Receptor-Independent Therapies for Forensic Detainees with Schizophrenia–Dementia Comorbidity
Abstract
:1. Introduction
2. The Neuroscience of Criminal Behavior
Interoceptive Awareness and Anosognosia
3. Schizophrenia as a Segmental Progeria
Microbial Translocation
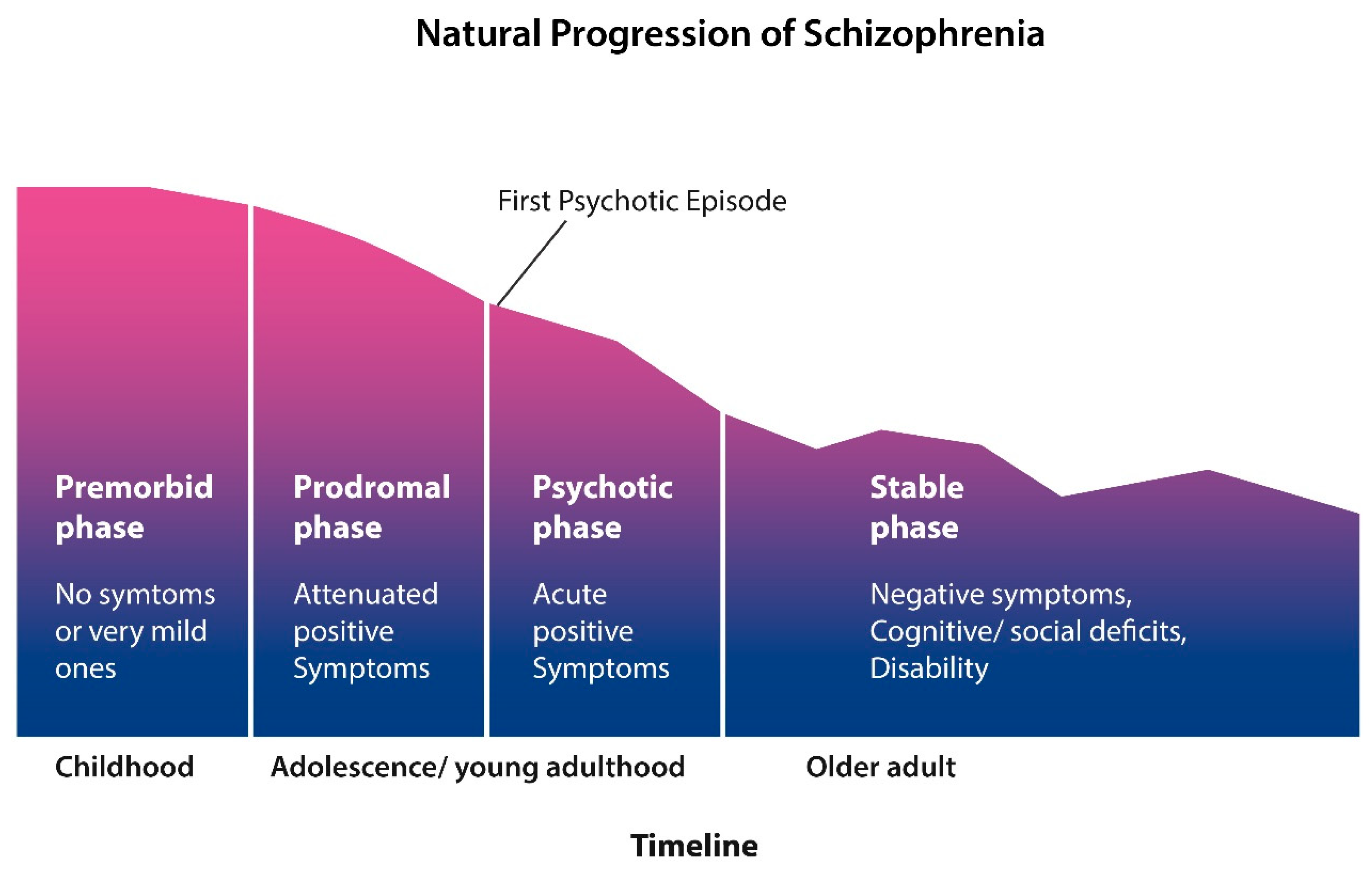
4. Schizophrenia Outcome Studies—Kraepelin Was Right!
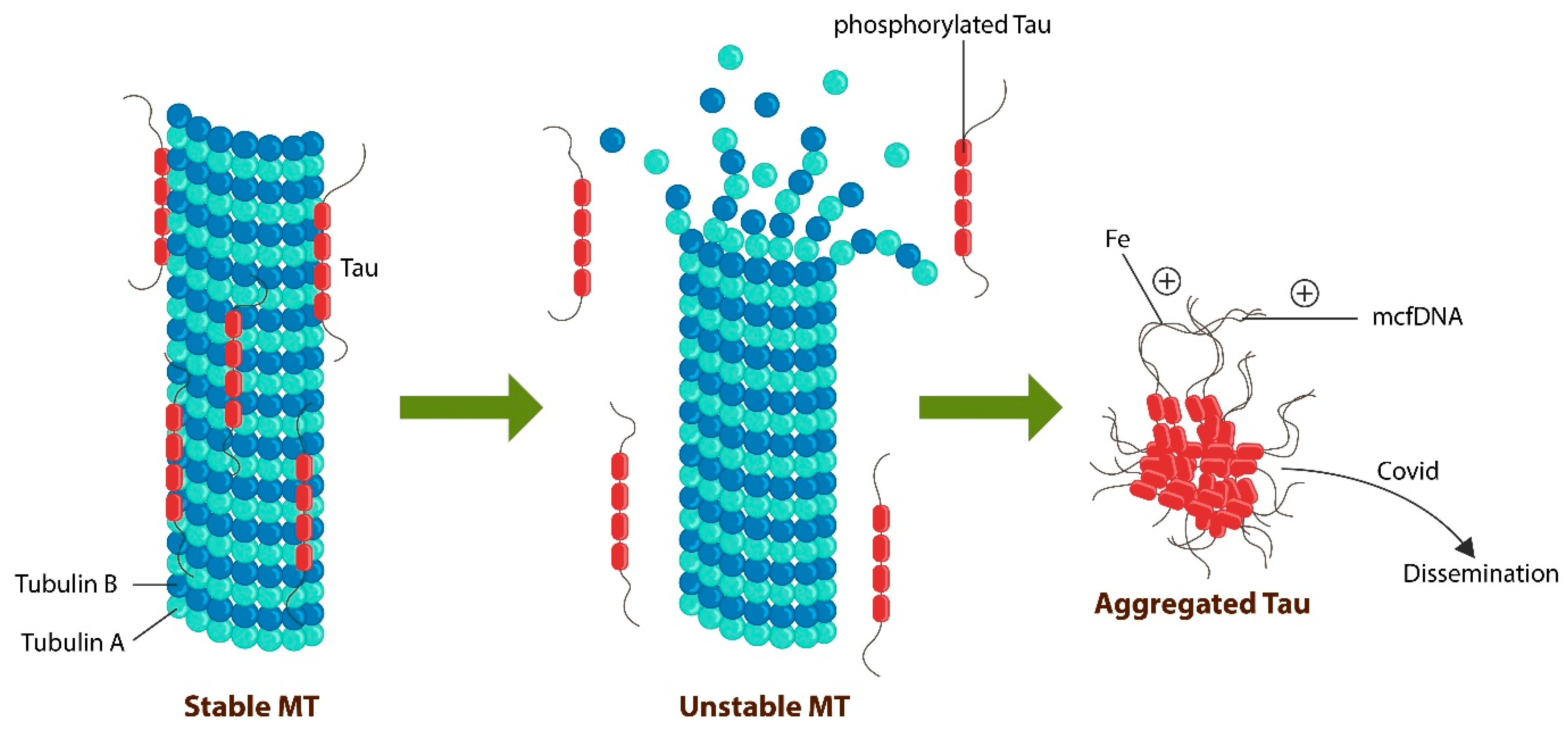
5. The Molecular Basis of SCZ and Dementia: Tau Protein Loss of Function
Chemo-Brain and Transplants
6. bvFTD: From Insight to Psychopathy
7. Neuropathological Basis of bvFTD
8. Dementia in People Living with Schizophrenia (PLWS), Potential Biomarkers
9. Chronic Traumatic Encephalopathy
10. Interventions: Receptor-Independent Antipsychotic Treatments
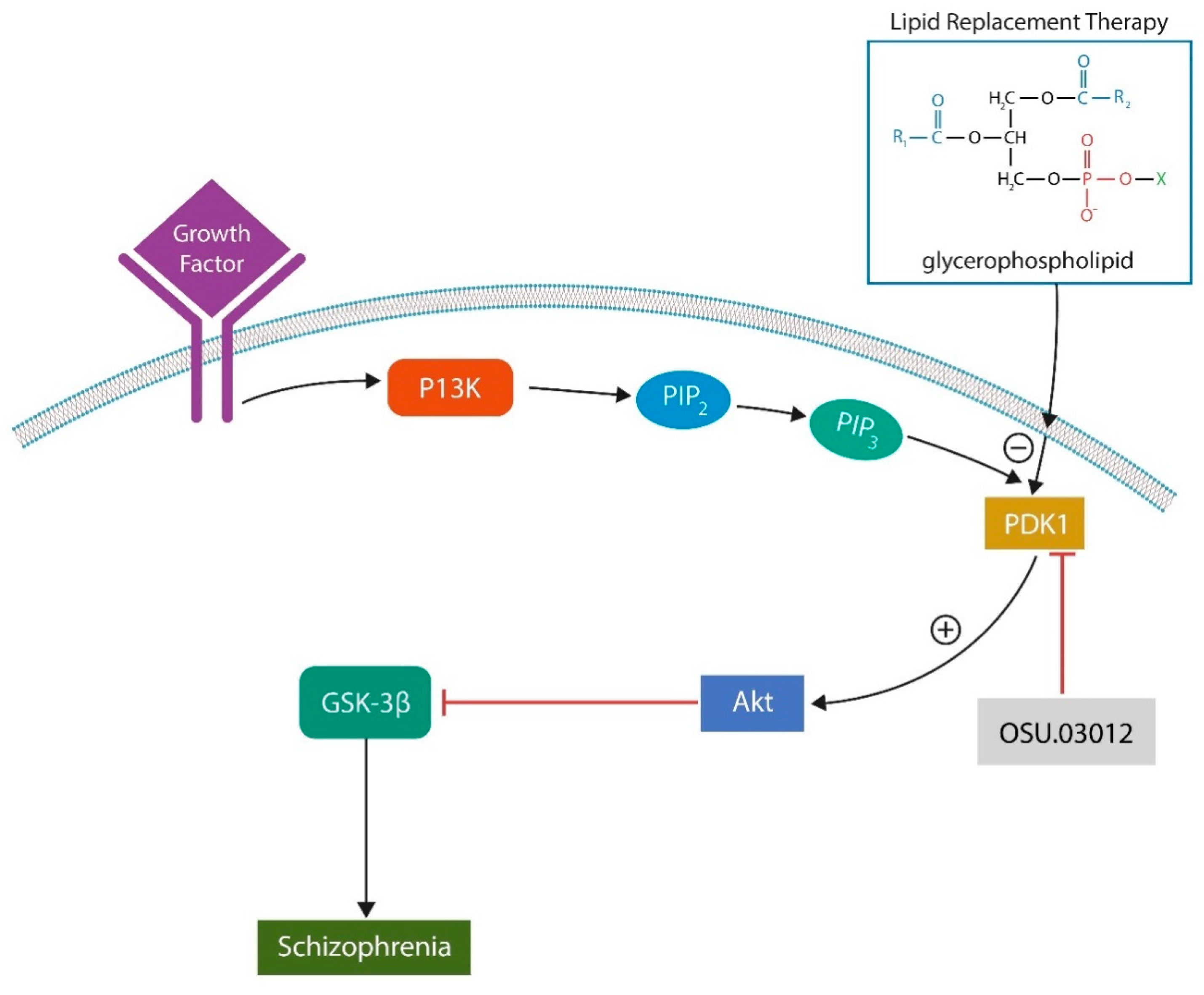
11. Phosphoinositide-Dependent Kinase 1 (PDK-1) Inhibitors
12. Recombinant Human Interleukin-22 (IL-22)
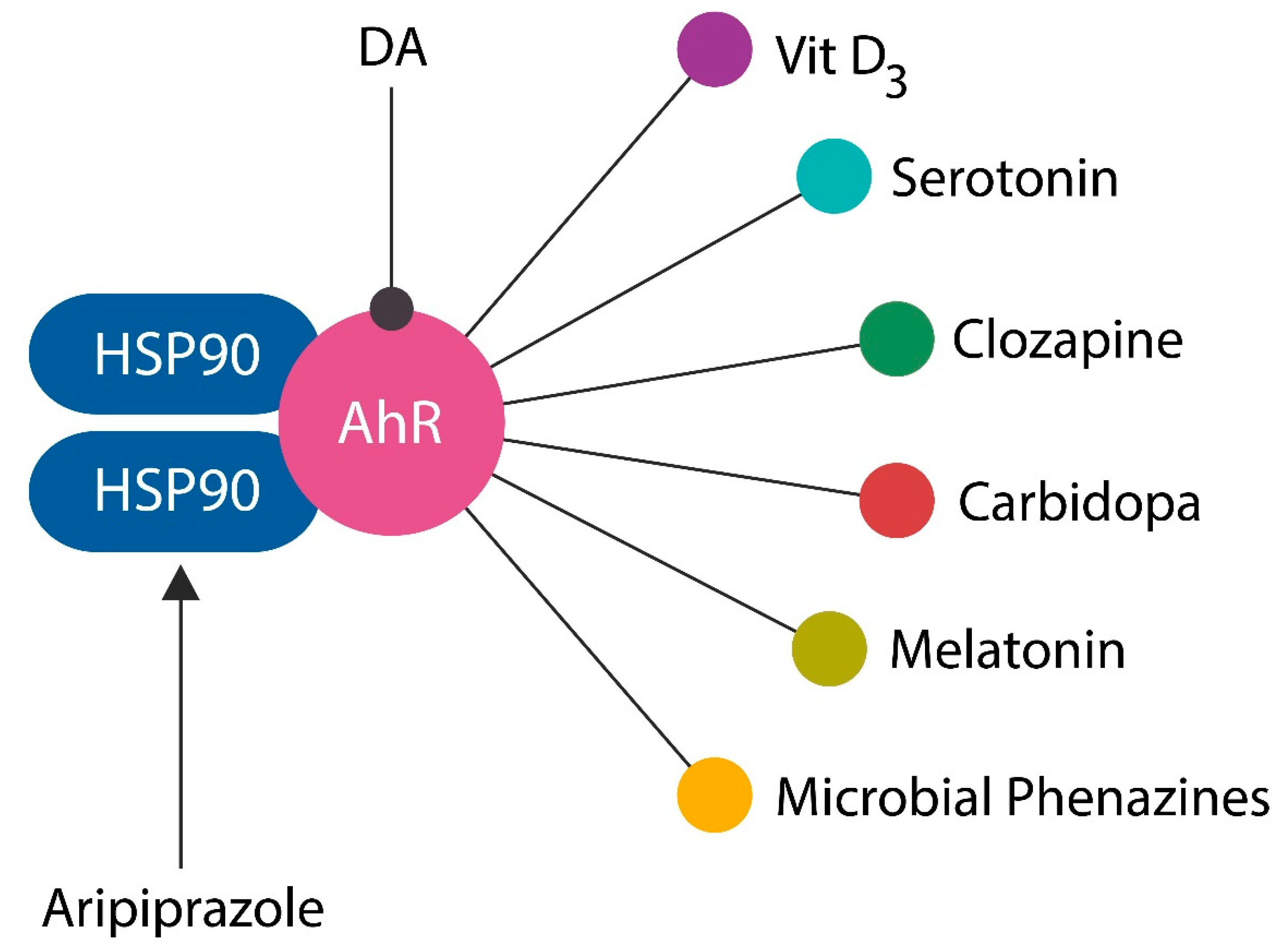
13. Aryl Hydrocarbon Receptor (AhR) Antagonists
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mendez, M.F. The neurobiology of moral behavior: Review and neuropsychiatric implications. CNS Spectr. 2009, 14, 608–620. [Google Scholar] [CrossRef]
- Savopoulos, P.; Lindell, A.K. Born criminal? Differences in structural, functional and behavioural lateralization between criminals and noncriminals. Laterality 2018, 23, 738–760. [Google Scholar] [CrossRef]
- Mendez, M.F.; Shapira, J.S.; Saul, R.E. The spectrum of sociopathy in dementia. J. Neuropsychiatry Clin. Neurosci. 2011, 23, 132–140. [Google Scholar] [CrossRef]
- Lehrer, D.S.; Lorenz, J. Anosognosia in schizophrenia: Hidden in plain sight. Innov. Clin. Neurosci. 2014, 11, 10–17. [Google Scholar]
- Räsänen, P.; Tiihonen, J.; Isohanni, M.; Rantakallio, P.; Lehtonen, J.; Moring, J. Schizophrenia, alcohol abuse, and violent behavior: A 26-year followup study of an unselected birth cohort. Schizophr. Bull. 1998, 24, 437–441. [Google Scholar] [CrossRef]
- Polat, H.; Uğur, K.; Aslanoğlu, E.; Yagin, F.H. The effect of functional remission and cognitive insight on criminal behavior in patients with schizophrenia. Arch. Psychiatr. Nurs. 2023, 45, 176–183. [Google Scholar] [CrossRef]
- Lien, Y.J.; Chang, H.A.; Kao, Y.C.; Tzeng, N.S.; Lu, C.W.; Loh, C.H. Insight, self-stigma and psychosocial outcomes in Schizophrenia: A structural equation modelling approach. Epidemiol. Psychiatr. Sci. 2018, 27, 176–185. [Google Scholar] [CrossRef]
- Lincoln, T.M.; Hodgins, S. Is lack of insight associated with physically aggressive behavior among people with schizophrenia living in the community? J. Nerv. Ment. Dis. 2008, 196, 62–66. [Google Scholar] [CrossRef]
- Buckley, P.F.; Hrouda, D.R.; Friedman, L.; Noffsinger, S.G.; Resnick, P.J.; Camlin-Shingler, K. Insight and its relationship to violent behavior in patients with schizophrenia. Am. J. Psychiatry 2004, 161, 1712–1714. [Google Scholar] [CrossRef]
- Zago, S.; Scarpazza, C.; Difonzo, T.; Arighi, A.; Hajhajate, D.; Torrente, Y.; Sartori, G. Behavioral Variant of Frontotemporal Dementia and Homicide in a Historical Case. J. Am. Acad. Psychiatry Law 2021, 49, 219–227. [Google Scholar] [CrossRef]
- Wander, C. Schizophrenia: Opportunities to improve outcomes and reduce economic burden through managed care. Am. J. Manag. Care 2020, 26 (Suppl. S3), S62–S68. [Google Scholar] [PubMed]
- Dregan, A.; McNeill, A.; Gaughran, F.; Jones, P.B.; Bazley, A.; Cross, S.; Lillywhite, K.; Armstrong, D.; Smith, S.; Osborn, D.P.J.; et al. Potential gains in life expectancy from reducing amenable mortality among people diagnosed with serious mental illness in the United Kingdom. PLoS ONE 2020, 15, e0230674. [Google Scholar] [CrossRef]
- Taipale, H.; Tanskanen, A.; Mehtälä, J.; Vattulainen, P.; Correll, C.U.; Tiihonen, J. 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry 2020, 19, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Stoliker, B.E.; Kerodal, A.G.; Jewell, L.M.; Brown, K.; Kent-Wilkinson, A.; Peacock, S.; O’Connell, M.E.; Wormith, J.S. Older people in custody in a forensic psychiatric facility, prevalence of dementia, and community reintegration needs: An exploratory analysis. Health Justice 2022, 10, 3. [Google Scholar] [CrossRef]
- Cai, L.; Huang, J. Schizophrenia and risk of dementia: A meta-analysis study. Neuropsychiatr. Dis. Treat. 2018, 14, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Kales, H.C.; Valenstein, M.; Kim, H.M.; McCarthy, J.F.; Ganoczy, D.; Cunningham, F.; Blow, F.C. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am. J. Psychiatry 2007, 164, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Bronskill, S.E.; Normand, S.L.; Anderson, G.M.; Sykora, K.; Lam, K.; Bell, C.M.; Lee, P.E.; Fischer, H.D.; Herrmann, N.; et al. Antipsychotic drug use and mortality in older adults with dementia. Ann. Intern. Med. 2007, 146, 775–786. [Google Scholar] [CrossRef]
- Randle, J.M.; Heckman, G.; Oremus, M.; Ho, J. Intermittent antipsychotic medication and mortality in institutionalized older adults: A scoping review. Int. J. Geriatr. Psychiatry 2019, 34, 906–920. [Google Scholar] [CrossRef]
- Desai, V.C.; Heaton, P.C.; Kelton, C.M. Impact of the Food and Drug Administration’s antipsychotic black box warning on psychotropic drug prescribing in elderly patients with dementia in outpatient and office-based settings. Alzheimers Dement. 2012, 8, 453–457. [Google Scholar] [CrossRef]
- Tessier, C.; Sweers, K.; Frajerman, A.; Bergaoui, H.; Ferreri, F.; Delva, C.; Lapidus, N.; Lamaziere, A.; Roiser, J.P.; De Hert, M.; et al. Membrane lipidomics in schizophrenia patients: A correlational study with clinical and cognitive manifestations. Transl. Psychiatry 2016, 6, e906. [Google Scholar] [CrossRef]
- Maxwell, C.R.; Kanes, S.J.; Abel, T.; Siegel, S.J. Phosphodiesterase inhibitors: A novel mechanism for receptor-independent antipsychotic medications. Neuroscience 2004, 129, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Shen, W.; Day, M.; Gertler, T.; Chan, S.; Tian, X.; Plotkin, J.L. The role of dopamine in modulating the structure and function of striatal circuits. Prog. Brain Res. 2010, 183, 149–167. [Google Scholar] [CrossRef]
- Shen, W.; Flajolet, M.; Greengard, P.; Surmeier, D.J. Dichotomous dopaminergic control of striatal synaptic plasticity. Science 2008, 321, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kai, L.; Day, M.; Ronesi, J.; Yin, H.H.; Ding, J.; Tkatch, T.; Lovinger, D.M.; Surmeier, D.J. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron 2006, 50, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.; Voineskos, D.; Daskalakis, Z.J.; Rajji, T.K.; Blumberger, D.M. A Review of Impaired Neuroplasticity in Schizophrenia Investigated with Non-invasive Brain Stimulation. Front. Psychiatry 2016, 7, 45. [Google Scholar] [CrossRef]
- Goel, P.; Chakrabarti, S.; Goel, K.; Bhutani, K.; Chopra, T.; Bali, S. Neuronal cell death mechanisms in Alzheimer’s disease: An insight. Front. Mol. Neurosci. 2022, 15, 937133. [Google Scholar] [CrossRef]
- Berry, A.S.; Shah, V.D.; Baker, S.L.; Vogel, J.W.; O’Neil, J.P.; Janabi, M.; Schwimmer, H.D.; Marks, S.M.; Jagust, W.J. Aging Affects Dopaminergic Neural Mechanisms of Cognitive Flexibility. J. Neurosci. 2016, 36, 12559–12569. [Google Scholar] [CrossRef] [PubMed]
- Braskie, M.N.; Wilcox, C.E.; Landau, S.M.; O’Neil, J.P.; Baker, S.L.; Madison, C.M.; Kluth, J.T.; Jagust, W.J. Relationship of striatal dopamine synthesis capacity to age and cognition. J. Neurosci. 2008, 28, 14320–14328. [Google Scholar] [CrossRef]
- Huber, M.; Beyer, L.; Prix, C.; Schönecker, S.; Palleis, C.; Rauchmann, B.S.; Morbelli, S.; Chincarini, A.; Bruffaerts, R.; Vandenberghe, R.; et al. Metabolic Correlates of Dopaminergic Loss in Dementia with Lewy Bodies. Mov. Disord. 2020, 35, 595–605. [Google Scholar] [CrossRef]
- Lubec, J.; Kalaba, P.; Hussein, A.M.; Feyissa, D.D.; Kotob, M.H.; Mahmmoud, R.R.; Wieder, O.; Garon, A.; Sagheddu, C.; Ilic, M.; et al. Reinstatement of synaptic plasticity in the aging brain through specific dopamine transporter inhibition. Mol. Psychiatry 2021, 26, 7076–7090. [Google Scholar] [CrossRef]
- Canfrán-Duque, A.; Pastor, Ó.; García-Seisdedos, D.; Molina, Y.L.; Babiy, B.; Lerma, M.; Sánchez-Castellano, C.; Martínez-Botas, J.; Gómez-Coronado, D.; Lasunción, M.A.; et al. The Antipsychotic Risperidone Alters Dihydroceramide and Ceramide Composition and Plasma Membrane Function in Leukocytes In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 3919. [Google Scholar] [CrossRef] [PubMed]
- Vantaggiato, C.; Panzeri, E.; Citterio, A.; Orso, G.; Pozzi, M. Antipsychotics Promote Metabolic Disorders Disrupting Cellular Lipid Metabolism and Trafficking. Trends Endocrinol. Metab. 2019, 30, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Au, E.; Agarwal, S.M.; Wright, D.C.; Hahn, M.K. Antipsychotic-Induced Alterations in Lipid Turnover. Endocrinology 2023, 164, bqad025. [Google Scholar] [CrossRef]
- Dietrich-Muszalska, A.; Kolińska-Łukaszuk, J. Comparative effects of aripiprazole and selected antipsychotic drugs on lipid peroxidation in plasma. Psychiatry Clin. Neurosci. 2018, 72, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, O.F.; Toprak, M.; Noory, M.A.; Robertson, G.P. Effect of lysosomotropic molecules on cellular homeostasis. Pharmacol. Res. 2017, 117, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Róg, T.; Girych, M.; Bunker, A. Mechanistic Understanding from Molecular Dynamics in Pharmaceutical Research 2: Lipid Membrane in Drug Design. Pharmaceuticals 2021, 14, 1062. [Google Scholar] [CrossRef] [PubMed]
- Elbaradei, A.; Wang, Z.; Malmstadt, N. Oxidation of Membrane Lipids Alters the Activity of the Human Serotonin 1A Receptor. Langmuir 2022, 38, 6798–6807. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, Y.; Wang, D.; Hu, X.; Jiang, J.; Qing, Y.; Yang, X.; Cui, G.; Wang, P.; Zhang, J.; et al. Impaired Membrane Lipid Homeostasis in Schizophrenia. Schizophr. Bull. 2022, 48, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.; Parikh, V.; Terry, A.V., Jr.; Mahadik, S.P. Long-term antipsychotic treatments and crossover studies in rats: Differential effects of typical and atypical agents on the expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J. Psychiatr. Res. 2007, 41, 372–386. [Google Scholar] [CrossRef]
- Oruch, R.; Lund, A.; Pryme, I.F.; Holmsen, H. An intercalation mechanism as a mode of action exerted by psychotropic drugs: Results of altered phospholipid substrate availabilities in membranes? J. Chem. Biol. 2010, 3, 67–88. [Google Scholar] [CrossRef]
- Joshi, Y.B.; Praticò, D. Lipid peroxidation in psychiatric illness: Overview of clinical evidence. Oxid. Med. Cell Longev. 2014, 2014, 828702. [Google Scholar] [CrossRef]
- Chong, S.A.; Mythily, M.B.B.S.; Remington, G. Tardive dyskinesia and iron status. J. Clin. Psychopharmacol. 2004, 24, 235–236. [Google Scholar] [CrossRef] [PubMed]
- Calarge, C.A.; Ziegler, E.E.; Del Castillo, N.; Aman, M.; McDougle, C.J.; Scahill, L.; McCracken, J.T.; Arnold, L.E. Iron homeostasis during risperidone treatment in children and adolescents. J. Clin. Psychiatry 2015, 76, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Dichtl, S.; Demetz, E.; Haschka, D.; Tymoszuk, P.; Petzer, V.; Nairz, M.; Seifert, M.; Hoffmann, A.; Brigo, N.; Würzner, R.; et al. Dopamine Is a Siderophore-Like Iron Chelator That Promotes Salmonella enterica Serovar Typhimurium Virulence in Mice. Mbio 2019, 10, e02624-18. [Google Scholar] [CrossRef]
- Jiang, P.; Gan, M.; Yen, S.H. Dopamine prevents lipid peroxidation-induced accumulation of toxic α-synuclein oligomers by preserving autophagy-lysosomal function. Front. Cell Neurosci. 2013, 7, 81. [Google Scholar] [CrossRef]
- Liao, J.; Wang, L.; Wu, Z.; Wang, Z.; Chen, J.; Zhong, Y.; Jiang, F.; Lu, Y. Identification of phenazine analogue as a novel scaffold for thioredoxin reductase I inhibitors against Hep G2 cancer cell lines. J. Enzym. Inhib. Med. Chem. 2019, 34, 1158–1163. [Google Scholar] [CrossRef]
- McRose, D.L.; Li, J.; Newman, D.K. The chemical ecology of coumarins and phenazines affects iron acquisition by pseudomonads. Proc. Natl. Acad. Sci. USA 2023, 120, e2217951120. [Google Scholar] [CrossRef]
- Vita, A.; De Peri, L.; Deste, G.; Barlati, S.; Sacchetti, E. The Effect of Antipsychotic Treatment on Cortical Gray Matter Changes in Schizophrenia: Does the Class Matter? A Meta-analysis and Meta-regression of Longitudinal Magnetic Resonance Imaging Studies. Biol. Psychiatry 2015, 78, 403–412. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Liao, J.; Jiang, S.; Yan, J.; Yue, W.; Zhang, D.; Yan, H. Progressive grey matter volume changes in patients with schizophrenia over 6 weeks of antipsychotic treatment and their relationship to clinical improvement. Neurosci. Bull. 2018, 34, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Nitta, Y.; Decarli, N.; de Sousa, L.E.; Stachelek, P.; Tohnai, N.; Minakata, S.; de Silva, P.; Data, P.; Takeda, Y. Revealing Internal Heavy Chalcogen Atom Effect on the Photophysics of Dibenzo[a,j]phenazine-Cored Donor–Acceptor–Donor Triad. ChemRxiv 2021. [Google Scholar] [CrossRef]
- Chen, Y.; Womer, F.Y.; Feng, R.; Zhang, X.; Zhang, Y.; Duan, J.; Chang, M.; Yin, Z.; Jiang, X.; Wei, S.; et al. A Real-World Observation of Antipsychotic Effects on Brain Volumes and Intrinsic Brain Activity in Schizophrenia. Front. Neurosci. 2022, 15, 749316. [Google Scholar] [CrossRef]
- Pustilnik, A.C. Violence on the Brain: A Critique of Neuroscience in Criminal Law. Faculty Scholarship. 2009. Available online: https://digitalcommons.law.umaryland.edu/fac_pubs/1035 (accessed on 23 October 2023).
- Insel, T. Rethinking schizophrenia. Nature 2010, 468, 187–193. [Google Scholar] [CrossRef]
- Warner, R. Recovery from schizophrenia and the recovery model. Curr. Opin. Psychiatry 2009, 22, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Sfera, A. Six Decades of Dopamine Hypothesis: Is Aryl Hydrocarbon Receptor the New D2? Reports 2023, 6, 36. [Google Scholar] [CrossRef]
- Williams, S.S. The terrorist inside my husband’s brain. Neurology 2016, 87, 1308–1311. [Google Scholar] [CrossRef]
- Sajous-Turner, A.; Anderson, N.E.; Widdows, M.; Nyalakanti, P.; Harenski, K.; Harenski, C.; Koenigs, M.; Decety, J.; Kiehl, K.A. Aberrant brain gray matter in murderers. Brain Imaging Behav. 2020, 14, 2050–2061. [Google Scholar] [CrossRef]
- Parton, A.; Malhotra, P.; Husain, M. Hemispatial neglect. J. Neurol. Neurosurg. Psychiatry 2004, 75, 13–21. [Google Scholar] [PubMed]
- Little, J.D.; Bell, E. Anosognosia and schizophrenia—A reminder. Australas Psychiatry 2021, 29, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Little, J.D. In schizophrenia, are lack of capacity and lack of insight more usefully understood as anosognosia? Australas Psychiatry 2021, 29, 346–348. [Google Scholar] [CrossRef]
- Torregrossa, L.J.; Amedy, A.; Roig, J.; Prada, A.; Park, S. Interoceptive functioning in schizophrenia and schizotypy. Schizophr. Res. 2022, 239, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Thakkar, K. Interoception abnormalities in schizophrenia: A review of preliminary evidence and an integration with Bayesian accounts of psychosis. Neurosci. Biobehav. Rev. 2022, 132, 757–773. [Google Scholar] [CrossRef] [PubMed]
- Ribolsi, M.; Daskalakis, Z.J.; Siracusano, A.; Koch, G. Abnormal asymmetry of brain connectivity in schizophrenia. Front. Hum. Neurosci. 2014, 8, 1010. [Google Scholar] [CrossRef] [PubMed]
- Brüne, M.; Schöbel, A.; Karau, R.; Benali, A.; Faustmann, P.M.; Juckel, G.; Petrasch-Parwez, E. Von Economo neuron density in the anterior cingulate cortex is reduced in early onset schizophrenia. Acta Neuropathol. 2010, 119, 771–778. [Google Scholar] [CrossRef]
- Brasso, C.; Stanziano, M.; Bosco, F.M.; Morese, R.; Valentini, M.C.; Vercelli, A.; Rocca, P. Alteration of the Functional Connectivity of the Cortical Areas Characterized by the Presence of Von Economo Neurons in Schizophrenia, a Pilot Study. J. Clin. Med. 2023, 12, 1377. [Google Scholar] [CrossRef] [PubMed]
- López-Ojeda, W.; Hurley, R.A. Von Economo Neuron Involvement in Social Cognitive and Emotional Impairments in Neuropsychiatric Disorders. J. Neuropsychiatry Clin. Neurosci. 2022, 34, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Kraepelin, E. Psychiatry; A Textbook for Students and Physicians; Metoui, H.; Ayed, S., Translators; Science History Publications: Canton, MA, USA, 1990; Volume 2. [Google Scholar]
- Voruz, P.; Cionca, A.; Jacot de Alcântara, I.; Nuber-Champier, A.; Allali, G.; Benzakour, L.; Thomasson, M.; Lalive, P.H.; Lövblad, K.O.; Braillard, O.; et al. Functional connectivity underlying cognitive and psychiatric symptoms in post-COVID-19 syndrome: Is anosognosia a key determinant? Brain Commun. 2022, 4, fcac057. [Google Scholar] [CrossRef] [PubMed]
- Juengst, S.; Skidmore, E.; Pramuka, M.; McCue, M.; Becker, J. Factors contributing to impaired self-awareness of cognitive functioning in an HIV positive and at-risk population. Disabil. Rehabil. 2012, 34, 19–25. [Google Scholar] [CrossRef]
- Allman, J.M.; Tetreault, N.A.; Hakeem, A.Y.; Park, S. The von Economo neurons in apes and humans. Am. J. Hum. Biol. 2011, 23, 5–21. [Google Scholar] [CrossRef]
- Dickman, M.S. von Economo encephalitis. Arch Neurol. 2001, 58, 1696–1698. [Google Scholar] [CrossRef]
- Vilensky, J.A.; Foley, P.; Gilman, S. Children and encephalitis lethargica: A historical review. Pediatr Neurol. 2007, 37, 79–84. [Google Scholar] [CrossRef]
- Deka, K.; Bhuyan, D.; Chaudhury, P.K. Conduct disorder-A sequelae of viral encephalitis. Indian J. Psychiatry 2006, 48, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, V. ‘A disease that makes criminals’: Encephalitis lethargica (EL) in children, mental deficiency, and the 1927 Mental Deficiency Act. Endeavour 2015, 39, 44–51. [Google Scholar] [CrossRef]
- Cauda, F.; Nani, A.; Costa, T.; Palermo, S.; Tatu, K.; Manuello, J.; Duca, S.; Fox, P.T.; Keller, R. The morphometric co-atrophy networking of schizophrenia, autistic and obsessive spectrum disorders. Hum. Brain Mapp. 2018, 39, 1898–1928. [Google Scholar] [CrossRef]
- Papanastasiou, E.; Gaughran, F.; Smith, S. Schizophrenia as segmental progeria. J. R. Soc. Med. 2011, 104, 475–484. [Google Scholar] [CrossRef]
- Yu, W.Y.; Chang, H.W.; Lin, C.H.; Cho, C.L. Short telomeres in patients with chronic schizophrenia who show a poor response to treatment. J. Psychiatry Neurosci. 2008, 33, 244–247, Erratum in J. Psychiatry Neurosci. 2008, 33, 343.. [Google Scholar]
- Laursen, T.M. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr. Res. 2011, 131, 101–104. [Google Scholar] [CrossRef]
- Peritogiannis, V.; Ninou, A.; Samakouri, M. Mortality in Schizophrenia-Spectrum Disorders: Recent Advances in Understanding and Management. Healthcare 2022, 10, 2366. [Google Scholar] [CrossRef]
- Feng, Y.; Shen, J.; He, J.; Lu, M. Schizophrenia and cell senescence candidate genes screening, machine learning, diagnostic models, and drug prediction. Front. Psychiatry 2023, 14, 1105987. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Ishida, I.; Ogura, J.; Aizawa, E.; Ota, M.; Hidese, S.; Yomogida, Y.; Matsuo, J.; Yoshida, S.; Kunugi, H. Gut permeability and its clinical relevance in schizophrenia. Neuropsychopharmacol. Rep. 2022, 42, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cong, L.; Lukiw, W.J. Lipopolysaccharide (LPS) Accumulates in Neocortical Neurons of Alzheimer’s Disease (AD) Brain and Impairs Transcription in Human Neuronal-Glial Primary Co-cultures. Front. Aging Neurosci. 2017, 9, 407. [Google Scholar] [CrossRef]
- Sung, K.Y.; Zhang, B.; Wang, H.E.; Bai, Y.M.; Tsai, S.J.; Su, T.P.; Chen, T.J.; Hou, M.C.; Lu, C.L.; Wang, Y.P.; et al. Schizophrenia and risk of new-onset inflammatory bowel disease: A nationwide longitudinal study. Aliment. Pharmacol. Ther. 2022, 55, 1192–1201. [Google Scholar] [CrossRef]
- Yanuck, S.F. Microglial Phagocytosis of Neurons: Diminishing Neuronal Loss in Traumatic, Infectious, Inflammatory, and Autoimmune CNS Disorders. Front. Psychiatry 2019, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Secher, T.; Samba-Louaka, A.; Oswald, E.; Nougayrède, J.P. Escherichia coli producing colibactin triggers premature and transmissible senescence in mammalian cells. PLoS ONE 2013, 8, e77157. [Google Scholar] [CrossRef]
- Ma, Q.; Gao, F.; Zhou, L.; Fan, Y.; Zhao, B.; Xi, W.; Wang, C.; Zhu, F.; Ma, X.; Wang, W.; et al. Characterizing serum amino acids in schizophrenic patients: Correlations with gut microbes. J. Psychiatr. Res. 2022, 153, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Dietrich-Muszalska, A.; Kolodziejczyk-Czepas, J.; Nowak, P. Comparative Study of the Effects of Atypical Antipsychotic Drugs on Plasma and Urine Biomarkers of Oxidative Stress in Schizophrenic Patients. Neuropsychiatr. Dis. Treat. 2021, 17, 555–565. [Google Scholar] [CrossRef]
- Khan, M.M.; Evans, D.R.; Gunna, V.; Scheffer, R.E.; Parikh, V.V.; Mahadik, S.P. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr. Res. 2002, 58, 1–10. [Google Scholar] [CrossRef]
- Lotan, A.; Luza, S.; Opazo, C.M.; Ayton, S.; Lane, D.J.R.; Mancuso, S.; Pereira, A.; Sundram, S.; Weickert, C.S.; Bousman, C.; et al. Perturbed iron biology in the prefrontal cortex of people with schizophrenia. Mol. Psychiatry 2023, 28, 2058–2070. [Google Scholar] [CrossRef] [PubMed]
- Killilea, D.W.; Atamna, H.; Liao, C.; Ames, B.N. Iron accumulation during cellular senescence in human fibroblasts in vitro. Antioxid. Redox Signal. 2003, 5, 507–516. [Google Scholar] [CrossRef]
- Santillo, A.F.; Nilsson, C.; Englund, E. von Economo neurones are selectively targeted in frontotemporal dementia. Neuropathol. Appl. Neurobiol. 2013, 39, 572–579. [Google Scholar] [CrossRef]
- Lin, L.C.; Nana, A.L.; Hepker, M.; Hwang, J.L.; Gaus, S.E.; Spina, S.; Cosme, C.G.; Gan, L.; Grinberg, L.T.; Geschwind, D.H.; et al. Preferential tau aggregation in von Economo neurons and fork cells in frontotemporal lobar degeneration with specific MAPT variants. Acta Neuropathol. Commun. 2019, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Guo, Z. Recent progress in ferroptosis: Inducers and inhibitors. Cell Death Discov. 2022, 8, 501. [Google Scholar] [CrossRef]
- Ma, H.; Dong, Y.; Chu, Y.; Guo, Y.; Li, L. The mechanisms of ferroptosis and its role in alzheimer’s disease. Front. Mol. Biosci. 2022, 9, 965064. [Google Scholar] [CrossRef]
- Nuñez, M.T.; Chana-Cuevas, P. New Perspectives in Iron Chelation Therapy for the Treatment of Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 109. [Google Scholar] [CrossRef]
- Huxley, P.; Krayer, A.; Poole, R.; Prendergast, L.; Aryal, S.; Warner, R. Schizophrenia outcomes in the 21st century: A systematic review. Brain Behav. 2021, 11, e02172. [Google Scholar] [CrossRef]
- Robinson, D.G.; Woerner, M.G.; McMeniman, M.; Mendelowitz, A.; Bilder, R.M. Symptomatic and functional recovery from a first episode of schizophrenia or schizoaffective disorder. Am. J. Psychiatry 2004, 161, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.; Hopper, K.; Craig, T.; Laska, E.; Siegel, C.; Wanderling, J.; Dube, K.C.; Ganev, K.; Giel, R.; Der Heiden, W.A.; et al. Recovery from psychotic illness: A 15- and 25-year international follow-up study. Br. J. Psychiatry 2001, 178, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.; Taipale, H.; Tanskanen, A.; Tiihonen, J.; Mitterdorfer-Rutz, E. Employment among people with schizophrenia or bipolar disorder: A population-based study using nationwide registers. Acta Psychiatr. Scand. 2020, 143, 61–71. [Google Scholar] [CrossRef]
- Lévesque, I.S.; Abdel-Baki, A. Homeless youth with first-episode psychosis: A 2-year outcome study. Schizophr. Res. 2019, 216, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, E.; Juola, P.; Hirvonen, N.; McGrath, J.J.; Saha, S.; Isohanni, M.; Veijola, J.; Miettunen, J. A Systematic Review and Meta-Analysis of Recovery in Schizophrenia. Schizophr. Bull. 2012, 39, 1296–1306. [Google Scholar] [CrossRef]
- Kotov, R.; Fochtmann, L.; Li, K.; Tanenberg-Karant, M.; Constantino, E.A.; Rubinstein, J.; Perlman, G.; Velthorst, E.; Fett, A.-K.J.; Carlson, G.; et al. One hundred years of schizophrenia: A meta-analysis of the outcome literature. Am. J. Psychiatry 1994, 151, 1409–1416. [Google Scholar]
- Vita, A.; De Peri, L.; Deste, G.; Sacchetti, E. Progressive loss of cortical gray matter in schizophrenia: A meta-analysis and meta-regression of longitudinal MRI studies. Transl. Psychiatry 2012, 2, e190. [Google Scholar] [CrossRef]
- Lieberman, J.A. Neurobiology and the natural history of schizophrenia. J. Clin. Psychiatry 2006, 67, e14. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Smieskova, R.; Kempton, M.; Ho, B.; Andreasen, N.; Borgwardt, S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci. Biobehav. Rev. 2013, 37, 1680–1691. [Google Scholar] [CrossRef]
- Ho, B.C.; Andreasen, N.C.; Ziebell, S.; Pierson, R.; Magnotta, V. Long-term antipsychotic treatment and brain volumes: A longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry 2011, 68, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Spalletta, G.; Piras, F.; Piras, F.; Sancesario, G.; Iorio, M.; Fratangeli, C.; Cacciari, C.; Caltagirone, C.; Orfei, M.D. Neuroanatomical correlates of awareness of illness in patients with amnestic mild cognitive impairment who will or will not convert to Alzheimer’s disease. Cortex 2014, 61, 183–195. [Google Scholar] [CrossRef]
- Ting, C.; Rajji, T.K.; Ismail, Z.; Tang-Wai, D.F.; Apanasiewicz, N.; Miranda, D.; Mamo, D.; Mulsant, B.H. Differentiating the cognitive profile of schizophrenia from that of Alzheimer disease and depression in late life. PLoS ONE 2010, 5, e10151. [Google Scholar] [CrossRef]
- Dehmelt, L.; Halpain, S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005, 6, 204. [Google Scholar] [CrossRef]
- Hameroff, S. Consciousness, Cognition and the Neuronal Cytoskeleton—A New Paradigm Needed in Neuroscience. Front. Mol. Neurosci. 2022, 15, 869935. [Google Scholar] [CrossRef]
- Tonello, L.; Cocchi, M.; Gabrielli, F.; Tuszynski, J.A. On the possible quantum role of serotonin in consciousness. J. Integr. Neurosci. 2015, 14, 295–308. [Google Scholar] [CrossRef]
- Craddock, T.J.; Priel, A.; Tuszynski, J.A. Keeping Time: Could Quantum Beating in Microtubules be the Basis for the Neural Synchrony Related to Consciousness? J. Integr. Neurosci. 2014, 13, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Planel, E.; Krishnamurthy, P.; Miyasaka, T.; Liu, L.; Herman, M.; Kumar, A.; Bretteville, A.; Figueroa, H.Y.; Yu, W.H.; Whittington, R.; et al. Anesthesia-induced hyperphosphorylation detaches 3-repeat tau from microtubules without affecting their stability in vivo. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 12798–12807. [Google Scholar] [CrossRef]
- Run, X.; Liang, Z.; Zhang, L.; Iqbal, K.; Grundke-Iqbal, I.; Gong, C.X. Anesthesia induces phosphorylation of tau. J. Alzheimers Dis. 2009, 16, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Rowinsky, E.K.; Chaudhry, V.; Cornblath, D.R.; Donehower, R.C. Neurotoxicity of Taxol. J. Natl. Cancer Inst. Monogr. 1993, 15, 107–115. [Google Scholar]
- Luciani, M.; Montalbano, M.; Troncone, L.; Bacchin, C.; Uchida, K.; Daniele, G.; Jacobs Wolf, B.; Butler, H.M.; Kiel, J.; Berto, S.; et al. Big tau aggregation disrupts microtubule tyrosination and causes myocardial diastolic dysfunction: From discovery to therapy. Eur. Heart J. 2023, 44, 1560–1570. [Google Scholar] [CrossRef]
- Bunzel, B.; Schmidl-Mohl, B.; Grundböck, A.; Wollenek, G. Does changing the heart mean changing personality? A retrospective inquiry on 47 heart transplant patients. Qual. Life Res. 1992, 1, 251–256. [Google Scholar] [CrossRef]
- Pearsall, P.; Schwartz, G.E.; Russek, L.G. Changes in heart transplant recipients that parallel the personalities of their donors. Integr. Med. 2000, 2, 65–72. [Google Scholar] [CrossRef]
- Prinzen, F.W.; Vernooy, K.; Cornelussen, R.N. Cardiac memory and cortical memory. Circulation 2004, 109, e226. [Google Scholar] [CrossRef]
- Liester, M.B. Personality changes following heart transplantation: The role of cellular memory. Med. Hypotheses 2020, 135, 109468. [Google Scholar] [CrossRef]
- Asgari, P.; Jackson, A.C.; Bahramnezhad, F. Adjustment to a New Heart: Concept Analysis Using a Hybrid Model. Iran. J. Nurs. Midwifery Res. 2021, 26, 89–96. [Google Scholar] [CrossRef]
- Letourneau, J.; Holmes, Z.C.; Dallow, E.P.; Durand, H.K.; Jiang, S.; Carrion, V.M.; Gupta, S.K.; Mincey, A.C.; Muehlbauer, M.J.; Bain, J.R.; et al. Ecological memory of prior nutrient exposure in the human gut microbiome. ISME J. 2022, 16, 2479–2490. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; Aussieker, T.; Holwerda, A.; Parise, G.; van Loon, L.J.C.; Verdijk, L.B. The concept of skeletal muscle memory: Evidence from animal and human studies. Acta Physiol. 2020, 229, e13465. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.; Dussutour, A. Direct Transfer of Learned Behaviour via Cell Fusion in Non-Neural Organisms. Proc. Biol. Sci. 2016, 283, 20162382. [Google Scholar] [CrossRef] [PubMed]
- Tetz, G.; Pinho, M.; Pritzkow, S.; Mendez, N.; Soto, C.; Tetz, V. Bacterial DNA promotes Tau aggregation. Sci. Rep. 2020, 10, 2369. [Google Scholar] [CrossRef] [PubMed]
- Andreou, D.; Jørgensen, K.N.; Nerland, S.; Smelror, R.E.; Wedervang-Resell, K.; Johannessen, C.H.; Myhre, A.M.; Andreassen, O.A.; Blennow, K.; Zetterberg, H.; et al. Lower plasma total tau in adolescent psychosis: Involvement of the orbitofrontal cortex. J Psychiatr Res. 2021, 144, 255–261. [Google Scholar] [CrossRef]
- Demirel, Ö.F.; Cetin, I.; Turan, Ş.; Yıldız, N.; Sağlam, T.; Duran, A. Total Tau and Phosphorylated Tau Protein Serum Levels in Patients with Schizophrenia Compared with Controls. Psychiatr. Q 2017, 88, 921–928. [Google Scholar] [CrossRef]
- Grubisha, M.J.; Sun, X.; MacDonald, M.L.; Garver, M.; Sun, Z.; Paris, K.A.; Patel, D.S.; DeGiosio, R.A.; Lewis, D.A.; Yates, N.A.; et al. MAP2 is differentially phosphorylated in schizophrenia, altering its function. Mol. Psychiatry 2021, 26, 5371–5388. [Google Scholar] [CrossRef]
- Craddock, T.J.; Hameroff, S.R.; Ayoub, A.T.; Klobukowski, M.; Tuszynski, J.A. Anesthetics act in quantum channels in brain microtubules to prevent consciousness. Curr. Top. Med. Chem. 2015, 15, 523–533. [Google Scholar] [CrossRef]
- Krause, M.; Theiss, C.; Brüne, M. Ultrastructural Alterations of Von Economo Neurons in the Anterior Cingulate Cortex in Schizophrenia. Anat. Rec. 2017, 300, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Boeve, B.F. Behavioral Variant Frontotemporal Dementia. Continuum 2022, 28, 702–725. [Google Scholar] [CrossRef]
- Seeley, W.W. Behavioral Variant Frontotemporal Dementia. Continuum 2019, 25, 76–100. [Google Scholar] [CrossRef]
- Dols, A.; van Liempt, S.; Gossink, F.; Krudop, W.A.; Sikkes, S.; Pijnenburg, Y.A.; Stek, M.L. Identifying specific clinical symptoms of behavioral variant frontotemporal dementia versus differential psychiatric disorders in patients presenting with a late-onset frontal lobe syndrome. J. Clin. Psychiatry 2016, 77, 1391–1395. [Google Scholar] [CrossRef]
- Cipriani, G.; Danti, S.; Nuti, A.; Di Fiorino, M.; Cammisuli, D.M. Is that schizophrenia or frontotemporal dementia? Supporting clinicians in making the right diagnosis. Acta Neurol. Belg. 2020, 120, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Bott, N.T.; Radke, A.; Stephens, M.L.; Kramer, J.H. Frontotemporal dementia: Diagnosis, deficits and management. Neurodegener. Dis. Manag. 2014, 4, 439–454. [Google Scholar] [CrossRef]
- Ducharme, S.; Dols, A.; Laforce, R.; Devenney, E.; Kumfor, F.; van den Stock, J.; Dallaire-Théroux, C.; Seelaar, H.; Gossink, F.; Vijverberg, E.; et al. Recommendations to distinguish behavioural variant frontotemporal dementia from psychiatric disorders. Brain 2020, 143, 1632–1650. [Google Scholar] [CrossRef]
- Woolley, J.D.; Khan, B.K.; Murthy, N.K.; Miller, B.L.; Rankin, K.P. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: Rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J. Clin. Psychiatry 2011, 72, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Santoro, J.; Bender, F.C.; Laterza, O.F. Quantitative determination of human interleukin 22 (IL-22) in serum using Singulex-Erenna® technology. J. Immunol. Methods 2013, 390, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Liljegren, M.; Naasan, G.; Temlett, J.; Perry, D.C.; Rankin, K.P.; Merrilees, J.; Grinberg, L.T.; Seeley, W.W.; Englund, E.; Miller, B.L. Criminal behavior in frontotemporal dementia and Alzheimer disease. JAMA Neurol. 2015, 72, 295–300. [Google Scholar] [CrossRef]
- Sfera, A.; Osorio, C.; Gradini, R.; Price, A. Neurodegeneration behind bars: From molecules to jurisprudence. Front. Psychiatry 2014, 5, 115. [Google Scholar] [CrossRef]
- Meeks, T.W.; Jeste, D.V. Beyond the Black Box: What is The Role for Antipsychotics in Dementia? Curr. Psychiatr. 2008, 7, 50–65. [Google Scholar]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J Inflamm Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef]
- Deming, P.; Cook, C.J.; Meyerand, M.E.; Kiehl, K.A.; Kosson, D.S.; Koenigs, M. Impaired salience network switching in psychopathy. Behav. Brain Res. 2023, 452, 114570. [Google Scholar] [CrossRef]
- Kohn, N.; Szopinska-Tokov, J.; Llera Arenas, A.; Beckmann, C.F.; Arias-Vasquez, A.; Aarts, E. Multivariate associative patterns between the gut microbiota and large-scale brain network connectivity. Gut Microbes 2021, 13, 2006586. [Google Scholar] [CrossRef] [PubMed]
- Mulder, D.; Aarts, E.; Arias Vasquez, A.; Bloemendaal, M. A systematic review exploring the association between the human gut microbiota and brain connectivity in health and disease. Mol. Psychiatry 2023. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, A.P.; Sanchez-Padilla, D.E.; Drew, J.C.; Oli, M.W.; Roesch, L.F.W.; Triplett, E.W. Saliva microbiome, dietary, and genetic markers are associated with suicidal ideation in university students. Sci. Rep. 2022, 12, 14306. [Google Scholar] [CrossRef] [PubMed]
- Koren, T.; Yifa, R.; Amer, M.; Krot, M.; Boshnak, N.; Ben-Shaanan, T.L.; Azulay-Debby, H.; Zalayat, I.; Avishai, E.; Hajjo, H.; et al. Insular cortex neurons encode and retrieve specific immune responses. Cell 2021, 184, 5902–5915.e17. [Google Scholar] [CrossRef] [PubMed]
- Rolls, A. Immunoception: The insular cortex perspective. Cell Mol. Immunol. 2023. [Google Scholar] [CrossRef]
- Bodea, L.G.; Eckert, A.; Ittner, L.M.; Piguet, O.; Götz, J. Tau physiology and pathomechanisms in frontotemporal lobar degeneration. J. Neurochem. 2016, 138 (Suppl. S1), 71–94. [Google Scholar] [CrossRef] [PubMed]
- Rademakers, R.; Cruts, M.; van Broeckhoven, C. The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum. Mutat. 2004, 24, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Marchisella, F.; Coffey, E.T.; Hollos, P. Microtubule and microtubule associated protein anomalies in psychiatric disease. Cytoskeleton 2016, 73, 596–611. [Google Scholar] [CrossRef]
- Arnold, S.E.; Lee, V.M.; Gur, R.E.; Trojanowski, J.Q. Abnormal expression of two microtubule-associated proteins (MAP2 and MAP5) in specific subfields of the hippocampal formation in schizophrenia. Proc. Natl. Acad. Sci. USA 1991, 88, 10850–10854. [Google Scholar] [CrossRef]
- Jones, L.B.; Johnson, N.; Byne, W. Alterations in MAP2 immunocytochemistry in areas 9 and 32 of schizophrenic prefrontal cortex. Psychiatry Res. 2002, 114, 137–148. [Google Scholar] [CrossRef]
- Eichenberger, E.M.; Degner, N.; Scott, E.R.; Ruffin, F.; Franzone, J.; Sharma-Kuinkel, B.; Shah, P.; Hong, D.; Dalai, S.C.; Blair, L.; et al. Microbial CellFree DNA Identifies the Causative Pathogen in Infective Endocarditis and Remains Detectable Longer Than Conventional Blood Culture in Patients with Prior Antibiotic Therapy. Clin. Infect. Dis. 2023, 76, e1492–e1500. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Stewart, R.; Park, W.Y.; Jhon, M.; Lee, J.Y.; Kim, S.Y.; Kim, J.M.; Amminger, P.; Chung, Y.C.; Yoon, J.S. Latent Iron Deficiency as a Marker of Negative Symptoms in Patients with First-Episode Schizophrenia Spectrum Disorder. Nutrients 2018, 10, 1707. [Google Scholar] [CrossRef]
- Wan, W.; Cao, L.; Kalionis, B.; Murthi, P.; Xia, S.; Guan, Y. Iron Deposition Leads to Hyperphosphorylation of Tau and Disruption of Insulin Signaling. Front. Neurol. 2019, 10, 607. [Google Scholar] [CrossRef]
- Rao, S.S.; Adlard, P.A. Untangling Tau and Iron: Exploring the Interaction Between Iron and Tau in Neurodegeneration. Front. Mol. Neurosci. 2018, 11, 276. [Google Scholar] [CrossRef]
- Sheelakumari, R.; Kesavadas, C.; Varghese, T.; Sreedharan, R.M.; Thomas, B.; Verghese, J.; Mathuranath, P.S. Assessment of Iron Deposition in the Brain in Frontotemporal Dementia and Its Correlation with Behavioral Traits. AJNR Am. J. Neuroradiol. 2017, 38, 1953–1958. [Google Scholar] [CrossRef]
- Tisdall, M.D.; Ohm, D.T.; Lobrovich, R.; Das, S.R.; Mizsei, G.; Prabhakaran, K.; Ittyerah, R.; Lim, S.; McMillan, C.T.; Wolk, D.A.; et al. Ex vivo MRI and histopathology detect novel iron-rich cortical inflammation in frontotemporal lobar degeneration with tau versus TDP-43 pathology. Neuroimage Clin. 2022, 33, 102913. [Google Scholar] [CrossRef] [PubMed]
- Kosyakovsky, J.; Fine, J.M.; Frey, W.H., II; Hanson, L.R. Mechanisms of Intranasal Deferoxamine in Neurodegenerative and Neurovascular Disease. Pharmaceuticals 2021, 14, 95. [Google Scholar] [CrossRef]
- Aaronson, A.L.; Bordelon, S.D.; Brakel, S.J.; Morrison, H. A Review of the Role of Chronic Traumatic Encephalopathy in Criminal Court. J. Am. Acad. Psychiatry Law 2021, 49, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Byard, R.; Tiemensma, M.; Buckland, M.E.; Vink, R. Chronic traumatic encephalopathy (CTE)—Features and forensic considerations. Forensic. Sci. Med. Pathol. 2023. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Stein, T.D.; Kiernan, P.T.; Alvarez, V.E. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015, 25, 350–364. [Google Scholar] [CrossRef]
- Katsumoto, A.; Takeuchi, H.; Tanaka, F. Tau Pathology in Chronic Traumatic Encephalopathy and Alzheimer’s Disease: Similarities and Differences. Front. Neurol. 2019, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Daglas, M.; Adlard, P.A. The Involvement of Iron in Traumatic Brain Injury and Neurodegenerative Disease. Front. Neurosci. 2018, 12, 981. [Google Scholar] [CrossRef]
- Han, X. Neurolipidomics: Challenges and developments. Front. Biosci. 2007, 12, 2601–2615. [Google Scholar] [CrossRef]
- Yoon, J.H.; Seo, Y.; Jo, Y.S.; Lee, S.; Cho, E.; Cazenave-Gassiot, A.; Shin, Y.S.; Moon, M.H.; An, H.J.; Wenk, M.R.; et al. Brain lipidomics: From functional landscape to clinical significance. Sci. Adv. 2022, 8, eadc9317. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Ash, M.E. Lipid Replacement Therapy: A natural medicine approach to replacing damaged lipids in cellular membranes and organelles and restoring function. Biochim. Biophys. Acta 2014, 1838, 1657–1679. [Google Scholar] [CrossRef] [PubMed]
- Hamsanathan, S.; Gurkar, A.U. Lipids as Regulators of Cellular Senescence. Front. Physiol. 2022, 13, 796850. [Google Scholar] [CrossRef]
- Horn, A.; Jaiswal, J.K. Structural and signaling role of lipids in plasma membrane repair. Curr. Top. Membr. 2019, 84, 67–98. [Google Scholar]
- Cadenas, C.; Vosbeck, S.; Hein, E.M.; Hellwig, B.; Langer, A.; Hayen, H.; Franckenstein, D.; Büttner, B.; Hammad, S.; Marchan, R.; et al. Glycerophospholipid profile in oncogene-induced senescence. Biochim. Biophys. Acta 2012, 1821, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.S.; Choi, D.H.; Choi, J.; Cho, S.Y.; Kim, S.H.; Baek, H.S.; Yoon, K.D.; Son, S.W.; Son, E.D.; et al. Kaempferol tetrasaccharides restore skin atrophy via PDK1 inhibition in human skin cells and tissues: Bench and clinical studies. Biomed. Pharmacother. 2022, 156, 113864. [Google Scholar] [CrossRef] [PubMed]
- Emamian, E.S. AKT/GSK3 signaling pathway and schizophrenia. Front. Mol. Neurosci. 2012, 5, 33. [Google Scholar] [CrossRef]
- Qattan, M.Y.; Khan, M.I.; Alharbi, S.H.; Verma, A.K.; Al-Saeed, F.A.; Abduallah, A.M.; Al Areefy, A.A. Therapeutic Importance of Kaempferol in the Treatment of Cancer through the Modulation of Cell Signalling Pathways. Molecules 2022, 27, 8864. [Google Scholar] [CrossRef]
- Maurya, A.K.; Vinayak, M. PI-103 and Quercetin Attenuate PI3K-AKT Signaling Pathway in T-Cell Lymphoma Exposed to Hydrogen Peroxide. PLoS ONE 2016, 11, e0160686. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, P. Molecular Docking Studies of Myricetin and Its Analogues against Human PDK-1 Kinase as Candidate Drugs for Cancer. Comput. Mol. Biosci. 2015, 5, 20. [Google Scholar] [CrossRef]
- Qin, J.; Fu, M.; Wang, J.; Huang, F.; Liu, H.; Huangfu, M.; Yu, D.; Liu, H.; Li, X.; Guan, X.; et al. PTEN/AKT/mTOR signaling mediates anticancer effects of epigallocatechin-3-gallate in ovarian cancer. Oncol. Rep. 2020, 43, 1885–1896. [Google Scholar] [CrossRef] [PubMed]
- Zughaibi, T.A.; Suhail, M.; Tarique, M.; Tabrez, S. Targeting PI3K/Akt/mTOR Pathway by Different Flavonoids: A Cancer Chemopreventive Approach. Int. J. Mol. Sci. 2021, 22, 12455. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yao, Z. Chronic over-nutrition and dysregulation of GSK3 in diseases. Nutr. Metab. 2016, 13, 49. [Google Scholar] [CrossRef]
- Issinger, O.G.; Guerra, B. Phytochemicals in cancer and their effect on the PI3K/AKT-mediated cellular signalling. Biomed. Pharmacother. 2021, 139, 111650. [Google Scholar] [CrossRef]
- Guerra, B.; Issinger, O.G. Natural Compounds and Derivatives as Ser/Thr Protein Kinase Modulators and Inhibitors. Pharmaceuticals 2019, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Lucas, N.; Cho, W. Phosphatidylserine binding is essential for plasma membrane recruitment and signaling function of 3-phosphoinositide-dependent kinase-1. J. Biol. Chem. 2011, 286, 41265–41272. [Google Scholar] [CrossRef] [PubMed]
- Noll, R. Kraepelin’s ‘lost biological psychiatry’? Autointoxication, organotherapy and surgery for dementia praecox. Hist. Psychiatry 2007, 18 Pt 3, 301–320. [Google Scholar] [CrossRef]
- Horrobin, D.F. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr. Res. 1998, 30, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Nazli, A.; Rojas, O.L.; Chege, D.; Alidina, Z.; Huibner, S.; Mujib, S.; Benko, E.; Kovacs, C.; Shin, L.Y.; et al. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol. 2012, 5, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Tsounis, E.P.; Triantos, C.; Konstantakis, C.; Marangos, M.; Assimakopoulos, S.F. Intestinal barrier dysfunction as a key driver of severe COVID-19. World J. Virol. 2023, 12, 68–90. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Ju, D.; Lin, Y.; Chen, W. The role of interleukin-22 in lung health and its therapeutic potential for COVID-19. Front. Immunol. 2022, 13, 951107. [Google Scholar] [CrossRef] [PubMed]
- Coronas, V.; Arnault, P.; Jégou, J.F.; Cousin, L.; Rabeony, H.; Clarhaut, S.; Harnois, T.; Lecron, J.C.; Morel, F. IL-22 Promotes Neural Stem Cell Self-Renewal in the Adult Brain. Stem Cells 2023, 41, 252–259. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Park, H.; Jin, U.H.; Karki, K.; Jayaraman, A.; Allred, C.; Michelhaugh, S.K.; Mittal, S.; Chapkin, R.S.; Safe, S. Dopamine is an aryl hydrocarbon receptor agonist. Biochem. J. 2020, 477, 3899–3910. [Google Scholar] [CrossRef]
- Fehsel, K.; Schwanke, K.; Kappel, B.A.; Fahimi, E.; Meisenzahl-Lechner, E.; Esser, C.; Hemmrich, K.; Haarmann-Stemmann, T.; Kojda, G.; Lange-Asschenfeldt, C. Activation of the aryl hydrocarbon receptor by clozapine induces preadipocyte differentiation and contributes to endothelial dysfunction. J. Psychopharmacol. 2022, 36, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Ishima, T.; Iyo, M.; Hashimoto, K. Neurite outgrowth mediated by the heat shock protein Hsp90α: A novel target for the antipsychotic drug aripiprazole. Transl. Psychiatry 2012, 2, e170. [Google Scholar] [CrossRef] [PubMed]
- Sun, L. Recent advances in the development of AHR antagonists in immuno-oncology. RSC Med. Chem. 2021, 12, 902–914. [Google Scholar] [CrossRef]
- De Juan, A.; Segura, E. Modulation of Immune Responses by Nutritional Ligands of Aryl Hydrocarbon Receptor. Front. Immunol. 2021, 12, 645168. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A. Aryl hydrocarbon receptor (AhR) reveals evidence of antagonistic pleiotropy in the regulation of the aging process. Cell Mol. Life Sci. 2022, 79, 489. [Google Scholar] [CrossRef]
- Ojo, E.S.; Tischkau, S.A. The Role of AhR in the Hallmarks of Brain Aging: Friend and Foe. Cells 2021, 10, 2729. [Google Scholar] [CrossRef] [PubMed]
- Eckers, A.; Jakob, S.; Heiss, C.; Haarmann-Stemmann, T.; Goy, C.; Brinkmann, V.; Cortese-Krott, M.M.; Sansone, R.; Esser, C.; Ale-Agha, N.; et al. The aryl hydrocarbon receptor promotes aging phenotypes across species. Sci. Rep. 2016, 6, 19618. [Google Scholar] [CrossRef] [PubMed]


| Interval | Sustained Recovery | Employed |
|---|---|---|
| 1901–1920 | 20% | 4.7% |
| 1921–1940 | 12% | 11.9% |
| 1941–1955 | 23% | 4.1% |
| 1956–1975 | 20% | 5.1% |
| 1976–1995 | 20% | 6.9% |
| Marker Type | Marker Assay | References |
|---|---|---|
| Integrity of gut barrier | IL-22 Singulex-Erenna®® | [127] |
| Translocated microbes | mcfDNA Karius Test®® | [139] |
| PDK1 Inhibitor | Plant | References |
|---|---|---|
| Kaempferol | Fruits, vegetables, and herbs | [175] |
| Quercetin | Onions, kale, broccoli | [176] |
| Myricetin | Oranges, berries, tomatoes, nuts, tea | [177] |
| Epigallcatechin-3 gallate | Green tea | [178] |
| Lupiwighteone isoflavone | Glycyrrhiza glabra; Lotus pedunculatus | [179] |
| Delphinidin | Citrus fruits | [180] |
| Honokiol | Cherries, berries, grapes | [181] |
| Delphinidin | Cranberries, concord grapes, pomegranates | [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfera, A.; Andronescu, L.; Britt, W.G.; Himsl, K.; Klein, C.; Rahman, L.; Kozlakidis, Z. Receptor-Independent Therapies for Forensic Detainees with Schizophrenia–Dementia Comorbidity. Int. J. Mol. Sci. 2023, 24, 15797. https://doi.org/10.3390/ijms242115797
Sfera A, Andronescu L, Britt WG, Himsl K, Klein C, Rahman L, Kozlakidis Z. Receptor-Independent Therapies for Forensic Detainees with Schizophrenia–Dementia Comorbidity. International Journal of Molecular Sciences. 2023; 24(21):15797. https://doi.org/10.3390/ijms242115797
Chicago/Turabian StyleSfera, Adonis, Luminita Andronescu, William G. Britt, Kiera Himsl, Carolina Klein, Leah Rahman, and Zisis Kozlakidis. 2023. "Receptor-Independent Therapies for Forensic Detainees with Schizophrenia–Dementia Comorbidity" International Journal of Molecular Sciences 24, no. 21: 15797. https://doi.org/10.3390/ijms242115797
APA StyleSfera, A., Andronescu, L., Britt, W. G., Himsl, K., Klein, C., Rahman, L., & Kozlakidis, Z. (2023). Receptor-Independent Therapies for Forensic Detainees with Schizophrenia–Dementia Comorbidity. International Journal of Molecular Sciences, 24(21), 15797. https://doi.org/10.3390/ijms242115797






