Q.; Li, B.; Zhu, R. PAQR5 Expression Is Suppressed by TGFβ1 and Associated With a Poor Survival Outcome in Renal Clear Cell Carcinoma. Front. Oncol. 2022, 11, 827344. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Liu, W.; Yang, Y.; Chu, Z.; Yang, C.; Yang, T.; Sun, J. TRIM16 overexpression inhibits the metastasis of colorectal cancer through mediating Snail degradation. Exp. Cell Res. 2021, 406, 112735. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.M.; Bell, J.L.; Koach, J.; Tan, O.; Kim, P.; Malyukova, A.; Thomas, W.; O Sekyere, E.; Liu, T.; Cunningham, A.M.; et al. TRIM16 acts as a tumour suppressor by inhibitory effects on cytoplasmic vimentin and nuclear E2F1 in neuroblastoma cells. Oncogene 2010, 29, 6172–6183. [Google Scholar] [CrossRef]
- Albensi, B.C. What is nuclear factor kappa B (NF-κB) doing in and to the mitochondrion. Front. Cell Dev. Biol. 2019, 7, 154. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef]
- González, R.; Francisco, J.; Molina-Ruiz, J.; Antonio, B.C.; Alicia, P.; Jordi, M. Regulation of cell survival, apoptosis, and epithelial-to-mesenchymal transition by nitric oxide-dependent post-translational modifications. Antioxid. Redox. Signal. 2018, 29, 1312–1332. [Google Scholar] [CrossRef]
- Della-Valle, V.; Roos-Weil, D.; Scourzic, L.; Mouly, E.; Aid, Z.; Darwiche, W.; Lecluse, Y.; Damm, F.; Mémet, S.; Mercher, T.; et al. Nfkbie-deficiency leads to increased susceptibility to develop B-cell lymphoproliferative disorders in aged mice. Blood Cancer J. 2020, 10, 38. [Google Scholar] [CrossRef]
- Olson, S.Y.; Garbán, H.J. Regulation of apoptosis-related genes by nitric oxide in cancer. Nitric Oxide 2008, 19, 170–176. [Google Scholar] [CrossRef]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-signaling pathway in cancer. OncoTargets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef]
- Bonini, P.; Kind, T.; Tsugawa, H.; Barupal, D.K.; Fiehn, O. Retip: Retention Time Prediction for Compound Annotation in Untargeted Metabolomics. Anal. Chem. 2020, 92, 7515–7522. [Google Scholar] [CrossRef]
- Stamm, A.; Reimers, K.; Strauß, S.; Vogt, P.; Scheper, T.; Pepelanova, I. In vitro wound healing assays—State of the art. BioNano-Mater. 2016, 17, 79–87. [Google Scholar] [CrossRef]
- Bolger, A.; Giorgi, F. Trimmomatic: A flexible read trimming tool for illumina NGS data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]





| S. No | Proposed Compounds | Formula | Rt [M − H] | Peak Area |
|---|---|---|---|---|
| Amino Acid Derivatives | ||||
| 1 | 4-Methyleneglutamine | C6H10N2O3 | 0.877 | 2,360,952,958 |
| 2 | D-(+)-Pyroglutamic Acid | C5H7NO3 | 1.901 | 816,414,433.5 |
| 3 | (2S)-3-(1H-Imidazol-4-yl) | C12H19N3O7 | 1.709 | 601,125,246 |
| 4 | DL-Arginine | C6H14N4O2 | 0.85 | 549,758,768 |
| 5 | DL-Histidine | C6H9N3O2 | 0.851 | 432,774,848.5 |
| 6 | DL-Arginine | C6H14N4O2 | 0.85 | 549,758,768 |
| 7 | Ornithine | C5H12N2O2 | 0.882 | 260,397,275 |
| 8 | D-(-)-Glutamine | C5H10N2O3 | 9.389 | 249,168,133.5 |
| 9 | N-Acetyl-L-Citrulline | C8H15N3O4 | 0.911 | 13,964,667.5 |
| 10 | L-(+)-Citrulline | C6H13N3O3 | 3.383 | 10,651,743 |
| 11 | Isoleucine | C6H13NO2 | 2.54 | 244,248,729 |
| 12 | L-Phenylalanine | C9H11NO2 | 3.718 | 133,133,714 |
| 13 | L-Glutamic acid, 5-[2-(4-carboxyphenyl)hydrazide] | C12H15N3O5 | 1.71 | 149,760,416 |
| 14 | L-(+)-Lactic acid | C3H6O3 | 10.516 | 89,115,500 |
| 15 | D-PANTOTHENIC ACID | C9H17NO5 | 3.845 | 143,296,593.5 |
| 16 | Valine | C5H11NO2 | 0.996 | 143,149,592 |
| 17 | L-Histidine | C6H9N3O2 | 0.837 | 18,285,235.5 |
| 18 | (2S)-2-Piperazinecarboxylic acid | C5H10N2O2 | 2.65 | 29,609,897.5 |
| 19 | N-Acetylglucosaminitol | C8H17NO6 | 0.858 | 23,771,288.5 |
| 20 | N-Acetyl-L-glutamic acid | C7H11NO5 | 1.983 | 22,064,841.5 |
| Acetylcarnitine | C9H17NO4 | 8.78 | 21,886,355.5 | |
| 21 | N-Acetyl-L-phenylalanine | C11H13NO3 | 5.26 | 5,322,091 |
| 22 | Organic Derivatives | |||
| 23 | DL-Malic acid | C4H6O5 | 0.98 | 3,630,710,449 |
| 24 | Isocitric acid | C6H8O7 | 1.822 | 1,381,987,018 |
| 25 | D-(+)-Pyroglutamic Acid | C5H7NO3 | 1.901 | 816,414,433.5 |
| 26 | Anthranilic acid | C7H7NO2 | 0.912 | 651,974,104 |
| 27 | 2-(alpha-d-mannosyl)-d-glyceric acid | C9H16O9 | 9.792 | 271,529,954.5 |
| 28 | 2-Formyl-1H-pyrrole | C5H5NO | 0.879 | 261,140,066.5 |
| 29 | 2-(1,3-Benzodioxol-5-yl)-4,5,6,7-tetramethyl-1H-benzimidazole | C18H18N2O2 | 8.301 | 86,427,528 |
| 30 | Gluconic acid | C6H12O7 | 0.881 | 219,928,281.5 |
| 31 | 2-Furoic acid | C5H4O3 | 1.433 | 136,738,393.5 |
| 32 | Citric acid | C6H8O7 | 1.822 | 1,381,987,018 |
| 33 | Glutarylcarnitine | C12H21NO6 | 2.963 | 209,483,451.5 |
| 34 | Benzyl ë?-primeveroside | C18H26O10 | 4.177 | 170,786,527 |
| 35 | Glucoheptonic Acid | C7H14O8 | 8.76 | 43,076,366 |
| 36 | 4-Hydroxybenzoic acid | C7H6O3 | 4.279 | 42,979,188 |
| 37 | Succinic anhydride | C4H4O3 | 2.332 | 33,805,020.5 |
| 38 | Itaconic acid | C5H6O4 | 1.451 | 24,903,345.5 |
| 39 | Mesaconic acid | C5H6O4 | 1.824 | 28,480,332 |
| 40 | Sorbic acid | C6H8O2 | 0.974 | 27,447,509.5 |
| 41 | Malondialdehyde | C3H4O2 | 0.859 | 21,556,194.5 |
| 42 | 2,6-Dimethoxybenzoquinone | C8H8O4 | 0.894 | 32,512,334 |
| 43 | Acetonedicarboxylic Acid | C5H6O5 | ||
| 44 | 6-Oxo-pipecolinic acid | C6H9NO3 | 1.702 | 23,925,387 |
| 45 | N-Acetyl-L-glutamic acid | C7H11NO5 | 1.983 | 22,064,841.5 |
| 46 | Acetylcarnitine | C9H17NO4 | 8.78 | 21,886,355.5 |
| 47 | Mevalonic acid | C6H12O4 | 6.95 | 21,755,237.5 |
| 48 | Malonic acid | |||
| 49 | 1,3,7-Trimethyluric acid | C8H10N4O3 | 10.067 | 18,133,176.5 |
| 50 | 5-Hydroxy-2-furoic acid | C5H4O4 | 1.805 | 50,812,245.5 |
| 51 | (±)-Malic Acid | C4H6O5 | 1.209 | 50,383,480 |
| 52 | Glucoheptonic Acid | C7H14O8 | 8.76 | 43,076,366 |
| 53 | Acetylcarnitine | C9H17NO4 | 8.78 | 21,886,355.5 |
| 54 | Itaconic acid | C5H6O4 | 1.451 | 24,903,345.5 |
| 55 | Mesaconic acid | C5H6O4 | 1.824 | 28,480,332 |
| 56 | Sorbic acid | C6H8O2 | 0.974 | 27,447,509.5 |
| Malondialdehyde | C3H4O2 | 0.859 | 21,556,194.5 | |
| 57 | Malonic acid | |||
| 58 | Sugar Derivatives | |||
| 59 | N-Acetylglucosamine | C17H27N3O17P2 | 1.264 | 2,146,760 |
| 60 | Maltose | C12H22O11 | 3.179 | 10,386,453 |
| Lactose | C12H22O11 | 0.981 | 289,280,035.5 | |
| 61 | Sucrose | C12H22O11 | 10.781 | 1,531,407 |
| Trehalose | C12H22O11 | 0.943 | 103,537,815 | |
| 62 | Raffinose | C18H32O16 | 1.32 | 5,170,093 |
| 63 | Hydroxycinnamic Acid Derivatives | |||
| 64 | Caffeic acid | C9H8O4 | 4.098 | 4,166,656 |
| 65 | p-Coumaric acid | C9H8O3 | 5.414 | 773,133 |
| Sample | No. of Raw PE Reads | No. of Filtered PE Reads | No. of Uniquely Mapped PE Reads | Mapping Percentage of Uniquely Mapped Reads |
|---|---|---|---|---|
| WMRC1 | 26,784,649 | 25,630,111 | 24,702,469 | 96.4 |
| WMRC2 | 27,470,824 | 26,302,571 | 25,318,621 | 96.3 |
| WMRC3 | 25,588,547 | 24,467,830 | 23,597,931 | 96.4 |
| WMRT1 | 32,424,898 | 31,024,773 | 29,939,262 | 96.5 |
| WMRT2 | 27,827,568 | 26,668,858 | 25,713,431 | 96.4 |
| WMRT3 | 31,744,771 | 30,391,428 | 29,270,759 | 96.3 |
| Pairwise comparison | Total DEGs | Upregulated | Downregulated | |
| Control vs. 44.8 MG | 186 | 149 | 37 | |
| S. No | Gene ID | Fold Change | p adj | Regulation | Annotation |
|---|---|---|---|---|---|
| 1 | NPTX1 | 3.822151586 | 1.40205 × 10−22 | UP | neuronal pentraxin 1(NPTX1) |
| 2 | JMA2 | 3.654043227 | 6.50348 × 10−7 | UP | junctional adhesion molecule 2(JAM2) |
| 3 | HMOX1 | 2.791739716 | 3.72984 × 10−9 | UP | heme oxygenase 1(HMOX1) |
| 4 | TRIM31 | 2.578310857 | 0.014206294 | UP | tripartite motif containing 31(TRIM31) |
| 5 | CXCL2 | 2.390913463 | 3.41557 × 10−11 | UP | C-X-C motif chemokine ligand 2(CXCL2) |
| 6 | KDF1 | 1.864605173 | 0.049178644 | UP | keratinocyte differentiation factor 1(KDF1) |
| 7 | TNFAIP3 | 1.77972605 | 1.3479 × 10−25 | UP | TNF alpha induced protein 3(TNFAIP3) |
| 8 | EFEMP2 | 1.753814923 | 0.045380994 | UP | EGF containing fibulin extracellular matrix protein 2(EFEMP2) |
| 9 | LINC00887 | 1.604240482 | 0.045386292 | UP | long intergenic non-protein coding RNA 887(LINC00887) |
| 10 | NFKBID | 1.4789834 | 1.08647 × 10−6 | UP | NFKB inhibitor delta(NFKBID) |
| 11 | LINC00472 | 1.467452434 | 0.000148121 | UP | long intergenic non-protein coding RNA 472(LINC00472) |
| 12 | PAQR5 | 1.355607471 | 9.19446 × 10−6 | UP | progestin and adipoQ receptor family member 5(PAQR5) |
| 13 | COL7A1 | 1.292376567 | 2.89259 × 10−43 | UP | collagen type VII alpha 1 chain(COL7A1) |
| 14 | RNF144B | 1.287668834 | 8.06605 × 10−6 | UP | ring finger protein 144B(RNF144B) |
| 15 | SOD2 | 1.24731498 | 6.67748 × 10−64 | UP | superoxide dismutase 2(SOD2) |
| 16 | ARRB1 | 1.244050952 | 0.00013318 | UP | arrestin beta 1(ARRB1) |
| 17 | BMF | 1.183513353 | 0.000755165 | UP | Bcl2 modifying factor(BMF) |
| 18 | NFKBIA | 1.140983535 | 1.77033 × 10−26 | UP | NFKB inhibitor alpha(NFKBIA) |
| 19 | TPD52L1 | 1.162282589 | 0.007265863 | UP | TPD52 like 1(TPD52L1) |
| 20 | RHBDL1 | 1.127426241 | 0.039940739 | UP | rhomboid like 1(RHBDL1) |
| 21 | CD82 | 1.109222868 | 3.56575 × 10−5 | UP | CD82 molecule(CD82) |
| 22 | CLIP4 | 1.105718912 | 0.004748612 | UP | CAP-Gly domain containing linker protein family member 4(CLIP4) |
| 23 | TRIM16L | 1.088105329 | 9.69946 × 10−41 | UP | tripartite motif containing 16 like(TRIM16L) |
| 24 | NFKBIE | 1.065145553 | 3.25882 × 10−15 | UP | NFKB inhibitor epsilon(NFKBIE) |
| 25 | TMEM158 | 1.061667088 | 1.49615 × 10−6 | UP | transmembrane protein 158(TMEM158) |
| 26 | ZC3H12A | 1.058928366 | 7.96899 × 10−18 | UP | s100 calcium binding protein A4(S100A4) |
| 27 | HSF4 | 1.058853794 | 0.005924004 | UP | heat shock transcription factor 4(HSF4) |
| 28 | AKR1C2 | 1.058573734 | 2.63416 × 10−21 | UP | aldo-keto reductase family 1 member C2(AKR1C2) |
| 29 | ADAMTS7 | 1.047212012 | 7.15087 ×10−14 | UP | ADAM metallopeptidase with thrombospondin type 1 motif 7(ADAMTS7) |
| 30 | KCNK3 | 1.026865059 | 3.68494 × 10−17 | UP | potassium two pore domain channel subfamily K member 3(KCNK3) |
| 31 | PGGHG | 1.026377088 | 9.09732 × 10−19 | UP | protein-glucosylgalactosylhydroxylysine glucosidase(PGGHG) |
| 32 | LACTB | 1.007908666 | 0.004812089 | UP | lactamase beta(LACTB) |
| 33 | ATG16L2 | 1.001999864 | 1.37778 × 10−5 | UP | autophagy related 16 like 2(ATG16L2) |
| 34 | CELF2 | −1.012165448 | 0.00091243 | DOWN | CUGBP Elav-like family member 2(CELF2) |
| 35 | KCNH1 | −1.01380309 | 0.020077479 | DOWN | potassium voltage-gated channel subfamily H member 1(KCNH1) |
| 36 | CDH6 | −1.044599459 | 1.57671 × 10−11 | DOWN | cadherin 6(CDH6) |
| 37 | DOCK2 | −1.058742034 | 4.76266 × 10−22 | DOWN | dedicator of cytokinesis 2(DOCK2) |
| 38 | PAX8-AS1 | −1.058885169 | 2.95114 × 10−8 | DOWN | PAX8 antisense RNA 1(PAX8-AS1) |
| 39 | ANKRD1 | −1.080370398 | 4.09198 × 10−9 | DOWN | ankyrin repeat domain 1(ANKRD1) |
| 40 | TAF1A-AS1 | −1.086638886 | 0.038122062 | DOWN | TAF1A antisense RNA 1(TAF1A-AS1) |
| 41 | RHOU | −1.10777548 | 0.048963406 | DOWN | ras homolog family member U(RHOU) |
| 42 | SLC16A9 | −1.1715508 | 0.010549451 | DOWN | solute carrier family 16 member 9(SLC16A9) |
| 43 | ENC1 | −1.17558341 | 0.000148121 | DOWN | ectodermal-neural cortex 1(ENC1) |
| 44 | GREB1 | −1.200291699 | 0.038699026 | DOWN | growth regulating estrogen receptor binding 1(GREB1) |
| 45 | PKHD1 | −1.311859039 | 4.96206 × 10−5 | DOWN | PKHD1 ciliary IPT domain containing fibrocystin/polyductin(PKHD1) |
| 46 | HORMAD2-AS1 | −1.375725708 | 0.042051375 | DOWN | HORMAD2 and MTMR3 antisense RNA 1(HORMAD2-AS1) |
| 47 | FGB | −1.441683784 | 1.07928 ×10−56 | DOWN | fibrinogen beta chain(FGB) |
| 48 | SLCO4C1 | −1.500449851 | 3.46892 × 10−5 | DOWN | solute carrier organic anion transporter family member 4C1(SLCO4C1) |
| 49 | NPNT | −1.529088042 | 5.81654 × 10−9 | DOWN | nephronectin(NPNT) |
| 50 | ARHGAP28 | −1.53301957 | 6.57424 × 10−6 | DOWN | Rho GTPase activating protein 28(ARHGAP28) |
| 51 | C1orf116 | −1.56932167 | 0.045320324 | DOWN | chromosome 1 open reading frame 116(C1orf116) |
| 52 | SLAMF7 | −1.840054142 | 0.024930087 | DOWN | SLAM family member 7(SLAMF7) |
| 53 | UNC13C | −1.944259269 | 0.03671916 | DOWN | unc-13 homolog C(UNC13C) |
| 54 | EPHA7 | −2.087931878 | 7.25051 × 10−81 | DOWN | EPH receptor A7(EPHA7) |
| 55 | SLC26A5-AS1 | −2.285084696 | 0.010311261 | DOWN | SLC26A5 antisense RNA 1(SLC26A5-AS1) |
| 56 | SULT1B1 | −2.333807748 | 3.9407 × 10−5 | DOWN | sulfotransferase family 1B member 1(SULT1B1) |
| 57 | FLT3 | −2.335935596 | 0.001480949 | DOWN | fms related receptor tyrosine kinase 3(FLT3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, C.S.; Natarajan, P.; Nimmakayala, P.; Hankins, G.R.; Reddy, U.K. From Fruit Waste to Medical Insight: The Comprehensive Role of Watermelon Rind Extract on Renal Adenocarcinoma Cellular and Transcriptomic Dynamics. Int. J. Mol. Sci. 2023, 24, 15615. https://doi.org/10.3390/ijms242115615
Reddy CS, Natarajan P, Nimmakayala P, Hankins GR, Reddy UK. From Fruit Waste to Medical Insight: The Comprehensive Role of Watermelon Rind Extract on Renal Adenocarcinoma Cellular and Transcriptomic Dynamics. International Journal of Molecular Sciences. 2023; 24(21):15615. https://doi.org/10.3390/ijms242115615
Chicago/Turabian StyleReddy, Chinreddy Subramanaym, Purushothaman Natarajan, Padma Nimmakayala, Gerald R. Hankins, and Umesh K. Reddy. 2023. "From Fruit Waste to Medical Insight: The Comprehensive Role of Watermelon Rind Extract on Renal Adenocarcinoma Cellular and Transcriptomic Dynamics" International Journal of Molecular Sciences 24, no. 21: 15615. https://doi.org/10.3390/ijms242115615







