Immunobiotic Ligilactobacillus salivarius FFIG58 Confers Long-Term Protection against Streptococcus pneumoniae
Abstract
:1. Introduction
2. Results
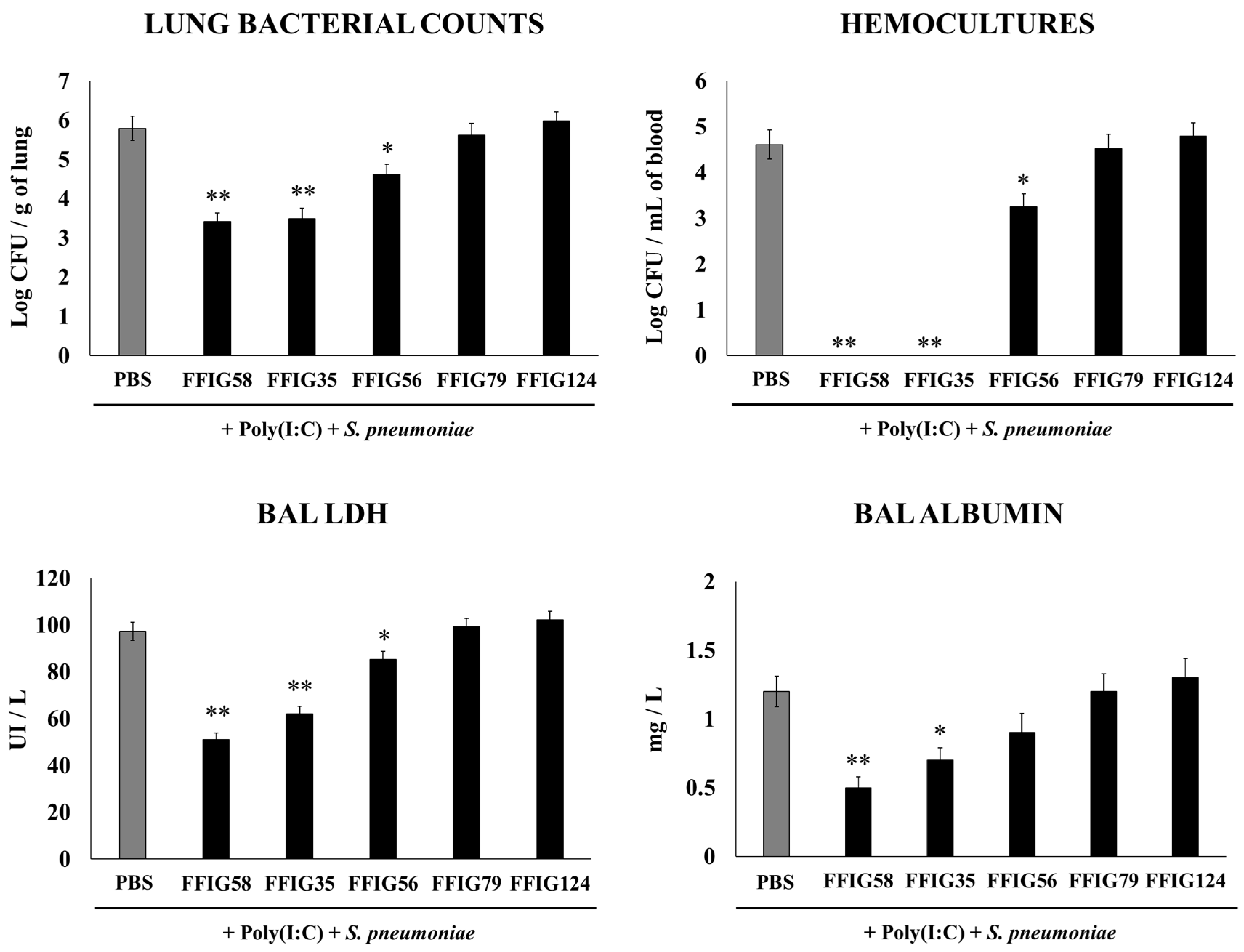
2.1. Effect of Porcine L. salivarius Strains against Secondary Pneumococcal Infection
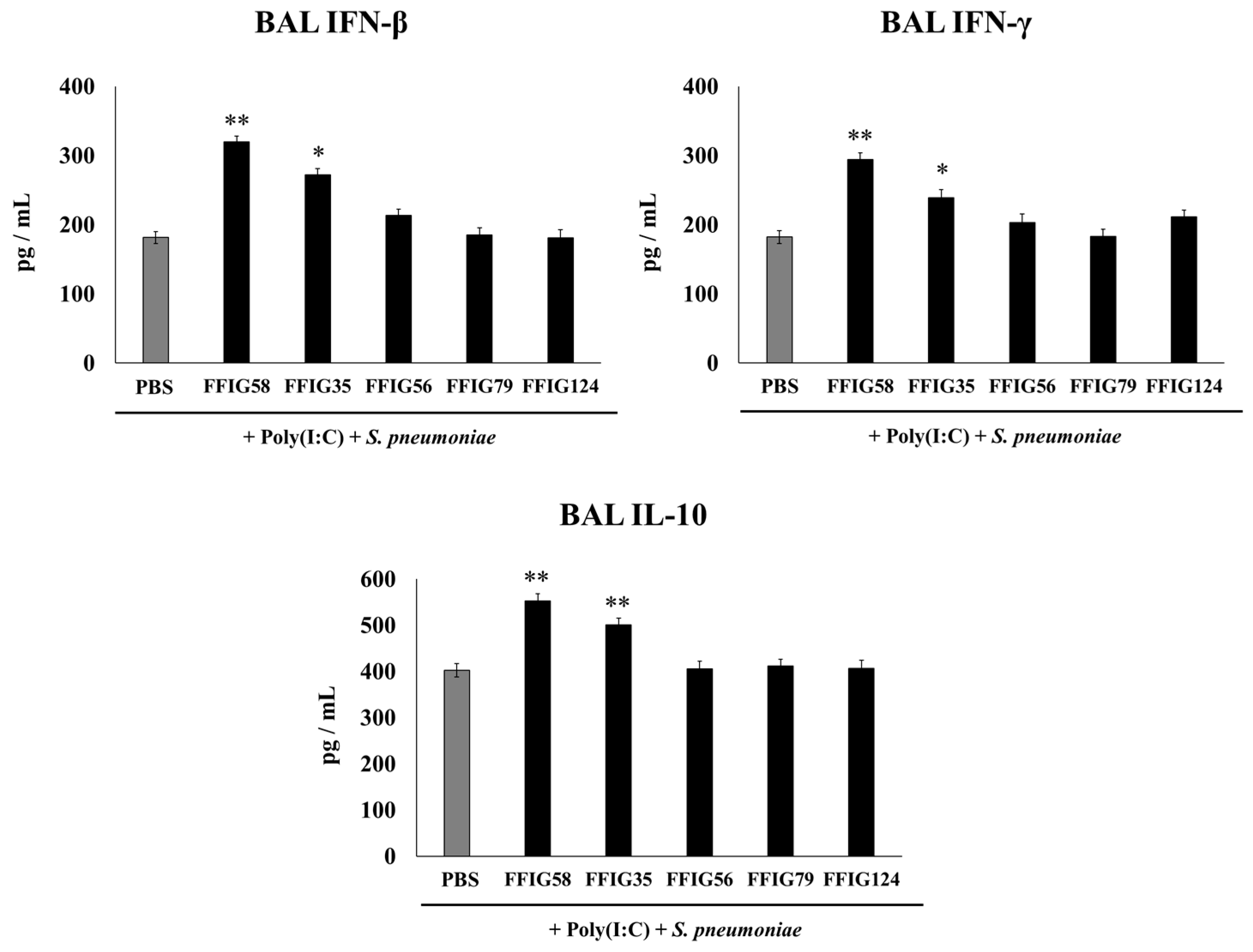
2.2. Effect of Porcine L. salivarius Strains on BAL Cytokine Profiles in Response to Secondary Pneumococcal Infection
2.3. Effects of Porcine L. salivarius FFIG58 on Alveolar Macrophages in Response to Secondary Pneumococcal Infection
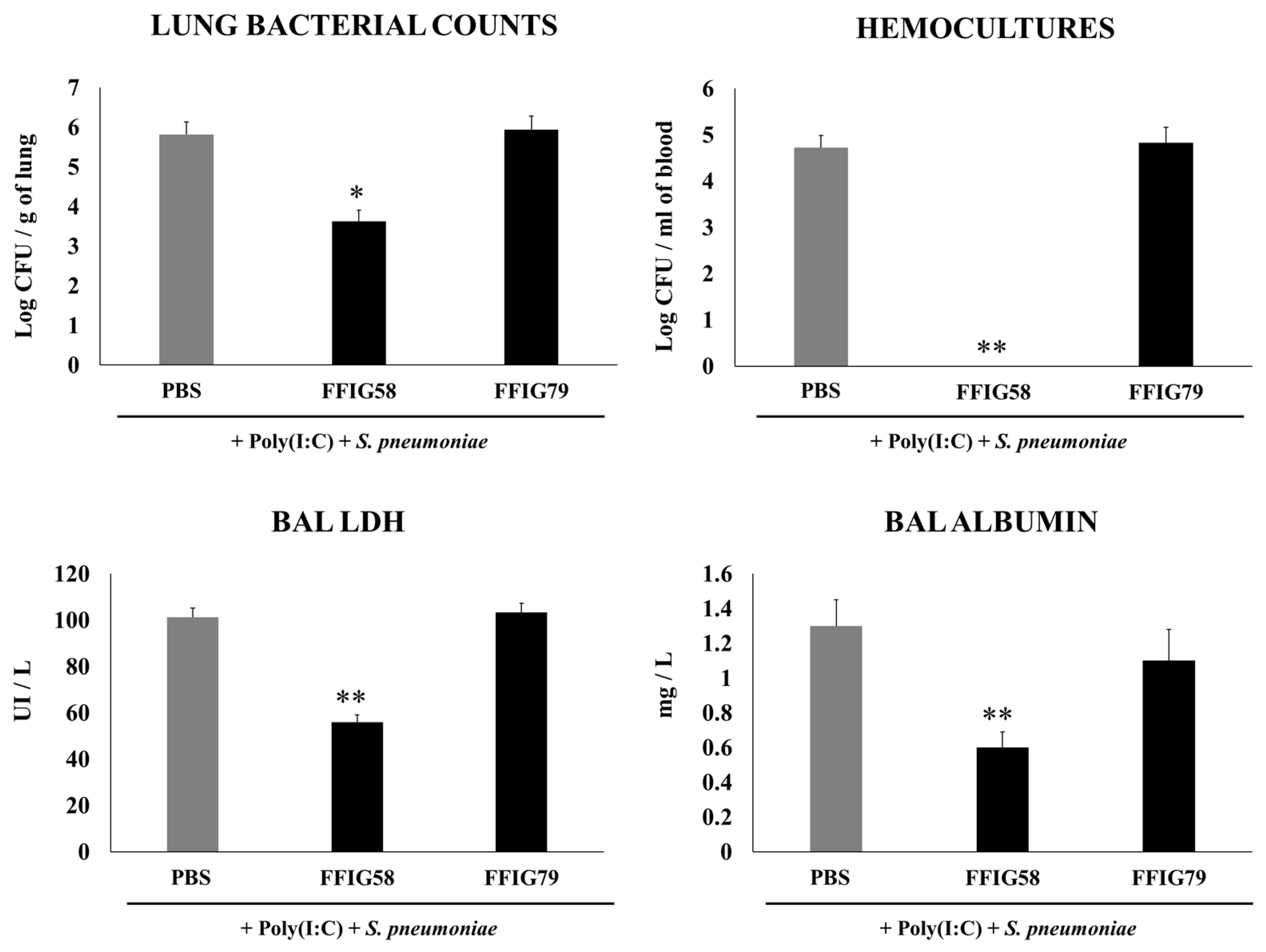
2.4. Long-Term Protection Conferred by Porcine L. salivarius FFIG58 against Secondary Pneumococcal Infection
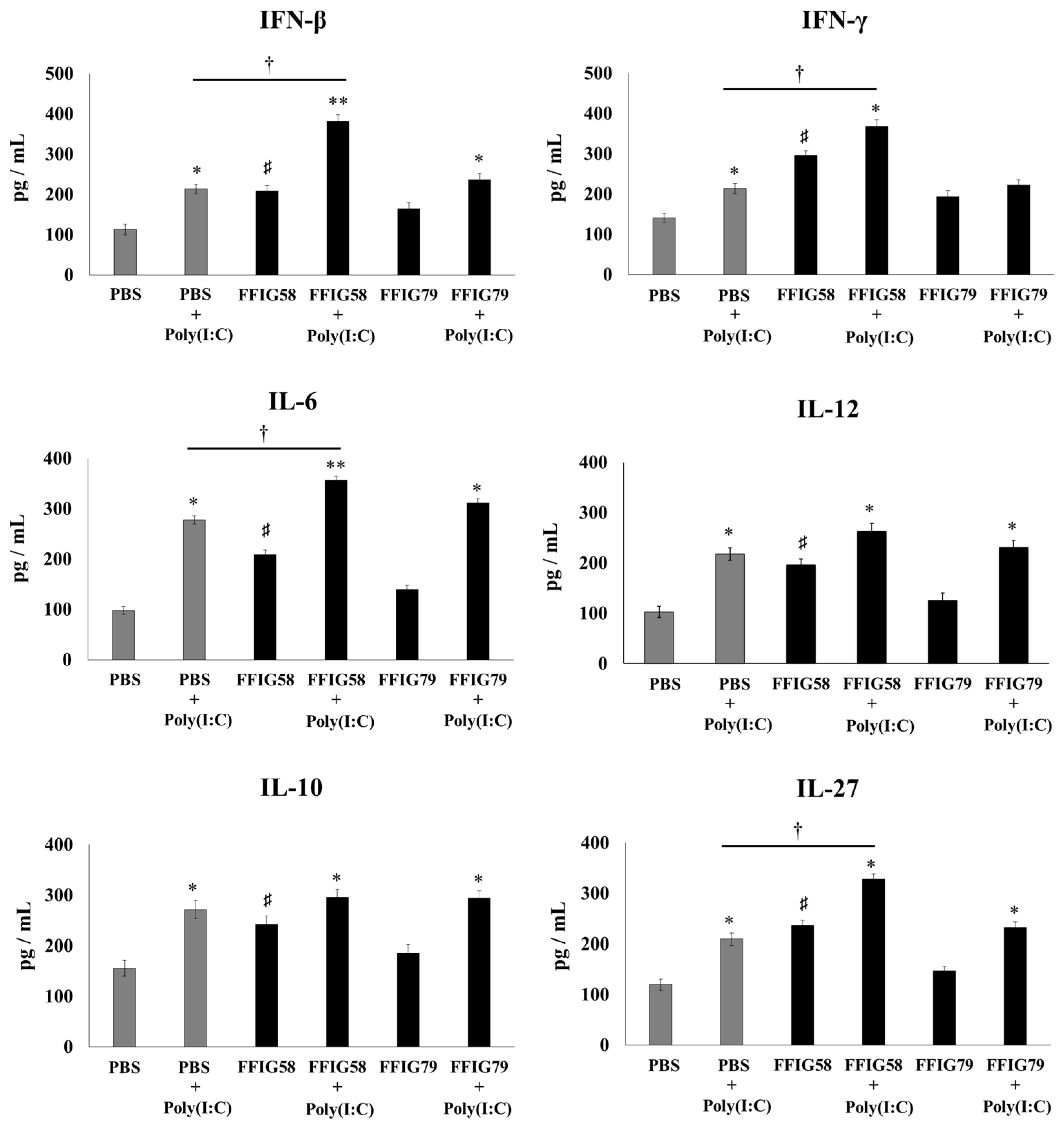
2.5. Long-Term Effects of L. salivarius FFIG58 on Alveolar Macrophages in Response to Secondary Pneumococcal Infection
3. Discussion
- (a)
- Immunobiotic strains can exert immunomodulatory properties in a different host and/or mucosal tissue from which they were isolated. The classic search for probiotics that can exert beneficial effects on mucosal tissues like the gastrointestinal, respiratory, or urogenital tracts is based on the isolation and characterization of strains taken from the same ecological niche in which the microorganism will be applied. This type of strategy is based on the concept that microorganisms from a certain niche have the necessary adaptations to colonize and interact positively with the host. However, there is considerable evidence that foreign microorganisms, isolated from different mucous membranes, foods, and even different species, can exert immunomodulatory activities beneficial to the host. An example of this phenomenon is the demonstrated ability of the probiotic strain Lacticaseibacillus rhamnosus CRL1505, originally isolated from goat milk, to modulate intestinal and respiratory immunity when administered orally [16] or nasally [17], respectively. Similarly, the well-characterized probiotic strain L. rhamnosus GG, isolated from the intestine, is able to modulate respiratory immunity when nasally administered [18]. In line with these studies, we showed, in this work, that the strain FFIG58 from porcine intestine is capable of modulating immunity in the respiratory tract of mice. In this way, strains with remarkable immunomodulatory properties and the ability to protect against infections in a specific mucosa could be evaluated in other mucosal tissues, to establish if they can also exert protective effects. Furthermore, considering that mice are often used as preclinical models for the application of probiotics for the improvement of human health, it would be interesting to evaluate whether L. salivarius FFIG58 can exert beneficial effects in the context of human respiratory infections. This is an interesting topic for future research.
- (b)
- Porcine L. salivarius strains differentially modulate the respiratory innate immune response in a strain-dependent manner. Using a mouse in vivo model of respiratory superinfection in which S. pneumoniae infects animals after the stimulation with poly(I:C) [15,20,21], we demonstrated that the immunomodulatory properties of the porcine L. salivarius strains and their potential to confer protection in the respiratory tract was a strain-specific characteristic. In our hands, the FFIG35 and FFIG58 strains had the ability to modulate respiratory immunity, while L. salivarius FFIG79 did not exert immunomodulatory effects, in accordance with the results obtained in intestinal epithelial cells [14]. These strains decreased lung and blood pneumococcal counts and reduced lung tissue damage evidenced by lower albumin levels and LDH activity. The nasal treatment of infant mice with L. salivarius FFIG35 or FFIG58 improved the production of IFN-β, IFN-γ, and IL-6 in the respiratory tract, factors that are critical to conferring protection against RSV [22] and pneumococci [23,24]. In this regard, it is well established that pneumococcal infections are more frequent and severe in the elderly. It was demonstrated that the production of IFN-β during S. pneumoniae infection was decreased in aged hosts compared to immunocompetent adults, and was associated with a reduced clearance of the pathogen [25]. Furthermore, this work demonstrated that despite similar levels of phagocytosis when compared to young hosts, aged macrophages produced significantly less IFN-β in response to pneumococcal infection. It was also shown that pneumococci defective in the virulence factor autolysin LytA were more efficiently phagocyted by macrophages than wild-type bacteria, leading to higher levels of cytosolic pneumococcal DNA accumulation, and higher expression of IFN-β, interferon-stimulated genes, TNF-α, and IL-1β, which promoted an improved clearance of S. pneumoniae [26]. Similarly, IFN-γ mediated protective effects against pneumococci, as mice deficient in this immune factor had impaired bacterial clearance [27,28]. On the other hand, it was shown that the impairment of IL-6 production in primary human monocytes cultures by influenza virus infection significantly increased their susceptibility to S. pneumoniae infection [29]. In line with those results, in vivo studies in IL-6−/− mice showed that the influenza–pneumococci co-infection was characterized by enhanced bacterial burden and dissemination as well as aggravated pulmonary lesions that correlated with high mortality in comparison with wild-type animals [30]. The work demonstrated that the protective effect of IL-6 was associated with appropriate macrophage function, since this cytokine has a key role in macrophage death and their capacity to control lung tissue inflammation [31].
- (c)
- Alveolar macrophages are a key respiratory immune cell population involved in the beneficial effects induced by L. salivarius FFIG58. AMs are the most abundant immune cell in the lungs, playing a critical role in homeostasis, host defense, and tissue remodeling [32]. Their phagocytic activity is crucial for the elimination of pathogens and infected cells during the course of infections. AMs also produce cytokines and chemokines that act on surrounding immune and epithelial cells inducing the transcription of immune factors that favors pathogens clearance [33]. Our previous studies demonstrated a key role of AMs in the beneficial effects induced in respiratory immunity by nasally administered probiotics [17,34]. In line with those previous studies, we observed, here, that L. salivarius FFIG58 improved the production of IFN-β, IFN-γ, and IL-6 in AMs in response to both poly(I:C) and S. pneumoniae challenges. The results indicate that AMs have a relevant role in the protective effect of the FFIG58 strain in the context of respiratory superinfection.
- (d)
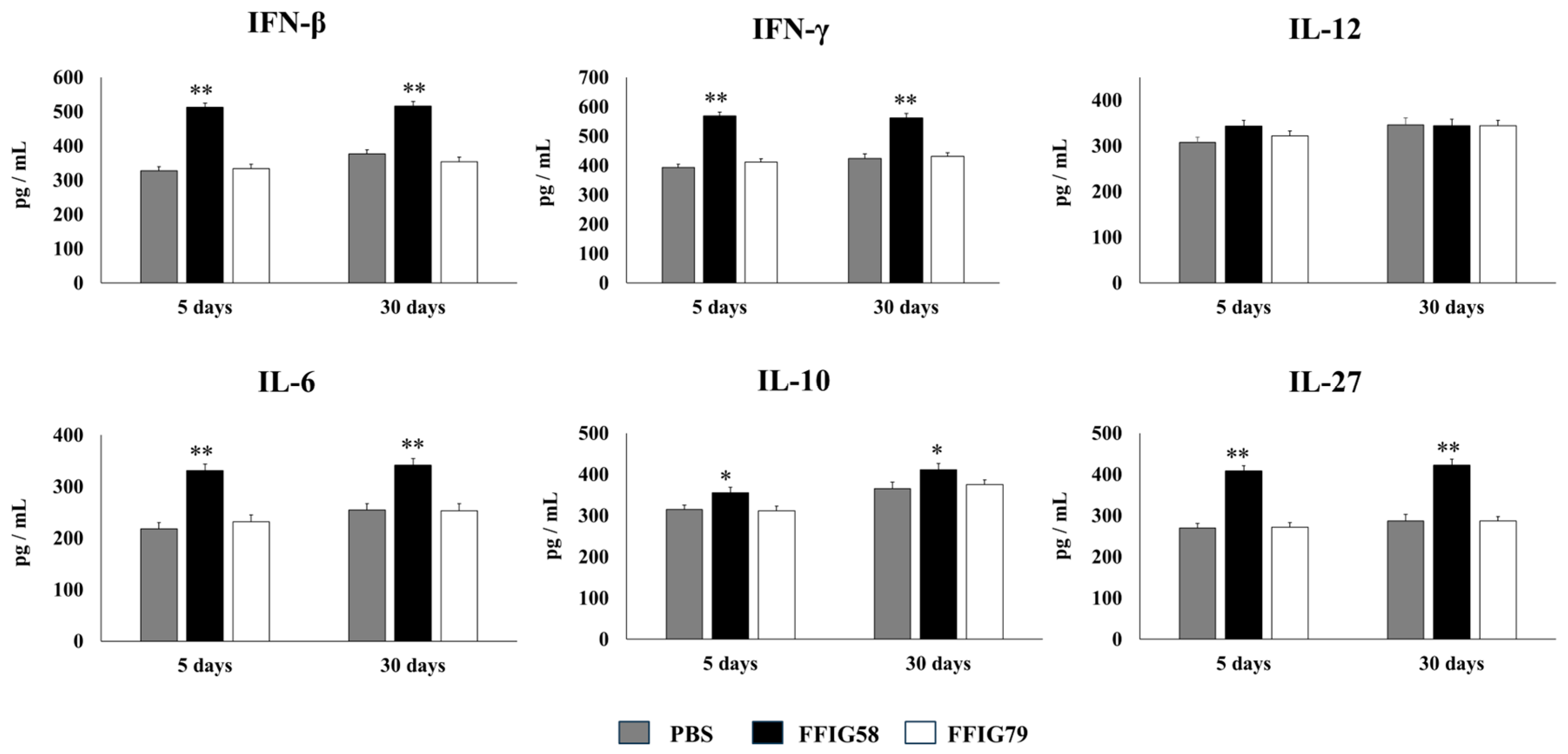
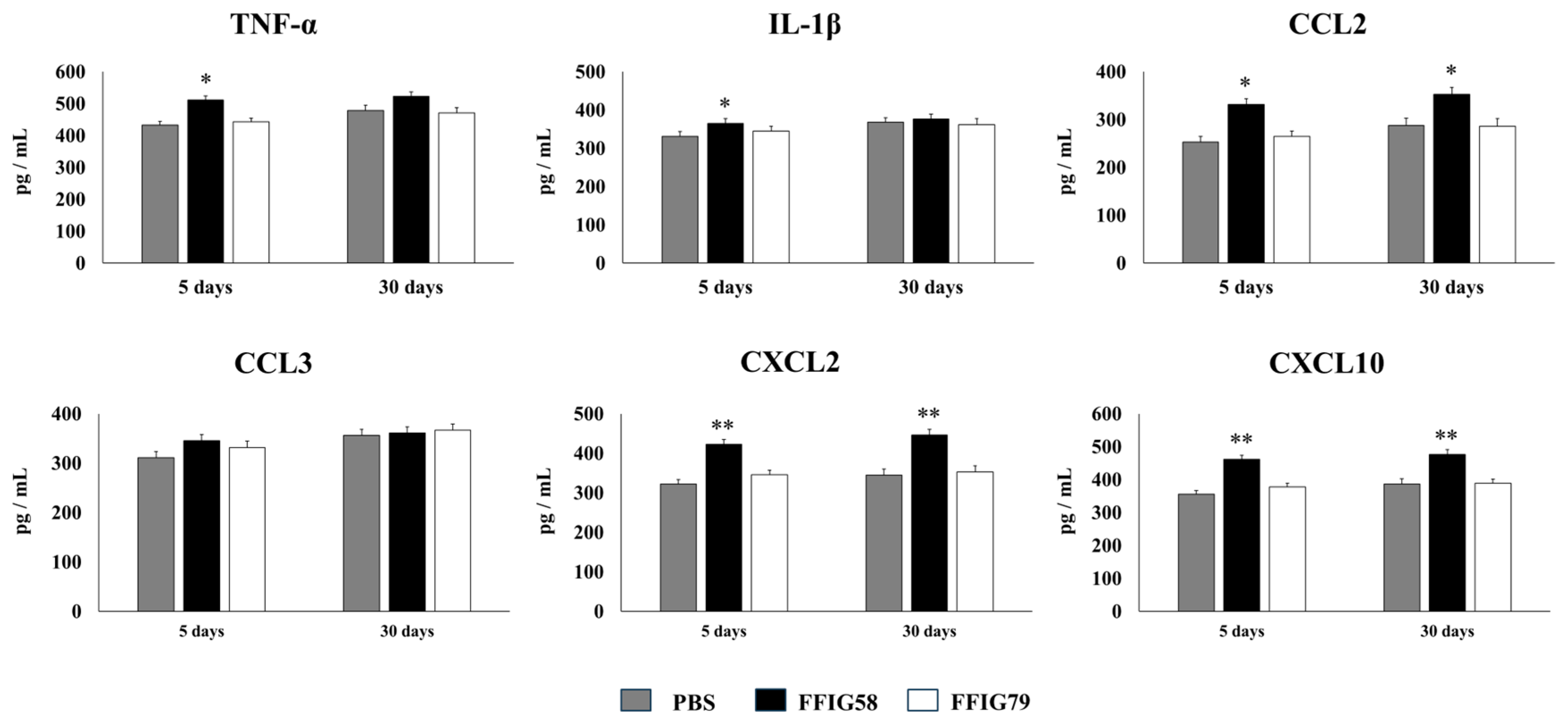
- The generation of “trained” alveolar macrophages would be involved in the beneficial effects induced by L. salivarius FFIG58 in the respiratory tract. Interestingly, we demonstrated, here, that the nasal priming of mice with L. salivarius FFIG58 can confer long-term protection against S. pneumoniae. Research over the last decade has shown that, after a first stimulus with an antigen, AMs can develop an innate immune memory phenotype [12,39,40,41]. This response, called “trained immunity”, stands out for its greater speed and enhanced capacity to produce cytokines after a second stimulus with the same or non-related antigen [12,40]. It has also been shown that the production of IFN-γ is essential for the generation of trained immunity in AMs [39]. Thus, we aimed to evaluate the ability of L. salivarius FFIG58 to generate trained immunity in mice, evaluating the protection against secondary pneumococcal pneumonia over the time. It was observed that the protective effect of the FFIG58 strain against secondary S. pneumoniae infection persisted for at least one month. Furthermore, the production of cytokines of AMs obtained from animals treated with FFIG58 and challenged with pneumococci after 5 and 30 days was comparable, demonstrating that the beneficial effects were maintained during this time. AMs from FFIG58-treated animals were capable of producing higher levels of IFN-β, IFN-γ, IL-6, IL-10, and IL-27 than controls after 5 and 30 days, which are important factors in the defense against pneumococci, as described previously. Furthermore, AMs produced higher levels of the inflammatory chemokines CCL2 (MCP-1), CXCL2 (MIP2-α), and CXCL10 (IP-10), both at 5 and at 30 days.
4. Materials and Methods
4.1. Strains and Culture Conditions
4.2. Animals and Treatments
4.3. Poly(I:C) Administration and Respiratory Infections
4.4. Lung Injury Parameters
4.5. Alveolar Macrophage Primary Cultures
4.6. Cytokine Concentrations in BAL and Culture Supernatants
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Troeger, C.; Blacker, B.; Khalil, I.A.; Rao, P.C.; Cao, J.; Zimsen, S.R.; Albertson, S.B.; Deshpande, A.; Farag, T.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef]
- Kaler, J.; Hussain, A.; Patel, K.; Hernandez, T.; Ray, S. Respiratory Syncytial Virus: A Comprehensive Review of Transmission, Pathophysiology, and Manifestation. Cureus 2023, 15, e36342. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Chang, C.; Gershwin, M.E.; Gershwin, L.J. Respiratory syncytial virus—A comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef] [PubMed]
- Laudanno, S.L.; Sanchez Yanotti, C.I.; Polack, F.P. RSV Lower Respiratory Tract Illness in Infants of Low- and Middle-income Countries. Acta Med. Acad. 2020, 49, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Young, M.; Smitherman, L. Socioeconomic Impact of RSV Hospitalization. Infect. Dis. Ther. 2021, 10, 35–45. [Google Scholar] [CrossRef]
- Shibata, T.; Makino, A.; Ogata, R.; Nakamura, S.; Ito, T.; Nagata, K.; Terauchi, Y.; Oishi, T.; Fujieda, M.; Takahashi, Y.; et al. Respiratory syncytial virus infection exacerbates pneumococcal pneumonia via Gas6/Axl-mediated macrophage polarization. J. Clin. Investig. 2020, 130, 3021–3037. [Google Scholar] [CrossRef]
- Hanada, S.; Pirzadeh, M.; Carver, K.Y.; Deng, J.C. Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia. Front. Immunol. 2018, 9, 2640. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Chughtai, A.A.; Barnes, M.; Ridda, I.; Seale, H.; Toms, R.; Heywood, A. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect. Dis. 2018, 18, 637. [Google Scholar] [CrossRef]
- Villena, J.; Kitazawa, H. The Modulation of Mucosal Antiviral Immunity by Immunobiotics: Could They Offer Any Benefit in the SARS-CoV-2 Pandemic? Front. Physiol. 2020, 11, 699. [Google Scholar] [CrossRef]
- Villena, J.; Li, C.; Vizoso-Pinto, M.G.; Sacur, J.; Ren, L.; Kitazawa, H. Lactiplantibacillus plantarum as a Potential Adjuvant and Delivery System for the Development of SARS-CoV-2 Oral Vaccines. Microorganisms 2021, 9, 683. [Google Scholar] [CrossRef]
- Jastrzab, R.; Graczyk, D.; Siedlecki, P. Molecular and Cellular Mechanisms Influenced by Postbiotics. Int. J. Mol. Sci. 2021, 22, 13475. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Masumizu, Y.; Zhou, B.; Kober, A.; Islam, M.A.; Iida, H.; Ikeda-Ohtsubo, W.; Suda, Y.; Albarracin, L.; Nochi, T.; Aso, H.; et al. Isolation and Immunocharacterization of Lactobacillus salivarius from the Intestine of Wakame-Fed Pigs to Develop Novel “Immunosynbiotics”. Microorganisms 2019, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Albarracin, L.; Indo, Y.; Arce, L.; Masumizu, Y.; Tomokiyo, M.; Islam, M.A.; Garcia-Castillo, V.; Ikeda-Ohtsubo, W.; Nochi, T.; et al. Selection of Immunobiotic Ligilactobacillus salivarius Strains from the Intestinal Tract of Wakame-Fed Pigs: Functional and Genomic Studies. Microorganisms 2020, 8, 1659. [Google Scholar] [CrossRef]
- Clua, P.; Kanmani, P.; Zelaya, H.; Tada, A.; Kober, A.; Salva, S.; Alvarez, S.; Kitazawa, H.; Villena, J. Peptidoglycan from Immunobiotic Lactobacillus rhamnosus Improves Resistance of Infant Mice to Respiratory Syncytial Viral Infection and Secondary Pneumococcal Pneumonia. Front. Immunol. 2017, 8, 948. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.; Chiba, E.; Vizoso-Pinto, M.G.; Tomosada, Y.; Takahashi, T.; Ishizuka, T.; Aso, H.; Salva, S.; Alvarez, S.; Kitazawa, H. Immunobiotic Lactobacillus rhamnosus strains differentially modulate antiviral immune response in porcine intestinal epithelial and antigen presenting cells. BMC Microbiol. 2014, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Raya Tonetti, F.; Clua, P.; Fukuyama, K.; Marcial, G.; Sacur, J.; Marranzino, G.; Tomokiyo, M.; Vizoso-Pinto, G.; Garcia-Cancino, A.; Kurata, S.; et al. The Ability of Postimmunobiotics from L. rhamnosus CRL1505 to Protect against Respiratory Syncytial Virus and Pneumococcal Super-Infection Is a Strain-Dependent Characteristic. Microorganisms 2022, 10, 2185. [Google Scholar] [CrossRef]
- Spacova, I.; Petrova, M.I.; Fremau, A.; Pollaris, L.; Vanoirbeek, J.; Ceuppens, J.L.; Seys, S.; Lebeer, S. Intranasal administration of probiotic Lactobacillus rhamnosus GG prevents birch pollen-induced allergic asthma in a murine model. Allergy 2019, 74, 100–110. [Google Scholar] [CrossRef]
- Indo, Y.; Kitahara, S.; Tomokiyo, M.; Araki, S.; Islam, M.A.; Zhou, B.; Albarracin, L.; Miyazaki, A.; Ikeda-Ohtsubo, W.; Nochi, T.; et al. Ligilactobacillus salivarius Strains Isolated From the Porcine Gut Modulate Innate Immune Responses in Epithelial Cells and Improve Protection Against Intestinal Viral-Bacterial Superinfection. Front. Immunol. 2021, 12, 652923. [Google Scholar] [CrossRef]
- Villena, J.; Chiba, E.; Tomosada, Y.; Salva, S.; Marranzino, G.; Kitazawa, H.; Alvarez, S. Orally administered Lactobacillus rhamnosus modulates the respiratory immune response triggered by the viral pathogen-associated molecular pattern poly(I:C). BMC Immunol. 2012, 13, 53. [Google Scholar] [CrossRef]
- Tomosada, Y.; Chiba, E.; Zelaya, H.; Takahashi, T.; Tsukida, K.; Kitazawa, H.; Alvarez, S.; Villena, J. Nasally administered Lactobacillus rhamnosus strains differentially modulate respiratory antiviral immune responses and induce protection against respiratory syncytial virus infection. BMC Immunol. 2013, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Bohmwald, K.; Espinoza, J.A.; Pulgar, R.A.; Jara, E.L.; Kalergis, A.M. Functional Impairment of Mononuclear Phagocyte System by the Human Respiratory Syncytial Virus. Front. Immunol. 2017, 8, 1643. [Google Scholar] [CrossRef] [PubMed]
- Koppe, U.; Hogner, K.; Doehn, J.M.; Muller, H.C.; Witzenrath, M.; Gutbier, B.; Bauer, S.; Pribyl, T.; Hammerschmidt, S.; Lohmeyer, J.; et al. Streptococcus pneumoniae stimulates a STING- and IFN regulatory factor 3-dependent type I IFN production in macrophages, which regulates RANTES production in macrophages, cocultured alveolar epithelial cells, and mouse lungs. J. Immunol. 2012, 188, 811–817. [Google Scholar] [CrossRef] [PubMed]
- LeMessurier, K.S.; Hacker, H.; Chi, L.; Tuomanen, E.; Redecke, V. Type I interferon protects against pneumococcal invasive disease by inhibiting bacterial transmigration across the lung. PLoS Pathog. 2013, 9, e1003727. [Google Scholar] [CrossRef]
- Mitzel, D.N.; Lowry, V.; Shirali, A.C.; Liu, Y.; Stout-Delgado, H.W. Age-enhanced endoplasmic reticulum stress contributes to increased Atg9A inhibition of STING-mediated IFN-beta production during Streptococcus pneumoniae infection. J. Immunol. 2014, 192, 4273–4283. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Dou, X.; Zhang, X.; Fang, Y.; Yang, Z.; Jiang, Y.; Hao, X.; Zhang, Z.; Wang, H. Streptococcus pneumoniae autolysin LytA inhibits ISG15 and ISGylation through decreasing bacterial DNA abnormally accumulated in the cytoplasm of macrophages. Mol. Immunol. 2021, 140, 87–96. [Google Scholar] [CrossRef]
- Sun, K.; Salmon, S.L.; Lotz, S.A.; Metzger, D.W. Interleukin-12 promotes gamma interferon-dependent neutrophil recruitment in the lung and improves protection against respiratory Streptococcus pneumoniae infection. Infect. Immun. 2007, 75, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Gomez, J.C.; Chugh, P.E.; Lowell, C.A.; Dinauer, M.C.; Dittmer, D.P.; Doerschuk, C.M. Interferon-gamma production by neutrophils during bacterial pneumonia in mice. Am. J. Respir. Crit. Care Med. 2011, 183, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Loughran, S.T.; Power, P.A.; Maguire, P.T.; McQuaid, S.L.; Buchanan, P.J.; Jonsdottir, I.; Newman, R.W.; Harvey, R.; Johnson, P.A. Influenza infection directly alters innate IL-23 and IL-12p70 and subsequent IL-17A and IFN-gamma responses to pneumococcus in vitro in human monocytes. PLoS ONE 2018, 13, e0203521. [Google Scholar] [CrossRef]
- Gou, X.; Yuan, J.; Wang, H.; Wang, X.; Xiao, J.; Chen, J.; Liu, S.; Yin, Y.; Zhang, X. IL-6 During Influenza-Streptococcus pneumoniae Co-Infected Pneumonia-A Protector. Front. Immunol. 2019, 10, 3102. [Google Scholar] [CrossRef]
- Gou, X.; Xu, W.; Liu, Y.; Peng, Y.; Xu, W.; Yin, Y.; Zhang, X. IL-6 Prevents Lung Macrophage Death and Lung Inflammation Injury by Inhibiting GSDME- and GSDMD-Mediated Pyroptosis during Pneumococcal Pneumosepsis. Microbiol. Spectr. 2022, 10, e0204921. [Google Scholar] [CrossRef]
- Hou, F.; Xiao, K.; Tang, L.; Xie, L. Diversity of Macrophages in Lung Homeostasis and Diseases. Front. Immunol. 2021, 12, 753940. [Google Scholar] [CrossRef]
- Hoffmann, H.H.; Schneider, W.M.; Rice, C.M. Interferons and viruses: An evolutionary arms race of molecular interactions. Trends Immunol. 2015, 36, 124–138. [Google Scholar] [CrossRef]
- Tomokiyo, M.; Tonetti, F.R.; Yamamuro, H.; Shibata, R.; Fukuyama, K.; Gobbato, N.; Albarracin, L.; Rajoka, M.S.R.; Kober, A.; Ikeda-Ohtsubo, W.; et al. Modulation of Alveolar Macrophages by Postimmunobiotics: Impact on TLR3-Mediated Antiviral Respiratory Immunity. Cells 2022, 11, 2986. [Google Scholar] [CrossRef]
- Branchett, W.J.; Lloyd, C.M. Regulatory cytokine function in the respiratory tract. Mucosal Immunol. 2019, 12, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.A.; Melo-Gonzalez, F.; Sebastian, V.P.; Vallejos, O.P.; Noguera, L.P.; Suazo, I.D.; Schultz, B.M.; Manosalva, A.H.; Penaloza, H.F.; Soto, J.A.; et al. Characterization of the Anti-Inflammatory Capacity of IL-10-Producing Neutrophils in Response to Streptococcus pneumoniae Infection. Front. Immunol. 2021, 12, 638917. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.E.; Jose, R.J.; Brown, J.S.; Chambers, R.C. Enhanced inflammation in aged mice following infection with Streptococcus pneumoniae is associated with decreased IL-10 and augmented chemokine production. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 308, L539–L549. [Google Scholar] [CrossRef]
- Pyle, C.J.; Uwadiae, F.I.; Swieboda, D.P.; Harker, J.A. Early IL-6 signalling promotes IL-27 dependent maturation of regulatory T cells in the lungs and resolution of viral immunopathology. PLoS Pathog. 2017, 13, e1006640. [Google Scholar] [CrossRef]
- Leopold Wager, C.M.; Hole, C.R.; Campuzano, A.; Castro-Lopez, N.; Cai, H.; Caballero Van Dyke, M.C.; Wozniak, K.L.; Wang, Y.; Wormley, F.L., Jr. IFN-gamma immune priming of macrophages in vivo induces prolonged STAT1 binding and protection against Cryptococcus neoformans. PLoS Pathog. 2018, 14, e1007358. [Google Scholar] [CrossRef]
- Yao, Y.; Jeyanathan, M.; Haddadi, S.; Barra, N.G.; Vaseghi-Shanjani, M.; Damjanovic, D.; Lai, R.; Afkhami, S.; Chen, Y.; Dvorkin-Gheva, A.; et al. Induction of Autonomous Memory Alveolar Macrophages Requires T Cell Help and Is Critical to Trained Immunity. Cell 2018, 175, 1634–1650 e1617. [Google Scholar] [CrossRef]
- Zahalka, S.; Starkl, P.; Watzenboeck, M.L.; Farhat, A.; Radhouani, M.; Deckert, F.; Hladik, A.; Lakovits, K.; Oberndorfer, F.; Lassnig, C.; et al. Trained immunity of alveolar macrophages requires metabolic rewiring and type 1 interferon signaling. Mucosal Immunol. 2022, 15, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Palomino, D.C.; Marti, L.C. Chemokines and immunity. Einstein Sao Paulo 2015, 13, 469–473. [Google Scholar] [CrossRef]
- Iwanaga, N.; Nakamura, S.; Oshima, K.; Kajihara, T.; Takazono, T.; Miyazaki, T.; Izumikawa, K.; Yanagihara, K.; Sugawara, A.; Sunazuka, T.; et al. Macrolides Promote CCL2-Mediated Macrophage Recruitment and Clearance of Nasopharyngeal Pneumococcal Colonization in Mice. J. Infect. Dis. 2015, 212, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; LaRose, M.I.; Hergott, C.B.; Leng, L.; Bucala, R.; Weiser, J.N. Macrophage migration inhibitory factor promotes clearance of pneumococcal colonization. J. Immunol. 2014, 193, 764–772. [Google Scholar] [CrossRef]
- Siegel, S.J.; Tamashiro, E.; Weiser, J.N. Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage Trafficking. PLoS Pathog. 2015, 11, e1005004. [Google Scholar] [CrossRef]
- Arora, A.; Singh, A. Exploring the role of neutrophils in infectious and noninfectious pulmonary disorders. Int. Rev. Immunol. 2023, 24, 1–21. [Google Scholar] [CrossRef]
- Palmer, C.S.; Kimmey, J.M. Neutrophil Recruitment in Pneumococcal Pneumonia. Front. Cell. Infect. Microbiol. 2022, 12, 894644. [Google Scholar] [CrossRef]
- Karimabad, M.N.; Kounis, N.G.; Hassanshahi, G.; Hassanshahi, F.; Mplani, V.; Koniari, I.; Hung, M.Y.; Nadimi, A.E. The Involvement of CXC Motif Chemokine Ligand 10 (CXCL10) and Its Related Chemokines in the Pathogenesis of Coronary Artery Disease and in the COVID-19 Vaccination: A Narrative Review. Vaccines 2021, 9, 1224. [Google Scholar] [CrossRef] [PubMed]
- Bruce, K.E.; Rued, B.E.; Tsui, H.T.; Winkler, M.E. The Opp (AmiACDEF) Oligopeptide Transporter Mediates Resistance of Serotype 2 Streptococcus pneumoniae D39 to Killing by Chemokine CXCL10 and Other Antimicrobial Peptides. J. Bacteriol. 2018, 200, 10-1128. [Google Scholar] [CrossRef]
- Kolli, D.; Gupta, M.R.; Sbrana, E.; Velayutham, T.S.; Chao, H.; Casola, A.; Garofalo, R.P. Alveolar macrophages contribute to the pathogenesis of human metapneumovirus infection while protecting against respiratory syncytial virus infection. Am. J. Respir. Cell Mol. Biol. 2014, 51, 502–515. [Google Scholar] [CrossRef]
- Fernandes, T.D.; Cunha, L.D.; Ribeiro, J.M.; Massis, L.M.; Lima-Junior, D.S.; Newton, H.J.; Zamboni, D.S. Murine Alveolar Macrophages Are Highly Susceptible to Replication of Coxiella burnetii Phase II In Vitro. Infect. Immun. 2016, 84, 2439–2448. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elean, M.; Raya Tonetti, F.; Fukuyama, K.; Arellano-Arriagada, L.; Namai, F.; Suda, Y.; Gobbato, N.; Nishiyama, K.; Villena, J.; Kitazawa, H. Immunobiotic Ligilactobacillus salivarius FFIG58 Confers Long-Term Protection against Streptococcus pneumoniae. Int. J. Mol. Sci. 2023, 24, 15773. https://doi.org/10.3390/ijms242115773
Elean M, Raya Tonetti F, Fukuyama K, Arellano-Arriagada L, Namai F, Suda Y, Gobbato N, Nishiyama K, Villena J, Kitazawa H. Immunobiotic Ligilactobacillus salivarius FFIG58 Confers Long-Term Protection against Streptococcus pneumoniae. International Journal of Molecular Sciences. 2023; 24(21):15773. https://doi.org/10.3390/ijms242115773
Chicago/Turabian StyleElean, Mariano, Fernanda Raya Tonetti, Kohtaro Fukuyama, Luciano Arellano-Arriagada, Fu Namai, Yoshihito Suda, Nadia Gobbato, Keita Nishiyama, Julio Villena, and Haruki Kitazawa. 2023. "Immunobiotic Ligilactobacillus salivarius FFIG58 Confers Long-Term Protection against Streptococcus pneumoniae" International Journal of Molecular Sciences 24, no. 21: 15773. https://doi.org/10.3390/ijms242115773
APA StyleElean, M., Raya Tonetti, F., Fukuyama, K., Arellano-Arriagada, L., Namai, F., Suda, Y., Gobbato, N., Nishiyama, K., Villena, J., & Kitazawa, H. (2023). Immunobiotic Ligilactobacillus salivarius FFIG58 Confers Long-Term Protection against Streptococcus pneumoniae. International Journal of Molecular Sciences, 24(21), 15773. https://doi.org/10.3390/ijms242115773







