Cadaveric Adipose-Derived Stem Cells for Regenerative Medicine and Research
Abstract
:1. Introduction
2. Results
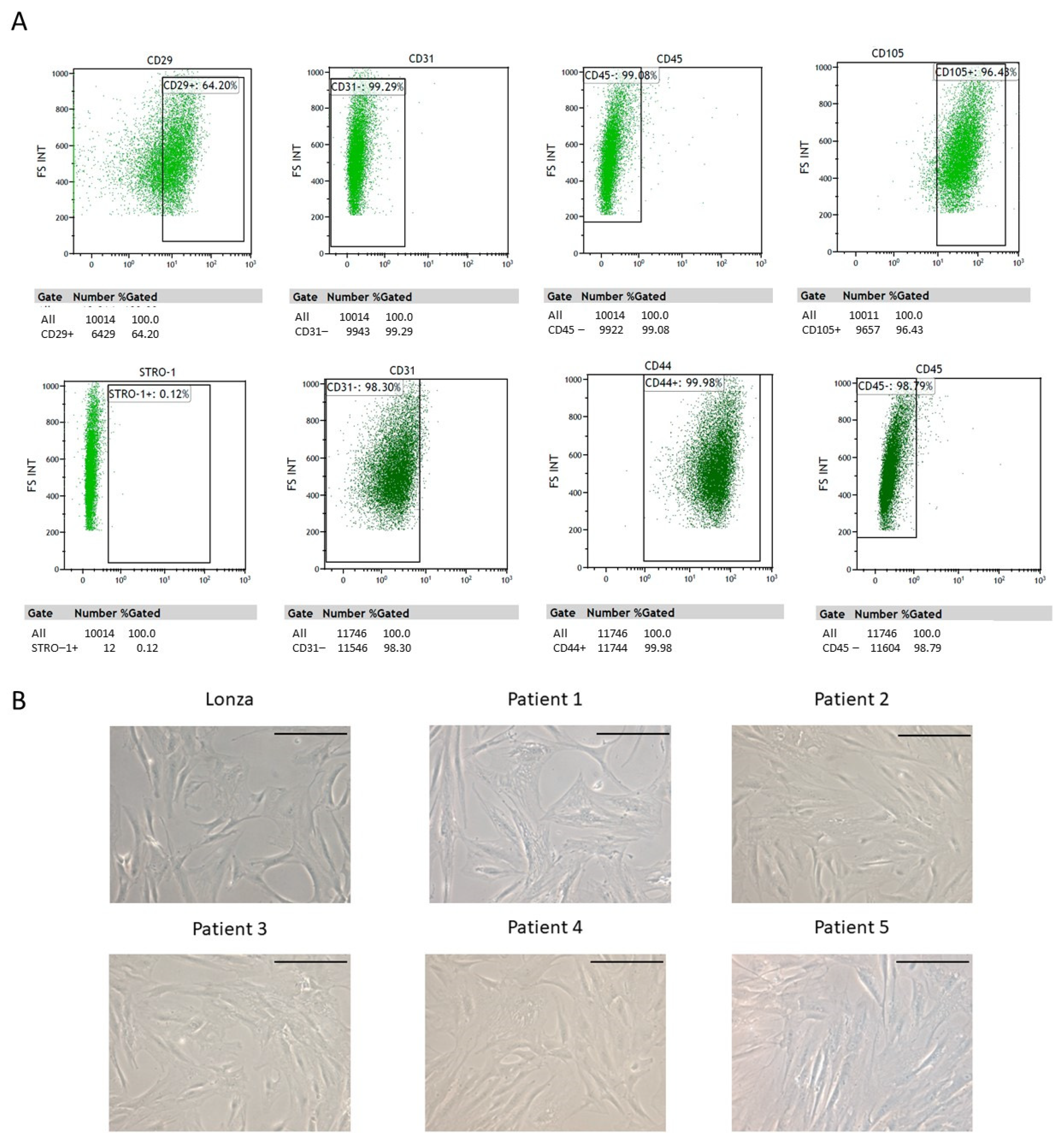
2.1. ADSC Isolation and Characterization
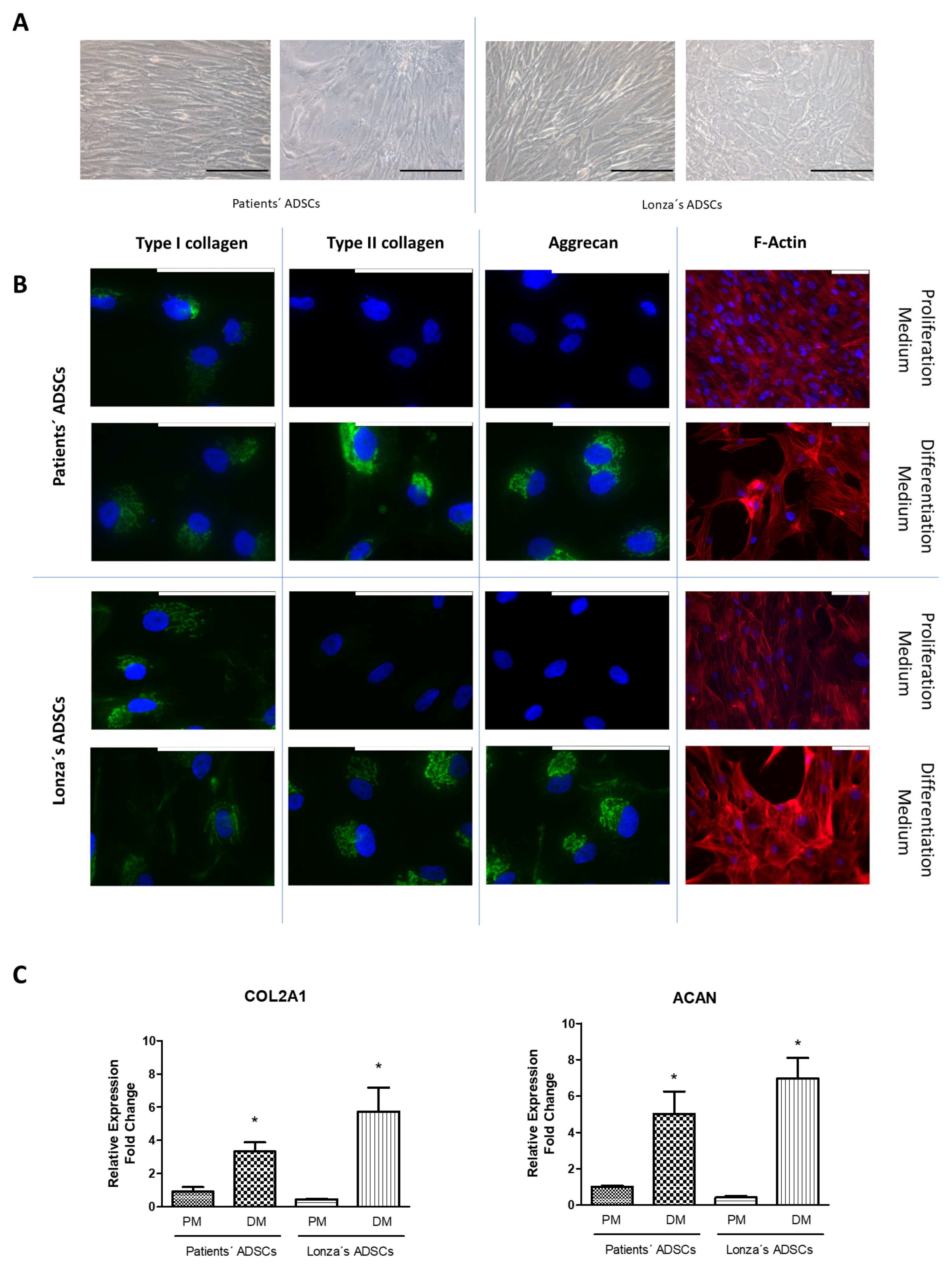
2.2. Evaluation of Chondrogenic Differentiation Capacity of ADSCs
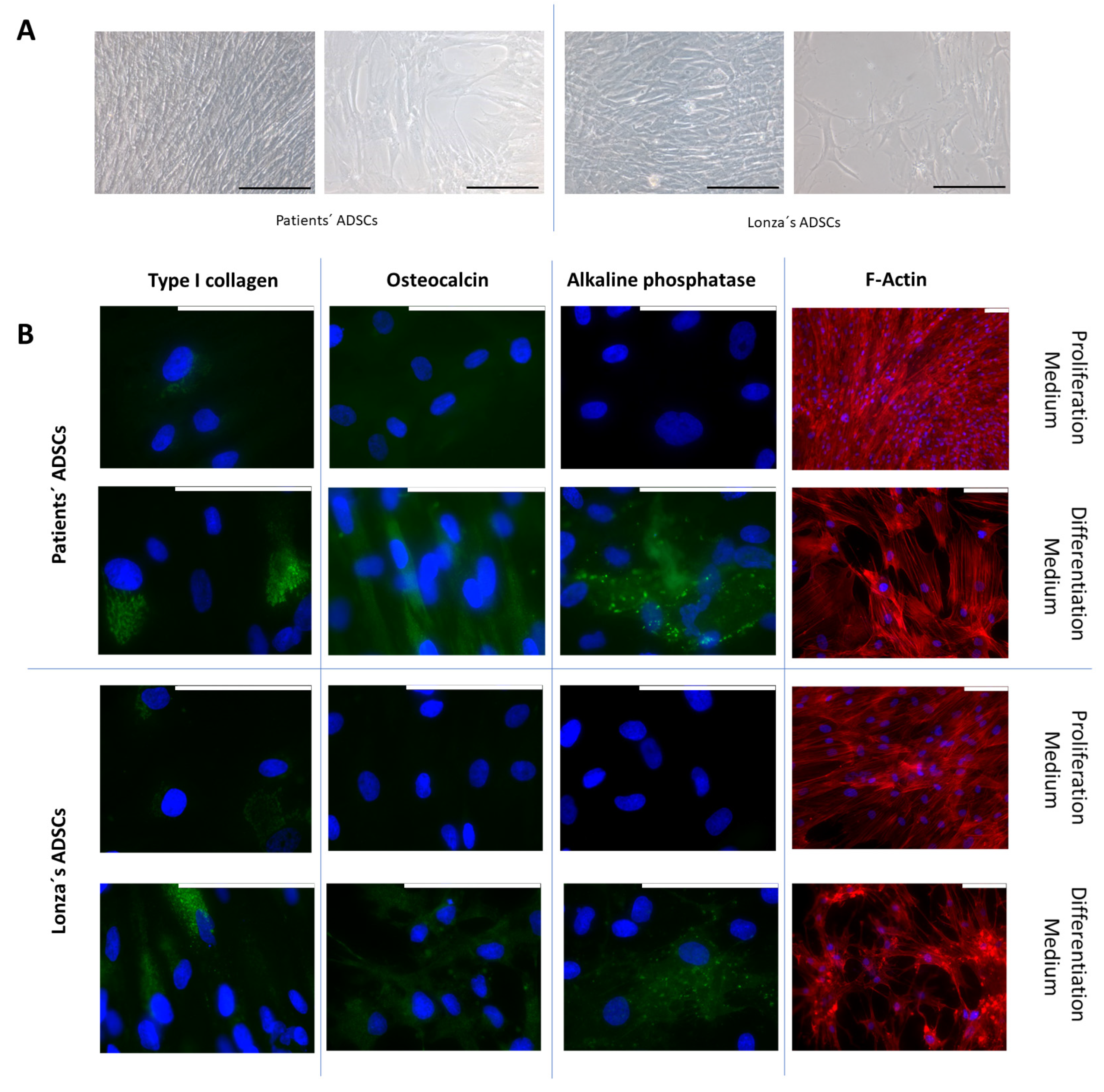
2.3. Evaluation of Osteogenic Differentiation Capacity of ADSCs
3. Discussion
4. Materials and Methods
4.1. Patient Enrolment
4.2. Samples Origin
4.3. ADSC Isolation and Characterization
4.4. ADSC Proliferation and Differentiation
4.5. Multiparametric Characterization of the Final Differentiated Cultures
4.6. Determination of Main Markers of Each Cell Type
4.6.1. Specific Proteins of Each Cell Type Using Immunofluorescence
4.6.2. Protocol to Quantify the Gene Expression of the Main Markers for Each Cell Type Using RT-qPCR
4.7. Data Presentation and Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem Cells: Past, Present, and Future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Frese, L.; Dijkman, P.E.; Hoerstrup, S.P. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Med. Hemother. 2016, 43, 268–274. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Meirelles, L.d.S.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal Stem Cells Reside in Virtually All Post-Natal Organs and Tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Bunnell, B.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-Derived Stem Cells: Isolation, Expansion and Differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef]
- Wankhade, U.D.; Shen, M.; Kolhe, R.; Fulzele, S. Advances in Adipose-Derived Stem Cells Isolation, Characterization, and Application in Regenerative Tissue Engineering. Stem Cells Int. 2016, 2016, 3206807. [Google Scholar] [CrossRef]
- Al Battah, F.; De Kock, J.; Vanhaecke, T.; Rogiers, V. Current Status of Human Adipose-Derived Stem Cells: Differentiation into Hepatocyte-like Cells. ScientificWorldJournal 2011, 11, 1568–1581. [Google Scholar] [CrossRef]
- Young, D.A.; DeQuach, J.A.; Christman, K.L. Human Cardiomyogenesis and the Need for Systems Biology Analysis. Wiley Interdiscip. Rev. Syst. Biol. Med. 2011, 3, 666–680. [Google Scholar] [CrossRef]
- Erba, P.; Terenghi, G.; Kingham, P.J. Neural Differentiation and Therapeutic Potential of Adipose Tissue Derived Stem Cells. Curr. Stem Cell Res. Ther. 2010, 5, 153–160. [Google Scholar] [CrossRef]
- Abdanipour, A.; Tiraihi, T.; Delshad, A. Trans-Differentiation of the Adipose Tissue-Derived Stem Cells into Neuron-like Cells Expressing Neurotrophins by Selegiline. Iran. Biomed. J. 2011, 15, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Alt, E. Myocardial Regeneration Potential of Adipose Tissue-Derived Stem Cells. Biochem. Biophys. Res. Commun. 2010, 401, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi-Ishihara, H.; Tobita, M.; Tajima, S.; Tanaka, R.; Oshita, T.; Tabata, Y.; Mizuno, H. Coadministration of Adipose-Derived Stem Cells and Control-Released Basic Fibroblast Growth Factor Facilitates Angiogenesis in a Murine Ischemic Hind Limb Model. J. Vasc. Surg. 2016, 64, 1825–1834.e1. [Google Scholar] [CrossRef]
- Salgado, A.J.; Oliveira, J.M.; Martins, A.; Teixeira, F.G.; Silva, N.A.; Neves, N.M.; Sousa, N.; Reis, R.L. Tissue Engineering and Regenerative Medicine: Past, Present, and Future. Int. Rev. Neurobiol. 2013, 108, 1–33. [Google Scholar] [CrossRef]
- Usuelli, F.G.; D’Ambrosi, R.; Maccario, C.; Indino, C.; Manzi, L.; Maffulli, N. Adipose-Derived Stem Cells in Orthopaedic Pathologies. Br. Med. Bull. 2017, 124, 31–54. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Huch, M. Disease Modelling in Human Organoids. Dis. Model. Mech. 2019, 12, dmm039347. [Google Scholar] [CrossRef] [PubMed]
- Aguado, B.A.; Grim, J.C.; Rosales, A.M.; Watson-Capps, J.J.; Anseth, K.S. Engineering Precision Biomaterials for Personalized Medicine. Sci. Transl. Med. 2018, 10, eaam8645. [Google Scholar] [CrossRef] [PubMed]
- Cieśla, J.; Tomsia, M. Cadaveric Stem Cells: Their Research Potential and Limitations. Front. Genet. 2021, 12, 798161. [Google Scholar] [CrossRef]
- Kami, D.; Kitani, T.; Nakata, M.; Gojo, S. Cardiac Mesenchymal Progenitors from Postmortem Cardiac Tissues Retained Cellular Characterization. Transplant. Proc. 2014, 46, 1194–1197. [Google Scholar] [CrossRef]
- Shikh Alsook, M.K.; Gabriel, A.; Piret, J.; Waroux, O.; Tonus, C.; Connan, D.; Baise, E.; Antoine, N. Tissues from Equine Cadaver Ligaments up to 72 Hours of Post-Mortem: A Promising Reservoir of Stem Cells. Stem Cell Res. Ther. 2015, 6, 253. [Google Scholar] [CrossRef] [PubMed]
- Latil, M.; Rocheteau, P.; Châtre, L.; Sanulli, S.; Mémet, S.; Ricchetti, M.; Tajbakhsh, S.; Chrétien, F. Skeletal Muscle Stem Cells Adopt a Dormant Cell State Post Mortem and Retain Regenerative Capacity. Nat. Commun. 2012, 3, 903. [Google Scholar] [CrossRef]
- Feldmann, R.E.; Mattern, R. The Human Brain and Its Neural Stem Cells Postmortem: From Dead Brains to Live Therapy. Int. J. Leg. Med. 2006, 120, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.H.; Bryant, P.J.; Fuja, T.J.; Su, H.; O’Dowd, D.K.; Klassen, H. Isolation and Characterization of Neural Progenitor Cells from Post-Mortem Human Cortex. J. Neurosci. Res. 2003, 74, 838–851. [Google Scholar] [CrossRef]
- Tormin, A.; Li, O.; Brune, J.C.; Walsh, S.; Schütz, B.; Ehinger, M.; Ditzel, N.; Kassem, M.; Scheding, S. CD146 Expression on Primary Nonhematopoietic Bone Marrow Stem Cells Is Correlated with in Situ Localization. Blood 2011, 117, 5067–5077. [Google Scholar] [CrossRef]
- Espagnolle, N.; Guilloton, F.; Deschaseaux, F.; Gadelorge, M.; Sensébé, L.; Bourin, P. CD146 Expression on Mesenchymal Stem Cells Is Associated with Their Vascular Smooth Muscle Commitment. J. Cell Mol. Med. 2014, 18, 104–114. [Google Scholar] [CrossRef]
- Li, X.; Guo, W.; Zha, K.; Jing, X.; Wang, M.; Zhang, Y.; Hao, C.; Gao, S.; Chen, M.; Yuan, Z.; et al. Enrichment of CD146 (+) Adipose-Derived Stem Cells in Combination with Articular Cartilage Extracellular Matrix Scaffold Promotes Cartilage Regeneration. Theranostics 2019, 9, 5105–5121. [Google Scholar] [CrossRef] [PubMed]
- Fitter, S.; Gronthos, S.; Ooi, S.S.; Zannettino, A.C.W. The Mesenchymal Precursor Cell Marker Antibody STRO-1 Binds to Cell Surface Heat Shock Cognate 70. Stem Cells 2017, 35, 940–951. [Google Scholar] [CrossRef]
- Psaltis, P.J.; Zannettino, A.C.W.; Worthley, S.G.; Gronthos, S. Concise Review: Mesenchymal Stromal Cells: Potential for Cardiovascular Repair. Stem Cells 2008, 26, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Barlian, A.; Judawisastra, H.; Alfarafisa, N.M.; Wibowo, U.A.; Rosadi, I. Chondrogenic Differentiation of Adipose-Derived Mesenchymal Stem Cells Induced by L-Ascorbic Acid and Platelet Rich Plasma on Silk Fibroin Scaffold. PeerJ 2018, 6, e5809. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Ferrándiz, M.; Milián, L.; Sancho-Tello, M.; Martín de Llano, J.J.; Gisbert Roca, F.; Martínez-Ramos, C.; Carda, C.; Mata, M. Alginate-Agarose Hydrogels Improve the In Vitro Differentiation of Human Dental Pulp Stem Cells in Chondrocytes. A Histological Study. Biomedicines 2021, 9, 834. [Google Scholar] [CrossRef]
- Salvador-Clavell, R.; Martín de Llano, J.J.; Milián, L.; Oliver, M.; Mata, M.; Carda, C.; Sancho-Tello, M. Chondrogenic Potential of Human Dental Pulp Stem Cells Cultured as Microtissues. Stem Cells Int. 2021, 2021, 7843798. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Tello, M.; Martorell, S.; Mata Roig, M.; Milián, L.; Gámiz-González, M.A.; Gómez Ribelles, J.L.; Carda, C. Human Platelet-Rich Plasma Improves the Nesting and Differentiation of Human Chondrocytes Cultured in Stabilized Porous Chitosan Scaffolds. J. Tissue Eng. 2017, 8, 2041731417697545. [Google Scholar] [CrossRef] [PubMed]
- Martin-Iglesias, S.; Milian, L.; Sancho-Tello, M.; Salvador-Clavell, R.; Martín de Llano, J.J.; Carda, C.; Mata, M. BMP-2 Enhances Osteogenic Differentiation of Human Adipose-Derived and Dental Pulp Stem Cells in 2D and 3D In Vitro Models. Stem Cells Int. 2022, 2022, 4910399. [Google Scholar] [CrossRef] [PubMed]
- Hanna, H.; Mir, L.M.; Andre, F.M. In Vitro Osteoblastic Differentiation of Mesenchymal Stem Cells Generates Cell Layers with Distinct Properties. Stem Cell Res. Ther. 2018, 9, 203. [Google Scholar] [CrossRef]
- Gould, N.R.; Torre, O.M.; Leser, J.M.; Stains, J.P. The Cytoskeleton and Connected Elements in Bone Cell Mechano-Transduction. Bone 2021, 149, 115971. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.-J.; Zeng, R.; Lu, J.-H.; Lai, W.-F.T.; Chen, W.-H.; Liu, H.-Y.; Chang, Y.-T.; Deng, W.-P. Adipose-Derived Stem Cells Promote Tumor Initiation and Accelerate Tumor Growth by Interleukin-6 Production. Oncotarget 2015, 6, 7713–7726. [Google Scholar] [CrossRef]
- Milian, L.; Mata, M.; Alcacer, J.; Oliver, M.; Sancho-Tello, M.; Martín de Llano, J.J.; Camps, C.; Galbis, J.; Carretero, J.; Carda, C. Cannabinoid Receptor Expression in Non-Small Cell Lung Cancer. Effectiveness of Tetrahydrocannabinol and Cannabidiol Inhibiting Cell Proliferation and Epithelial-Mesenchymal Transition in Vitro. PLoS ONE 2020, 15, e0228909. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milián, L.; Molina, P.; Oliver-Ferrándiz, M.; Fernández-Sellers, C.; Monzó, A.; Sánchez-Sánchez, R.; Braza-Boils, A.; Mata, M.; Zorio, E. Cadaveric Adipose-Derived Stem Cells for Regenerative Medicine and Research. Int. J. Mol. Sci. 2023, 24, 15696. https://doi.org/10.3390/ijms242115696
Milián L, Molina P, Oliver-Ferrándiz M, Fernández-Sellers C, Monzó A, Sánchez-Sánchez R, Braza-Boils A, Mata M, Zorio E. Cadaveric Adipose-Derived Stem Cells for Regenerative Medicine and Research. International Journal of Molecular Sciences. 2023; 24(21):15696. https://doi.org/10.3390/ijms242115696
Chicago/Turabian StyleMilián, Lara, Pilar Molina, María Oliver-Ferrándiz, Carlos Fernández-Sellers, Ana Monzó, Rafael Sánchez-Sánchez, Aitana Braza-Boils, Manuel Mata, and Esther Zorio. 2023. "Cadaveric Adipose-Derived Stem Cells for Regenerative Medicine and Research" International Journal of Molecular Sciences 24, no. 21: 15696. https://doi.org/10.3390/ijms242115696
APA StyleMilián, L., Molina, P., Oliver-Ferrándiz, M., Fernández-Sellers, C., Monzó, A., Sánchez-Sánchez, R., Braza-Boils, A., Mata, M., & Zorio, E. (2023). Cadaveric Adipose-Derived Stem Cells for Regenerative Medicine and Research. International Journal of Molecular Sciences, 24(21), 15696. https://doi.org/10.3390/ijms242115696







