Newly Synthesized CoFe2−xDyxO4 (x = 0; 0.1; 0.2; 0.4) Nanoparticles Reveal Promising Anticancer Activity against Melanoma (A375) and Breast Cancer (MCF-7) Cells
Abstract
:1. Introduction
2. Results
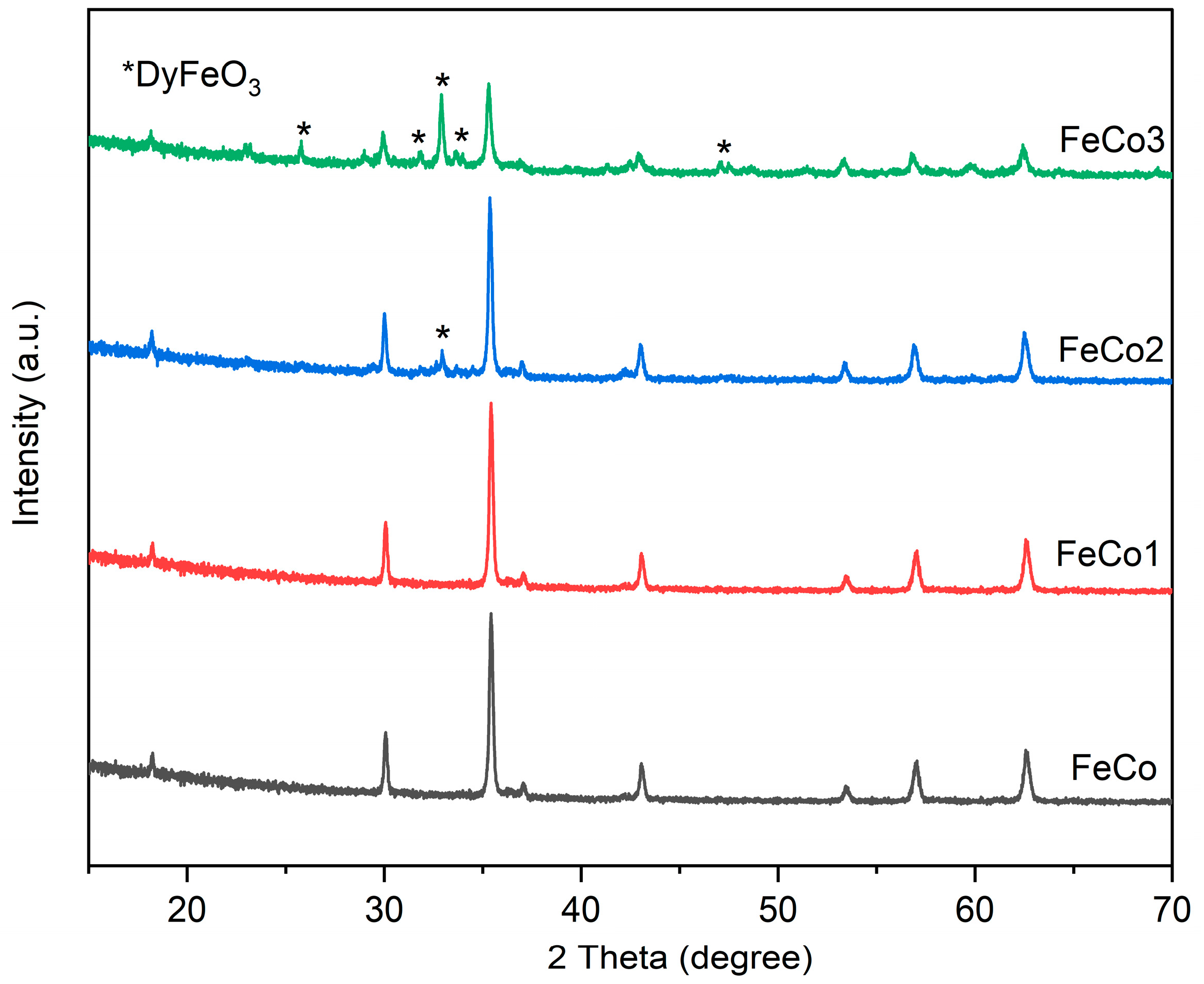
2.1. X-ray Diffraction (XRD)
2.2. Fourier Transform Infrared Spectroscopy (FTIR)
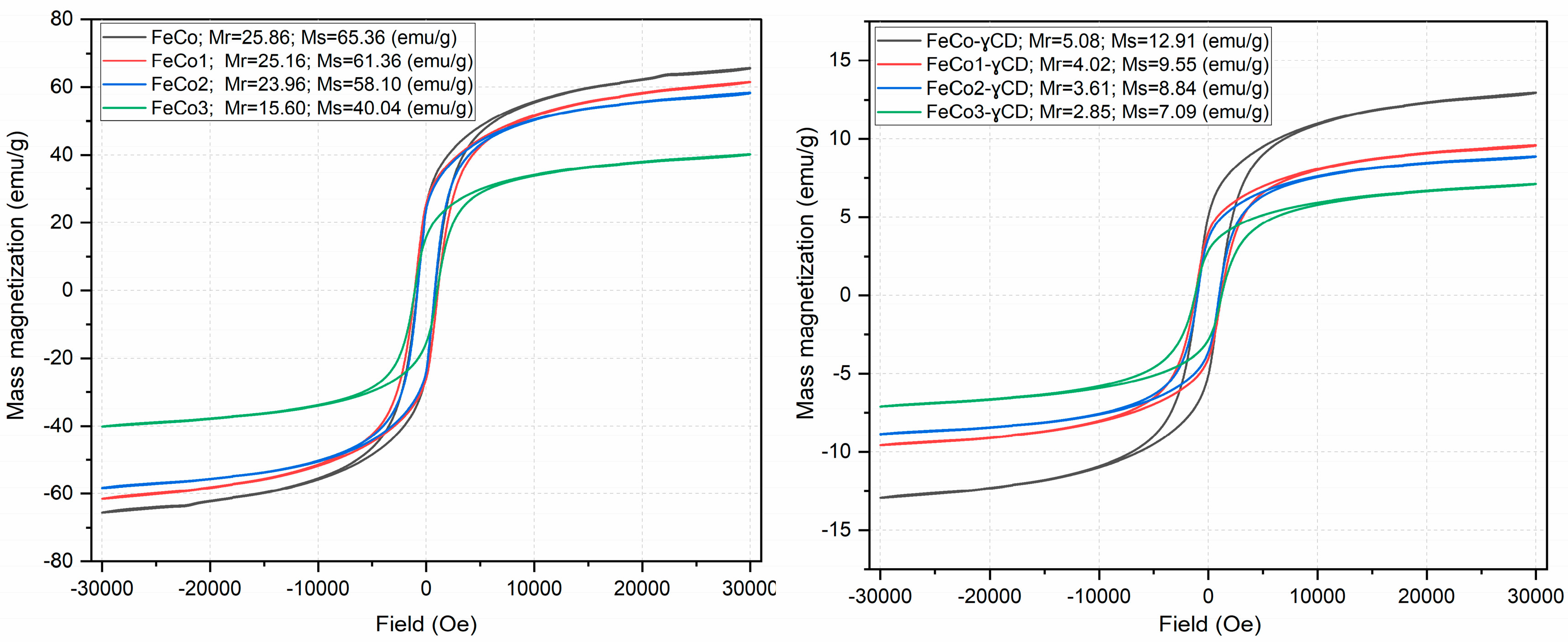
2.3. Investigation of Magnetic Properties
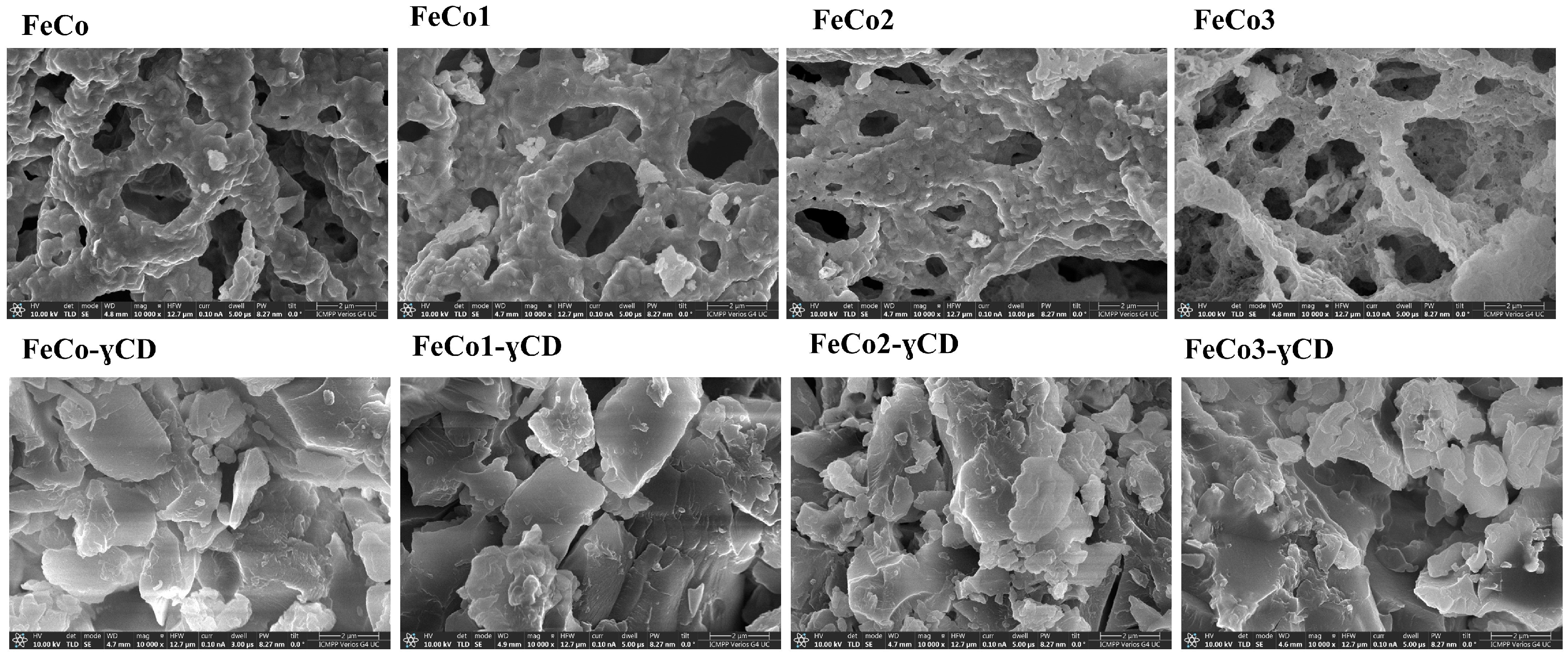
2.4. Scanning Electron Microscopy (SEM)
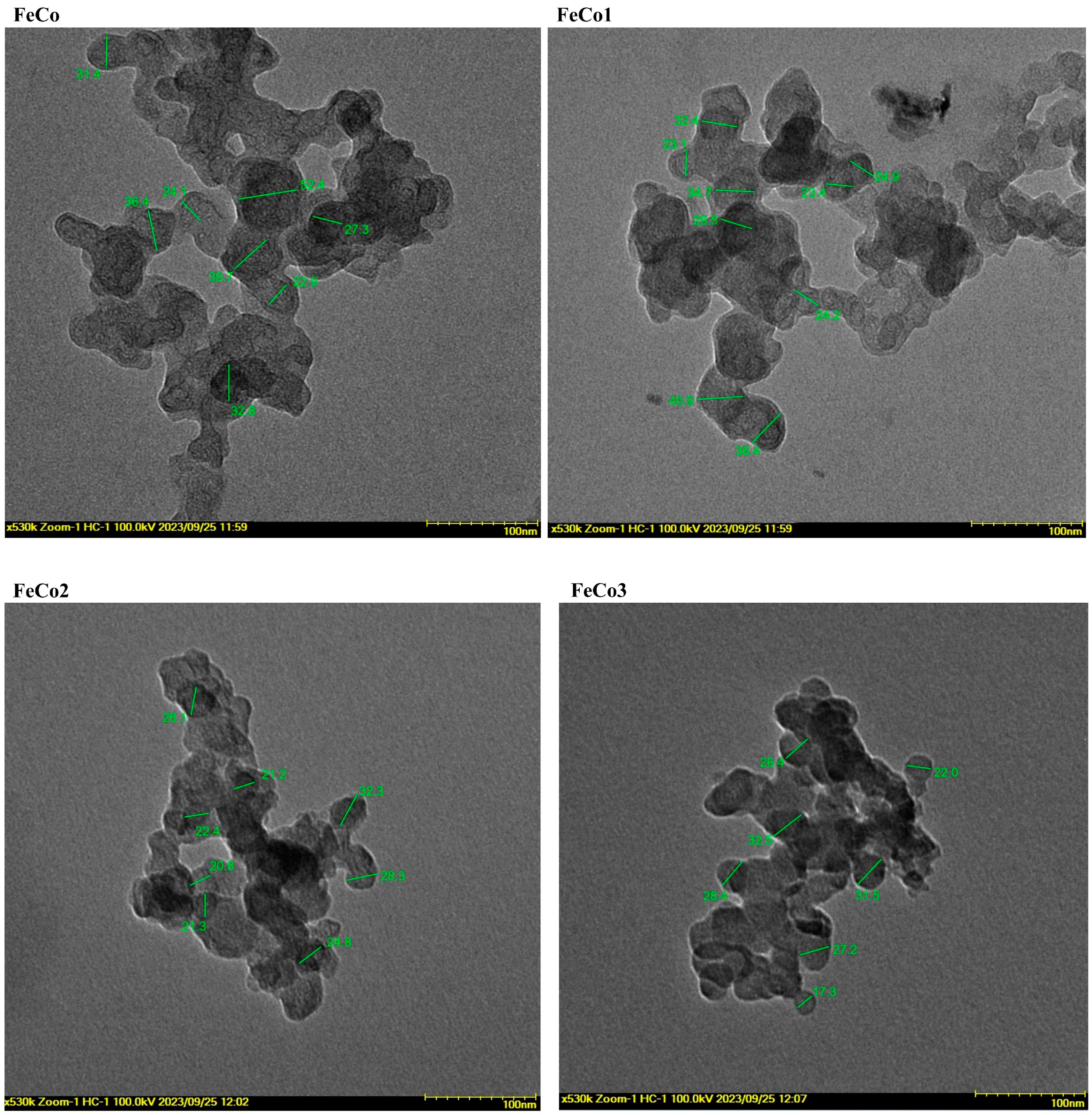
2.5. Transmitted Electron Microscopy (TEM)
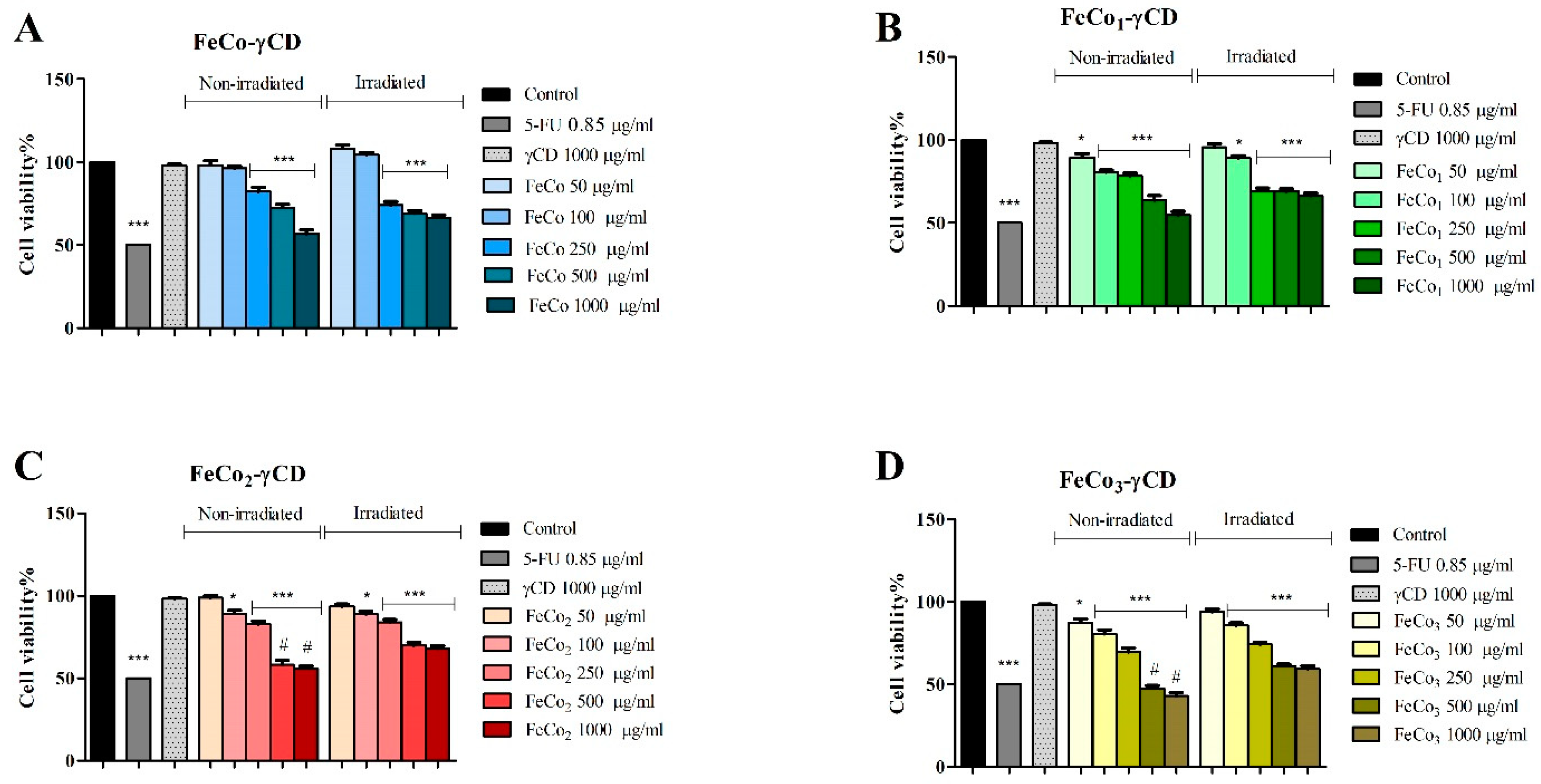
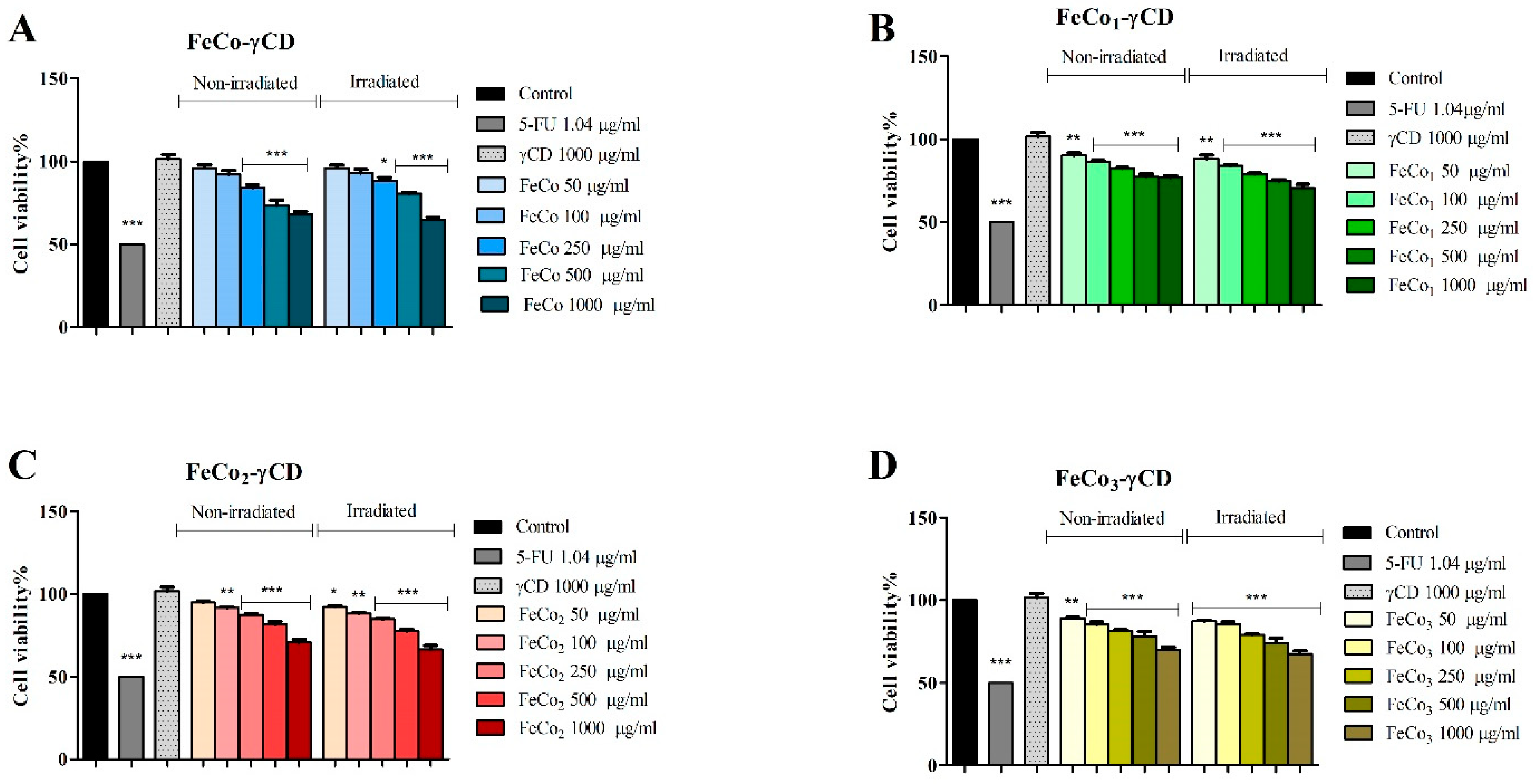
2.6. The Effect of RL and γ-Cyclodextrin FeCo Complexes on Cell Viability
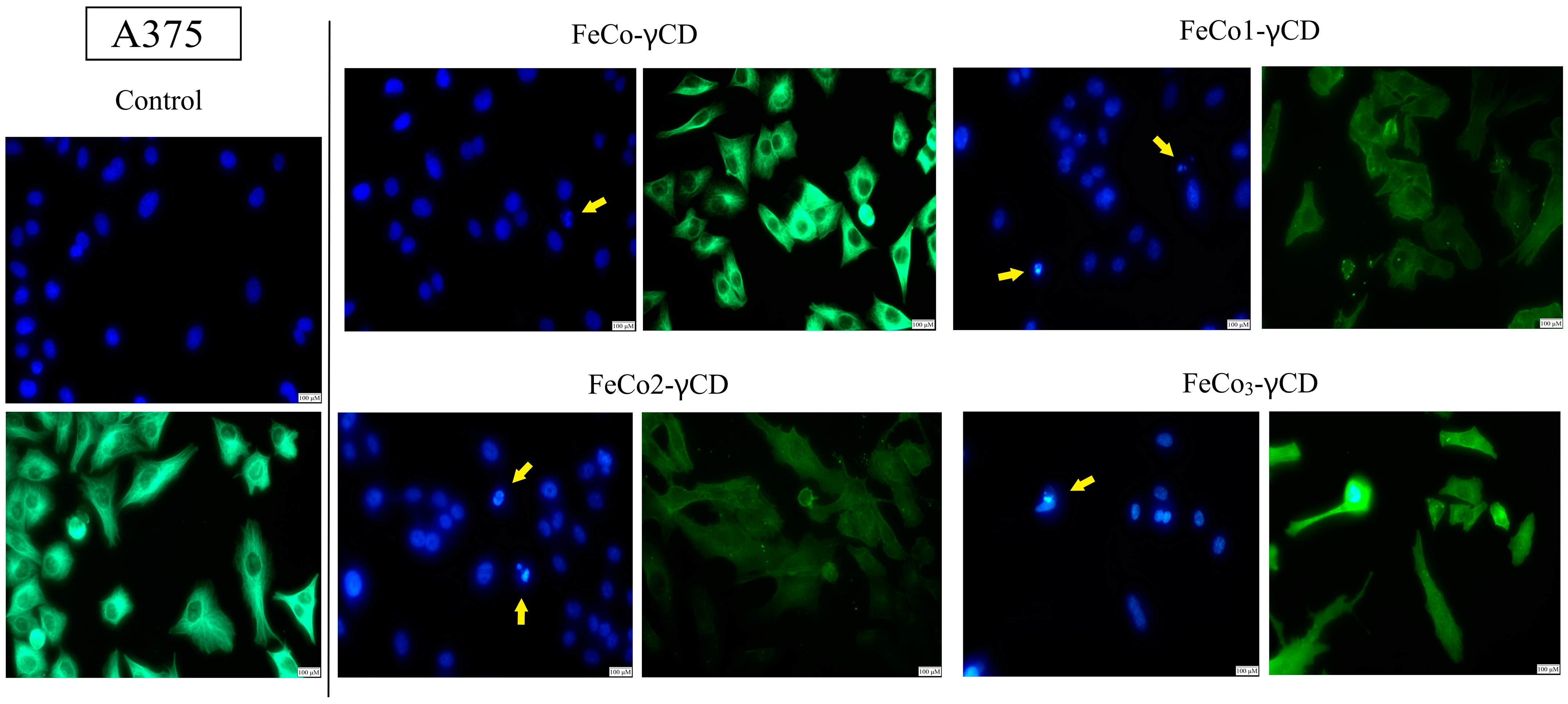
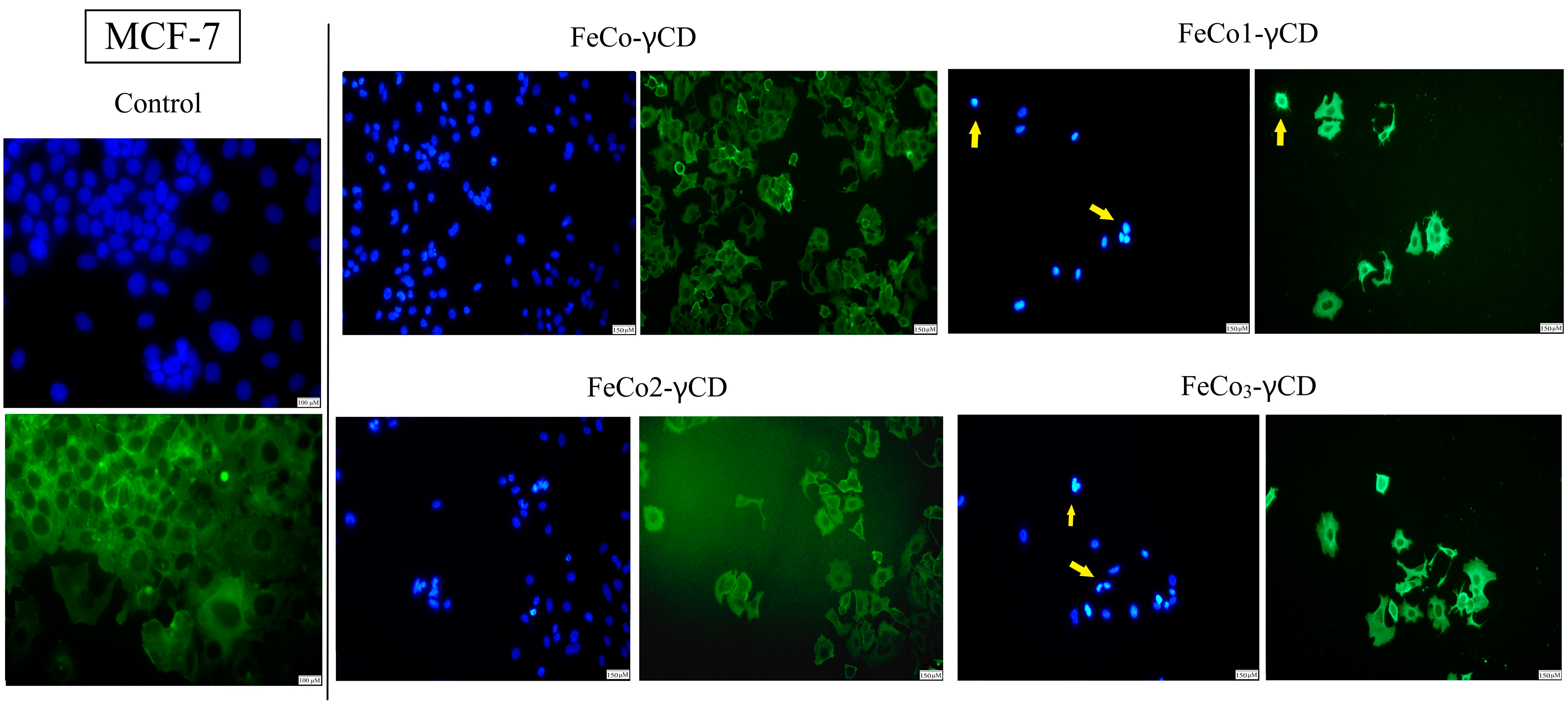
2.7. Morphological Changes of Cell Nuclei and Cytoskeleton—Evaluation of Apoptotic Features
2.8. Western Blot Analysis of Cyclin D1, p53 and Caspase-9
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis by Combustion of Cobalt Ferrite—FeCo
4.3. Synthesis of Cobalt Ferrite Doped with Dysprosium
4.4. Cyclodextrin Complex Formation
4.5. Characterization Methods
4.6. Biological Assessment
4.6.1. Cell Culture
4.6.2. Cell Viability Assay
4.6.3. Red Light (RL) Experimental Protocol
4.6.4. Detection of Apoptotic Morphological Changes
4.6.5. Western Blot
4.6.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y.K. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef] [PubMed]
- Tatarchuk, T.; Bououdina, M.; Judith Vijaya, J.; John Kennedy, L. Spinel Ferrite Nanoparticles: Synthesis, Crystal Structure, Properties, and Perspective Applications. In Springer Proceedings in Physics; Springer Science and Business Media, LLC: Berlin/Heidelberg, Germany, 2017; Volume 195, pp. 305–325. [Google Scholar]
- Amiri, M.; Salavati-Niasari, M.; Akbari, A. Magnetic Nanocarriers: Evolution of Spinel Ferrites for Medical Applications. Adv. Colloid Interface Sci. 2019, 265, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Barani, M.; Rahdar, A.; Mukhtar, M.; Razzaq, S.; Qindeel, M.; Hosseini Olam, S.A.; Paiva-Santos, A.C.; Ajalli, N.; Sargazi, S.; Balakrishnan, D.; et al. Recent Application of Cobalt Ferrite Nanoparticles as a Theranostic Agent. Mater. Today Chem. 2022, 26, 101131. [Google Scholar] [CrossRef]
- Alghamdi, N.; Stroud, J.; Przybylski, M.; Żukrowski, J.; Cruz Hernandez, A.; Brown, J.M.; Hankiewicz, J.H.; Celinski, Z. Structural, Magnetic and Toxicity Studies of Ferrite Particles Employed as Contrast Agents for Magnetic Resonance Imaging Thermometry. J. Magn. Magn. Mater. 2020, 497, 165981. [Google Scholar] [CrossRef]
- Ranga, R.; Kumar, A.; Kumari, P.; Singh, P.; Madaan, V.; Kumar, K. Ferrite Application as an Electrochemical Sensor: A Review. Mater. Charact. 2021, 178, 111269. [Google Scholar] [CrossRef]
- Cheng, R.; Yin, L.; Wen, Y.; Zhai, B.; Guo, Y.; Zhang, Z.; Liao, W.; Xiong, W.; Wang, H.; Yuan, S.; et al. Ultrathin Ferrite Nanosheets for Room-Temperature Two-Dimensional Magnetic Semiconductors. Nat. Commun. 2022, 13, 5241. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Taneja, S.; Chahar, D.; Ravelo, B.; Thakur, A. Recent Advances on Synthesis, Characterization and High Frequency Applications of Ni-Zn Ferrite Nanoparticles. J. Magn. Magn. Mater. 2021, 530, 167925. [Google Scholar] [CrossRef]
- Dumitru, I.; Caltun, O.F. Ferrites Use in Magnetic Recording. In Ferrite Nanostructured Magnetic Materials: Technologies and Applications; Woodhead Publishing: Sawston, UK, 2023; pp. 733–745. ISBN 9780128237175. [Google Scholar]
- Dubey, S.; Vedrtnam, A. A Review of Hexagonal and Spinel Ferrites for Data Storage Applications. Invertis J. Sci. Technol. 2022, 15, 14–40. [Google Scholar] [CrossRef]
- Ansari, S.M.; Bhor, R.D.; Pai, K.R.; Mazumder, S.; Sen, D.; Kolekar, Y.D.; Ramana, C.V. Size and Chemistry Controlled Cobalt-Ferrite Nanoparticles and Their Anti-Proliferative Effect against the MCF-7 Breast Cancer Cells. ACS Biomater. Sci. Eng. 2016, 2, 2139–2152. [Google Scholar] [CrossRef] [PubMed]
- Hathout, A.S.; Aljawish, A.; Sabry, B.A.; El-Nekeety, A.A.; Roby, M.H.; Deraz, N.M.; Aly, S.E.; Abdel-Wahhab, M.A. Synthesis and Characterization of Cobalt Ferrites Nanoparticles with Cytotoxic and Antimicrobial Properties. J. Appl. Pharm. Sci. 2017, 7, 86–92. [Google Scholar] [CrossRef]
- Balakrishnan, P.B.; Silvestri, N.; Fernandez-Cabada, T.; Marinaro, F.; Fernandes, S.; Fiorito, S.; Miscuglio, M.; Serantes, D.; Ruta, S.; Livesey, K.; et al. Exploiting Unique Alignment of Cobalt Ferrite Nanoparticles, Mild Hyperthermia, and Controlled Intrinsic Cobalt Toxicity for Cancer Therapy. Adv. Mater. 2020, 32, 2003712. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Banerjee, J.; Chakravarty, S.; Samanta, S.; Nath, M.; Chattopadhyay, S.; Sarkar, S.; Mitra Banerjee, S.; Chowdhury, S.; Dash, S.K.; et al. Exploration of Structural and Magnetic Aspects of Biocompatible Cobalt Ferrite Nanoparticles with Canted Spin Configuration and Assessment of Their Selective Anti-Leukemic Efficacy. J. Magn. Magn. Mater. 2022, 563, 169957. [Google Scholar] [CrossRef]
- Chakraborty, S.; Menon, D.; Akhil Varri, V.S.; Sahoo, M.; Ranganathan, R.; Zhang, P.; Misra, S.K. Does the Doping Strategy of Ferrite Nanoparticles Create a Correlation between Reactivity and Toxicity? Environ. Sci. Nano 2023, 10, 1553–1569. [Google Scholar] [CrossRef]
- Cadar, O.; Dippong, T.; Senila, M.; Levei, E.-A. Progress, Challenges and Opportunities in Divalent Transition Metal-Doped Cobalt Ferrites Nanoparticles Applications. In Advanced Functional Materials; IntechOpen: London, UK, 2020; ISBN 978-1-83962-480-3. [Google Scholar]
- Kumar, H.; Singh, J.P.; Srivastava, R.C.; Negi, P.; Agrawal, H.M.; Asokan, K. FTIR and Electrical Study of Dysprosium Doped Cobalt Ferrite Nanoparticles. J. Nanosci. 2014, 2014, 862415. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Sertkol, M.; Khan, F.A.; Nawaz, M.; Tombuloglu, H.; Al-Suhaimi, E.A.; Baykal, A. Ce–Nd Co-Substituted Nanospinel Cobalt Ferrites: An Investigation of Their Structural, Magnetic, Optical, and Apoptotic Properties. Ceram. Int. 2019, 45, 16147–16156. [Google Scholar] [CrossRef]
- Kumar, H.; Srivastava, R.C.; Pal Singh, J.; Negi, P.; Agrawal, H.M.; Das, D.; Hwa Chae, K. Structural and Magnetic Study of Dysprosium Substituted Cobalt Ferrite Nanoparticles. J. Magn. Magn. Mater. 2016, 401, 16–21. [Google Scholar] [CrossRef]
- Aziz, C.; Azhdar, B. Synthesis of Dysprosium Doped Cobalt Ferrites Nanoparticles by Solgel Auto-Combustion Method and Influence of Grinding Techniques on Structural, Morphological, and Magnetic Properties. J. Magn. Magn. Mater. 2022, 542, 168577. [Google Scholar] [CrossRef]
- Varalakshmi, G.S.; Pawar, C.S.; Manikantan, V.; Pillai, A.S.; Alexander, A.; Akash, B.A.; Prasad, N.R.; Enoch, I.V.M.V. Dysprosium-Containing Cobalt Sulfide Nanoparticles as Anticancer Drug Carriers. Curr. Drug Deliv. 2023, 21–22. [Google Scholar] [CrossRef]
- Sri Varalakshmi, G.; Pawar, C.; Selvam, R.; Gem Pearl, W.; Manikantan, V.; Sumohan Pillai, A.; Alexander, A.; Rajendra Prasad, N.; Enoch, I.V.M.V.; Dhanaraj, P. Nickel Sulfide and Dysprosium-Doped Nickel Sulfide Nanoparticles: Dysprosium-Induced Variation in Properties, in Vitro Chemo-Photothermal Behavior, and Antibacterial Activity. Int. J. Pharm. 2023, 643, 123282. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.S.; Havlica, J.; Kuřitka, I.; Kozakova, Z.; Palou, M.; Bartoníčková, E.; Boháč, M.; Frajkorová, F.; Masilko, J.; Kalina, L.; et al. Magnetic Properties of Dysprosium-Doped Cobalt Ferrite Nanoparticles Synthesized by Starch-Assisted Sol-Gel Auto-Combustion Method. J. Supercond. Nov. Magn. 2015, 28, 2097–2107. [Google Scholar] [CrossRef]
- Mota, L.R.; Motta, L.J.; Duarte, I.D.S.; Horliana, A.C.R.T.; Silva, D.D.F.T.D.; Pavani, C. Efficacy of Phototherapy to Treat Facial Ageing When Using a Red versus an Amber LED: A Protocol for a Randomised Controlled Trial. BMJ Open 2018, 8, e021419. [Google Scholar] [CrossRef] [PubMed]
- Wunsch, A.; Matuschka, K. A Controlled Trial to Determine the Efficacy of Red and Near-Infrared Light Treatment in Patient Satisfaction, Reduction of Fine Lines, Wrinkles, Skin Roughness, and Intradermal Collagen Density Increase. Photomed. Laser Surg. 2014, 32, 93–100. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Hamblin, M.R.; Abrahamse, H. Effect of Red Light and near Infrared Laser on the Generation of Reactive Oxygen Species in Primary Dermal Fibroblasts. J. Photochem. Photobiol. B Biol. 2018, 188, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Austin, E.; Huang, A.; Wang, J.Y.; Cohen, M.; Heilman, E.; Maverakis, E.; Michl, J.; Jagdeo, J. Red Light Phototherapy Using Light-Emitting Diodes Inhibits Melanoma Proliferation and Alters Tumor Microenvironments. Front. Oncol. 2022, 12, 928484. [Google Scholar] [CrossRef] [PubMed]
- Gyanani, V.; Haley, J.C.; Goswami, R. Challenges of Current Anticancer Treatment Approaches with Focus on Liposomal Drug Delivery Systems. Pharmaceuticals 2021, 14, 835. [Google Scholar] [CrossRef]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current Trends and Challenges in Cancer Management and Therapy Using Designer Nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Maaz, K.; Mumtaz, A.; Hasanain, S.K.; Ceylan, A. Synthesis and Magnetic Properties of Cobalt Ferrite (CoFe2O4) Nanoparticles Prepared by Wet Chemical Route. J. Magn. Magn. Mater. 2007, 308, 289–295. [Google Scholar] [CrossRef]
- Srinivasan, S.Y.; Paknikar, K.M.; Bodas, D.; Gajbhiye, V. Applications of Cobalt Ferrite Nanoparticles in Biomedical Nanotechnology. Nanomedicine 2018, 13, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Han, J.T.; Huang, K.J.; Li, J.; Liu, Y.M.; Yu, M. β-Cyclodextrin-Cobalt Ferrite Nanocomposite as Enhanced Sensing Platform for Catechol Determination. Colloids Surf. B Biointerfaces 2012, 98, 58–62. [Google Scholar] [CrossRef]
- Chen, P.; Song, H.; Yao, S.; Tu, X.; Su, M.; Zhou, L. Magnetic Targeted Nanoparticles Based on β-Cyclodextrin and Chitosan for Hydrophobic Drug Delivery and a Study of Their Mechanism. RSC Adv. 2017, 7, 29025–29034. [Google Scholar] [CrossRef]
- Muankaew, C.; Loftsson, T. Cyclodextrin-Based Formulations: A Non-Invasive Platform for Targeted Drug Delivery. Basic Clin. Pharmacol. Toxicol. 2018, 122, 46–55. [Google Scholar] [CrossRef]
- Lin, Q.; He, Y.; Lin, J.; Yang, F.; Wang, L.; Dong, J. Structural and Magnetic Studies of Mg Substituted Cobalt Composite Oxide Catalyst Co1−xMgxFe2O4. J. Magn. Magn. Mater. 2019, 469, 89–94. [Google Scholar] [CrossRef]
- Hadela, A.; Lakić, M.; Potočnik, M.; Košak, A.; Gutmaher, A.; Lobnik, A. Novel Reusable Functionalized Magnetic Cobalt Ferrite Nanoparticles as Oil Adsorbents. Adsorpt. Sci. Technol. 2020, 38, 168–190. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Zeng, Y.; Wang, G.; Han, S.; Yang, Z.; Li, B.; Wang, X.; Gao, J.; Zheng, L.; et al. Leucine-Coated Cobalt Ferrite Nanoparticles: Synthesis, Characterization and Potential Biomedical Applications for Drug Delivery. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2020, 384, 126600. [Google Scholar] [CrossRef]
- Zorai, A.; Souici, A.; Dragoe, D.; Rivière, E.; Ouhenia, S.; Belloni, J.; Mostafavi, M. Superparamagnetic Cobalt Ferrite Nanoparticles Synthesized by Gamma Irradiation. New J. Chem. 2022, 47, 2626–2634. [Google Scholar] [CrossRef]
- Sangeetha, K.; Ashok, M.; Girija, E.K. Development of Multifunctional Cobalt Ferrite/Hydroxyapatite Nanocomposites by Microwave Assisted Wet Precipitation Method: A Promising Platform for Synergistic Chemo-Hyperthermia Therapy. Ceram. Int. 2019, 45, 12860–12869. [Google Scholar] [CrossRef]
- Li, X.H.; Xu, C.L.; Han, X.H.; Qiao, L.; Wang, T.; Li, F.S. Synthesis and Magnetic Properties of Nearly Monodisperse CoFe2O4 Nanoparticles through a Simple Hydrothermal Condition. Nanoscale Res. Lett. 2010, 5, 1039–1044. [Google Scholar] [CrossRef]
- Panda, J.; Das, S.; Kumar, S.; Tudu, B.; Sarkar, R. Investigation of Antibacterial, Antioxidant, and Anticancer Properties of Hydrothermally Synthesized Cobalt Ferrite Nanoparticles. Appl. Phys. A Mater. Sci. Process. 2022, 128, 562. [Google Scholar] [CrossRef]
- Prabhakaran, T.; Hemalatha, J. Combustion Synthesis and Characterization of Cobalt Ferrite Nanoparticles. Ceram. Int. 2016, 42, 14113–14120. [Google Scholar] [CrossRef]
- Pop, D.; Buzatu, R.; Moacă, E.A.; Watz, C.G.; Cîntă-Pînzaru, S.; Tudoran, L.B.; Nekvapil, F.; Avram, Ș.; Dehelean, C.A.; Crețu, M.O.; et al. Development and Characterization of Fe3O4@carbon Nanoparticles and Their Biological Screening Related to Oral Administration. Materials 2021, 14, 3556. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhou, C.; Qin, H.; Hu, J. High-Performance Acetone Gas Sensor Based on Ferrite–DyFeO3. J. Mater. Sci. 2020, 55, 16300–16310. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, H.; Xi, X.; Luo, B.; Zhu, P.; Li, G.; Li, B.; Zhou, J. Tuning Absorption of Terahertz Dielectric Ceramics through Spin-Reorientation Transitions. Ceram. Int. 2022, 48, 16273–16279. [Google Scholar] [CrossRef]
- Xavier, S.; Sebastian, R.M.; Mohammed, E.M. Effect of Dysprosium Substitution on Structural and Magnetic Properties of Cobalt Ferrite Nanoparticles. Certif. Int. J. Eng. Sci. Innov. Technol. 2008, 9001, 2319–5967. [Google Scholar]
- Zhao, L.; Yang, H.; Zhao, X.; Yu, L.; Cui, Y.; Feng, S. Magnetic Properties of CoFe2O4 Ferrite Doped with Rare Earth Ion. Mater. Lett. 2006, 60, 1–6. [Google Scholar] [CrossRef]
- Javed, F.; Abbas, M.A.; Asad, M.I.; Ahmed, N.; Naseer, N.; Saleem, H.; Errachid, A.; Lebaz, N.; Elaissari, A.; Ahmad, N.M. Gd3+ Doped Cofe2o4 Nanoparticles for Targeted Drug Delivery and Magnetic Resonance Imaging. Magnetochemistry 2021, 7, 47. [Google Scholar] [CrossRef]
- Khizar, S.; Ahmad, N.M.; Ahmed, N.; Manzoor, S.; Hamayun, M.A.; Naseer, N.; Tenório, M.K.L.; Lebaz, N.; Elaissari, A. Aminodextran Coated CoFe2O4 Nanoparticles for Combined Magnetic Resonance Imaging and Hyperthermia. Nanomaterials 2020, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Tamhankar, P.M.; Kulkarni, A.M.; Watawe, S.C. Functionalization of Cobalt Ferrite Nanoparticles with Alginate Coating for Biocompatible Applications. Mater. Sci. Appl. 2011, 02, 1317–1321. [Google Scholar] [CrossRef]
- Allaedini, G.; Tasirin, S.M.; Aminayi, P. Magnetic Properties of Cobalt Ferrite Synthesized by Hydrothermal Method. Int. Nano Lett. 2015, 5, 183–186. [Google Scholar] [CrossRef]
- Ansari, M.M.N.; Khan, S.; Ahmad, N. Influence of Dy3+ and Cu Substitution on the Structural, Electrical and Dielectric Properties of CoFe2O4 Nanoferrites. J. Mater. Sci. Mater. Electron. 2019, 30, 17630–17642. [Google Scholar] [CrossRef]
- Dabagh, S.; Dini, G. Synthesis of Silica-Coated Silver-Cobalt Ferrite Nanoparticles for Biomedical Applications. J. Supercond. Nov. Magn. 2019, 32, 3865–3872. [Google Scholar] [CrossRef]
- Guan, D.; Zhong, J.; Xu, H.; Huang, Y.C.; Hu, Z.; Chen, B.; Zhang, Y.; Ni, M.; Xu, X.; Zhou, W.; et al. A Universal Chemical-Induced Tensile Strain Tuning Strategy to Boost Oxygen-Evolving Electrocatalysis on Perovskite Oxides. Appl. Phys. Rev. 2022, 9, 011422. [Google Scholar] [CrossRef]
- Abbas, N.; Rubab, N.; Sadiq, N.; Manzoor, S.; Khan, M.I.; Garcia, J.F.; Aragao, I.B.; Tariq, M.; Akhtar, Z.; Yasmin, G. Aluminum-Doped Cobalt Ferrite as an Efficient Photocatalyst for the Abatement of Methylene Blue. Water 2020, 12, 2285. [Google Scholar] [CrossRef]
- Han, G.; Li, M.; Yu, Y.; Yang, W.; Li, J.; Xu, S.; Feng, M.; Li, H. Structure and Magnetic Properties of Cobalt Ferrite Foam with Low Mass Density. J. Alloys Compd. 2019, 790, 947–954. [Google Scholar] [CrossRef]
- Lin, Q.; Lin, J.; He, Y.; Wang, R.; Dong, J. The Structural and Magnetic Properties of Gadolinium Doped CoFe2O4 Nanoferrites. J. Nanomater. 2015, 2015, 294239. [Google Scholar] [CrossRef]
- Dixit, G.; Pal Singh, J.; Srivastava, R.C.; Agrawal, H.M. Magnetic Resonance Study of Ce and Gd Doped NiFe2O4 Nanoparticles. J. Magn. Magn. Mater. 2012, 324, 479–483. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 558493. [Google Scholar] [CrossRef]
- Tang, L.; Yang, X.; Yin, Q.; Cai, K.; Wang, H.; Chaudhury, I.; Yao, C.; Zhou, Q.; Kwon, M.; Hartman, J.A.; et al. Investigating the Optimal Size of Anticancer Nanomedicine. Proc. Natl. Acad. Sci. USA 2014, 111, 15344–15349. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Song, M.; Fang, Z.; Zheng, L.; Huang, X.; Liu, K. Applications and Challenges of Ultra-Small Particle Size Nanoparticles in Tumor Therapy. J. Control. Release 2023, 353, 699–712. [Google Scholar] [CrossRef]
- Abudayyak, M.; Altinçekiç Gürkaynak, T.; Özhan, G. Kobalt Ferrit Nanopartiküllerinin Böbrek Hücresi Üzerine Güvenliğinin in Vitro Değerlendirmesi. Turkish J. Pharm. Sci. 2017, 14, 169–173. [Google Scholar] [CrossRef]
- Oliveira, A.B.B.; De Moraes, F.R.; Candido, N.M.; Sampaio, I.; Paula, A.S.; De Vasconcellos, A.; Silva, T.C.; Miller, A.H.; Rahal, P.; Nery, J.G.; et al. Metabolic Effects of Cobalt Ferrite Nanoparticles on Cervical Carcinoma Cells and Nontumorigenic Keratinocytes. J. Proteome Res. 2016, 15, 4337–4348. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, X.; Li, H.; Dong, D.; Zuo, X.; Wu, C. wei Magnetic Nanoparticles with Low Curie Temperature and High Heating Efficiency for Self-Regulating Temperature Hyperthermia. J. Magn. Magn. Mater. 2019, 489, 165382. [Google Scholar] [CrossRef]
- Ayuk, S.M.; Houreld, N.N.; Abrahamse, H. Effect of 660 Nm Visible Red Light on Cell Proliferation and Viability in Diabetic Models in Vitro under Stressed Conditions. Lasers Med. Sci. 2018, 33, 1085–1093. [Google Scholar] [CrossRef]
- Panda, J.; Satapathy, B.S.; Mandal, B.; Sen, R.; Mukherjee, B.; Sarkar, R.; Tudu, B. Anticancer Potential of Docetaxel-Loaded Cobalt Ferrite Nanocarrier: An In Vitro Study on MCF-7 and MDA-MB-231 Cell Lines. J. Microencapsul. 2021, 38, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Eshrati Yeganeh, F.; Eshrati Yeganeh, A.; Fatemizadeh, M.; Farasati Far, B.; Quazi, S.; Safdar, M. In Vitro Cytotoxicity and Anti-Cancer Drug Release Behavior of Methionine-Coated Magnetite Nanoparticles as Carriers. Med. Oncol. 2022, 39, 252. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Monier, B.; Suzanne, M. Orchestration of Force Generation and Nuclear Collapse in Apoptotic Cells. Int. J. Mol. Sci. 2021, 22, 10257. [Google Scholar] [CrossRef]
- Sundram, S.; Baskar, S.; Subramanian, A. Green Synthesized Nickel Doped Cobalt Ferrite Nanoparticles Exhibit Antibacterial Activity and Induce Reactive Oxygen Species Mediated Apoptosis in MCF-7 Breast Cancer Cells through Inhibition of PI3K/Akt/MTOR Pathway. Environ. Toxicol. 2022, 37, 2877–2888. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Rehman, S.; Khan, F.A.; Güngüneş, D.; Güner, S.; Shirsath, S.E.; Baykal, A. Magnetic Properties, Anticancer and Antibacterial Effectiveness of Sonochemically Produced Ce3+/Dy3+ Co-Activated Mn-Zn Nanospinel Ferrites. Arab. J. Chem. 2020, 13, 7403–7417. [Google Scholar] [CrossRef]
- Horev-Azaria, L.; Baldi, G.; Beno, D.; Bonacchi, D.; Golla-Schindler, U.; Kirkpatrick, J.C.; Kolle, S.; Landsiedel, R.; Maimon, O.; Marche, P.N.; et al. Predictive Toxicology of Cobalt Ferrite Nanoparticles: Comparative in-Vitro Study of Different Cellular Models Using Methods of Knowledge Discovery from Data. Part. Fibre Toxicol. 2013, 10, 32. [Google Scholar] [CrossRef]
- Kanagesan, S.; Hashim, M.; Tamilselvan, S.; Alitheen, N.B.; Ismail, I.; Syazwan, M.; Zuikimi, M.M.M. Sol-Gel Auto-Combustion Synthesis of Cobalt Ferrite and It’s Cytotoxicity Properties. Dig. J. Nanomater. Biostructures 2013, 8, 1601–1610. [Google Scholar]
- Li, P.; Zhou, L.; Zhao, T.; Liu, X.; Zhang, P.; Liu, Y.; Zheng, X.; Li, Q. Caspase-9: Structure, Mechanisms and Clinical Application. Oncotarget 2017, 8, 23996–24008. [Google Scholar] [CrossRef]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. P53 Signaling in Cancer Progression and Therapy. Cancer Cell Int. 2021, 21, 703. [Google Scholar] [CrossRef] [PubMed]
- Al-Qubaisi, M.S.; Rasedee, A.; Flaifel, M.H.; Ahmad, S.H.; Hussein-Al-Ali, S.; Hussein, M.Z.; Zainal, Z.; Alhassan, F.H.; Taufiq-Yap, Y.H.; Eid, E.E.M.; et al. Induction of Apoptosis in Cancer Cells by NiZn Ferrite Nanoparticles through Mitochondrial Cytochrome C Release. Int. J. Nanomed. 2013, 8, 4115–4130. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Akhtar, M.J.; Siddiqui, M.A.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; AlSalhi, M.S.; Alrokayan, S.A. Oxidative Stress Mediated Apoptosis Induced by Nickel Ferrite Nanoparticles in Cultured A549 Cells. Toxicology 2011, 283, 101–108. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Alhadlaq, H.A.; Khan, M.A.M.; Alrokayan, S.A. Comparative Cytotoxic Response of Nickel Ferrite Nanoparticles in Human Liver HepG2 and Breast MFC-7 Cancer Cells. Chemosphere 2015, 135, 278–288. [Google Scholar] [CrossRef]
- Alao, J.P. The Regulation of Cyclin D1 Degradation: Roles in Cancer Development and the Potential for Therapeutic Invention. Mol. Cancer 2007, 6, 24. [Google Scholar] [CrossRef]
- Vishnubhakthula, S.; Elupula, R.; Durán-Lara, E.F. Recent Advances in Hydrogel-Based Drug Delivery for Melanoma Cancer Therapy: A Mini Review. J. Drug Deliv. 2017, 2017, 7275985. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, Y.; Liu, X.L.; Ma, H.; Li, G.; Li, Y.; Gao, F.; Peng, M.; Fan, H.M.; Liang, X.J. Fe3O4-Pd Janus Nanoparticles with Amplified Dual-Mode Hyperthermia and Enhanced ROS Generation for Breast Cancer Treatment. Nanoscale Horiz. 2019, 4, 1450–1459. [Google Scholar] [CrossRef]
- Ocampo Osorio, F.; Augusto Jativa Herrera, J.; Moscoso Londoño, O.; Leandro Londoño Calderón, C. Nanoferrites-Based Drug Delivery Systems as Adjuvant Therapy for Cancer Treatments. Current Challenges and Future Perspectives. In Ferrites—Synthesis and Applications; IntechOpen: London, UK, 2021; ISBN 978-1-83962-887-0. [Google Scholar]



| Sample | DyCl3·6H2O (Moles) | Fe(NO3)3·9H2O (Moles) |
|---|---|---|
| FeCo1 | 0.002 | 0.038 |
| FeCo2 | 0.004 | 0.036 |
| FeCo3 | 0.008 | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotunjanu, S.; Racoviceanu, R.; Mioc, A.; Milan, A.; Negrea-Ghiulai, R.; Mioc, M.; Marangoci, N.L.; Şoica, C. Newly Synthesized CoFe2−xDyxO4 (x = 0; 0.1; 0.2; 0.4) Nanoparticles Reveal Promising Anticancer Activity against Melanoma (A375) and Breast Cancer (MCF-7) Cells. Int. J. Mol. Sci. 2023, 24, 15733. https://doi.org/10.3390/ijms242115733
Rotunjanu S, Racoviceanu R, Mioc A, Milan A, Negrea-Ghiulai R, Mioc M, Marangoci NL, Şoica C. Newly Synthesized CoFe2−xDyxO4 (x = 0; 0.1; 0.2; 0.4) Nanoparticles Reveal Promising Anticancer Activity against Melanoma (A375) and Breast Cancer (MCF-7) Cells. International Journal of Molecular Sciences. 2023; 24(21):15733. https://doi.org/10.3390/ijms242115733
Chicago/Turabian StyleRotunjanu, Slaviţa, Roxana Racoviceanu, Alexandra Mioc, Andreea Milan, Roxana Negrea-Ghiulai, Marius Mioc, Narcisa Laura Marangoci, and Codruţa Şoica. 2023. "Newly Synthesized CoFe2−xDyxO4 (x = 0; 0.1; 0.2; 0.4) Nanoparticles Reveal Promising Anticancer Activity against Melanoma (A375) and Breast Cancer (MCF-7) Cells" International Journal of Molecular Sciences 24, no. 21: 15733. https://doi.org/10.3390/ijms242115733
APA StyleRotunjanu, S., Racoviceanu, R., Mioc, A., Milan, A., Negrea-Ghiulai, R., Mioc, M., Marangoci, N. L., & Şoica, C. (2023). Newly Synthesized CoFe2−xDyxO4 (x = 0; 0.1; 0.2; 0.4) Nanoparticles Reveal Promising Anticancer Activity against Melanoma (A375) and Breast Cancer (MCF-7) Cells. International Journal of Molecular Sciences, 24(21), 15733. https://doi.org/10.3390/ijms242115733



.jpg)



