Effects of Persimmon (Diospyros kaki L. cv. Mopan) Polysaccharide and Their Carboxymethylated Derivatives on Lactobacillus Strains Proliferation and Gut Microbiota: A Comparative Study
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of PFP
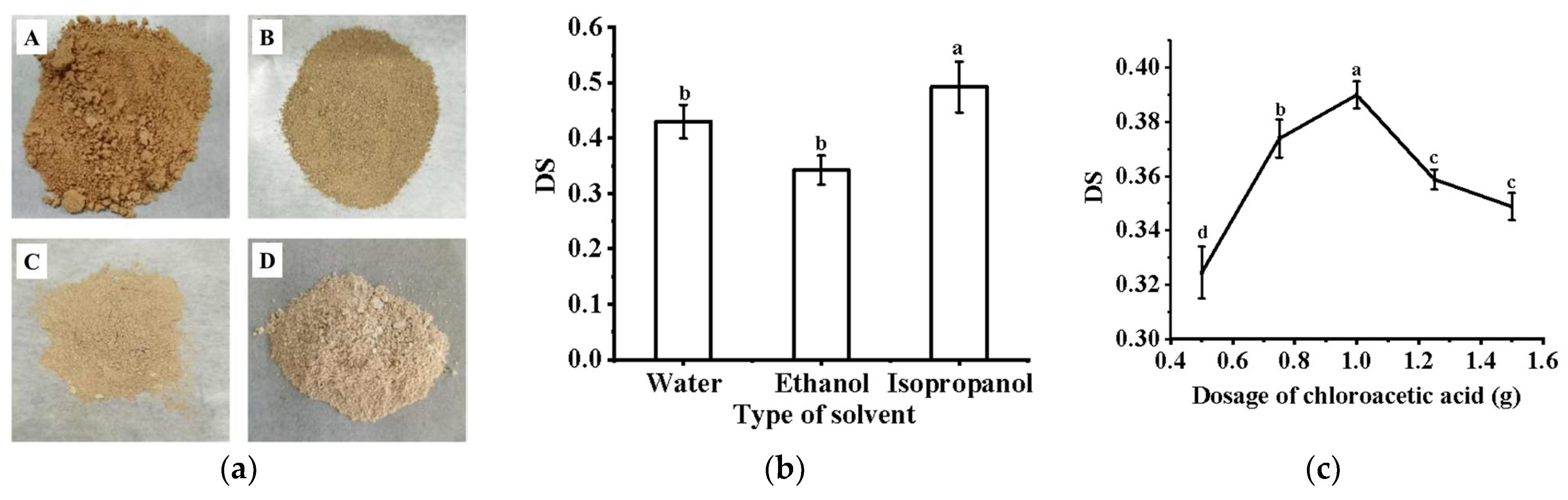
2.2. Analysis of Carboxymethylation Modification of PFP
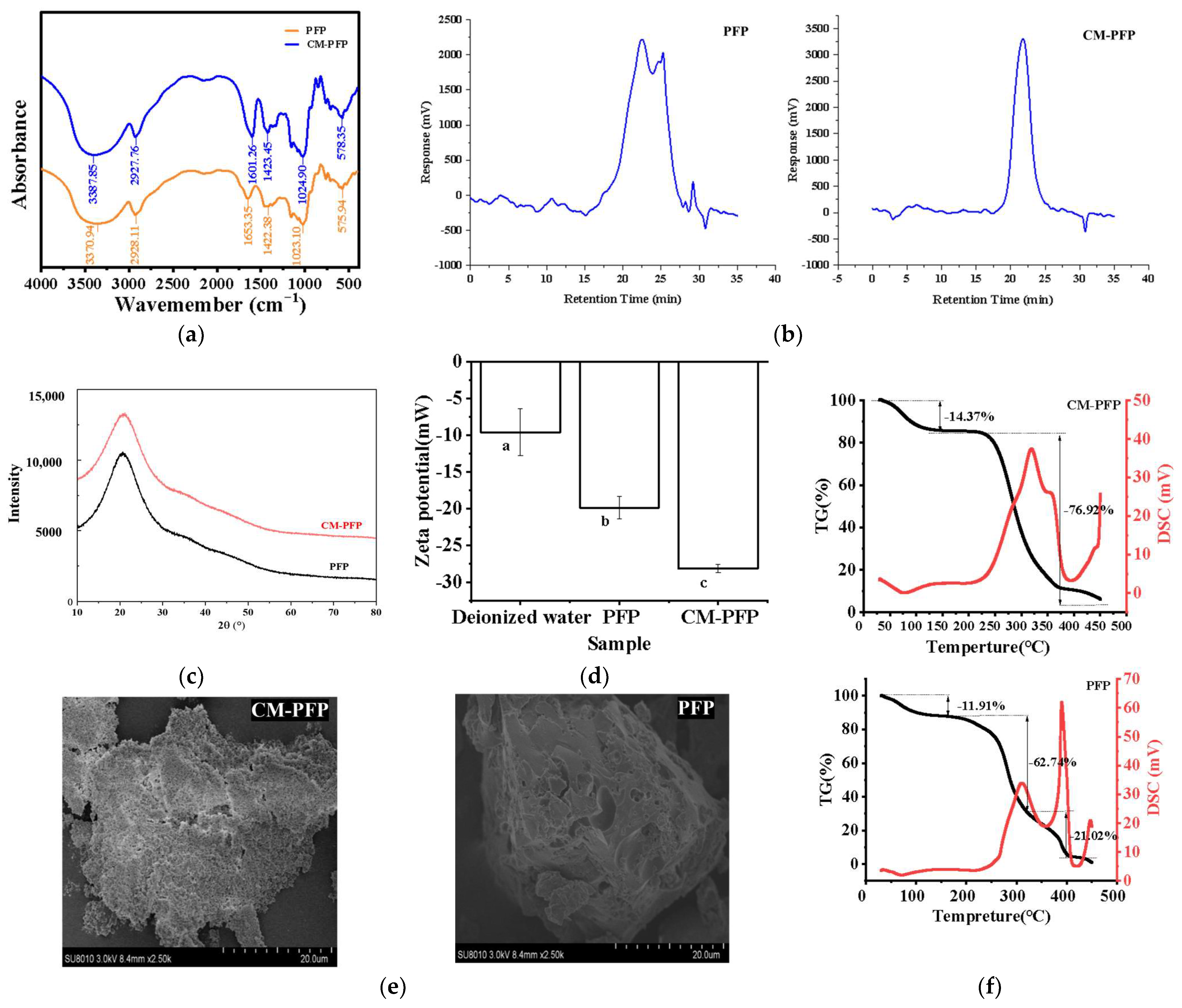
2.3. Physicochemical Properties and Structural Characterization of PFP and CM-PFP
2.3.1. The Fourier Transform Infrared (FTIR) Spectra of PFP and CM-PFP Analysis
2.3.2. The Mw of PFP and CM-PFP Analysis
2.3.3. Monosaccharide Composition of PFP and CM-PFP Analysis
2.3.4. X-ray Diffraction (XRD)
2.3.5. Zeta Potential of PFP and CM-PFP
2.3.6. Thermal Stability Analysis of PFP and CM-PFP
2.3.7. Scanning Electron Microscopy (SEM) of PFP and CM-PFP
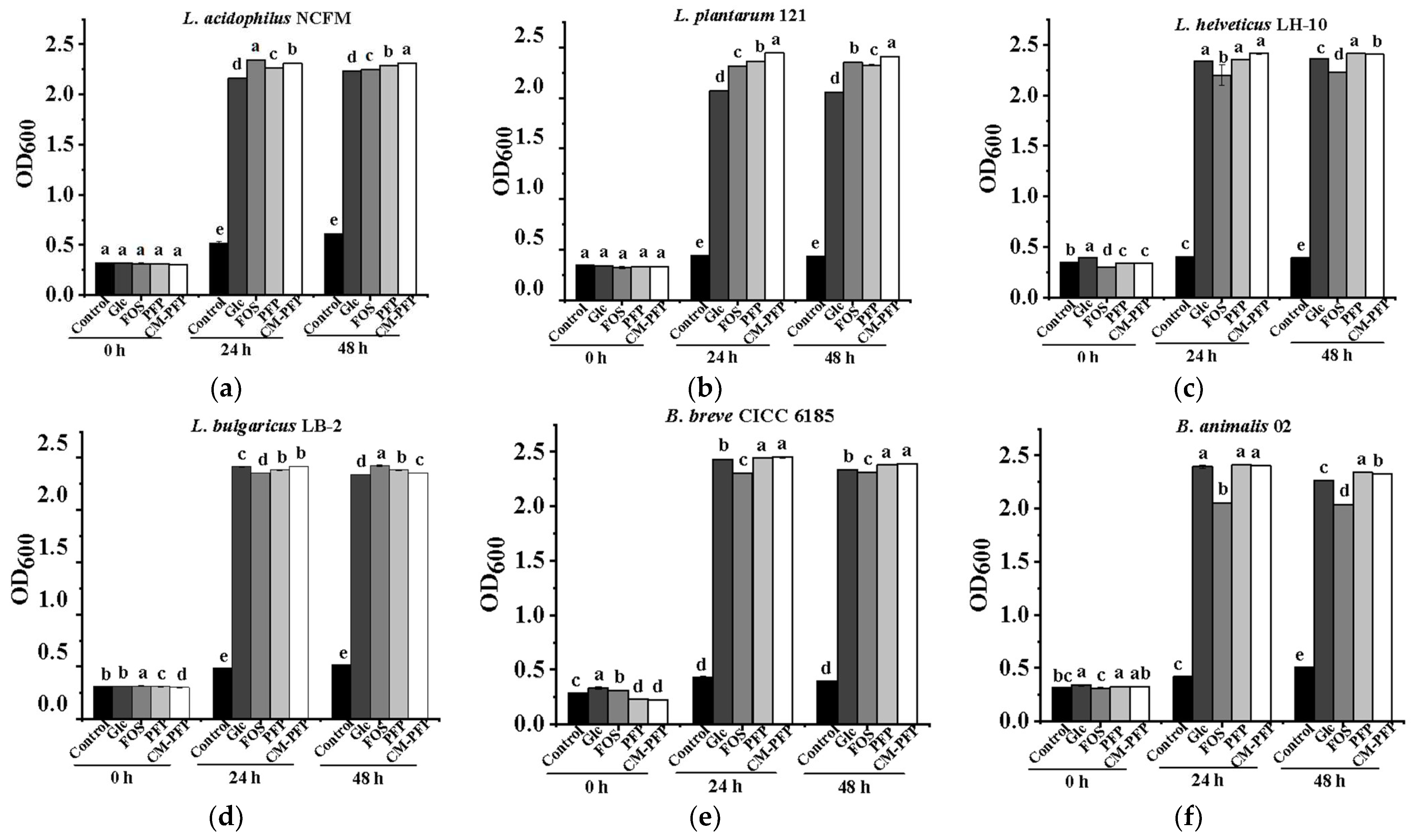
2.4. The Effect of PFP and CM-PFP on Lactobacillus Proliferation
2.5. Inhibitory Effects on Escherichia coli and Staphylococcus aureus
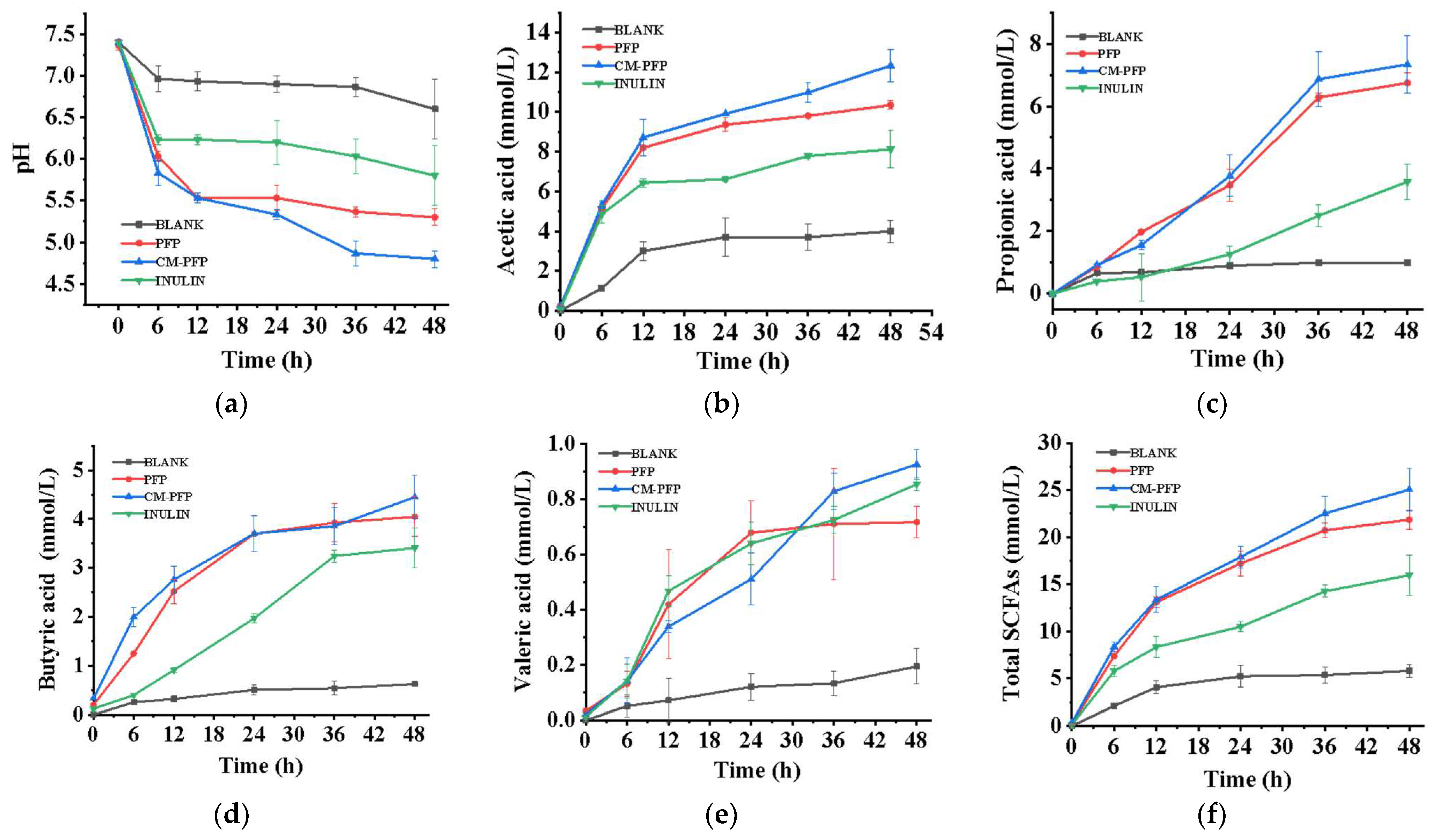
2.6. In Vitro Fecal Fermentation
2.6.1. SCFAs Produced in PFP and CM-PFP Medium
2.6.2. Phylum-Level Species Relative Abundances
2.6.3. Genus-Level Species Relative Abundance
2.6.4. Linear Discriminant Analysis Effect Size (LEfSe) Abundance Difference Analysis
3. Discussion
3.1. Carboxymethylation of PFP
3.2. Physicochemical Properties and Structural Characterization of PFP and CM-PFP
3.3. In Vitro Proliferation of Lactobacillus Bacteria
3.4. Inhibitory Effects on Escherichia coli and Staphylococcus aureus
3.5. In Vitro Fecal Fermentation
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Extraction and Purification of PFP
4.3. Carboxymethylation of PFP
4.4. Physicochemical Properties and Structural Characterization of PFP and CM-PFP
4.4.1. The FTIR Spectra of PFP and CM-PFP Analysis
4.4.2. Measurement of Mw of PFP and CM-PFP
4.4.3. Monosaccharide Composition of PFP and CM-PFP
4.4.4. X-ray Diffraction (XRD) Analysis
4.4.5. Zeta Potential Determination
4.4.6. Thermal Analysis
4.4.7. SEM Analysis
4.5. In Vitro Proliferation of Lactobacillus Bacteria
4.6. Inhibitory Effects of PFP and CM-PFP on E. coli and S. aureus
4.7. In Vitro Fecal Fermentation
4.7.1. Analysis of SCFA Production Ability
4.7.2. 16S rRNA Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tardugno, R.; Gervasi, T.; Nava, V.; Cammilleri, G.; Ferrantelli, V.; Cicero, N. Nutritional and mineral composition of persimmon fruits (Diospyros kaki L.) from Central and Southern Italy. Nat. Prod. Res. 2022, 36, 5168–5173. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Sultan, M.T.; Aziz, M.; Naz, A.; Ahmed, W.; Kumar, N.; Imran, M. Persimmon (Diospyros kaki) fruit: Hidden phytochemicals and health claims. EXCLI J. 2015, 14, 542–561. [Google Scholar] [CrossRef]
- Furukawa, R.; Kitabatake, M.; Ouji-Sageshima, N.; Suzuki, Y.; Nakano, A.; Matsumura, Y.; Nakano, R.; Kasahara, K.; Kubo, K.; Kayano, S.I.; et al. Persimmon-derived tannin has antiviral effects and reduces the severity of infection and transmission of SARS-CoV-2 in a Syrian hamster model. Sci. Rep. 2021, 11, 23695. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, P.; Li, X.; Shen, S.; Li, K. Persimmon proanthocyanidins with different degrees of polymerization possess distinct activities in models of high fat diet induced obesity. Nutrients 2022, 14, 3718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, X.; Fu, Z.; Wang, Z.; Zhang, J. Sulphated modification of a polysaccharide obtained from fresh persimmon (Diospyros kaki L.) fruit and antioxidant activities of the sulphated derivatives. Food Chem. 2011, 127, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Mo, X.; Guo, H.; Zhang, Y. Sulfation modification and anticoagulant activity of the polysaccharides obtained from persimmon (Diospyros kaki L.) fruits. Int. J. Biol. Macromol. 2012, 51, 1189–1195. [Google Scholar] [CrossRef]
- Hwang, K.C.; Shin, H.Y.; Kim, W.J.; Seo, M.S.; Kim, H. Effects of a high-molecular-weight polysaccharides isolated from Korean persimmon on the antioxidant, anti-inflammatory, and antiwrinkle activity. Molecules 2021, 26, 1600. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, M.; Li, Q.; Wang, T.; Zhang, B.; Zhao, H.; Fu, J. The beneficial effects of polysaccharide obtained from persimmon (Diospyros kaki L.) on the proliferation of Lactobacillus and gut microbiota. Int. J. Biol. Macromol. 2021, 182, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Hong, R.; Yi, Y.; Bai, Y.; Dong, L.; Jia, X.; Zhang, R.; Wang, G.; Zhang, M.; Wu, J. In vitro digestion and human gut microbiota fermentation of longan pulp polysaccharides as affected by Lactobacillus fermentum fermentation. Int. J. Biol. Macromol. 2020, 147, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Li, Y.; Hou, X.; Zeng, F. Correlation of structure to antitumor activities of five derivatives of a beta-glucan from Poria cocos sclerotium. Carbohydr. Res. 2004, 339, 2567–2574. [Google Scholar] [CrossRef] [PubMed]
- Yiasmin, M.N.; Islam, M.S.; Md, E.; Yang, R.; Yanjun, T.; Hua, X. Fermentability of Maitake polysaccharides processed by various hydrothermal conditions and fermented with probiotic (Lactobacillus). Int. J. Biol. Macromol. 2022, 209, 1075–1087. [Google Scholar] [CrossRef]
- Zou, X.; Xiao, J.; Chi, J.; Zhang, M.; Zhang, R.; Jia, X.; Mei, D.; Dong, L.; Yi, Y.; Huang, F. Physicochemical properties and prebiotic activities of polysaccharides from Zizyphus jujube based on different extraction techniques. Int. J. Biol. Macromol. 2022, 223, 663–672. [Google Scholar] [CrossRef]
- de Nooy, A.E.; Rori, V.; Masci, G.; Dentini, M.; Crescenzi, V. Synthesis and preliminary characterisation of charged derivatives and hydrogels from scleroglucan. Carbohydr. Res. 2000, 324, 116–126. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G. Extraction, derivatization and antioxidant activity of bitter gourd polysaccharide. Int. J. Biol. Macromol. 2019, 141, 14–20. [Google Scholar] [CrossRef]
- Li, Y.T.; Chen, B.J.; Wu, W.D.; Ge, K.; Wei, X.Y.; Kong, L.M.; Xie, Y.Y.; Gu, J.P.; Zhang, J.C.; Zhou, T. Antioxidant and antimicrobial evaluation of carboxymethylated and hydroxamated degraded polysaccharides from Sargassum fusiforme. Int. J. Biol. Macromol. 2018, 118, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Huang, Z.; Qin, L.; Yu, Q.; Chen, Y.; Zhu, H.; Xie, J. Effects of sulfation and carboxymethylation on Cyclocarya paliurus polysaccharides: Physicochemical properties, antitumor activities and protection against cellular oxidative stress. Int. J. Biol. Macromol. 2022, 204, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.Y.; Sun, P.P.; Ji, Y.P.; Wang, X.T.; Dai, S.H.; Zhu, Z.Y. Carboxymethylation and acetylation of the polysaccharide from Cordyceps militaris and their alpha-glucosidase inhibitory activities. Nat. Prod. Res. 2020, 34, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Sejersen, M.T.; Salomonsen, T.; Ipsen, R.; Clark, R.; Rolin, C.; Engelsen, S.B. Zeta potential of pectin-stabilised casein aggregates in acidified milk drinks. Int. Dairy J. 2007, 17, 302–307. [Google Scholar] [CrossRef]
- Joseph, E.; Singhvi, G. Chapter 4—Multifunctional nanocrystals for cancer therapy: A potential nanocarrier. In Nanomaterials for Drug Delivery and Therapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 91–116. [Google Scholar]
- Pei, F.; Li, W.; Ni, X.; Sun, X.; Yao, Y.; Fang, Y.; Yang, W.; Hu, Q. Effect of cooked rice with added fructo-oligosaccharide on faecal microorganisms investigated by in vitro digestion and fermentation. Food Sci. Hum. Wellness 2023, 12, 662–668. [Google Scholar] [CrossRef]
- Wu, D.T.; An, L.Y.; Liu, W.; Hu, Y.C.; Wang, S.P.; Zou, L. In vitro fecal fermentation properties of polysaccharides from Tremella fuciformis and related modulation effects on gut microbiota. Food Res. Int. 2022, 156, 111185. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Liu, H.; Li, X.; Liu, J.; Chen, L.; Jin, X.; Chen, T.; Wang, W.; Liu, Z.; Zhang, M.; et al. Carboxymethylation modification, characterization, antioxidant activity and anti-UVC ability of Sargassum fusiforme polysaccharide. Carbohydr. Res. 2022, 515, 108555. [Google Scholar] [CrossRef]
- Duan, S.; Zhao, M.; Wu, B.; Wang, S.; Yang, Y.; Xu, Y.; Wang, L. Preparation, characteristics, and antioxidant activities of carboxymethylated polysaccharides from blackcurrant fruits. Int. J. Biol. Macromol. 2020, 155, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Konduri, M.K.; Fatehi, P. Synthesis and characterization of carboxymethylated xylan and its application as a dispersant. Carbohydr. Polym. 2016, 146, 26–35. [Google Scholar] [CrossRef]
- Castañeda-Salazar, A.; Figueroa-Cárdenas, J.D.; López, M.G.; Mendoza, S. Physicochemical and functional characterization of agave fructans modified by cationization and carboxymethylation. Carbohydr. Polym. Technol. Appl. 2023, 5, 100284. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, Y.; Wang, S.; Wang, S.; Yang, J.; Ismael, M.; Wang, X.; Lu, X. Purification, structural characterization and antioxidant activities of two neutral polysaccharides from persimmon peel. Int. J. Biol. Macromol. 2023, 225, 241–254. [Google Scholar] [CrossRef]
- Zhou, F.; Su, L.; Xu, J.; Wang, M.; Fan, J.; Zhang, B.; Li, Y. Sulphated and carboxymethylated polysaccharides from Lycium barbarum L. leaves suppress the gelatinisation, retrogradation and digestibility of potato starch. Int. J. Food Sci. Technol. 2023, 58, 94–106. [Google Scholar] [CrossRef]
- Chen, G.J.; Hong, Q.Y.; Ji, N.; Wu, W.N.; Ma, L.Z. Influences of different drying methods on the structural characteristics and prebiotic activity of polysaccharides from bamboo shoot (Chimonobambusa quadrangularis) residues. Int. J. Biol. Macromol. 2020, 155, 674–684. [Google Scholar] [CrossRef]
- Huang, F.; Hong, R.; Zhang, R.; Dong, L.; Bai, Y.; Liu, L.; Jia, X.; Wang, G.; Zhang, M. Dynamic variation in biochemical properties and prebiotic activities of polysaccharides from longan pulp during fermentation process. Int. J. Biol. Macromol. 2019, 132, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Heydarian, M.; Jooyandeh, H.; Nasehi, B.; Noshad, M. Characterization of Hypericum perforatum polysaccharides with antioxidant and antimicrobial activities: Optimization based statistical modeling. Int. J. Biol. Macromol. 2017, 104, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.; You, S.; Luo, J.; Lin, Z.; Chen, T.; Peng, X.; Qiu, B. Antibacterial mechanism of polysaccharides from the leaves of Lindera aggregata (Sims) Kosterm. by metabolomics based on HPLC/MS. Int. J. Biol. Macromol. 2022, 221, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, X.; Liang, X.; Zheng, X.; Shu, Z.; Sun, Q.; Wang, Q.; Li, N. Antioxidant and antibacterial activities of a polysaccharide produced by Chaetomium globosum CGMCC 6882. Int. J. Biol. Macromol. 2023, 233, 123628. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Peng, Y.; Rui, Y.; Zeng, X.; Ye, H. Simulated digestion and fermentation in vitro with human gut microbiota of polysaccharides from Coralline pilulifera. LWT 2019, 100, 167–174. [Google Scholar] [CrossRef]
- Hu, J.L.; Nie, S.P.; Li, C.; Xie, M.Y. In vitro fermentation of polysaccharide from the seeds of Plantago asiatica L. by human fecal microbiota. Food Hydrocoll. 2013, 33, 384–392. [Google Scholar] [CrossRef]
- Liu, C.; Du, P.; Cheng, Y.L.; Guo, Y.H.; Hu, B.; Yao, W.R.; Zhu, X.; Qian, H. Study on fecal fermentation characteristics of aloe polysaccharides in vitro and their predictive modeling. Carbohydr. Polym. 2021, 256, 117571. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhou, Q.; Gao, Z.; Lin, X.; Zhou, K.; Cheng, X.; Chitrakar, B.; Chen, H.; Zhao, W. In vitro digestion and fecal fermentation behaviors of polysaccharides from Ziziphus Jujuba cv. Pozao and its interaction with human gut microbiota. Food Res. Int. 2022, 162, 112022. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; Fu, Y.; Guo, H.; Yuan, Q.; Nie, X.R.; Wang, S.P.; Gan, R.Y. In vitro simulated digestion and fecal fermentation of polysaccharides from loquat leaves: Dynamic changes in physicochemical properties and impacts on human gut microbiota. Int. J. Biol. Macromol. 2021, 168, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.J.; Xie, M.H.; Wan, P.; Chen, D.; Ye, H.; Chen, L.G.; Zeng, X.X.; Liu, Z.H. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.; Peng, Y.; Chen, D.; Mi, J.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int. J. Biol. Macromol. 2019, 125, 751–760. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, L.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lu, X. Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]



| Sample | Total Sugar Content (%) | Content of Protein (%) | L* | a* | b* |
|---|---|---|---|---|---|
| Crude PFP | 42.8 ± 0.21 | 11.8 ± 0.12 | 7.24 | 77.30 | 11.90 |
| Deproteinized PFP | 64.3 ± 0.33 | 3.1 ± 0.04 | 18.68 | 50.19 | 24.28 |
| PFP | 77.24 ± 0.07 | 2.9 ± 0.41 | 30.80 | 20.17 | 23.19 |
| CM-PFP | 76.58 ± 0.22 | 2.8 ± 0.03 | 32.63 | 18.90 | 21.86 |
| Sample | 0–5000 Da | 5000–10,000 Da | 10,000–20,000 Da | Mw | Mn | PD (Mw/Mn) |
|---|---|---|---|---|---|---|
| PFP | 51.668% | 18.868% | 18.132% | 13,327 Da | 2164 Da | 6.126 |
| CM-PFP | 8.767% | 64.506% | 35.064% | 16,566 Da | 9502 Da | 1.743 |
| Monosaccharides | PFP | CM-PFP |
|---|---|---|
| Ara | 0.23% | 0.10% |
| Gal | 0.38% | 0.16% |
| Glc | 95.62% | 97.61% |
| Man | 0.99% | 0.62% |
| Rib | 0.00% | 1.08% |
| GalA | 1.54% | 0.18% |
| GulA | 0.66% | 0.25% |
| GlcA | 0.58% | 0.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Xie, S.; Zhang, H.; Wang, T.; Zhang, B.; Zhao, H. Effects of Persimmon (Diospyros kaki L. cv. Mopan) Polysaccharide and Their Carboxymethylated Derivatives on Lactobacillus Strains Proliferation and Gut Microbiota: A Comparative Study. Int. J. Mol. Sci. 2023, 24, 15730. https://doi.org/10.3390/ijms242115730
Shen X, Xie S, Zhang H, Wang T, Zhang B, Zhao H. Effects of Persimmon (Diospyros kaki L. cv. Mopan) Polysaccharide and Their Carboxymethylated Derivatives on Lactobacillus Strains Proliferation and Gut Microbiota: A Comparative Study. International Journal of Molecular Sciences. 2023; 24(21):15730. https://doi.org/10.3390/ijms242115730
Chicago/Turabian StyleShen, Xiaowei, Shanshan Xie, Huixin Zhang, Tao Wang, Bolin Zhang, and Hongfei Zhao. 2023. "Effects of Persimmon (Diospyros kaki L. cv. Mopan) Polysaccharide and Their Carboxymethylated Derivatives on Lactobacillus Strains Proliferation and Gut Microbiota: A Comparative Study" International Journal of Molecular Sciences 24, no. 21: 15730. https://doi.org/10.3390/ijms242115730





