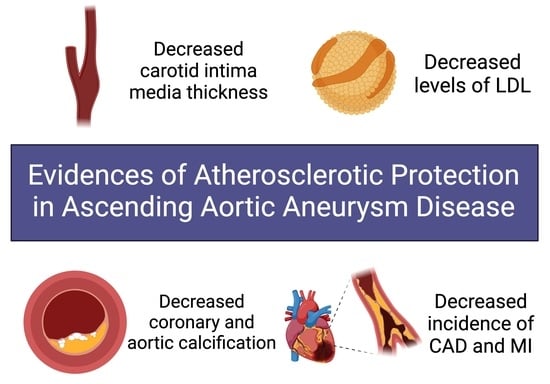
Evidence Accumulates: Patients with Ascending Aneurysms Are Strongly Protected from Atherosclerotic Disease
Abstract
1. Introduction
2. Embryology of the Aorta
3. Our Investigations
3.1. Intimal Medial Thickness
3.2. Lipid Profiles
3.3. Coronary Artery and Aortic Calcification
3.4. CAD/Total Protection from MI
4. Aortic Histology
5. Discussion: Potential Mechanisms of Anti-Atherogenic Protection
5.1. Aortic Vascular Smooth Muscle Cells
5.2. Phenotypic Switch Defect in Thoracic Aortopathy Discourages Atherosclerosis
5.3. MMP/TMP Dysregulation Accompanies VSMC Disturbances
5.4. TGF- Provides Anti-Atherogenic Contribution
5.5. Hemodynamic Changes
6. Conclusions/Future Steps/Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zarins, C.K.; Xu, C.; Glagov, S. Atherosclerotic enlargement of the human abdominal aorta. Atherosclerosis 2001, 155, 157–164. [Google Scholar] [CrossRef]
- Singh, K.; Bonaa, K.H.; Jacobsen, B.K.; Bjork, L.; Solberg, S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: The Tromso Study. Am. J. Epidemiol. 2001, 154, 236–244. [Google Scholar] [CrossRef]
- Tung, W.S.; Lee, J.K.; Thompson, R.W. Simultaneous analysis of 1176 gene products in normal human aorta and abdominal aortic aneurysms using a membrane-based complementary DNA expression array. J. Vasc. Surg. 2001, 34, 143–150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuhlencordt, P.J.; Gyurko, R.; Han, F.; Scherrer-Crosbie, M.; Aretz, T.H.; Hajjar, R.; Picard, M.H.; Huang, P.L. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation 2001, 104, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Biddinger, A.; Rocklin, M.; Coselli, J.; Milewicz, D.M. Familial thoracic aortic dilatations and dissections: A case control study. J. Vasc. Surg. 1997, 25, 506–511. [Google Scholar] [CrossRef]
- Ostberg, N.P.; Zafar, M.A.; Ziganshin, B.A.; Elefteriades, J.A. The Genetics of Thoracic Aortic Aneurysms and Dissection: A Clinical Perspective. Biomolecules 2020, 10, 182. [Google Scholar] [CrossRef]
- Schmidt, J.; Sunesen, K.; Kornum, J.B.; Duhaut, P.; Thomsen, R.W. Predictors for pathologically confirmed aortitis after resection of the ascending aorta: A 12-year Danish nationwide population-based cross-sectional study. Arthritis Res. Ther. 2011, 13, R87. [Google Scholar] [CrossRef]
- Evans, J.M.; O’Fallon, W.M.; Hunder, G.G. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann. Intern. Med. 1995, 122, 502–507. [Google Scholar] [CrossRef]
- Roberts, W.C.; Barbin, C.M.; Weissenborn, M.R.; Ko, J.M.; Henry, A.C. Syphilis as a Cause of Thoracic Aortic Aneurysm. Am. J. Cardiol. 2015, 116, 1298–1303. [Google Scholar] [CrossRef]
- Elefteriades, J.A.; Ziganshin, B.A.; Halperin, J.L. Diseases of the Aorta. In Fuster and Hurst’s the Heart, 15e; Fuster, V., Narula, J., Vaishnava, P., Leon, M.B., Callans, D.J., Rumsfeld, J., Poppas, A., Eds.; McGraw-Hill Education: New York, NY, USA, 2022. [Google Scholar]
- Ruddy, J.M.; Jones, J.A.; Spinale, F.G.; Ikonomidis, J.S. Regional heterogeneity within the aorta: Relevance to aneurysm disease. J. Thorac. Cardiovasc. Surg. 2008, 136, 1123–1130. [Google Scholar] [CrossRef]
- Elefteriades, J.A.; Farkas, E.A. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J. Am. Coll. Cardiol. 2010, 55, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, J.M.; Jones, J.A.; Ikonomidis, J.S. Pathophysiology of thoracic aortic aneurysm (TAA): Is it not one uniform aorta? Role of embryologic origin. Prog. Cardiovasc. Dis. 2013, 56, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Grewal, N.; Gittenberger-de Groot, A.C.; Lindeman, J.H.; Klautz, A.; Driessen, A.; Klautz, R.J.M.; Poelmann, R.E. Normal and abnormal development of the aortic valve and ascending aortic wall: A comprehensive overview of the embryology and pathology of the bicuspid aortic valve. Ann. Cardiothorac. Surg. 2022, 11, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Halloran, B.G.; Davis, V.A.; McManus, B.M.; Lynch, T.G.; Baxter, B.T. Localization of aortic disease is associated with intrinsic differences in aortic structure. J. Surg. Res. 1995, 59, 17–22. [Google Scholar] [CrossRef]
- Bots, M.L.; Grobbee, D.E. Intima media thickness as a surrogate marker for generalised atherosclerosis. Cardiovasc. Drugs Ther. 2002, 16, 341–351. [Google Scholar] [CrossRef]
- Crouse, J.R.; Goldbourt, U.; Evans, G.; Pinsky, J.; Sharrett, A.R.; Sorlie, P.; Riley, W.; Heiss, G. Risk factors and segment-specific carotid arterial enlargement in the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1996, 27, 69–75. [Google Scholar] [CrossRef]
- Bots, M.L.; Hoes, A.W.; Koudstaal, P.J.; Hofman, A.; Grobbee, D.E. Common carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation 1997, 96, 1432–1437. [Google Scholar] [CrossRef]
- O’Leary, D.H.; Polak, J.F.; Kronmal, R.A.; Manolio, T.A.; Burke, G.L.; Wolfson, S.K., Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N. Engl. J. Med. 1999, 340, 14–22. [Google Scholar] [CrossRef]
- Urbina, E.M.; Srinivasan, S.R.; Tang, R.; Bond, M.G.; Kieltyka, L.; Berenson, G.S.; Bogalusa Heart, S. Impact of multiple coronary risk factors on the intima-media thickness of different segments of carotid artery in healthy young adults (The Bogalusa Heart Study). Am. J. Cardiol. 2002, 90, 953–958. [Google Scholar] [CrossRef]
- Salonen, J.T.; Salonen, R. Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation 1993, 87, II56–II65. [Google Scholar]
- Johnsen, S.H.; Mathiesen, E.B.; Joakimsen, O.; Stensland, E.; Wilsgaard, T.; Lochen, M.L.; Njolstad, I.; Arnesen, E. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: A 6-year follow-up study of 6226 persons: The Tromso Study. Stroke 2007, 38, 2873–2880. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Corrado, E.; Coppola, G.; Muratori, I.; Novo, G.; Novo, S. Prediction of cardio- and cerebro-vascular events in patients with subclinical carotid atherosclerosis and low HDL-cholesterol. Atherosclerosis 2008, 200, 389–395. [Google Scholar] [CrossRef]
- Novo, S.; Peritore, A.; Trovato, R.L.; Guarneri, F.P.; Di Lisi, D.; Muratori, I.; Novo, G. Preclinical atherosclerosis and metabolic syndrome increase cardio- and cerebrovascular events rate: A 20-year follow up. Cardiovasc. Diabetol. 2013, 12, 155. [Google Scholar] [CrossRef]
- Stein, J.H.; Fraizer, M.C.; Aeschlimann, S.E.; Nelson-Worel, J.; McBride, P.E.; Douglas, P.S. Vascular age: Integrating carotid intima-media thickness measurements with global coronary risk assessment. Clin. Cardiol. 2004, 27, 388–392. [Google Scholar] [CrossRef]
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiogr. 2008, 21, 93–111, quiz 189–190. [Google Scholar] [CrossRef]
- Ali, Y.S.; Rembold, K.E.; Weaver, B.; Wills, M.B.; Tatar, S.; Ayers, C.R.; Rembold, C.M. Prediction of major adverse cardiovascular events by age-normalized carotid intimal medial thickness. Atherosclerosis 2006, 187, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Gepner, A.D.; Keevil, J.G.; Wyman, R.A.; Korcarz, C.E.; Aeschlimann, S.E.; Busse, K.L.; Stein, J.H. Use of carotid intima-media thickness and vascular age to modify cardiovascular risk prediction. J. Am. Soc. Echocardiogr. 2006, 19, 1170–1174. [Google Scholar] [CrossRef]
- Ludwig, M.; von Petzinger-Kruthoff, A.; von Buquoy, M.; Stumpe, K.O. Intima-Media-Dicke der Karotisarterien: Fruher Indikator fur Arteriosklerose und therapeutischer Endpunkt. [Intima media thickness of the carotid arteries: Early pointer to arteriosclerosis and therapeutic endpoint]. Ultraschall Med. 2003, 24, 162–174. [Google Scholar] [CrossRef]
- Baldassarre, D.; Amato, M.; Bondioli, A.; Sirtori, C.R.; Tremoli, E. Carotid artery intima-media thickness measured by ultrasonography in normal clinical practice correlates well with atherosclerosis risk factors. Stroke 2000, 31, 2426–2430. [Google Scholar] [CrossRef]
- Poredos, P. Intima-media thickness: Indicator of cardiovascular risk and measure of the extent of atherosclerosis. Vasc. Med. 2004, 9, 46–54. [Google Scholar] [CrossRef]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Chambless, L.E.; Heiss, G.; Folsom, A.R.; Rosamond, W.; Szklo, M.; Sharrett, A.R.; Clegg, L.X. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: The Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am. J. Epidemiol. 1997, 146, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Greenland, P.; Alpert, J.S.; Beller, G.A.; Benjamin, E.J.; Budoff, M.J.; Fayad, Z.A.; Foster, E.; Hlatky, M.A.; Hodgson, J.M.; Kushner, F.G.; et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2010, 56, e50–e103. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.F.; O’Leary, D.H.; Kronmal, R.A.; Wolfson, S.K.; Bond, M.G.; Tracy, R.P.; Gardin, J.M.; Kittner, S.J.; Price, T.R.; Savage, P.J. Sonographic evaluation of carotid artery atherosclerosis in the elderly: Relationship of disease severity to stroke and transient ischemic attack. Radiology 1993, 188, 363–370. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, D.H.; Polak, J.F.; Kronmal, R.A.; Savage, P.J.; Borhani, N.O.; Kittner, S.J.; Tracy, R.; Gardin, J.M.; Price, T.R.; Furberg, C.D. Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Cardiovascular Health Study Collaborative Research Group. Stroke 1996, 27, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.F.; Szklo, M.; O’Leary, D.H. Carotid Intima-Media Thickness Score, Positive Coronary Artery Calcium Score, and Incident Coronary Heart Disease: The Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2017, 6, e004612. [Google Scholar] [CrossRef]
- Kim, G.H.; Youn, H.J. Is Carotid Artery Ultrasound Still Useful Method for Evaluation of Atherosclerosis? Korean Circ. J. 2017, 47, 1–8. [Google Scholar] [CrossRef]
- Saba, L.; Jamthikar, A.; Gupta, D.; Khanna, N.N.; Viskovic, K.; Suri, H.S.; Gupta, A.; Mavrogeni, S.; Turk, M.; Laird, J.R.; et al. Global perspective on carotid intima-media thickness and plaque: Should the current measurement guidelines be revisited? Int. Angiol. 2019, 38, 451–465. [Google Scholar] [CrossRef]
- Hung, A.; Zafar, M.; Mukherjee, S.; Tranquilli, M.; Scoutt, L.M.; Elefteriades, J.A. Carotid intima-media thickness provides evidence that ascending aortic aneurysm protects against systemic atherosclerosis. Cardiology 2012, 123, 71–77. [Google Scholar] [CrossRef]
- Norrgard, O.; Angquist, K.A.; Dahlen, G. High concentrations of Lp(a) lipoprotein in serum are common among patients with abdominal aortic aneurysms. Int. Angiol. 1988, 7, 46–49. [Google Scholar]
- Papagrigorakis, E.; Iliopoulos, D.; Asimacopoulos, P.J.; Safi, H.J.; Weilbaecher, D.J.; Ghazzaly, K.G.; Nava, M.L.; Gaubatz, J.W.; Morrisett, J.D. Lipoprotein(a) in plasma, arterial wall, and thrombus from patients with aortic aneurysm. Clin. Genet. 1997, 52, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, M.; Domanovits, H.; Ignatescu, M.; Exner, M.; Bayegan, K.; Sedivy, R.; Polterauer, P.; Laggner, A.N.; Minar, E.; Kostner, K. Lipoprotein (a) in patients with aortic aneurysmal disease. J. Vasc. Surg. 2002, 36, 25–30. [Google Scholar] [CrossRef]
- Naydeck, B.L.; Sutton-Tyrrell, K.; Schiller, K.D.; Newman, A.B.; Kuller, L.H. Prevalence and risk factors for abdominal aortic aneurysms in older adults with and without isolated systolic hypertension. Am. J. Cardiol. 1999, 83, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Cushing, G.L.; Gaubatz, J.W.; Nava, M.L.; Burdick, B.J.; Bocan, T.M.; Guyton, J.R.; Weilbaecher, D.; DeBakey, M.E.; Lawrie, G.M.; Morrisett, J.D. Quantitation and localization of apolipoproteins [a] and B in coronary artery bypass vein grafts resected at re-operation. Arteriosclerosis 1989, 9, 593–603. [Google Scholar] [CrossRef]
- Kostner, G.M.; Avogaro, P.; Cazzolato, G.; Marth, E.; Bittolo-Bon, G.; Qunici, G.B. Lipoprotein Lp(a) and the risk for myocardial infarction. Atherosclerosis 1981, 38, 51–61. [Google Scholar] [CrossRef]
- Kostner, K.M.; Oberbauer, R.; Hoffmann, U.; Stefenelli, T.; Maurer, G.; Watschinger, B. Urinary excretion of apo(a) in patients after kidney transplantation. Nephrol. Dial. Transplant. 1997, 12, 2673–2678. [Google Scholar] [CrossRef]
- Murai, A.; Miyahara, T.; Fujimoto, N.; Matsuda, M.; Kameyama, M. Lp(a) lipoprotein as a risk factor for coronary heart disease and cerebral infarction. Atherosclerosis 1986, 59, 199–204. [Google Scholar] [CrossRef]
- Berg, K.; Dahlen, G.; Frick, M.H. Lp(a) lipoprotein and pre-beta1-lipoprotein in patients with coronary heart disease. Clin. Genet. 1974, 6, 230–235. [Google Scholar] [CrossRef]
- Armstrong, V.W.; Cremer, P.; Eberle, E.; Manke, A.; Schulze, F.; Wieland, H.; Kreuzer, H.; Seidel, D. The association between serum Lp(a) concentrations and angiographically assessed coronary atherosclerosis. Dependence on serum LDL levels. Atherosclerosis 1986, 62, 249–257. [Google Scholar] [CrossRef]
- Weininger, G.; Ostberg, N.; Shang, M.; Zafar, M.; Ziganshin, B.A.; Liu, S.; Erben, Y.; Elefteriades, J.A. Lipid profiles help to explain protection from systemic atherosclerosis in patients with ascending aortic aneurysm. J. Thorac. Cardiovasc. Surg. 2022, 163, e129–e132. [Google Scholar] [CrossRef]
- Kita, T.; Kume, N.; Minami, M.; Hayashida, K.; Murayama, T.; Sano, H.; Moriwaki, H.; Kataoka, H.; Nishi, E.; Horiuchi, H.; et al. Role of oxidized LDL in atherosclerosis. Ann. N. Y. Acad. Sci. 2001, 947, 199–205, discussion 205–196. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecher, U.P.; Zhang, H.F.; Lougheed, M. Role of oxidatively modified LDL in atherosclerosis. Free Radic. Biol. Med. 1990, 9, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Morabito, G.; Urban, L.; Ioannone, F.; Serafini, M. Oxidative stress in atherosclerosis development: The central role of LDL and oxidative burst. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 351–360. [Google Scholar] [CrossRef]
- Saigusa, T.; Izawa, A.; Miura, T.; Ebisawa, S.; Shiba, Y.; Miyashita, Y.; Tomita, T.; Koyama, J.; Fukui, D.; Takano, T.; et al. Low levels of high-density lipoprotein cholesterol predict the presence of coronary artery disease in patients with aortic aneurysms. Angiology 2014, 65, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.A.; Criqui, M.H.; Wright, C.M. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Solberg, L.A.; Eggen, D.A. Localization and sequence of development of atherosclerotic lesions in the carotid and vertebral arteries. Circulation 1971, 43, 711–724. [Google Scholar] [CrossRef]
- Rifkin, R.D.; Parisi, A.F.; Folland, E. Coronary calcification in the diagnosis of coronary artery disease. Am. J. Cardiol. 1979, 44, 141–147. [Google Scholar] [CrossRef]
- Sangiorgi, G.; Rumberger, J.A.; Severson, A.; Edwards, W.D.; Gregoire, J.; Fitzpatrick, L.A.; Schwartz, R.S. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: A histologic study of 723 coronary artery segments using nondecalcifying methodology. J. Am. Coll. Cardiol. 1998, 31, 126–133. [Google Scholar] [CrossRef]
- Doherty, T.M.; Detrano, R.C.; Mautner, S.L.; Mautner, G.C.; Shavelle, R.M. Coronary calcium: The good, the bad, and the uncertain. Am. Heart J. 1999, 137, 806–814. [Google Scholar] [CrossRef]
- Doherty, T.M.; Fitzpatrick, L.A.; Shaheen, A.; Rajavashisth, T.B.; Detrano, R.C. Genetic determinants of arterial calcification associated with atherosclerosis. Mayo Clin. Proc. 2004, 79, 197–210. [Google Scholar] [CrossRef]
- Watson, K.E. Pathophysiology of coronary calcification. J. Cardiovasc. Risk 2000, 7, 93–97. [Google Scholar] [CrossRef]
- Kuller, L.H.; Matthews, K.A.; Sutton-Tyrrell, K.; Edmundowicz, D.; Bunker, C.H. Coronary and aortic calcification among women 8 years after menopause and their premenopausal risk factors: The healthy women study. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2189–2198. [Google Scholar] [CrossRef]
- McCullough, P.A.; Soman, S. Cardiovascular calcification in patients with chronic renal failure: Are we on target with this risk factor? Kidney Int. Suppl. 2004, 66, S18–S24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Newman, A.B.; Naydeck, B.L.; Sutton-Tyrrell, K.; Edmundowicz, D.; O’Leary, D.; Kronmal, R.; Burke, G.L.; Kuller, L.H. Relationship between coronary artery calcification and other measures of subclinical cardiovascular disease in older adults. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1674–1679. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shaw, L.J.; Raggi, P.; Schisterman, E.; Berman, D.S.; Callister, T.Q. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology 2003, 228, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R.; Partridge, J. Coronary calcification score: The coronary-risk impact factor. Lancet 2004, 363, 557–559. [Google Scholar] [CrossRef]
- Danielsen, R.; Sigvaldason, H.; Thorgeirsson, G.; Sigfusson, N. Predominance of aortic calcification as an atherosclerotic manifestation in women: The Reykjavik study. J. Clin. Epidemiol. 1996, 49, 383–387. [Google Scholar] [CrossRef]
- Iribarren, C.; Sidney, S.; Sternfeld, B.; Browner, W.S. Calcification of the aortic arch: Risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA 2000, 283, 2810–2815. [Google Scholar] [CrossRef]
- Li, J.; Galvin, H.K.; Johnson, S.C.; Langston, C.S.; Sclamberg, J.; Preston, C.A. Aortic calcification on plain chest radiography increases risk for coronary artery disease. Chest 2002, 121, 1468–1471. [Google Scholar] [CrossRef]
- Symeonidis, G.; Papanas, N.; Giannakis, I.; Mavridis, G.; Lakasas, G.; Kyriakidis, G.; Artopoulos, I. Gravity of aortic arch calcification as evaluated in adult Greek patients. Int. Angiol. 2002, 21, 233–236. [Google Scholar]
- Takasu, J.; Mao, S.; Budoff, M.J. Aortic atherosclerosis detected with electron-beam CT as a predictor of obstructive coronary artery disease. Acad. Radiol. 2003, 10, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Shavelle, D.; Takasu, J.; Lu, B.; Mao, S.S.; Fischer, H.; Budoff, M.J. Valvular and thoracic aortic calcium as a marker of the extent and severity of angiographic coronary artery disease. Am. Heart J. 2003, 146, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Takasu, J.; Yamamoto, R.; Yokoyama, K.; Taguchi, R.; Itani, Y.; Imai, H.; Koizumi, T.; Nomoto, K.; Sato, N.; et al. Assessment of aortic atherosclerosis and carotid atherosclerosis in coronary artery disease. Jpn. Circ. J. 2000, 64, 745–749. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Achenbach, S.; Ropers, D.; Mohlenkamp, S.; Schmermund, A.; Muschiol, G.; Groth, J.; Kusus, M.; Regenfus, M.; Daniel, W.G.; Erbel, R.; et al. Variability of repeated coronary artery calcium measurements by electron beam tomography. Am. J. Cardiol. 2001, 87, 210–213, A218. [Google Scholar] [CrossRef] [PubMed]
- Janowitz, W.R.; Agatston, A.S.; Kaplan, G.; Viamonte, M., Jr. Differences in prevalence and extent of coronary artery calcium detected by ultrafast computed tomography in asymptomatic men and women. Am. J. Cardiol. 1993, 72, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, D.; Toulgaridis, T.; Davlouros, P.; Christodoulou, J.; Sitafidis, G.; Hahalis, G.; Vagenakis, A.G. Prognostic significance of coronary artery calcium in asymptomatic subjects with usual cardiovascular risk. Am. Heart J. 2003, 145, 542–548. [Google Scholar] [CrossRef]
- Jayalath, R.W.; Mangan, S.H.; Golledge, J. Aortic calcification. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 476–488. [Google Scholar] [CrossRef]
- O’Malley, P.G.; Taylor, A.J.; Jackson, J.L.; Doherty, T.M.; Detrano, R.C. Prognostic value of coronary electron-beam computed tomography for coronary heart disease events in asymptomatic populations. Am. J. Cardiol. 2000, 85, 945–948. [Google Scholar] [CrossRef]
- Achneck, H.; Modi, B.; Shaw, C.; Rizzo, J.; Albornoz, G.; Fusco, D.; Elefteriades, J. Ascending thoracic aneurysms are associated with decreased systemic atherosclerosis. Chest 2005, 128, 1580–1586. [Google Scholar] [CrossRef]
- Islamoglu, F.; Atay, Y.; Can, L.; Kara, E.; Ozbaran, M.; Yuksel, M.; Buket, S. Diagnosis and treatment of concomitant aortic and coronary disease: A retrospective study and brief review. Tex. Heart Inst. J. 1999, 26, 182–188. [Google Scholar]
- Agmon, Y.; Khandheria, B.K.; Meissner, I.; Schwartz, G.L.; Sicks, J.D.; Fought, A.J.; O’Fallon, W.M.; Wiebers, D.O.; Tajik, A.J. Is aortic dilatation an atherosclerosis-related process? Clinical, laboratory, and transesophageal echocardiographic correlates of thoracic aortic dimensions in the population with implications for thoracic aortic aneurysm formation. J. Am. Coll. Cardiol. 2003, 42, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Suwa, S.; Fujiwara, Y.; Inoue, K.; Mineda, Y.; Ohta, H.; Tokano, T.; Nakata, Y. Incidence and severity of coronary artery disease in patients with acute aortic dissection: Comparison with abdominal aortic aneurysm and arteriosclerosis obliterans. J. Cardiol. 2001, 37, 165–171. [Google Scholar] [PubMed]
- Nakashima, Y.; Kurozumi, T.; Sueishi, K.; Tanaka, K. Dissecting aneurysm: A clinicopathologic and histopathologic study of 111 autopsied cases. Hum. Pathol. 1990, 21, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Yeager, R.A.; Weigel, R.M.; Murphy, E.S.; McConnell, D.B.; Sasaki, T.M.; Vetto, R.M. Application of clinically valid cardiac risk factors to aortic aneurysm surgery. Arch. Surg. 1986, 121, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Langanay, T.; Valla, J.; Le Du, J.; Verhoye, J.P.; Leguerrier, A.; Lelong, B.; Menestret, P.; Rioux, C.; Logeais, Y. Insuffisance coronaire chez les patients ayant un anevrysme de l’aorte abdominale. A propos d’une serie consecutive de 172 operes. [Coronary artery disease in patients with aortic abdominal aneurysm. Apropos of a consecutive series of 172 cases]. Arch. Mal. Coeur Vaiss. 1996, 89, 211–218. [Google Scholar]
- Ruby, S.T.; Whittemore, A.D.; Couch, N.P.; Collins, J.J.; Cohn, L.; Shemin, R.; Mannick, J.A. Coronary artery disease in patients requiring abdominal aortic aneurysm repair. Selective use of a combined operation. Ann. Surg. 1985, 201, 758–764. [Google Scholar] [CrossRef]
- Kishi, K.; Ito, S.; Hiasa, Y. Risk factors and incidence of coronary artery lesions in patients with abdominal aortic aneurysms. Intern. Med. 1997, 36, 384–388. [Google Scholar] [CrossRef][Green Version]
- Chau, K.; Elefteriades, J.A. Ascending thoracic aortic aneurysms protect against myocardial infarctions. Int. J. Angiol. 2014, 23, 177–182. [Google Scholar] [CrossRef]
- Dolmaci, O.B.; El Mathari, S.; Driessen, A.H.G.; Klautz, R.J.M.; Poelmann, R.E.; Lindeman, J.H.N.; Grewal, N. Are Thoracic Aortic Aneurysm Patients at Increased Risk for Cardiovascular Diseases? J. Clin. Med. 2022, 12, 272. [Google Scholar] [CrossRef]
- Jackson, V.; Eriksson, M.J.; Caidahl, K.; Eriksson, P.; Franco-Cereceda, A. Ascending aortic dilatation is rarely associated with coronary artery disease regardless of aortic valve morphology. J. Thorac. Cardiovasc. Surg. 2014, 148, 2973–2980.e2971. [Google Scholar] [CrossRef]
- Khoury, Z.; Gottlieb, S.; Stern, S.; Keren, A. Frequency and distribution of atherosclerotic plaques in the thoracic aorta as determined by transesophageal echocardiography in patients with coronary artery disease. Am. J. Cardiol. 1997, 79, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Grewal, N.; Dolmaci, O.; Jansen, E.; Klautz, R.; Driessen, A.; Lindeman, J.; Poelmann, R.E. Are acute type A aortic dissections atherosclerotic? Front. Cardiovasc. Med. 2022, 9, 1032755. [Google Scholar] [CrossRef] [PubMed]
- Hashiyama, N.; Goda, M.; Uchida, K.; Isomatsu, Y.; Suzuki, S.; Mo, M.; Nishida, T.; Masuda, M. Stanford type B aortic dissection is more frequently associated with coronary artery atherosclerosis than type A. J. Cardiothorac. Surg. 2018, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Corsini, A.; Pacini, D.; Corti, B.; Lorenzini, M.; Laus, V.; Foa, A.; Bacchi Reggiani, M.L.; Di Marco, L.; Rapezzi, C. The complex interplay among atherosclerosis, inflammation, and degeneration in ascending thoracic aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2020, 160, 1434–1443.e1436. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Pacini, D.; Foa, A.; Corsini, A.; Agostini, V.; Corti, B.; Di Marco, L.; Leone, A.; Lorenzini, M.; Reggiani, L.B.; et al. Redefining the histopathologic profile of acute aortic syndromes: Clinical and prognostic implications. J. Thorac. Cardiovasc. Surg. 2018, 156, 1776–1785.e1776. [Google Scholar] [CrossRef]
- Albini, P.T.; Segura, A.M.; Liu, G.; Minard, C.G.; Coselli, J.S.; Milewicz, D.M.; Shen, Y.H.; LeMaire, S.A. Advanced atherosclerosis is associated with increased medial degeneration in sporadic ascending aortic aneurysms. Atherosclerosis 2014, 232, 361–368. [Google Scholar] [CrossRef]
- Stejskal, V.; Karalko, M.; Krbal, L. Histopathological findings of diseased ascending aortae with clinicopathological correlation—A single-centre study of 160 cases. Pathol. Res. Pract. 2023, 246, 154526. [Google Scholar] [CrossRef]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef]
- He, X.; Lian, Z.; Yang, Y.; Wang, Z.; Fu, X.; Liu, Y.; Li, M.; Tian, J.; Yu, T.; Xin, H. Long Non-coding RNA PEBP1P2 Suppresses Proliferative VSMCs Phenotypic Switching and Proliferation in Atherosclerosis. Mol. Ther. Nucleic Acids 2020, 22, 84–98. [Google Scholar] [CrossRef]
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef]
- Chin, D.D.; Poon, C.; Wang, J.; Joo, J.; Ong, V.; Jiang, Z.; Cheng, K.; Plotkin, A.; Magee, G.A.; Chung, E.J. miR-145 micelles mitigate atherosclerosis by modulating vascular smooth muscle cell phenotype. Biomaterials 2021, 273, 120810. [Google Scholar] [CrossRef]
- Grewal, N.; Gittenberger-de Groot, A.C.; Poelmann, R.E.; Klautz, R.J.; Lindeman, J.H.; Goumans, M.J.; Palmen, M.; Mohamed, S.A.; Sievers, H.H.; Bogers, A.J.; et al. Ascending aorta dilation in association with bicuspid aortic valve: A maturation defect of the aortic wall. J. Thorac. Cardiovasc. Surg. 2014, 148, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Grewal, N.; Gittenberger-de Groot, A.C. Pathogenesis of aortic wall complications in Marfan syndrome. Cardiovasc. Pathol. 2018, 33, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Grewal, N.; Klautz, R.J.M.; Poelmann, R.E. Intrinsic histological and morphological abnormalities of the pediatric thoracic aorta in bicuspid aortic valve patients are predictive for future aortopathy. Pathol. Res. Pract. 2023, 248, 154620. [Google Scholar] [CrossRef]
- Rabkin, S.W. The Role Matrix Metalloproteinases in the Production of Aortic Aneurysm. Prog. Mol. Biol. Transl. Sci. 2017, 147, 239–265. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Vandooren, J.; Van den Steen, P.E.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.M.; Swingler, T.E.; Sampieri, C.L.; Edwards, D.R. The regulation of matrix metalloproteinases and their inhibitors. Int. J. Biochem. Cell Biol. 2008, 40, 1362–1378. [Google Scholar] [CrossRef]
- Bode, W.; Fernandez-Catalan, C.; Tschesche, H.; Grams, F.; Nagase, H.; Maskos, K. Structural properties of matrix metalloproteinases. Cell. Mol. Life Sci. 1999, 55, 639–652. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, J.S.; Ivey, C.R.; Wheeler, J.B.; Akerman, A.W.; Rice, A.; Patel, R.K.; Stroud, R.E.; Shah, A.A.; Hughes, C.G.; Ferrari, G.; et al. Plasma biomarkers for distinguishing etiologic subtypes of thoracic aortic aneurysm disease. J. Thorac. Cardiovasc. Surg. 2013, 145, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Koullias, G.J.; Ravichandran, P.; Korkolis, D.P.; Rimm, D.L.; Elefteriades, J.A. Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann. Thorac. Surg. 2004, 78, 2106–2110, discussion 2110–2101. [Google Scholar] [CrossRef]
- Huusko, T.; Salonurmi, T.; Taskinen, P.; Liinamaa, J.; Juvonen, T.; Paakko, P.; Savolainen, M.; Kakko, S. Elevated messenger RNA expression and plasma protein levels of osteopontin and matrix metalloproteinase types 2 and 9 in patients with ascending aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2013, 145, 1117–1123. [Google Scholar] [CrossRef][Green Version]
- Mi, T.; Nie, B.; Zhang, C.; Zhou, H. The elevated expression of osteopontin and NF-kappaB in human aortic aneurysms and its implication. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 602. [Google Scholar] [CrossRef]
- Ishii, T.; Asuwa, N. Collagen and elastin degradation by matrix metalloproteinases and tissue inhibitors of matrix metalloproteinase in aortic dissection. Hum. Pathol. 2000, 31, 640–646. [Google Scholar] [CrossRef]
- Tscheuschler, A.; Meffert, P.; Beyersdorf, F.; Heilmann, C.; Kocher, N.; Uffelmann, X.; Discher, P.; Siepe, M.; Kari, F.A. MMP-2 Isoforms in Aortic Tissue and Serum of Patients with Ascending Aortic Aneurysms and Aortic Root Aneurysms. PLoS ONE 2016, 11, e0164308. [Google Scholar] [CrossRef]
- Wang, C.; Chang, Q.; Sun, X.; Qian, X.; Liu, P.; Pei, H.; Guo, X.; Liu, W. Angiotensin II Induces an Increase in Matrix Metalloproteinase 2 Expression in Aortic Smooth Muscle Cells of Ascending Thoracic Aortic Aneurysms Through JNK, ERK1/2, and p38 MAPK Activation. J. Cardiovasc. Pharmacol. 2015, 66, 285–293. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Z.; Wu, H.; Yang, Z.; Jiang, W.; Li, L.; Hu, X. Ang II enhances noradrenaline release from sympathetic nerve endings thus contributing to the up-regulation of metalloprotease-2 in aortic dissection patients’ aorta wall. PLoS ONE 2013, 8, e76922. [Google Scholar] [CrossRef]
- Khanafer, K.; Ghosh, A.; Vafai, K. Correlation between MMP and TIMP levels and elastic moduli of ascending thoracic aortic aneurysms. Cardiovasc. Revasc. Med. 2019, 20, 324–327. [Google Scholar] [CrossRef]
- Rabkin, S.W. Differential expression of MMP-2, MMP-9 and TIMP proteins in thoracic aortic aneurysm—Comparison with and without bicuspid aortic valve: A meta-analysis. Vasa 2014, 43, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, A.; Yoshimura, K.; Suzuki, R.; Mikamo, A.; Yamashita, O.; Ikeda, Y.; Tsuchida, M.; Hamano, K. Important role of the angiotensin II pathway in producing matrix metalloproteinase-9 in human thoracic aortic aneurysms. J. Surg. Res. 2013, 183, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Del Porto, F.; di Gioia, C.; Tritapepe, L.; Ferri, L.; Leopizzi, M.; Nofroni, I.; De Santis, V.; Della Rocca, C.; Mitterhofer, A.P.; Bruno, G.; et al. The multitasking role of macrophages in Stanford type A acute aortic dissection. Cardiology 2014, 127, 123–129. [Google Scholar] [CrossRef]
- Song, Y.; Xie, Y.; Liu, F.; Zhao, C.; Yu, R.; Ban, S.; Ye, Q.; Wen, J.; Wan, H.; Li, X.; et al. Expression of matrix metalloproteinase-12 in aortic dissection. BMC Cardiovasc. Disord. 2013, 13, 34. [Google Scholar] [CrossRef]
- Kimura, N.; Futamura, K.; Arakawa, M.; Okada, N.; Emrich, F.; Okamura, H.; Sato, T.; Shudo, Y.; Koyano, T.K.; Yamaguchi, A.; et al. Gene expression profiling of acute type A aortic dissection combined with in vitro assessment. Eur. J. Cardiothorac. Surg. 2017, 52, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Aikawa, M. Many faces of matrix metalloproteinases in aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Vacek, T.P.; Rehman, S.; Neamtu, D.; Yu, S.; Givimani, S.; Tyagi, S.C. Matrix metalloproteinases in atherosclerosis: Role of nitric oxide, hydrogen sulfide, homocysteine, and polymorphisms. Vasc. Health Risk Manag. 2015, 11, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.H.; Cho, C.H.; Kim, H.O.; Jo, Y.H.; Yoon, K.S.; Lee, J.H.; Park, J.C.; Park, K.C.; Ahn, T.B.; Chung, K.C.; et al. Plaque rupture is a determinant of vascular events in carotid artery atherosclerotic disease: Involvement of matrix metalloproteinases 2 and 9. J. Clin. Neurol. 2011, 7, 69–76. [Google Scholar] [CrossRef]
- Rossignol, P.; Ho-Tin-Noe, B.; Vranckx, R.; Bouton, M.C.; Meilhac, O.; Lijnen, H.R.; Guillin, M.C.; Michel, J.B.; Angles-Cano, E. Protease nexin-1 inhibits plasminogen activation-induced apoptosis of adherent cells. J. Biol. Chem. 2004, 279, 10346–10356. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Liapis, C.D. Matrix metalloproteinases: Contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysms. Curr. Med. Res. Opin. 2004, 20, 419–432. [Google Scholar] [CrossRef]
- Pyo, R.; Lee, J.K.; Shipley, J.M.; Curci, J.A.; Mao, D.; Ziporin, S.J.; Ennis, T.L.; Shapiro, S.D.; Senior, R.M.; Thompson, R.W. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Investig. 2000, 105, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Longo, G.M.; Xiong, W.; Greiner, T.C.; Zhao, Y.; Fiotti, N.; Baxter, B.T. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J. Clin. Investig. 2002, 110, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Davis, V.; Persidskaia, R.; Baca-Regen, L.; Itoh, Y.; Nagase, H.; Persidsky, Y.; Ghorpade, A.; Baxter, B.T. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Freestone, T.; Turner, R.J.; Coady, A.; Higman, D.J.; Greenhalgh, R.M.; Powell, J.T. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1145–1151. [Google Scholar] [CrossRef]
- Newman, K.M.; Jean-Claude, J.; Li, H.; Scholes, J.V.; Ogata, Y.; Nagase, H.; Tilson, M.D. Cellular localization of matrix metalloproteinases in the abdominal aortic aneurysm wall. J. Vasc. Surg. 1994, 20, 814–820. [Google Scholar] [CrossRef]
- Newman, K.M.; Malon, A.M.; Shin, R.D.; Scholes, J.V.; Ramey, W.G.; Tilson, M.D. Matrix metalloproteinases in abdominal aortic aneurysm: Characterization, purification, and their possible sources. Connect. Tissue Res. 1994, 30, 265–276. [Google Scholar] [CrossRef]
- Reeps, C.; Pelisek, J.; Seidl, S.; Schuster, T.; Zimmermann, A.; Kuehnl, A.; Eckstein, H.H. Inflammatory infiltrates and neovessels are relevant sources of MMPs in abdominal aortic aneurysm wall. Pathobiology 2009, 76, 243–252. [Google Scholar] [CrossRef]
- Gandhi, R.H.; Irizarry, E.; Cantor, J.O.; Keller, S.; Nackman, G.B.; Halpern, V.J.; Newman, K.M.; Tilson, M.D. Analysis of elastin cross-linking and the connective tissue matrix of abdominal aortic aneurysms. Surgery 1994, 115, 617–620. [Google Scholar]
- Kuzuya, M.; Nakamura, K.; Sasaki, T.; Cheng, X.W.; Itohara, S.; Iguchi, A. Effect of MMP-2 deficiency on atherosclerotic lesion formation in apoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1120–1125. [Google Scholar] [CrossRef]
- Bendeck, M.P.; Zempo, N.; Clowes, A.W.; Galardy, R.E.; Reidy, M.A. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ. Res. 1994, 75, 539–545. [Google Scholar] [CrossRef]
- Zempo, N.; Kenagy, R.D.; Au, Y.P.; Bendeck, M.; Clowes, M.M.; Reidy, M.A.; Clowes, A.W. Matrix metalloproteinases of vascular wall cells are increased in balloon-injured rat carotid artery. J. Vasc. Surg. 1994, 20, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Forough, R.; Koyama, N.; Hasenstab, D.; Lea, H.; Clowes, M.; Nikkari, S.T.; Clowes, A.W. Overexpression of tissue inhibitor of matrix metalloproteinase-1 inhibits vascular smooth muscle cell functions in vitro and in vivo. Circ. Res. 1996, 79, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Southgate, K.M.; Fisher, M.; Banning, A.P.; Thurston, V.J.; Baker, A.H.; Fabunmi, R.P.; Groves, P.H.; Davies, M.; Newby, A.C. Upregulation of basement membrane-degrading metalloproteinase secretion after balloon injury of pig carotid arteries. Circ. Res. 1996, 79, 1177–1187. [Google Scholar] [CrossRef]
- Beck, L., Jr.; D’Amore, P.A. Vascular development: Cellular and molecular regulation. FASEB J. 1997, 11, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Hara, H.; Fujiwara, T.; Kanaya, T.; Maemura, S.; Komuro, I. TGF-beta Signaling-Related Genes and Thoracic Aortic Aneurysms and Dissections. Int. J. Mol. Sci. 2018, 19, 2125. [Google Scholar] [CrossRef]
- Cook, J.R.; Clayton, N.P.; Carta, L.; Galatioto, J.; Chiu, E.; Smaldone, S.; Nelson, C.A.; Cheng, S.H.; Wentworth, B.M.; Ramirez, F. Dimorphic effects of transforming growth factor-beta signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for Marfan syndrome. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 911–917. [Google Scholar] [CrossRef]
- Gomez, D.; Al Haj Zen, A.; Borges, L.F.; Philippe, M.; Gutierrez, P.S.; Jondeau, G.; Michel, J.B.; Vranckx, R. Syndromic and non-syndromic aneurysms of the human ascending aorta share activation of the Smad2 pathway. J. Pathol. 2009, 218, 131–142. [Google Scholar] [CrossRef]
- Habashi, J.P.; Judge, D.P.; Holm, T.M.; Cohn, R.D.; Loeys, B.L.; Cooper, T.K.; Myers, L.; Klein, E.C.; Liu, G.; Calvi, C.; et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006, 312, 117–121. [Google Scholar] [CrossRef]
- King, V.L.; Lin, A.Y.; Kristo, F.; Anderson, T.J.; Ahluwalia, N.; Hardy, G.J.; Owens, A.P., 3rd; Howatt, D.A.; Shen, D.; Tager, A.M.; et al. Interferon-gamma and the interferon-inducible chemokine CXCL10 protect against aneurysm formation and rupture. Circulation 2009, 119, 426–435. [Google Scholar] [CrossRef]
- Zilberberg, L.; Phoon, C.K.; Robertson, I.; Dabovic, B.; Ramirez, F.; Rifkin, D.B. Genetic analysis of the contribution of LTBP-3 to thoracic aneurysm in Marfan syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 14012–14017. [Google Scholar] [CrossRef]
- Chen, X.; Rateri, D.L.; Howatt, D.A.; Balakrishnan, A.; Moorleghen, J.J.; Cassis, L.A.; Daugherty, A. TGF-beta Neutralization Enhances AngII-Induced Aortic Rupture and Aneurysm in Both Thoracic and Abdominal Regions. PLoS ONE 2016, 11, e0153811. [Google Scholar] [CrossRef]
- Wang, Y.; Ait-Oufella, H.; Herbin, O.; Bonnin, P.; Ramkhelawon, B.; Taleb, S.; Huang, J.; Offenstadt, G.; Combadiere, C.; Renia, L.; et al. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J. Clin. Investig. 2010, 120, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, J.; Murtada, S.I.; Li, G.; Jiao, Y.; Uman, S.; Ting, M.Y.; Tellides, G.; Humphrey, J.D. Pharmacologically Improved Contractility Protects Against Aortic Dissection in Mice With Disrupted Transforming Growth Factor-beta Signaling Despite Compromised Extracellular Matrix Properties. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Q.; Jiao, Y.; Qin, L.; Ali, R.; Zhou, J.; Ferruzzi, J.; Kim, R.W.; Geirsson, A.; Dietz, H.C.; et al. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J. Clin. Investig. 2014, 124, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.H.; Wei, H.; Jaffe, M.; Airhart, N.; Du, L.; Angelov, S.N.; Yan, J.; Allen, J.K.; Kang, I.; Wight, T.N.; et al. Postnatal Deletion of the Type II Transforming Growth Factor-beta Receptor in Smooth Muscle Cells Causes Severe Aortopathy in Mice. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Hu, J.H.; Angelov, S.N.; Fox, K.; Yan, J.; Enstrom, R.; Smith, A.; Dichek, D.A. Aortopathy in a Mouse Model of Marfan Syndrome Is Not Mediated by Altered Transforming Growth Factor beta Signaling. J. Am. Heart Assoc. 2017, 6, e004968. [Google Scholar] [CrossRef]
- Gadson, P.F., Jr.; Dalton, M.L.; Patterson, E.; Svoboda, D.D.; Hutchinson, L.; Schram, D.; Rosenquist, T.H. Differential response of mesoderm- and neural crest-derived smooth muscle to TGF-beta1: Regulation of c-myb and alpha1 (I) procollagen genes. Exp. Cell Res. 1997, 230, 169–180. [Google Scholar] [CrossRef]
- Thieszen, S.L.; Dalton, M.; Gadson, P.F.; Patterson, E.; Rosenquist, T.H. Embryonic lineage of vascular smooth muscle cells determines responses to collagen matrices and integrin receptor expression. Exp. Cell Res. 1996, 227, 135–145. [Google Scholar] [CrossRef]
- El-Hamamsy, I.; Yacoub, M.H. Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat. Rev. Cardiol. 2009, 6, 771–786. [Google Scholar] [CrossRef]
- Grainger, D.J. Transforming growth factor beta and atherosclerosis: So far, so good for the protective cytokine hypothesis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 399–404. [Google Scholar] [CrossRef]
- McCaffrey, T.A. TGF-betas and TGF-beta receptors in atherosclerosis. Cytokine Growth Factor. Rev. 2000, 11, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Neptune, E.R.; Frischmeyer, P.A.; Arking, D.E.; Myers, L.; Bunton, T.E.; Gayraud, B.; Ramirez, F.; Sakai, L.Y.; Dietz, H.C. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003, 33, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Nataatmadja, M.; West, J.; West, M. Overexpression of transforming growth factor-beta is associated with increased hyaluronan content and impairment of repair in Marfan syndrome aortic aneurysm. Circulation 2006, 114, I371–I377. [Google Scholar] [CrossRef] [PubMed]
- Lutgens, E.; Daemen, M.J. Transforming growth factor-beta: A local or systemic mediator of plaque stability? Circ. Res. 2001, 89, 853–855. [Google Scholar] [CrossRef][Green Version]
- Mallat, Z.; Gojova, A.; Marchiol-Fournigault, C.; Esposito, B.; Kamate, C.; Merval, R.; Fradelizi, D.; Tedgui, A. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ. Res. 2001, 89, 930–934. [Google Scholar] [CrossRef]
- McCaffrey, T.A.; Consigli, S.; Du, B.; Falcone, D.J.; Sanborn, T.A.; Spokojny, A.M.; Bush, H.L., Jr. Decreased type II/type I TGF-beta receptor ratio in cells derived from human atherosclerotic lesions. Conversion from an antiproliferative to profibrotic response to TGF-beta1. J. Clin. Investig. 1995, 96, 2667–2675. [Google Scholar] [CrossRef]
- Andreotti, F.; Porto, I.; Crea, F.; Maseri, A. Inflammatory gene polymorphisms and ischaemic heart disease: Review of population association studies. Heart 2002, 87, 107–112. [Google Scholar] [CrossRef]
- Humphries, S.E.; Luong, L.A.; Talmud, P.J.; Frick, M.H.; Kesaniemi, Y.A.; Pasternack, A.; Taskinen, M.R.; Syvanne, M. The 5A/6A polymorphism in the promoter of the stromelysin-1 (MMP-3) gene predicts progression of angiographically determined coronary artery disease in men in the LOCAT gemfibrozil study. Lopid Coronary Angiography Trial. Atherosclerosis 1998, 139, 49–56. [Google Scholar] [CrossRef]
- Kempf, K.; Haltern, G.; Futh, R.; Herder, C.; Muller-Scholze, S.; Gulker, H.; Martin, S. Increased TNF-alpha and decreased TGF-beta expression in peripheral blood leukocytes after acute myocardial infarction. Horm. Metab. Res. 2006, 38, 346–351. [Google Scholar] [CrossRef]
- Koch, W.; Hoppmann, P.; Mueller, J.C.; Schomig, A.; Kastrati, A. Association of transforming growth factor-beta1 gene polymorphisms with myocardial infarction in patients with angiographically proven coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1114–1119. [Google Scholar] [CrossRef]
- Vaughan, C.J.; Casey, M.; He, J.; Veugelers, M.; Henderson, K.; Guo, D.; Campagna, R.; Roman, M.J.; Milewicz, D.M.; Devereux, R.B.; et al. Identification of a chromosome 11q23.2-q24 locus for familial aortic aneurysm disease, a genetically heterogeneous disorder. Circulation 2001, 103, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Eriksson, P.; Hamsten, A.; Kurkinen, M.; Humphries, S.E.; Henney, A.M. Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin-1 promoter which results in reduced gene expression. J. Biol. Chem. 1996, 271, 13055–13060. [Google Scholar] [CrossRef] [PubMed]
- Yokota, M.; Ichihara, S.; Lin, T.L.; Nakashima, N.; Yamada, Y. Association of a T29-->C polymorphism of the transforming growth factor-beta1 gene with genetic susceptibility to myocardial infarction in Japanese. Circulation 2000, 101, 2783–2787. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Al-Sammarraie, N.; Gebere, M.G.; Bhattacharya, A.; Chopra, S.; Johnson, J.; Pena, E.A.; Eberth, J.F.; Poelmann, R.E.; Gittenberger-de Groot, A.C.; et al. Transforming Growth Factor Beta3 is Required for Cardiovascular Development. J. Cardiovasc. Dev. Dis. 2020, 7, 19. [Google Scholar] [CrossRef]
- Grewal, N.; Girdauskas, E.; Idhrees, M.; Velayudhan, B.; Klautz, R.; Driessen, A.; Poelmann, R.E. Structural abnormalities in the non-dilated ascending aortic wall of bicuspid aortic valve patients. Cardiovasc. Pathol. 2023, 62, 107478. [Google Scholar] [CrossRef]
- Moncada, S. Adventures in vascular biology: A tale of two mediators. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 735–759. [Google Scholar] [CrossRef]
- Griffith, T.M. Endothelial control of vascular tone by nitric oxide and gap junctions: A haemodynamic perspective. Biorheology 2002, 39, 307–318. [Google Scholar]
- Pohl, U.; Holtz, J.; Busse, R.; Bassenge, E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 1986, 8, 37–44. [Google Scholar] [CrossRef]
- Davies, P.F. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 1995, 75, 519–560. [Google Scholar] [CrossRef]
- Garcia-Cardena, G.; Comander, J.I.; Blackman, B.R.; Anderson, K.R.; Gimbrone, M.A. Mechanosensitive endothelial gene expression profiles: Scripts for the role of hemodynamics in atherogenesis? Ann. N. Y. Acad. Sci. 2001, 947, 1–6. [Google Scholar] [CrossRef]
- Suo, J.; Oshinski, J.N.; Giddens, D.P. Blood flow patterns in the proximal human coronary arteries: Relationship to atherosclerotic plaque occurrence. Mol. Cell. Biomech. 2008, 5, 9–18. [Google Scholar] [PubMed]
- Davies, P.F.; Polacek, D.C.; Handen, J.S.; Helmke, B.P.; DePaola, N. A spatial approach to transcriptional profiling: Mechanotransduction and the focal origin of atherosclerosis. Trends Biotechnol. 1999, 17, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Steinman, D.A.; Taylor, C.A. Flow imaging and computing: Large artery hemodynamics. Ann. Biomed. Eng. 2005, 33, 1704–1709. [Google Scholar] [CrossRef]
- Hajra, L.; Evans, A.I.; Chen, M.; Hyduk, S.J.; Collins, T.; Cybulsky, M.I. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc. Natl. Acad. Sci. USA 2000, 97, 9052–9057. [Google Scholar] [CrossRef] [PubMed]
- Passerini, A.G.; Polacek, D.C.; Shi, C.; Francesco, N.M.; Manduchi, E.; Grant, G.R.; Pritchard, W.F.; Powell, S.; Chang, G.Y.; Stoeckert, C.J., Jr.; et al. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc. Natl. Acad. Sci. USA 2004, 101, 2482–2487. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Kim, S.H. Pulse Wave Velocity in Atherosclerosis. Front. Cardiovasc. Med. 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Witteman, J.C.; Grobbee, D.E.; Valkenburg, H.A.; van Hemert, A.M.; Stijnen, T.; Burger, H.; Hofman, A. J-shaped relation between change in diastolic blood pressure and progression of aortic atherosclerosis. Lancet 1994, 343, 504–507. [Google Scholar] [CrossRef]
- Dao, H.H.; Essalihi, R.; Bouvet, C.; Moreau, P. Evolution and modulation of age-related medial elastocalcinosis: Impact on large artery stiffness and isolated systolic hypertension. Cardiovasc. Res. 2005, 66, 307–317. [Google Scholar] [CrossRef]
- Ohyama, Y.; Ambale-Venkatesh, B.; Noda, C.; Kim, J.Y.; Tanami, Y.; Teixido-Tura, G.; Chugh, A.R.; Redheuil, A.; Liu, C.Y.; Wu, C.O.; et al. Aortic Arch Pulse Wave Velocity Assessed by Magnetic Resonance Imaging as a Predictor of Incident Cardiovascular Events: The MESA (Multi-Ethnic Study of Atherosclerosis). Hypertension 2017, 70, 524–530. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waldron, C.; Zafar, M.A.; Ziganshin, B.A.; Weininger, G.; Grewal, N.; Elefteriades, J.A. Evidence Accumulates: Patients with Ascending Aneurysms Are Strongly Protected from Atherosclerotic Disease. Int. J. Mol. Sci. 2023, 24, 15640. https://doi.org/10.3390/ijms242115640
Waldron C, Zafar MA, Ziganshin BA, Weininger G, Grewal N, Elefteriades JA. Evidence Accumulates: Patients with Ascending Aneurysms Are Strongly Protected from Atherosclerotic Disease. International Journal of Molecular Sciences. 2023; 24(21):15640. https://doi.org/10.3390/ijms242115640
Chicago/Turabian StyleWaldron, Christina, Mohammad A. Zafar, Bulat A. Ziganshin, Gabe Weininger, Nimrat Grewal, and John A. Elefteriades. 2023. "Evidence Accumulates: Patients with Ascending Aneurysms Are Strongly Protected from Atherosclerotic Disease" International Journal of Molecular Sciences 24, no. 21: 15640. https://doi.org/10.3390/ijms242115640
APA StyleWaldron, C., Zafar, M. A., Ziganshin, B. A., Weininger, G., Grewal, N., & Elefteriades, J. A. (2023). Evidence Accumulates: Patients with Ascending Aneurysms Are Strongly Protected from Atherosclerotic Disease. International Journal of Molecular Sciences, 24(21), 15640. https://doi.org/10.3390/ijms242115640