Transmembrane Helices 7 and 8 Confer Aggregation Sensitivity to the Cystic Fibrosis Transmembrane Conductance Regulator
Abstract
:1. Introduction
2. Results
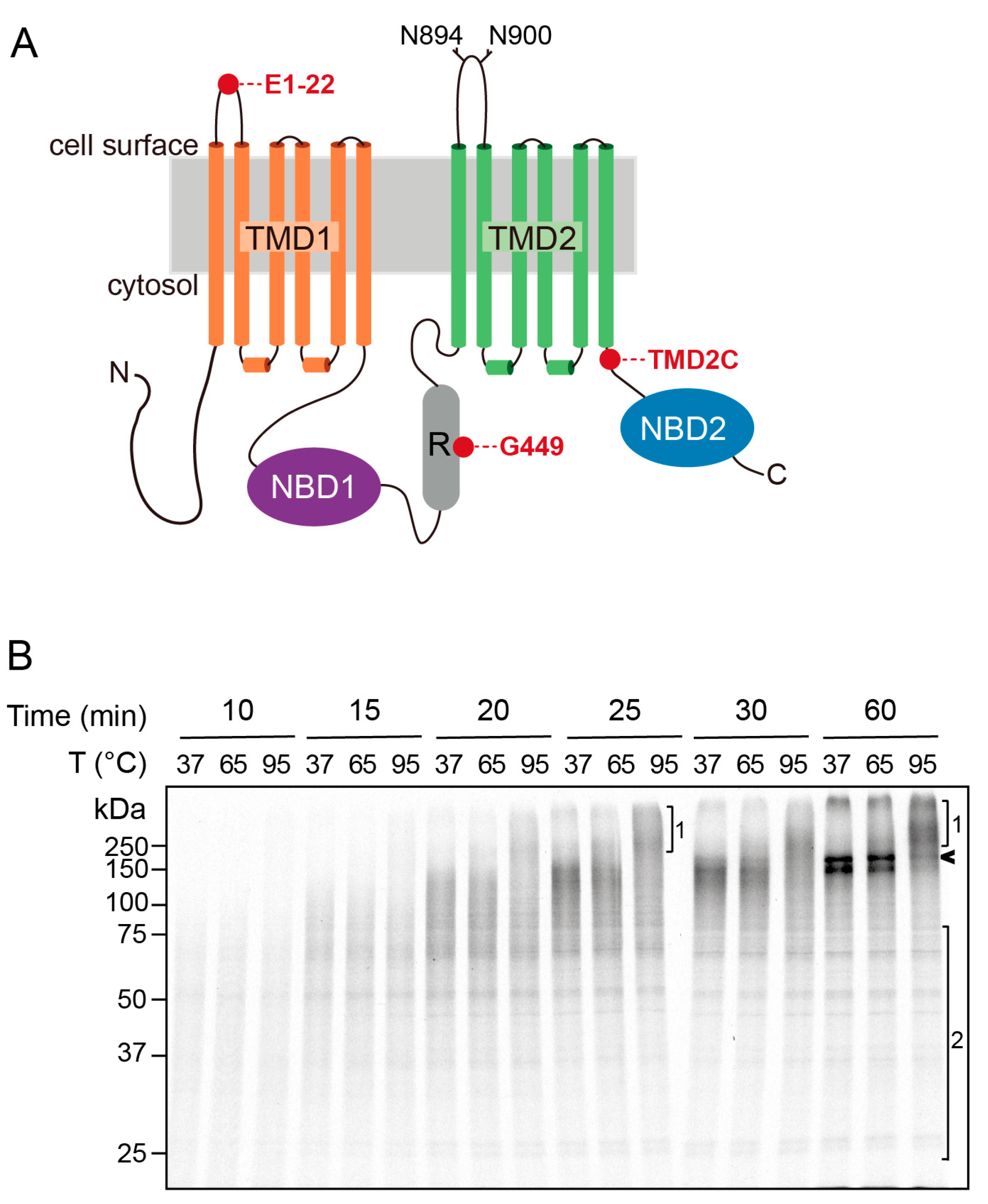
2.1. Aggregation Sensitivity of CFTR Nascent Chains
2.2. Aggregation Propensity Is Increased When TMD2 Is Present in the Nascent Chain
2.3. Aggregation Propensity of TMD1 and TMD2 Is Independent of Other Domains in CFTR
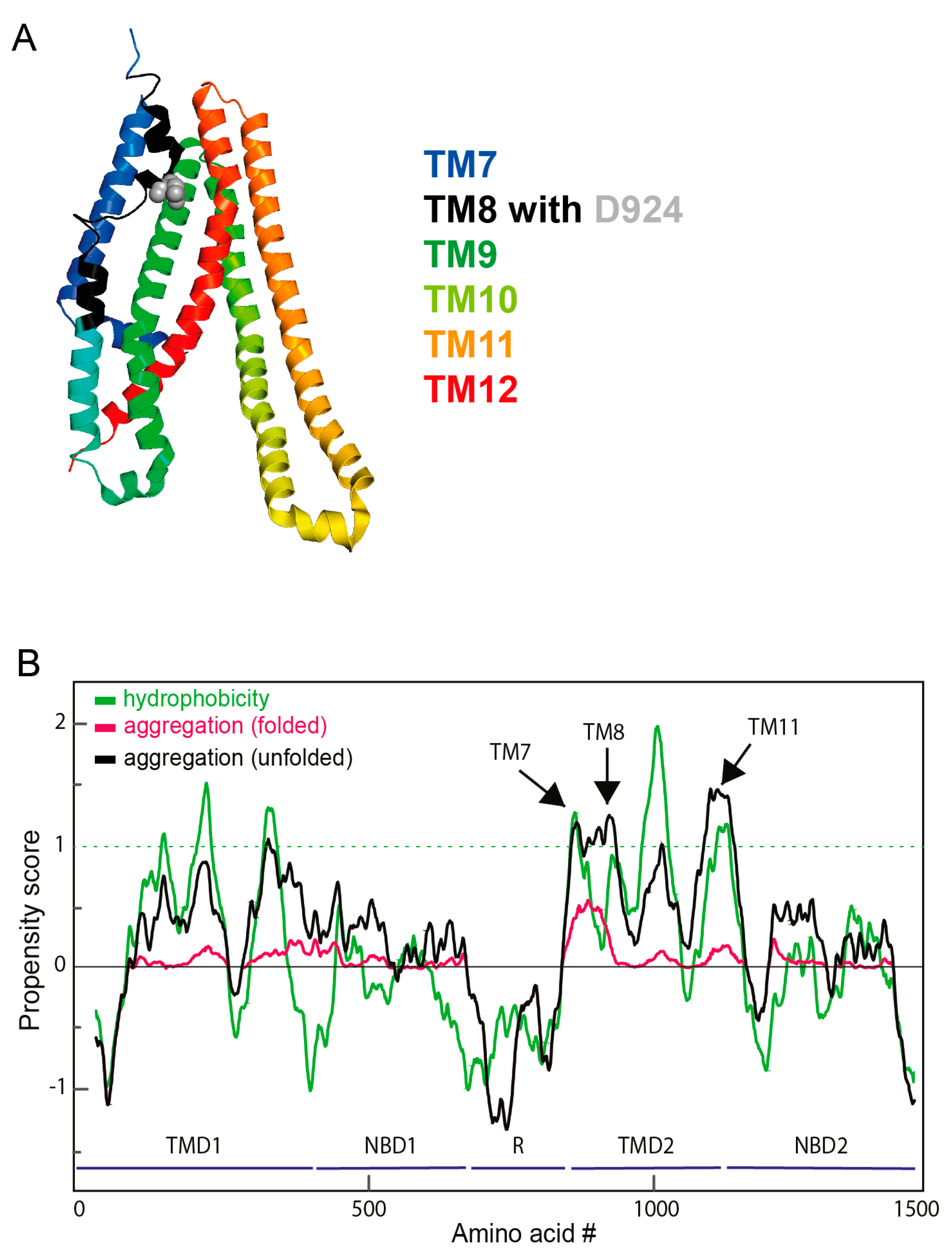
2.4. Identification of Protease-Resistant Aggregation-Prone R-TMD2 Fragments
2.5. Aggregation Propensity Is Distributed along the Entire TMD2 Sequence
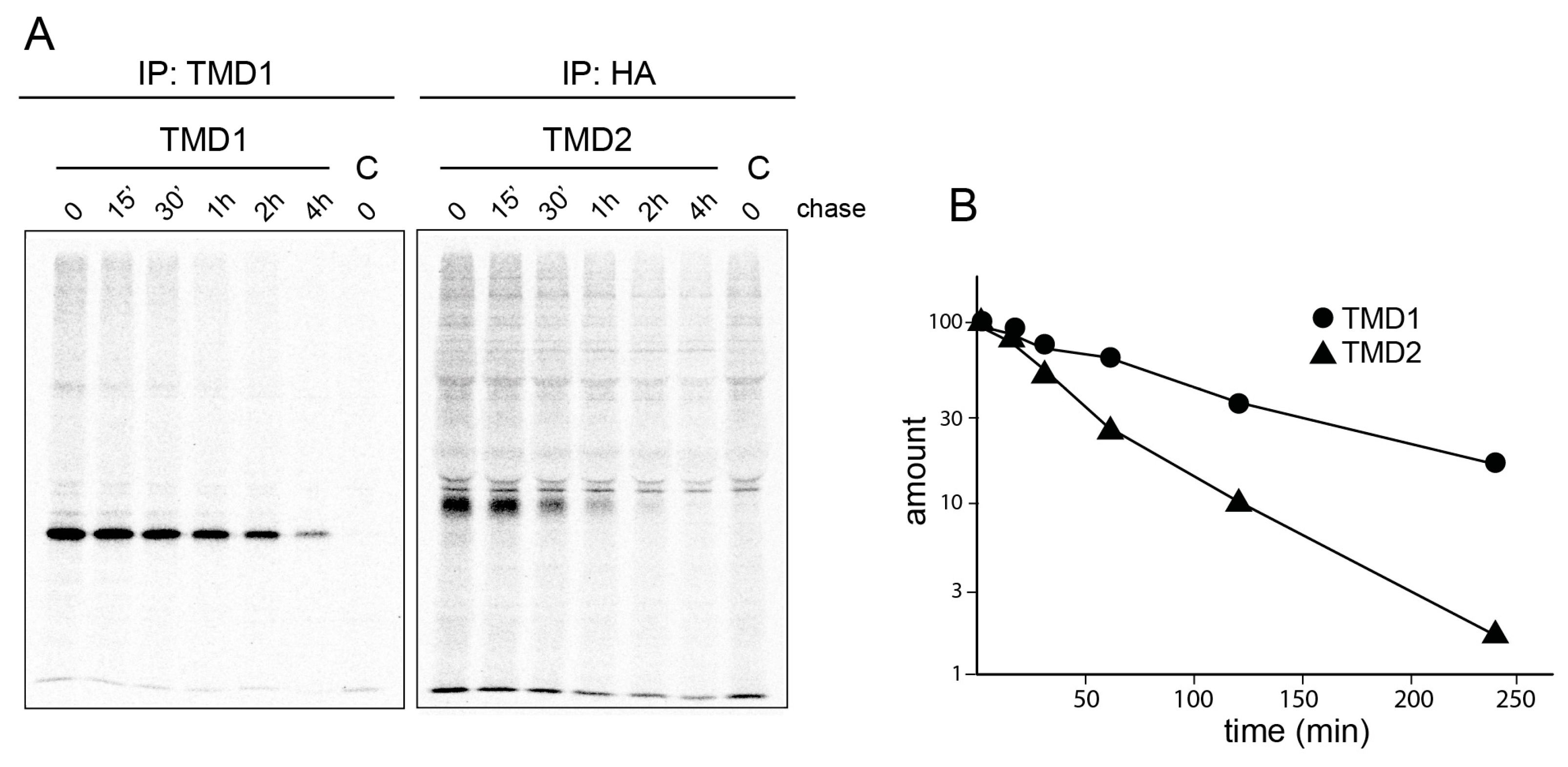
2.6. TMD2 Has a Higher Turnover Rate and Is Less Stable than TMD1 In Vivo
3. Discussion
4. Material and Methods
4.1. Cell Lines and Transfection
4.2. Expression Constructs and Antibodies
4.3. Preparation of Semi-Permeabilized HT1080 Cells
4.4. In Vitro Translocation–Translocation
4.5. Radioactive Pulse Chase Assay
4.6. Limited Proteolysis
4.7. Immunoprecipitation
4.8. Endoglycosidase H Treatment
4.9. Thermal Aggregation Assay
4.10. Quantitation and Statistical Analysis
4.11. Prediction of Aggregation-Prone Sequences
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, R.; Ursu, O.; Gaulton, A.; Bento, A.P.; Donadi, R.S.; Bologa, C.G.; Karlsson, A.; Al-Lazikani, B.; Hersey, A.; Oprea, T.I.; et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017, 16, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Peterson, S.M.; Müller, S.; Reichelt, M.; Amador, C.M.; Martinez-Martin, N. A membrane protein display platform for receptor interactome discovery. Proc. Natl. Acad. Sci. USA 2021, 118, e2025451118. [Google Scholar] [CrossRef] [PubMed]
- Marinko, J.T.; Huang, H.; Penn, W.D.; Capra, J.A.; Schlebach, J.P.; Sanders, C.R. Folding and Misfolding of Human Membrane Proteins in Health and Disease: From Single Molecules to Cellular Proteostasis. Chem. Rev. 2019, 119, 5537–5606. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, H.H.; Russell, D.W.; Brown, M.S.; Goldstein, J.L. The LDL receptor locus in familial hypercholesterolemia: Mutational analysis of a membrane protein. Annu. Rev. Genet. 1990, 24, 133–170. [Google Scholar] [CrossRef] [PubMed]
- Mulders, S.M.; Bichet, D.G.; Rijss, J.P.; Kamsteeg, E.J.; Arthus, M.F.; Lonergan, M.; Fujiwara, M.; Morgan, K.; Leijendekker, R.; van der Sluijs, P.; et al. An aquaporin-2 water channel mutant which causes autosomal dominant nephrogenic diabetes insipidus is retained in the Golgi complex. J. Clin. Investig. 1998, 102, 57–66. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef]
- Riordan, J.R.; Rommens, J.M.; Kerem, B.-S.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J.-L.; et al. Identification of the Cystic Fibrosis Gene: Cloning and Characterization of Complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef]
- Ward, C.L.; Omura, S.; Kopito, R.R. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell 1995, 83, 121–127. [Google Scholar] [CrossRef]
- Jensen, T.J.; Loo, M.A.; Pind, S.; Williams, D.B.; Goldberg, A.L.; Riordan, J.R. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 1995, 83, 129–135. [Google Scholar] [CrossRef]
- Xia, D.; Qu, L.; Li, G.; Hongdu, B.; Xu, C.; Lin, X.; Lou, Y.; He, Q.; Ma, D.; Chen, Y. MARCH2 regulates autophagy by promoting CFTR ubiquitination and degradation and PIK3CA-AKT-MTOR signaling. Autophagy 2016, 12, 1614–1630. [Google Scholar] [CrossRef]
- He, L.; Kennedy, A.S.; Houck, S.; Aleksandrov, A.; Quinney, N.L.; Cyr-Scully, A.; Cholon, D.M.; Gentzsch, M.; Randell, S.H.; Ren, H.Y.; et al. DNAJB12 and Hsp70 triage arrested intermediates of N1303K-CFTR for endoplasmic reticulum-associated autophagy. Mol. Biol. Cell 2021, 32, 538–553. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.A.; Ward, C.L.; Kopito, R.R. Aggresomes: A Cellular Response to Misfolded Proteins. J. Cell Biol. 1998, 143, 1883–1898. [Google Scholar] [CrossRef] [PubMed]
- Wigley, W.C.; Fabunmi, R.P.; Lee, M.G.; Marino, C.R.; Muallem, S.; DeMartino, G.N.; Thomas, P.J. Dynamic Association of Proteasomal Machinery with the Centrosome. J. Cell Biol. 1999, 145, 481–490. [Google Scholar] [CrossRef]
- Varga, K.; Jurkuvenaite, A.; Wakefield, J.; Hong, J.S.; Guimbellot, J.S.; Venglarik, C.J.; Niraj, A.; Mazur, M.; Sorscher, E.J.; Collawn, J.F.; et al. Efficient Intracellular Processing of the Endogenous Cystic Fibrosis Transmembrane Conductance Regulator in Epithelial Cell Lines. J. Biol. Chem. 2004, 279, 22578–22584. [Google Scholar] [CrossRef]
- Kleizen, B.; van Willigen, M.; Mijnders, M.; Peters, F.; Grudniewska, M.; Hillenaar, T.; Thomas, A.; Kooijman, L.; Peters, K.W.; Frizzell, R.; et al. Co-Translational Folding of the First Transmembrane Domain of ABC-Transporter CFTR is Supported by Assembly with the First Cytosolic Domain. J. Mol. Biol. 2021, 433, 166955. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Benharouga, M.; Hu, W.; Lukacs, G.L. Conformational and temperature-sensitive stability defects of the ΔF508 cystic fibrosis transmembrane conductance regulator in post-endoplasmic reticulum compartments. J. Biol. Chem. 2001, 276, 8942–8950. [Google Scholar] [CrossRef]
- Zhang, L.; Aleksandrov, L.A.; Zhao, Z.; Birtley, J.R.; Riordan, J.R.; Ford, R.C. Architecture of the cystic fibrosis transmembrane conductance regulator protein and structural changes associated with phosphorylation and nucleotide binding. J. Struct. Biol. 2009, 167, 242–251. [Google Scholar] [CrossRef]
- Hildebrandt, E.; Zhang, Q.; Cant, N.; Ding, H.; Dai, Q.; Peng, L.; Fu, Y.; DeLucas, L.J.; Ford, R.; Kappes, J.C.; et al. A survey of detergents for the purification of stable, active human cystic fibrosis transmembrane conductance regulator (CFTR). Biochim. Biophys. Acta 2014, 1838, 2825–2837. [Google Scholar] [CrossRef]
- Aleksandrov, L.A.; Jensen, T.J.; Cui, L.; Kousouros, J.N.; He, L.; Aleksandrov, A.A.; Riordan, J.R. Thermal stability of purified and reconstituted CFTR in a locked open channel conformation. Protein Expr. Purif. 2015, 116, 159–166. [Google Scholar] [CrossRef]
- Meng, X.; Clews, J.; Kargas, V.; Wang, X.; Ford, R.C. The cystic fibrosis transmembrane conductance regulator (CFTR) and its stability. Cell. Mol. Life Sci. 2017, 74, 23–38. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J. Atomic Structure of the Cystic Fibrosis Transmembrane Conductance Regulator. Cell 2016, 167, 1586–1597.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, F.; Chen, J. Molecular structure of the ATP-bound, phosphorylated human CFTR. Proc. Natl. Acad. Sci. USA 2018, 115, 12757–12762. [Google Scholar] [CrossRef] [PubMed]
- Riordan, J.R. CFTR Function and Prospects for Therapy. Annu. Rev. Biochem. 2008, 77, 701–726. [Google Scholar] [CrossRef]
- Mendoza, J.L.; Schmidt, A.; Li, Q.; Nuvaga, E.; Barrett, T.; Bridges, R.J.; Feranchak, A.P.; Brautigam, C.A.; Thomas, P.J. Requirements for Efficient Correction of ΔF508 CFTR Revealed by Analyses of Evolved Sequences. Cell 2012, 148, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Rabeh, W.M.; Bossard, F.; Xu, H.; Okiyoneda, T.; Bagdany, M.; Mulvihill, C.M.; Du, K.; di Bernardo, S.; Liu, Y.; Konermann, L.; et al. Correction of Both NBD1 Energetics and Domain Interface Is Required to Restore ΔF508 CFTR Folding and Function. Cell 2012, 148, 150–163. [Google Scholar] [CrossRef]
- Im, J.; Hillenaar, T.; Yeoh, H.Y.; Sahasrabudhe, P.; Mijnders, M.; van Willigen, M.; Hagos, A.; de Mattos, E.; van der Sluijs, P.; Braakman, I. ABC-transporter CFTR folds with high fidelity through a modular, stepwise pathway. Cell. Mol. Life Sci. 2023, 80, 33. [Google Scholar] [CrossRef]
- Kleizen, B.; van Vlijmen, T.; de Jonge, H.R.; Braakman, I. Folding of CFTR Is Predominantly Cotranslational. Mol. Cell 2005, 20, 277–287. [Google Scholar] [CrossRef]
- Wilson, R.; Allen, A.J.; Oliver, J.; Brookman, J.L.; High, S.; Bulleid, N.J. The translocation, folding, assembly and redox-dependent degradation of secretory and membrane proteins in semi-permeabilized mammalian cells. Biochem. J. 1995, 307, 679–687. [Google Scholar] [CrossRef]
- Sagné, C.; Isambert, M.-F.; Henry, J.-P.; Gasnier, B. SDS-resistant aggregation of membrane proteins: Application to the purification of the vesicular monoamine transporter. Biochem. J. 1996, 316, 825–831. [Google Scholar] [CrossRef]
- Bullis, B.L.; Li, X.; Rieder, C.V.; Singh, D.N.; Berthiaume, L.G.; Fliegel, L. Properties of the Na+/H+ exchanger protein. Detergent-resistant aggregation and membrane microdistribution. Eur. J. Biochem. 2002, 269, 4887–4895. [Google Scholar] [CrossRef]
- Manley, D.M.; McComb, M.E.; Perreault, H.; Donald, L.J.; Duckworth, H.W.; O’Neil, J.D. Secondary structure and oligomerization of the E. coli glycerol facilitator. Biochemistry 2000, 39, 12303–12311. [Google Scholar] [CrossRef] [PubMed]
- Ostedgaard, L.S.; Rich, D.P.; DeBerg, L.G.; Welsh, M.J. Association of Domains within the Cystic Fibrosis Transmembrane Conductance Regulator. Biochemistry 1997, 36, 1287–1294. [Google Scholar] [CrossRef]
- Tanford, C.; Reynolds, J.A. Characterization of membrane proteins in detergent solutions. Biochim. Biophys. Acta 1976, 457, 133–170. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, S.J. The structural aspects of limited proteolysis of native proteins. Biochim. Biophys. Acta 1998, 1382, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; De Laureto, P.P.; Spolaore, B.; Frare, E.; Picotti, P.; Zambonin, M. Probing protein structure by limited proteolysis. Acta Biochim. Pol. 2004, 51, 299–321. [Google Scholar] [CrossRef] [PubMed]
- de Souza, N.; Picotti, P. Mass spectrometry analysis of the structural proteome. Curr. Opin. Struct. Biol. 2020, 60, 57–65. [Google Scholar] [CrossRef]
- Zhang, F.; Kartner, N.; Lukacs, G.L. Limited proteolysis as a probe for arrested conformational maturation of ΔF508 CFTR. Nat. Struct. Biol. 1998, 5, 180–183. [Google Scholar] [CrossRef]
- Du, K.; Sharma, M.; Lukacs, G.L. The ΔF508 cystic fibrosis mutation impairs domain-domain interactions and arrests post-translational folding of CFTR. Nat. Struct. Mol. Biol. 2005, 12, 17–25. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Cohn, J.; Bertuzzi, G.; Greengard, P.; Nairn, A. Phosphorylation of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 1992, 267, 12742–12752. [Google Scholar] [CrossRef]
- Hillenaar, T.; Beekman, J.; van der Sluijs, P.; Braakman, I. Redefining Hypo- and Hyper-Responding Phenotypes of CFTR Mutants for Understanding and Therapy. Int. J. Mol. Sci. 2022, 23, 15170. [Google Scholar] [CrossRef]
- Cui, L.; Aleksandrov, L.; Chang, X.-B.; Hou, Y.-X.; He, L.; Hegedus, T.; Gentzsch, M.; Aleksandrov, A.; Balch, W.E.; Riordan, J.R. Domain Interdependence in the Biosynthetic Assembly of CFTR. J. Mol. Biol. 2007, 365, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Loo, T.W.; Bartlett, M.C.; Clarke, D.M. Corrector VX-809 stabilizes the first transmembrane domain of CFTR. Biochem. Pharmacol. 2013, 86, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, Z.; Csanády, L.; Gadsby, D.C.; Chen, J. Molecular Structure of the Human CFTR Ion Channel. Cell 2017, 169, 85–95.e8. [Google Scholar] [CrossRef]
- Tartaglia, G.G.; Pawar, A.P.; Campioni, S.; Dobson, C.M.; Chiti, F.; Vendruscolo, M. Prediction of Aggregation-Prone Regions in Structured Proteins. J. Mol. Biol. 2008, 380, 425–436. [Google Scholar] [CrossRef]
- Tartaglia, G.G.; Vendruscolo, M. The Zyggregator method for predicting protein aggregation propensities. Chem. Soc. Rev. 2008, 37, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Bruley, A.; Mornon, J.-P.; Duprat, E.; Callebaut, I. Digging into the 3D Structure Predictions of AlphaFold2 with Low Confidence: Disorder and Beyond. Biomolecules 2022, 12, 1467. [Google Scholar] [CrossRef]
- Ruff, K.M.; Pappu, R.V. AlphaFold and Implications for Intrinsically Disordered Proteins. J. Mol. Biol. 2021, 433, 167208. [Google Scholar] [CrossRef]
- Sharma, M.; Pampinella, F.; Nemes, C.; Benharouga, M.; So, J.; Du, K.; Bache, K.G.; Papsin, B.; Zerangue, N.; Stenmark, H.; et al. Misfolding diverts CFTR from recycling to degradation: Quality control at early endosomes. J. Cell Biol. 2004, 164, 923–933. [Google Scholar] [CrossRef]
- Loo, T.W.; Bartlett, M.C.; Shi, L.; Clarke, D.M. Corrector-mediated rescue of misprocessed CFTR mutants can be reduced by the P-glycoprotein drug pump. Biochem. Pharmacol. 2012, 83, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Winogradoff, D.; John, S.; Aksimentiev, A. Protein unfolding by SDS: The microscopic mechanisms and the properties of the SDS-protein assembly. Nanoscale 2020, 12, 5422–5434. [Google Scholar] [CrossRef] [PubMed]
- Shusta, E.V.; Holler, P.D.; Kieke, M.C.; Kranz, D.M.; Wittrup, K. Directed evolution of a stable scaffold for T-cell receptor engineering. Nat. Biotechnol. 2000, 18, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Shusta, E.V.; Kieke, M.C.; Parke, E.; Kranz, D.M.; Wittrup, K. Yeast polypeptide fusion surface display levels predict thermal stability and soluble secretion efficiency. J. Mol. Biol. 1999, 292, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Milewski, M.I.; Mickle, J.E.; Forrest, J.K.; Stafford, D.M.; Moyer, B.D.; Cheng, J.; Guggino, W.B.; Stanton, B.A.; Cutting, G.R. A PDZ-binding motif is essential but not sufficient to localize the C terminus of CFTR to the apical membrane. J. Cell Sci. 2001, 114, 719–726. [Google Scholar] [CrossRef]
- Milewski, M.I.; Mickle, J.E.; Forrest, J.K.; Stanton, B.A.; Cutting, G.R. Aggregation of Misfolded Proteins Can Be a Selective Process Dependent upon Peptide Composition. J. Biol. Chem. 2002, 277, 34462–34470. [Google Scholar] [CrossRef]
- Bąk, D.; Cutting, G.R.; Milewski, M. The CFTR-derived peptides as a model of sequence-specific protein aggregation. Cell. Mol. Biol. Lett. 2007, 12, 435–447. [Google Scholar] [CrossRef]
- Tector, M.; Hartl, F.U. An unstable transmembrane segment in the cystic fibrosis transmembrane conductance regulator. EMBO J. 1999, 18, 6290–6298. [Google Scholar] [CrossRef]
- Vernon, R.M.; Chong, P.A.; Lin, H.; Yang, Z.; Zhou, Q.; Aleksandrov, A.A.; Dawson, J.E.; Riordan, J.R.; Brouillette, C.G.; Thibodeau, P.H.; et al. Stabilization of a nucleotide-binding domain of the cystic fibrosis transmembrane conductance regulator yields insight into disease-causing mutations. J. Biol. Chem. 2017, 292, 14147–14164. [Google Scholar] [CrossRef]
- Enquist, K.; Fransson, M.; Boekel, C.; Bengtsson, I.; Geiger, K.; Lang, L.; Pettersson, A.; Johansson, S.; von Heijne, G.; Nilsson, I. Membrane-integration Characteristics of Two ABC Transporters, CFTR and P-glycoprotein. J. Mol. Biol. 2009, 387, 1153–1164. [Google Scholar] [CrossRef]
- Hessa, T.; Meindl-Beinker, N.M.; Bernsel, A.; Kim, H.; Sato, Y.; Lerch-Bader, M.; Nilsson, I.; White, S.H.; von Heijne, G. Molecular code for transmembrane-helix recognition by the Sec61 translocon. Nature 2007, 450, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Ostedgaard, L.S.; Baldursson, O.; Vermeer, D.W.; Welsh, M.J.; Robertson, A.D. A functional R domain from cystic fibrosis transmembrane conductance regulator is predominantly unstructured in solution. Proc. Natl. Acad. Sci. USA 2000, 97, 5657–5662. [Google Scholar] [CrossRef] [PubMed]
- Fay, J.F.; Aleksandrov, L.A.; Jensen, T.J.; Cui, L.L.; Kousouros, J.N.; He, L.; Aleksandrov, A.A.; Gingerich, D.S.; Riordan, J.R.; Chen, J.Z. Cryo-EM Visualization of an Active High Open Probability CFTR Anion Channel. Biochemistry 2018, 57, 6234–6246. [Google Scholar] [CrossRef]
- Bozoky, Z.; Krzeminski, M.; Muhandiram, R.; Birtley, J.R.; Al-Zahrani, A.; Thomas, P.J.; Frizzell, R.A.; Ford, R.C.; Forman-Kay, J.D. Regulatory R region of the CFTR chloride channel is a dynamic integrator of phospho-dependent intra- and intermolecular interactions. Proc. Natl. Acad. Sci. USA 2013, 110, E4427–E4436. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.R.; Hudson, R.P.; Kanelis, V.; Choy, W.-Y.; Thibodeau, P.H.; Thomas, P.J.; Forman-Kay, J.D. CFTR regulatory region interacts with NBD1 predominantly via multiple transient helices. Nat. Struct. Mol. Biol. 2007, 14, 738–745. [Google Scholar] [CrossRef]
- Naren, A.P.; Cormet-Boyaka, E.; Fu, J.; Villain, M.; Blalock, J.E.; Quick, M.W.; Kirk, K.L. CFTR Chloride Channel Regulation by an Interdomain Interaction. Science 1999, 286, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Seavilleklein, G.; Amer, N.; Evagelidis, A.; Chappe, F.; Irvine, T.; Hanrahan, J.W.; Chappe, V. PKC phosphorylation modulates PKA-dependent binding of the R domain to other domains of CFTR. Am. J. Physiol. Cell Physiol. 2008, 295, C1366–C1375. [Google Scholar] [CrossRef]
- Wang, G.; Duan, D.D. Regulation of Activation and Processing of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) by a Complex Electrostatic Interaction between the Regulatory Domain and Cytoplasmic Loop 3. J. Biol. Chem. 2012, 287, 40484–40492. [Google Scholar] [CrossRef]
- Kanelis, V.; Hudson, R.P.; Thibodeau, P.H.; Thomas, P.J.; Forman-Kay, J.D. NMR evidence for differential phosphorylation-dependent interactions in WT and ΔF508 CFTR. EMBO J. 2010, 29, 263–277. [Google Scholar] [CrossRef]
- King, S.A.; Sorscher, E.J. R-Domain Interactions with Distal Regions of CFTR Lead to Phosphorylation and Activation. Biochemistry 2000, 39, 9868–9875. [Google Scholar] [CrossRef]
- Pitonzo, D.; Yang, Z.; Matsumura, Y.; Johnson, A.E.; Skach, W.R.; Patrick, A.E.; Karamyshev, A.L.; Millen, L.; Thomas, P.J.; Gilmore, M.E.R.; et al. Sequence-specific Retention and Regulated Integration of a Nascent Membrane Protein by the Endoplasmic Reticulum Sec61 Translocon. Mol. Biol. Cell 2009, 20, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Carveth, K.; Buck, T.; Anthony, V.; Skach, W.R. Cooperativity and Flexibility of Cystic Fibrosis Transmembrane Conductance Regulator Transmembrane Segments Participate in Membrane Localization of a Charged Residue. J. Biol. Chem. 2002, 277, 39507–39514. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, M.M.; Aleksandrov, A.A.; Chang, X.-B.; Riordan, J.R. A novel CFTR disease-associated mutation causes addition of an extra N-linked oligosaccharide. Glycoconj. J. 2000, 17, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Corradi, V.; Gu, R.-X.; Vergani, P.; Tieleman, D.P. Structure of Transmembrane Helix 8 and Possible Membrane Defects in CFTR. Biophys. J. 2018, 114, 1751–1754. [Google Scholar] [CrossRef]
- Negoda, A.; Hogan, M.S.; Cowley, E.A.; Linsdell, P. Contribution of the eighth transmembrane segment to the function of the CFTR chloride channel pore. Cell. Mol. Life Sci. 2019, 76, 2411–2423. [Google Scholar] [CrossRef]
- Choma, C.; Lear, J.D.; DeGrado, W.F.; Gratkowski, H. Asparagine-mediated self-association of a model transmembrane helix. Nat. Struct. Biol. 2000, 7, 161–166. [Google Scholar]
- Zhou, F.X.; Cocco, M.J.; Russ, W.P.; Brunger, A.T.; Engelman, D.M. Interhelical hydrogen bonding drives strong interactions in membrane proteins. Nat. Struct. Biol. 2000, 7, 154–160. [Google Scholar] [CrossRef]
- White, S.H.; Wimley, W.C. Membrane Protein Folding and Stability: Physical Principles. Annu. Rev. Biophys. Biomol. Struct. 1999, 28, 319–365. [Google Scholar] [CrossRef]
- Popot, J.-L.; Engelman, D.M. Helical Membrane Protein Folding, Stability, and Evolution. Annu. Rev. Biochem. 2000, 69, 881–922. [Google Scholar] [CrossRef]
- Thirumalai, D.; Klimov, D.K.; Dima, R.I. Emerging ideas on the molecular basis of protein and peptide aggregation. Curr. Opin. Struct. Biol. 2003, 13, 146–159. [Google Scholar] [CrossRef]
- Ramjeesingh, M.; Ugwu, F.; Li, C.; Dhani, S.; Huan, L.J.; Wang, Y.; Bear, C.E. Stable dimeric assembly of the second membrane-spanning domain of CFTR (cystic fibrosis transmembrane conductance regulator) reconstitutes a chloride-selective pore. Biochem. J. 2003, 375, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Itskanov, S.; Park, E. Mechanism of Protein Translocation by the Sec61 Translocon Complex. Cold Spring Harb. Perspect. Biol. 2023, 15, a041250. [Google Scholar] [CrossRef] [PubMed]
- Schuldiner, M.; Metz, J.; Schmid, V.; Denic, V.; Rakwalska, M.; Schmitt, H.D.; Schwappach, B.; Weissman, J.S. The GET Complex Mediates Insertion of Tail-Anchored Proteins into the ER Membrane. Cell 2008, 134, 634–645. [Google Scholar] [CrossRef]
- Chitwood, P.J.; Juszkiewicz, S.; Guna, A.; Shao, S.; Hegde, R.S. EMC Is Required to Initiate Accurate Membrane Protein Topogenesis. Cell 2018, 175, 1507–1519.e16. [Google Scholar] [CrossRef]
- McGilvray, P.T.; Anghel, S.A.; Sundaram, A.; Zhong, F.; Trnka, M.J.; Fuller, J.R.; Hu, H.; Burlingame, A.L.; Keenan, R.J. An ER translocon for multi-pass membrane protein biogenesis. Elife 2020, 9, e56889. [Google Scholar] [CrossRef] [PubMed]
- Aviram, N.; Ast, T.; Costa, E.A.; Arakel, E.C.; Chuartzman, S.G.; Jan, C.H.; Haßdenteufel, S.; Dudek, J.; Jung, M.; Schorr, S.; et al. The SND proteins constitute an alternative targeting route to the endoplasmic reticulum. Nature 2016, 540, 134–138. [Google Scholar] [CrossRef]
- Sundaram, A.; Yamsek, M.; Zhong, F.; Hooda, Y.; Hegde, R.S.; Keenan, R.J. Substrate-driven assembly of a translocon for multipass membrane proteins. Nature 2022, 611, 167–172. [Google Scholar] [CrossRef]
- Smalinskaitė, L.; Kim, M.K.; Lewis, A.J.O.; Keenan, R.J.; Hegde, R.S. Mechanism of an intramembrane chaperone for multipass membrane proteins. Nature 2022, 611, 161–166. [Google Scholar] [CrossRef]
- Schamel, W.W.; Kuppig, S.; Becker, B.; Gimborn, K.; Hauri, H.-P.; Reth, M. A high-molecular-weight complex of membrane proteins BAP29/BAP31 is involved in the retention of membrane-bound IgD in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 2003, 100, 9861–9866. [Google Scholar] [CrossRef]
- Lambert, G.; Becker, B.; Schreiber, R.; Boucherot, A.; Reth, M.; Kunzelmann, K. Control of Cystic Fibrosis Transmembrane Conductance Regulator Expression by BAP31. J. Biol. Chem. 2001, 276, 20340–20345. [Google Scholar] [CrossRef]
- Wang, B.; Heath-Engel, H.; Zhang, D.; Thomas, D.Y.; Hanrahan, J.W.; Shore, G.C. BAP31 interacts with Sec61 translocons and promotes retrotranslocation of CFTRΔF508 via the derlin-1 complex. Cell 2008, 133, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Ellgaard, L.; Helenius, A. ER quality control: Towards an understanding at the molecular level. Curr. Opin. Cell Biol. 2001, 13, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Pind, S.; Riordan, J.R.; Williams, D.B. Participation of the endoplasmic reticulum chaperone calnexin (p88, IP90) in the biogenesis of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 1994, 269, 12784–12788. [Google Scholar] [CrossRef] [PubMed]
- Farinha, C.M.; Amaral, M.D. Most F508del-CFTR Is Targeted to Degradation at an Early Folding Checkpoint and Independently of Calnexin. Mol. Cell. Biol. 2005, 25, 5242–5252. [Google Scholar] [CrossRef]
- Rosser, M.F.N.; Grove, D.E.; Chen, L.; Cyr, D.M. Assembly and Misassembly of Cystic Fibrosis Transmembrane Conductance Regulator: Folding Defects Caused by Deletion of F508 Occur Before and After the Calnexin-dependent Association of Membrane Spanning Domain (MSD) 1 and MSD2. Mol. Biol. Cell 2008, 19, 4570–4579. [Google Scholar] [CrossRef]
- Glozman, R.; Okiyoneda, T.; Mulvihill, C.M.; Rini, J.M.; Barriere, H.; Lukacs, G.L. N-glycans are direct determinants of CFTR folding and stability in secretory and endocytic membrane traffic. J. Cell Biol. 2009, 184, 847–862. [Google Scholar] [CrossRef]
- Cannon, K.S.; Cresswell, P. Quality control of transmembrane domain assembly in the tetraspanin CD82. EMBO J. 2001, 20, 2443–2453. [Google Scholar] [CrossRef]
- Swanton, E.; High, S.; Woodman, P. Role of calnexin in the glycan-independent quality control of proteolipid protein. EMBO J. 2003, 22, 2948–2958. [Google Scholar] [CrossRef]
- Bloemeke, N.; Meighen-Berger, K.; Hitzenberger, M.; Bach, N.C.; Parr, M.; Coelho, J.P.; Frishman, D.; Zacharias, M.; Sieber, S.A.; Feige, M.J. Intramembrane client recognition potentiates the chaperone functions of calnexin. EMBO J. 2022, 41, e110959. [Google Scholar] [CrossRef]
- Guay, K.P.; Williams, R.V.; Hebert, D.N. Calnexin reveals a sugar-free taste within the lipid bilayer. EMBO J. 2022, 41, e113003. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Z.; Levit, A.; Levring, J.; Touhara, K.K.; Shoichet, B.K.; Chen, J. Structural identification of a hotspot on CFTR for potentiation. Science 2019, 364, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, J.; Bruneau, A.; Hoffmann, B.; Durand-Schneider, A.; Barbu, V.; Jacquemin, E.; Maurice, M.; Housset, C.; Callebaut, I.; Aït-Slimane, T. Functional defect of variants in the adenosine triphosphate–binding sites of ABCB4 and their rescue by the cystic fibrosis transmembrane conductance regulator potentiator, ivacaftor (VX-770). Hepatology 2017, 65, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Mareux, E.; Lapalus, M.; Amzal, R.; Almes, M.; Aït-Slimane, T.; Delaunay, J.; Adnot, P.; Collado-Hilly, M.; Davit-Spraul, A.; Falguières, T.; et al. Functional rescue of an ABCB11 mutant by ivacaftor: A new targeted pharmacotherapy approach in bile salt export pump deficiency. Liver Int. 2020, 40, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Sjöstedt, N.; Santo, M.; Neuvonen, M.; Niemi, M.; Kidron, H. Novel inhibitors of breast cancer resistance protein (BCRP, ABCG2) among marketed drugs. Eur. J. Pharm. Sci. 2023, 181, 106362. [Google Scholar] [CrossRef]
- Yeh, H.-I.; Sohma, Y.; Conrath, K.; Hwang, T.-C. A common mechanism for CFTR potentiators. J. Gen. Physiol. 2017, 149, 1105–1118. [Google Scholar] [CrossRef]
- Yeh, H.-I.; Qiu, L.; Sohma, Y.; Conrath, K.; Zou, X.; Hwang, T.-C. Identifying the molecular target sites for CFTR potentiators GLPG1837 and VX-770. J. Gen. Physiol. 2019, 151, 912–928. [Google Scholar] [CrossRef]
- Sheppard, D.N.; Ostedgaard, L.S.; Rich, D.P.; Welsh, M.J. The amino-terminal portion of CFTR forms a regulated Cl− channel. Cell 1994, 76, 1091–1098. [Google Scholar] [CrossRef]
- Xiong, X.; Bragin, A.; Widdicombe, J.H.; Cohn, J.; Skach, W.R. Structural cues involved in endoplasmic reticulum degradation of G85E and G91R mutant cystic fibrosis transmembrane conductance regulator. J. Clin. Investig. 1997, 100, 1079–1088. [Google Scholar] [CrossRef]
- Meacham, G.C.; Lu, Z.; King, S.; Sorscher, E.; Tousson, A.; Cyr, D.M. The Hdj-2/Hsc70 chaperone pair facilitates early steps in CFTR biogenesis. EMBO J. 1999, 18, 1492–1505. [Google Scholar] [CrossRef]
- Schildknegt, D.; Lodder, N.; Pandey, A.; Chatsisvili, A.; Egmond, M.; Pena, F.; Braakman, I.; van der Sluijs, P. Characterization of CNPY5 and its family members. Protein Sci. 2019, 28, 1276–1289. [Google Scholar] [CrossRef]
- Benham, A.M.; Cabibbo, A.; Fassio, A.; Bulleid, N.; Sitia, R.; Braakman, I. The CXXCXXC motif determines the folding, structure and stability of human Ero1-Lα. EMBO J. 2000, 19, 4493–4502. [Google Scholar] [CrossRef] [PubMed]
- Hoelen, H.; Kleizen, B.; Schmidt, A.; Richardson, J.; Charitou, P.; Thomas, P.J.; Braakman, I. The Primary Folding Defect and Rescue of ΔF508 CFTR Emerge during Translation of the Mutant Domain. PLoS ONE 2010, 5, e15458. [Google Scholar] [CrossRef] [PubMed]
- Rupert, M.M.J.; Zacco, E.; Tartaglia, G.G. A Computational Approach Reveals the Ability of Amyloids to Sequester RNA: The Alpha Synuclein Case. Nucleic Acid Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Armaos, A.; Fantini, M.; Pastore, A.; Tartaglia, G.G. Aggregation is a Context-Dependent Constraint on Protein Evolution. Front. Mol. Biosci. 2021, 8, 678115. [Google Scholar] [CrossRef]
- Dobson, C.M. Principles of protein folding, misfolding and aggregation. Semin. Cell Dev. Biol. 2004, 15, 3–16. [Google Scholar] [CrossRef]
- Heinig, M.; Frishman, D. STRIDE: A web server for secondary structure assignment from known atomic coordinates of proteins. Nucleic Acids Res. 2004, 32, W500–W502. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleizen, B.; de Mattos, E.; Papaioannou, O.; Monti, M.; Tartaglia, G.G.; van der Sluijs, P.; Braakman, I. Transmembrane Helices 7 and 8 Confer Aggregation Sensitivity to the Cystic Fibrosis Transmembrane Conductance Regulator. Int. J. Mol. Sci. 2023, 24, 15741. https://doi.org/10.3390/ijms242115741
Kleizen B, de Mattos E, Papaioannou O, Monti M, Tartaglia GG, van der Sluijs P, Braakman I. Transmembrane Helices 7 and 8 Confer Aggregation Sensitivity to the Cystic Fibrosis Transmembrane Conductance Regulator. International Journal of Molecular Sciences. 2023; 24(21):15741. https://doi.org/10.3390/ijms242115741
Chicago/Turabian StyleKleizen, Bertrand, Eduardo de Mattos, Olga Papaioannou, Michele Monti, Gian Gaetano Tartaglia, Peter van der Sluijs, and Ineke Braakman. 2023. "Transmembrane Helices 7 and 8 Confer Aggregation Sensitivity to the Cystic Fibrosis Transmembrane Conductance Regulator" International Journal of Molecular Sciences 24, no. 21: 15741. https://doi.org/10.3390/ijms242115741
APA StyleKleizen, B., de Mattos, E., Papaioannou, O., Monti, M., Tartaglia, G. G., van der Sluijs, P., & Braakman, I. (2023). Transmembrane Helices 7 and 8 Confer Aggregation Sensitivity to the Cystic Fibrosis Transmembrane Conductance Regulator. International Journal of Molecular Sciences, 24(21), 15741. https://doi.org/10.3390/ijms242115741






