A Strainer-Based Platform for the Collection and Immunolabeling of Porcine Epidemic Diarrhea Virus-Infected Porcine Intestinal Organoid
Abstract
:1. Introduction
2. Results
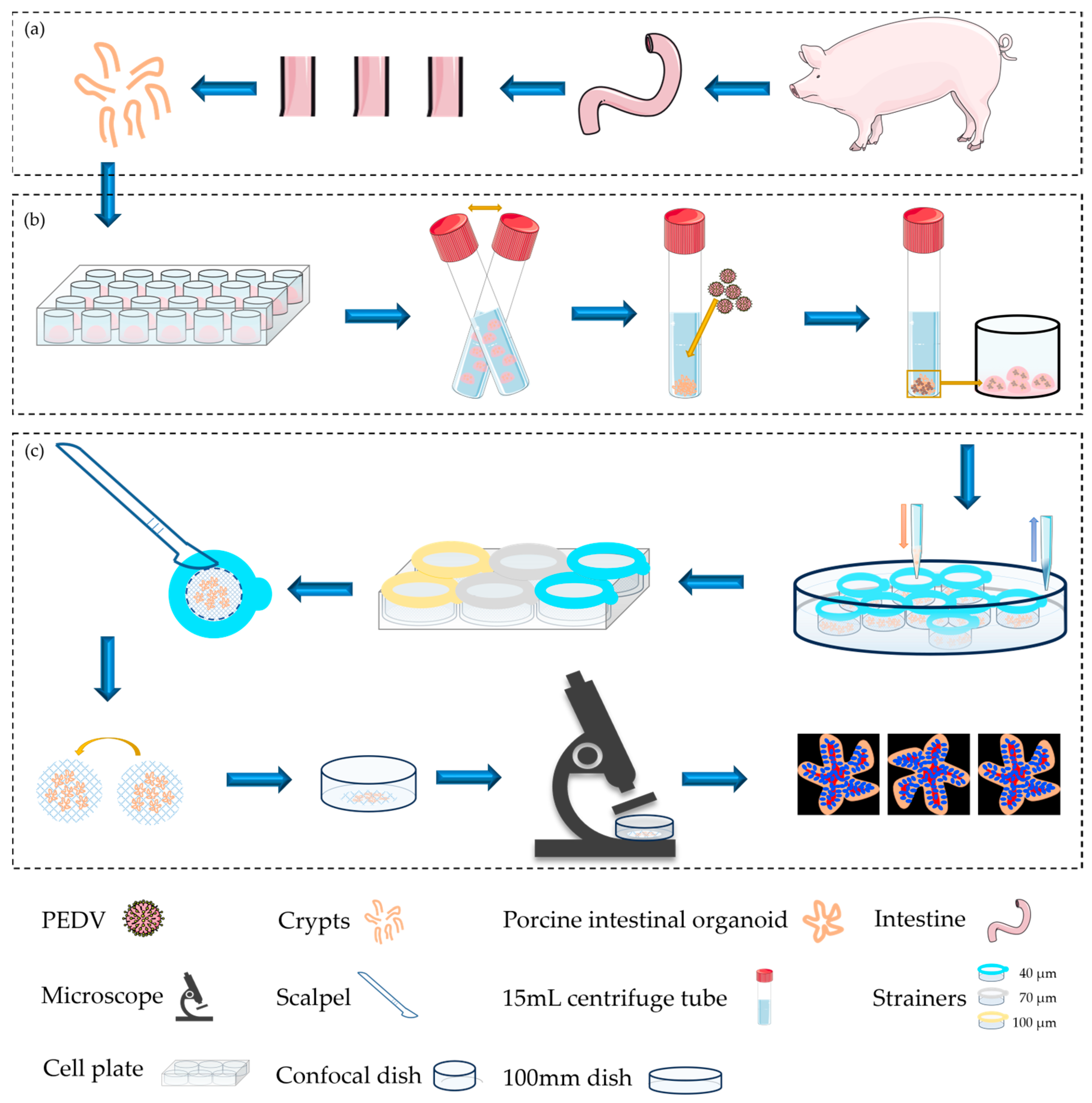
2.1. Summarization of Porcine Intestinal Organoids: Culturalization, Infection, Collection, and Fluorescence Imaging
2.2. Development of Intestinal Organoids from Porcine Inoculated
2.3. Collection, Labeling, and Imaging of Porcine Intestinal Organoids Using Cell Strainers with Different Aperture Sizes
2.4. Visualization of Porcine Intestinal Organoids Infected with PEDV
3. Discussion
4. Materials and Methods
4.1. Acquisition and Culture of Porcine Intestinal Organoids
4.2. Porcine Intestinal Organoids Infected with Porcine Epidemic Diarrhea Virus
4.3. Detection via Western Blotting
4.4. The Collection and Loading of Porcine Intestinal Organoids with the Strainer Platform
4.5. Labeling Process of PEDV-Infected or Uninfected Porcine Intestinal Organoids
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahapatra, C.; Lee, R.; Paul, M.K. Emerging role and promise of nanomaterials in organoid research. Drug Discov. Today 2022, 27, 890–899. [Google Scholar] [CrossRef]
- Fujii, M.; Matano, M.; Toshimitsu, K.; Takano, A.; Mikami, Y.; Nishikori, S.; Sugimoto, S.; Sato, T. Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell 2018, 23, 787–793.e6. [Google Scholar] [CrossRef]
- Serra, D.; Mayr, U.; Boni, A.; Lukonin, I.; Rempfler, M.; Challet Meylan, L.; Stadler, M.B.; Strnad, P.; Papasaikas, P.; Vischi, D.; et al. Self-organization and symmetry breaking in intestinal organoid development. Nature 2019, 569, 66–72. [Google Scholar] [CrossRef]
- He, J.; Zhang, X.; Xia, X.; Han, M.; Li, F.; Li, C.; Li, Y.; Gao, D. Organoid technology for tissue engineering. J. Mol. Cell Biol. 2020, 12, 569–579. [Google Scholar] [CrossRef]
- Wilson, H.V. A new method by which sponges may be artificially reared. Science 1907, 25, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Y.; Wu, S.; Wang, D.; Chu, C.; Hong, Y.; Tao, M.; Hu, H.; Xu, M.; Guo, X.; Liu, Y. Human organoids in basic research and clinical applications. Signal Transduct. Target. Ther. 2022, 7, 168. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Geng, Z.; Su, J. The horizon of bone organoid: A perspective on construction and application. Bioact. Mater. 2022, 18, 15–25. [Google Scholar] [CrossRef]
- Dutta, D.; Heo, I.; Clevers, H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol. Med. 2017, 23, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Hu, W.; Matulay, J.T.; Silva, M.V.; Owczarek, T.B.; Kim, K.; Chua, C.W.; Barlow, L.J.; Kandoth, C.; Williams, A.B.; et al. Tumor Evolution and Drug Response in Patient-Derived Organoid Models of Bladder Cancer. Cell 2018, 173, 515–528.e17. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Reza, H.A.; Okabe, R.; Takebe, T. Organoid transplant approaches for the liver. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2021, 34, 2031–2045. [Google Scholar] [CrossRef]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordóñez-Morán, P.; Clevers, H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef]
- Mizutani, T.; Clevers, H. Primary Intestinal Epithelial Organoid Culture. Methods Mol. Biol. 2020, 2171, 185–200. [Google Scholar] [PubMed]
- Wakisaka, Y.; Sugimoto, S.; Sato, T. Organoid Medicine for Inflammatory Bowel Disease. Stem Cells 2022, 40, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Drost, J.; van Jaarsveld, R.H.; Ponsioen, B.; Zimberlin, C.; van Boxtel, R.; Buijs, A.; Sachs, N.; Overmeer, R.M.; Offerhaus, G.J.; Begthel, H.; et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 2015, 521, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Beyaz, S.; Mana, M.D.; Roper, J.; Kedrin, D.; Saadatpour, A.; Hong, S.J.; Bauer-Rowe, K.E.; Xifaras, M.E.; Akkad, A.; Arias, E.; et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 2016, 531, 53–58. [Google Scholar] [CrossRef]
- Xing, C.; Liang, G.; Yu, X.; Zhang, A.; Luo, X.; Liu, Y.; Tang, Z.; Wu, B.; Song, Z.; Lan, D. Establishment of Epithelial Inflammatory Injury Model Using Intestinal Organoid Cultures. Stem Cells Int. 2023, 2023, 3328655. [Google Scholar] [CrossRef]
- Fujimichi, Y.; Otsuka, K.; Tomita, M.; Iwasaki, T. Ionizing radiation alters organoid forming potential and replenishment rate in a dose/dose-rate dependent manner. J. Radiat. Res. 2022, 63, 166–173. [Google Scholar] [CrossRef]
- Puschhof, J.; Pleguezuelos-Manzano, C.; Martinez-Silgado, A.; Akkerman, N.; Saftien, A.; Boot, C.; de Waal, A.; Beumer, J.; Dutta, D.; Heo, I.; et al. Intestinal organoid cocultures with microbes. Nat. Protoc. 2021, 16, 4633–4649. [Google Scholar] [CrossRef]
- Ma, L.; Yu, J.; Zhang, H.; Zhao, B.; Zhang, J.; Yang, D.; Luo, F.; Wang, B.; Jin, B.; Liu, J. Effects of Immune Cells on Intestinal Stem Cells: Prospects for Therapeutic Targets. Stem Cell Rev. Rep. 2022, 18, 2296–2314. [Google Scholar] [CrossRef]
- Sun, J. Intestinal organoid as an in vitro model in studying host-microbial interactions. Front. Biol. 2017, 12, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hou, Q.; Ye, L.; Yang, Q.; Yu, Q. Crosstalk between H9N2 avian influenza virus and crypt-derived intestinal organoids. Vet. Res. 2017, 48, 71. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Blanc, F.; Cherbuy, C.; Egidy, G.; Giuffra, E.; Lacroix-Lamandé, S.; Wiedemann, A. Intestinal organoids in farm animals. Vet. Res. 2021, 52, 33. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, Y.; Fu, Y.; Li, Y.; Yang, S.; Chen, J.; Liu, G. Transmissible Gastroenteritis Virus Infection Promotes the Self-Renewal of Porcine Intestinal Stem Cells via Wnt/β-Catenin Pathway. J. Virol. 2022, 96, e0096222. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fu, F.; Guo, S.; Wang, H.; He, X.; Xue, M.; Yin, L.; Feng, L.; Liu, P. Porcine Intestinal Enteroids: A New Model for Studying Enteric Coronavirus Porcine Epidemic Diarrhea Virus Infection and the Host Innate Response. J. Virol. 2019, 93, e01682-18. [Google Scholar] [CrossRef]
- Li, Y.; Yang, N.; Chen, J.; Huang, X.; Zhang, N.; Yang, S.; Liu, G.; Liu, G. Next-Generation Porcine Intestinal Organoids: An Apical-Out Organoid Model for Swine Enteric Virus Infection and Immune Response Investigations. J. Virol. 2020, 94, e01006-20. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Lee, H.J.; Gu, N.Y.; Park, Y.R.; Kim, E.J.; Kang, S.J.; Hyun, B.H.; Yang, D.K. Evaluation of porcine intestinal organoids as an in vitro model for mammalian orthoreovirus 3 infection. J. Vet. Sci. 2023, 24, e53. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, L.; Cai, H.; Li, Y.; Gao, F.; Yu, L.; Jiang, Y.; Tong, W.; Li, L.; Li, G.; et al. Long-Term Expansion of Porcine Intestinal Organoids Serves as an in vitro Model for Swine Enteric Coronavirus Infection. Front. Microbiol. 2022, 13, 865336. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Liu, Y.; Li, W.; Chen, G.; Fang, Y.; He, X.; Fu, B.; Jing, Z. A Strainer-Based Platform for the Collection and Immunolabeling of Mouse Intestinal Organoids. Int. J. Mol. Sci. 2023, 24, 13568. [Google Scholar] [CrossRef]
- Zander, R.; Schauder, D.; Xin, G.; Nguyen, C.; Wu, X.; Zajac, A.; Cui, W. CD4(+) T Cell Help Is Required for the Formation of a Cytolytic CD8(+) T Cell Subset that Protects against Chronic Infection and Cancer. Immunity 2019, 51, 1028–1042.e4. [Google Scholar] [CrossRef]
- Harda, Z.; Misiołek, K.; Klimczak, M.; Chrószcz, M.; Rodriguez Parkitna, J. C57BL/6N mice show a sub-strain specific resistance to the psychotomimetic effects of ketamine. Front. Behav. Neurosci. 2022, 16, 1057319. [Google Scholar] [CrossRef]
- Casey, M.J.; Stumpf, P.S.; MacArthur, B.D. Theory of cell fate. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1471. [Google Scholar] [CrossRef]
- Matich, P.; Kiszka, J.J.; Heithaus, M.R.; Le Bourg, B.; Mourier, J. Inter-individual differences in ontogenetic trophic shifts among three marine predators. Oecologia 2019, 189, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Brown, P.C.; Chow, E.C.Y.; Ewart, L.; Ferguson, S.S.; Fitzpatrick, S.; Freedman, B.S.; Guo, G.L.; Hedrich, W.; Heyward, S.; et al. 3D cell culture models: Drug pharmacokinetics, safety assessment, and regulatory consideration. Clin. Transl. Sci. 2021, 14, 1659–1680. [Google Scholar] [CrossRef]
- Corsini, N.S.; Knoblich, J.A. Human organoids: New strategies and methods for analyzing human development and disease. Cell 2022, 185, 2756–2769. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Hatano, Y.; Sato, T. Intestinal epithelial organoids: Regeneration and maintenance of the intestinal epithelium. Curr. Opin. Genet. Dev. 2022, 76, 101977. [Google Scholar] [CrossRef]
- Prior, N.; Inacio, P.; Huch, M. Liver organoids: From basic research to therapeutic applications. Gut 2019, 68, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, K.; Feng, Q.; Liao, Y. Cardiac Organoids: A 3D Technology for Modeling Heart Development and Disease. Stem Cell Rev. Rep. 2022, 18, 2593–2605. [Google Scholar] [CrossRef]
- Bein, A.; Shin, W.; Jalili-Firoozinezhad, S.; Park, M.H.; Sontheimer-Phelps, A.; Tovaglieri, A.; Chalkiadaki, A.; Kim, H.J.; Ingber, D.E. Microfluidic Organ-on-a-Chip Models of Human Intestine. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 659–668. [Google Scholar] [CrossRef]
- Saorin, G.; Caligiuri, I.; Rizzolio, F. Microfluidic organoids-on-a-chip: The future of human models. Semin. Cell Dev. Biol. 2023, 144, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gehart, H.; Artegiani, B.; LÖpez-Iglesias, C.; Dekkers, F.; Basak, O.; van Es, J.; Chuva de Sousa Lopes, S.M.; Begthel, H.; Korving, J.; et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell 2018, 175, 1591–1606.e19. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.P.; Baumgarten, S.F.; Verma, R.; Lunov, O.; Dejneka, A.; Sullivan, G.J. Liver Organoids: Recent Developments, Limitations and Potential. Front. Med. 2021, 8, 574047. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Tan, J.; Zhang, N.; Li, W.; Fu, B. A Strainer-Based Platform for the Collection and Immunolabeling of Porcine Epidemic Diarrhea Virus-Infected Porcine Intestinal Organoid. Int. J. Mol. Sci. 2023, 24, 15671. https://doi.org/10.3390/ijms242115671
Liu Y, Tan J, Zhang N, Li W, Fu B. A Strainer-Based Platform for the Collection and Immunolabeling of Porcine Epidemic Diarrhea Virus-Infected Porcine Intestinal Organoid. International Journal of Molecular Sciences. 2023; 24(21):15671. https://doi.org/10.3390/ijms242115671
Chicago/Turabian StyleLiu, Yinju, Jinlong Tan, Nianzhang Zhang, Wenhui Li, and Baoquan Fu. 2023. "A Strainer-Based Platform for the Collection and Immunolabeling of Porcine Epidemic Diarrhea Virus-Infected Porcine Intestinal Organoid" International Journal of Molecular Sciences 24, no. 21: 15671. https://doi.org/10.3390/ijms242115671




