Chemical Chaperone 4-PBA Mitigates Tumor Necrosis Factor Alpha-Induced Endoplasmic Reticulum Stress in Human Airway Smooth Muscle
Abstract
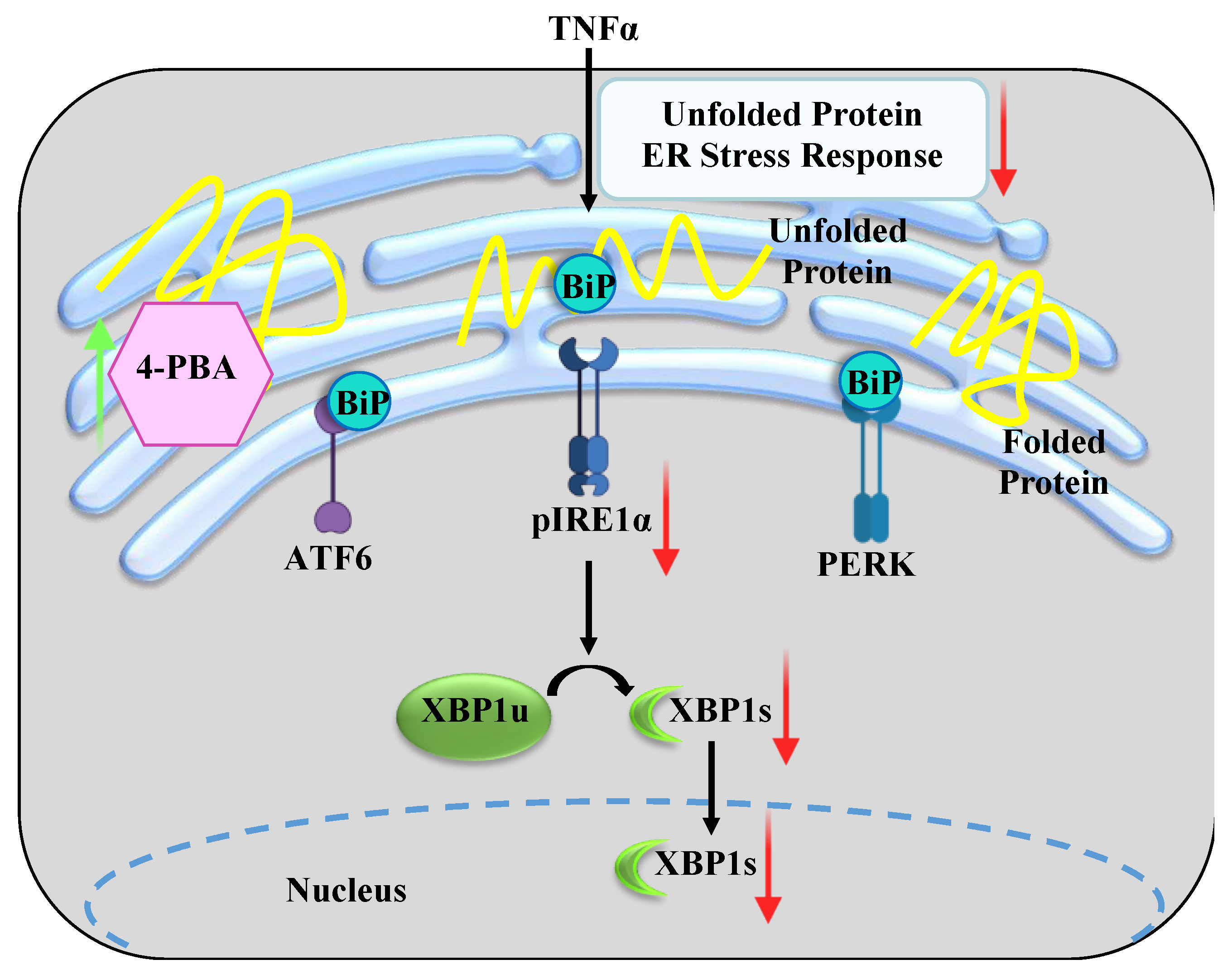
1. Introduction
2. Results
2.1. TNFα Induces Phosphorylation of IRE1α in hASM Cells
2.2. Chemical Chaperone 4-PBA Mitigates TNFα-Induced Phosphorylation of IRE1α in hASM Cells
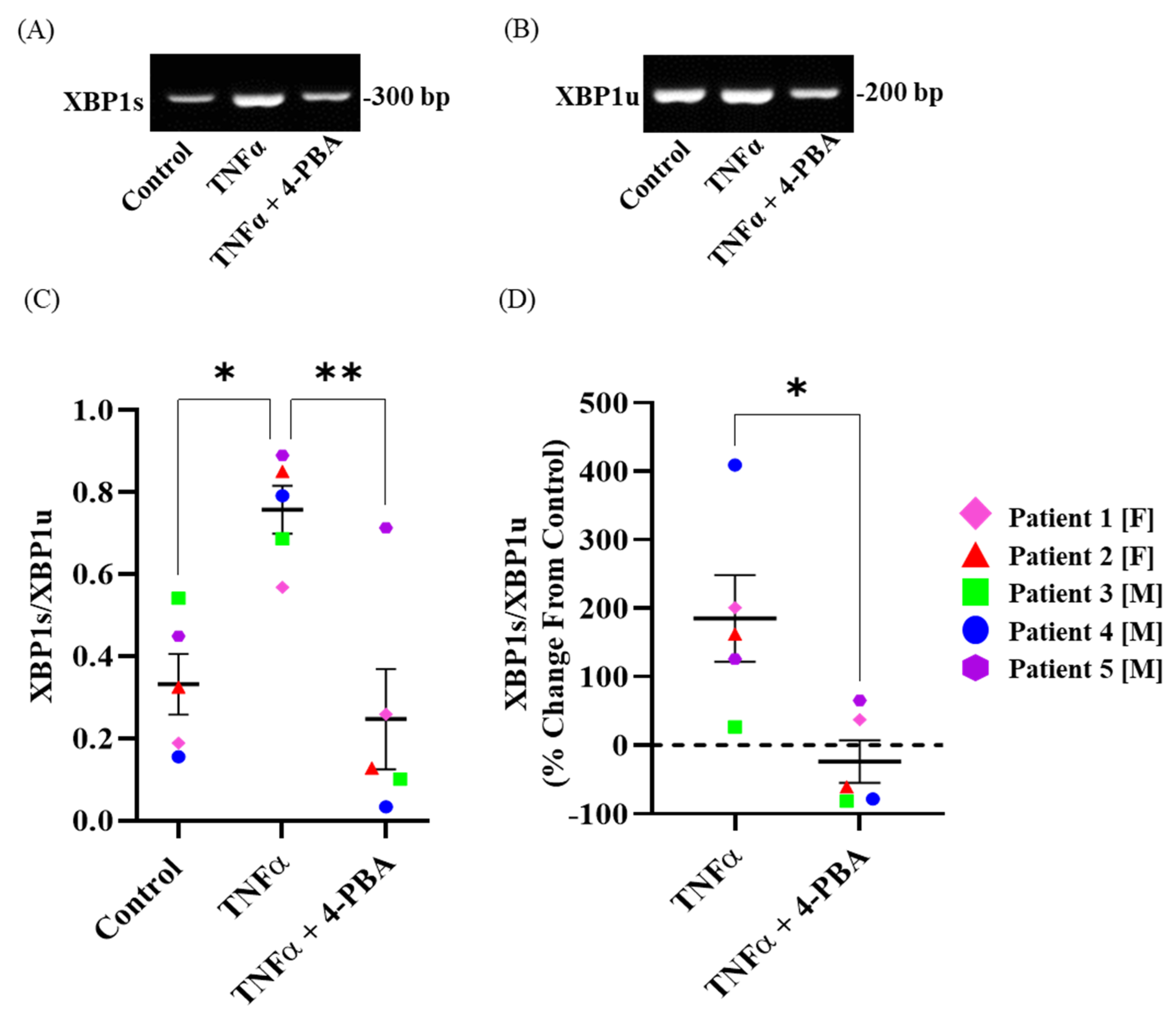
2.3. TNFα Induces Splicing of XBP1 mRNA in hASM Cells
2.4. Chemical Chaperone 4-PBA Mitigates TNFα-Induced Splicing of XBP1 mRNA in hASM Cells
2.5. 4-PBA Alone Has No Effect on Phosphorylation of IRE1α and XBP1 Splicing in hASM Cells
3. Discussion
3.1. TNFα Induces Phosphorylation of IRE1α and mRNA Splicing of XBP1 at 12 h
3.2. 4-PBA Mitigates ER Stress in hASM Cells
3.3. Study Limitations
3.4. Clinical Significance
4. Material and Methods
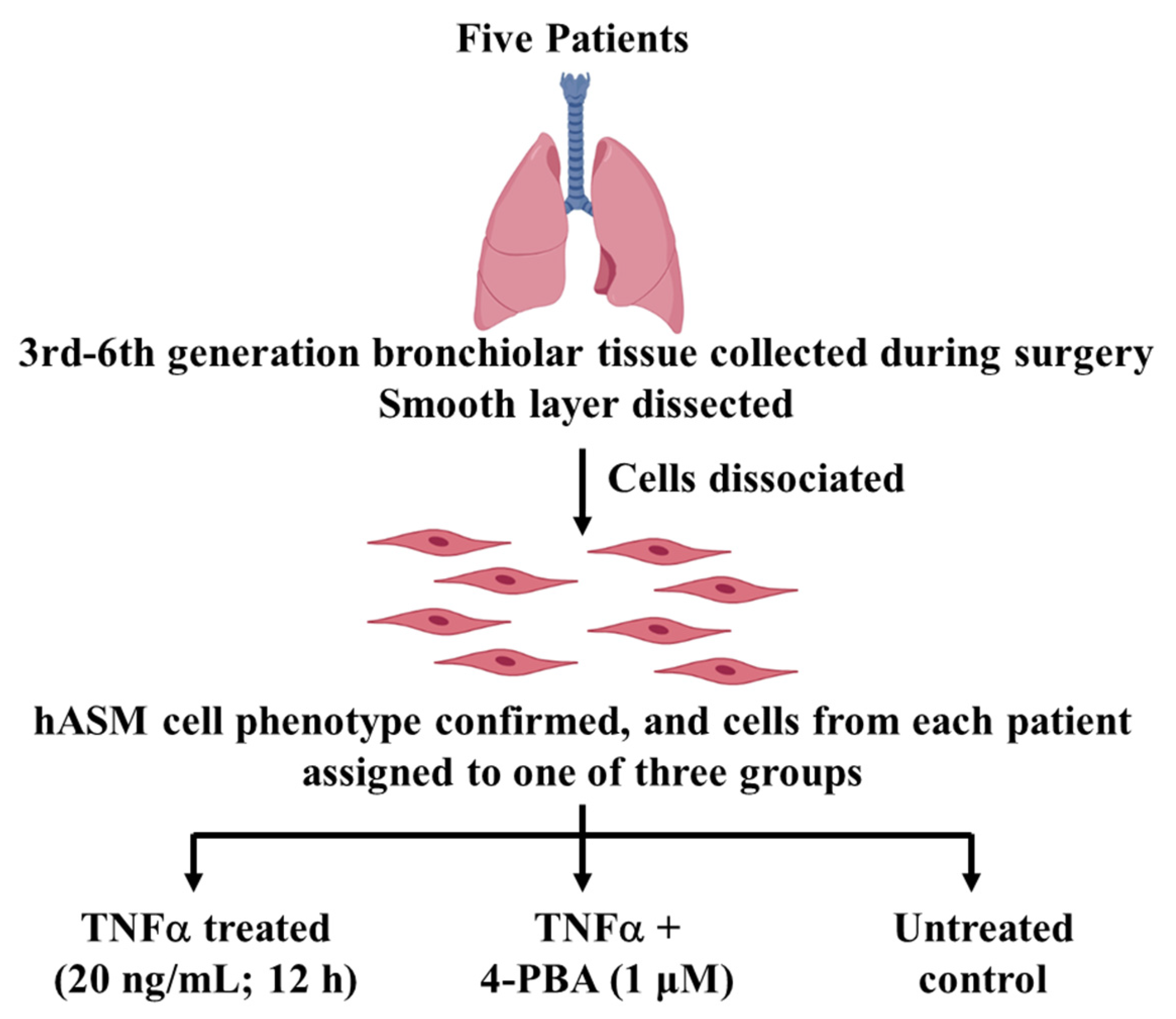
4.1. Experimental Design
4.1.1. Patient Samples
4.1.2. Dissociation of Cells from Bronchiolar Tissue Samples
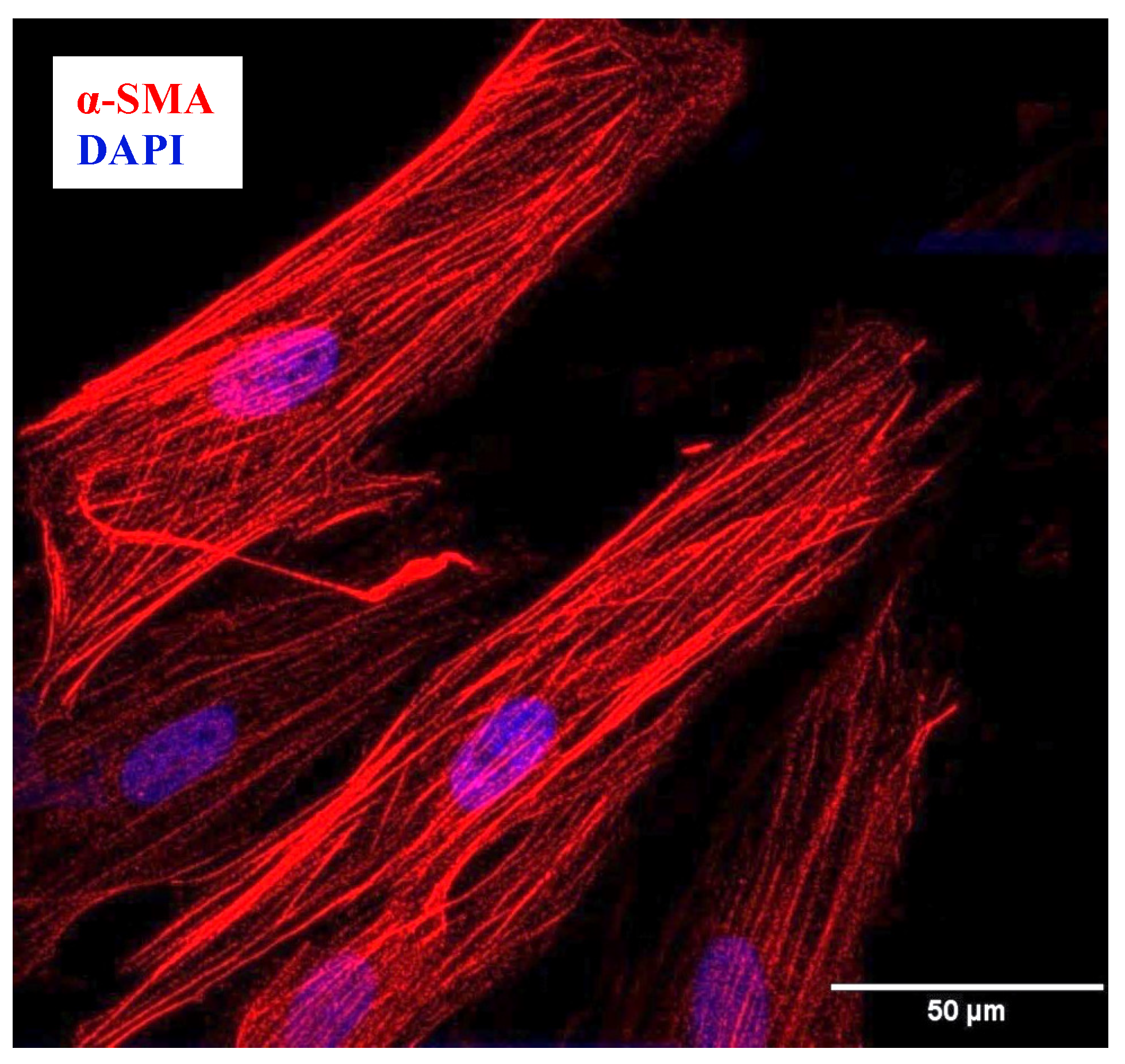
4.1.3. Confirmation of hASM Phenotype
4.1.4. Treatment Groups
4.2. Determining pIRE1αS724 and XBP1s ER Stress Response
4.2.1. pIRE1αS724 and Total IRE1α Protein Expression
4.2.2. Measuring the Splicing of XBP1 mRNA
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Busse, W.W.; Calhoun, W.F.; Sedgwick, J.D. Mechanism of airway inflammation in asthma. Am. Rev. Respir. Dis. 1993, 147, S20–S24. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Ferri, S.; Pepys, J.; Poto, R.; Spadaro, G.; Nappi, E.; Paoletti, G.; Virchow, J.C.; Heffler, E.; Canonica, W.G. Biologics and airway remodeling in severe asthma. Allergy 2022, 77, 3538–3552. [Google Scholar] [CrossRef] [PubMed]
- Eapen, M.S.; Myers, S.; Walters, E.H.; Sohal, S.S. Airway inflammation in chronic obstructive pulmonary disease (COPD): A true paradox. Expert Rev. Respir. Med. 2017, 11, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, L.; Fossali, T.; Frangipane, V.; Bozzini, S.; Morosini, M.; D’Amato, M.; Lettieri, S.; Urtis, M.; Di Toro, A.; Saracino, L.; et al. Broncho-alveolar inflammation in COVID-19 patients: A correlation with clinical outcome. BMC Pulm. Med. 2020, 20, 301. [Google Scholar] [CrossRef] [PubMed]
- Snoeck-Stroband, J.B.; Lapperre, T.S.; Gosman, M.M.; Boezen, H.M.; Timens, W.; ten Hacken, N.H.; Sont, J.K.; Sterk, P.J.; Hiemstra, P.S. Chronic bronchitis sub-phenotype within COPD: Inflammation in sputum and biopsies. Eur. Respir. J. 2008, 31, 70–77. [Google Scholar] [CrossRef]
- Dasgupta, D.; Delmotte, P.; Sieck, G.C. Inflammation-Induced Protein Unfolding in Airway Smooth Muscle Triggers a Homeostatic Response in Mitochondria. Int. J. Mol. Sci. 2020, 22, 363. [Google Scholar] [CrossRef]
- Yap, J.; Chen, X.; Delmotte, P.; Sieck, G.C. TNFα selectively activates the IRE1α/XBP1 endoplasmic reticulum stress pathway in human airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L483–L493. [Google Scholar] [CrossRef] [PubMed]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta. 2013, 1833, 3460–3470. [Google Scholar] [CrossRef]
- Wu, Z.H.; Chiu, C.H.; Chen, C.C.; Chyau, C.C.; Cheng, C.H. Amelioration of Cyclosporine A-Induced Acute Nephrotoxicity by Cordyceps cicadae Mycelia via Mg(+2) Reabsorption and the Inhibition of GRP78-IRE1-CHOP Pathway: In Vivo and In Vitro. Int. J. Mol. Sci. 2023, 24, 772. [Google Scholar] [CrossRef]
- Nozaki, J.; Kubota, H.; Yoshida, H.; Naitoh, M.; Goji, J.; Yoshinaga, T.; Mori, K.; Koizumi, A.; Nagata, K. The endoplasmic reticulum stress response is stimulated through the continuous activation of transcription factors ATF6 and XBP1 in Ins2+/Akita pancreatic beta cells. Genes Cells 2004, 9, 261–270. [Google Scholar] [CrossRef]
- Dromparis, P.; Paulin, R.; Stenson, T.H.; Haromy, A.; Sutendra, G.; Michelakis, E.D. Attenuating endoplasmic reticulum stress as a novel therapeutic strategy in pulmonary hypertension. Circulation 2013, 127, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Engin, F.; Hotamisligil, G.S. Restoring endoplasmic reticulum function by chemical chaperones: An emerging therapeutic approach for metabolic diseases. Diabetes Obes. Metab. 2010, 12 (Suppl. S2), 108–115. [Google Scholar] [CrossRef]
- Rubenstein, R.C.; Zeitlin, P.L. A pilot clinical trial of oral sodium 4-phenylbutyrate (Buphenyl) in deltaF508-homozygous cystic fibrosis patients: Partial restoration of nasal epithelial CFTR function. Am. J. Respir. Crit. Care Med. 1998, 157, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, P.L.; Diener-West, M.; Rubenstein, R.C.; Boyle, M.P.; Lee, C.K.; Brass-Ernst, L. Evidence of CFTR function in cystic fibrosis after systemic administration of 4-phenylbutyrate. Mol. Ther. 2002, 6, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Bertini, E.; Messina, S.; Solari, A.; D’Amico, A.; Angelozzi, C.; Battini, R.; Berardinelli, A.; Boffi, P.; Bruno, C.; et al. Randomized, double-blind, placebo-controlled trial of phenylbutyrate in spinal muscular atrophy. Neurology 2007, 68, 51–55. [Google Scholar] [CrossRef]
- Ayaub, E.A.; Kolb, P.S.; Mohammed-Ali, Z.; Tat, V.; Murphy, J.; Bellaye, P.S.; Shimbori, C.; Boivin, F.J.; Lai, R.; Lynn, E.G.; et al. GRP78 and CHOP modulate macrophage apoptosis and the development of bleomycin-induced pulmonary fibrosis. J. Pathol. 2016, 239, 411–425. [Google Scholar] [CrossRef]
- Heijink, I.H.; Brandenburg, S.M.; Noordhoek, J.A.; Postma, D.S.; Slebos, D.J.; van Oosterhout, A.J. Characterisation of cell adhesion in airway epithelial cell types using electric cell-substrate impedance sensing. Eur. Respir. J. 2010, 35, 894–903. [Google Scholar] [CrossRef]
- Kenche, H.; Baty, C.J.; Vedagiri, K.; Shapiro, S.D.; Blumental-Perry, A. Cigarette smoking affects oxidative protein folding in endoplasmic reticulum by modifying protein disulfide isomerase. FASEB J. 2013, 27, 965–977. [Google Scholar] [CrossRef]
- van‘t Wout, E.F.; van Schadewijk, A.; van Boxtel, R.; Dalton, L.E.; Clarke, H.J.; Tommassen, J.; Marciniak, S.J.; Hiemstra, P.S. Virulence Factors of Pseudomonas aeruginosa Induce Both the Unfolded Protein and Integrated Stress Responses in Airway Epithelial Cells. PLoS Pathog. 2015, 11, e1004946. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.; Lin, Y.; Liu, Y.; YouYou; Yin, W. IRE1α-TRAF2-ASK1 pathway is involved in CSTMP-induced apoptosis and ER stress in human non-small cell lung cancer A549 cells. Biomed. Pharmacother. 2016, 82, 281–289. [Google Scholar] [CrossRef]
- Han, D.; Lerner, A.G.; Vande Walle, L.; Upton, J.P.; Xu, W.; Hagen, A.; Backes, B.J.; Oakes, S.A.; Papa, F.R. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 2009, 138, 562–575. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, C.Y.; Back, S.H.; Clark, R.L.; Peisach, D.; Xu, Z.; Kaufman, R.J. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc. Natl. Acad. Sci. USA 2006, 103, 14343–14348. [Google Scholar] [CrossRef] [PubMed]
- Aragon, T.; van Anken, E.; Pincus, D.; Serafimova, I.M.; Korennykh, A.V.; Rubio, C.A.; Walter, P. Messenger RNA targeting to endoplasmic reticulum stress signalling sites. Nature 2009, 457, 736–740. [Google Scholar] [CrossRef]
- Acosta-Alvear, D.; Zhou, Y.; Blais, A.; Tsikitis, M.; Lents, N.H.; Arias, C.; Lennon, C.J.; Kluger, Y.; Dynlacht, B.D. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell 2007, 27, 53–66. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, S.; Sha, H.; Liu, Z.; Yang, L.; Xue, Z.; Chen, H.; Qi, L. Emerging roles for XBP1, a sUPeR transcription factor. Gene Expr. 2010, 15, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Kanemoto, S.; Kondo, S.; Ogata, M.; Murakami, T.; Urano, F.; Imaizumi, K. XBP1 activates the transcription of its target genes via an ACGT core sequence under ER stress. Biochem. Biophys. Res. Commun. 2005, 331, 1146–1153. [Google Scholar] [CrossRef]
- Tokutake, Y.; Yamada, K.; Hayashi, S.; Arai, W.; Watanabe, T.; Yonekura, S. IRE1-XBP1 Pathway of the Unfolded Protein Response Is Required during Early Differentiation of C2C12 Myoblasts. Int. J. Mol. Sci. 2019, 21, 182. [Google Scholar] [CrossRef]
- Wang, X.Z.; Harding, H.P.; Zhang, Y.; Jolicoeur, E.M.; Kuroda, M.; Ron, D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998, 17, 5708–5717. [Google Scholar] [CrossRef]
- Urano, F.; Wang, X.; Bertolotti, A.; Zhang, Y.; Chung, P.; Harding, H.P.; Ron, D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000, 287, 664–666. [Google Scholar] [CrossRef]
- Kolb, P.S.; Ayaub, E.A.; Zhou, W.; Yum, V.; Dickhout, J.G.; Ask, K. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int. J. Biochem. Cell Biol. 2015, 61, 45–52. [Google Scholar] [CrossRef]
- Pao, H.P.; Liao, W.I.; Tang, S.E.; Wu, S.Y.; Huang, K.L.; Chu, S.J. Suppression of Endoplasmic Reticulum Stress by 4-PBA Protects Against Hyperoxia-Induced Acute Lung Injury via Up-Regulating Claudin-4 Expression. Front. Immunol. 2021, 12, 674316. [Google Scholar] [CrossRef] [PubMed]
- Papp, E.; Csermely, P. Chemical chaperones: Mechanisms of action and potential use. In Molecular Chaperones in Health and Disease; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2006; pp. 405–416. [Google Scholar] [CrossRef]
- Basseri, S.; Lhoták, S.; Sharma, A.M.; Austin, R.C. The chemical chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response. J. Lipid. Res. 2009, 50, 2486–2501. [Google Scholar] [CrossRef]
- Lu, H.Y.; Zhang, J.; Wang, Q.X.; Tang, W.; Zhang, L.J. Activation of the endoplasmic reticulum stress pathway involving CHOP in the lungs of rats with hyperoxia-induced bronchopulmonary dysplasia. Mol. Med. Rep. 2015, 12, 4494–4500. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dasgupta, D.; Mahadev Bhat, S.; Price, A.L.; Delmotte, P.; Sieck, G.C. Molecular Mechanisms Underlying TNFalpha-Induced Mitochondrial Biogenesis in Human Airway Smooth Muscle. Int. J. Mol. Sci. 2023, 24, 5788. [Google Scholar] [CrossRef] [PubMed]
- Delmotte, P.; Zavaletta, V.A.; Thompson, M.A.; Prakash, Y.S.; Sieck, G.C. TNFα decreases mitochondrial movement in human airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L166–L176. [Google Scholar] [CrossRef]
- Prakash, Y.S.; Kannan, M.S.; Sieck, G.C. Regulation of intracellular calcium oscillations in porcine tracheal smooth muscle cells. Am. J. Physiol. 1997, 272, C966–C975. [Google Scholar] [CrossRef]
- Delmotte, P.; Marin Mathieu, N.; Sieck, G.C. TNFalpha induces mitochondrial fragmentation and biogenesis in human airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L137–L151. [Google Scholar] [CrossRef]
- Meylan, P.; Dreos, R.; Ambrosini, G.; Groux, R.; Bucher, P. EPD in 2020: Enhanced data visualization and extension to ncRNA promoters. Nucleic. Acids. Res. 2020, 48, D65–D69. [Google Scholar] [CrossRef]

| Patient No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Sex | F | F | M | M | M |
| Age (years) | 64 | 61 | 71 | 75 | 34 |
| Asthma | No | No | No | No | No |
| COPD | No | No | No | No | No |
| Pulmonary Fibrosis | No | No | No | No | |
| Pulmonary Hypertension | No | No | No | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delmotte, P.; Yap, J.Q.; Dasgupta, D.; Sieck, G.C. Chemical Chaperone 4-PBA Mitigates Tumor Necrosis Factor Alpha-Induced Endoplasmic Reticulum Stress in Human Airway Smooth Muscle. Int. J. Mol. Sci. 2023, 24, 15816. https://doi.org/10.3390/ijms242115816
Delmotte P, Yap JQ, Dasgupta D, Sieck GC. Chemical Chaperone 4-PBA Mitigates Tumor Necrosis Factor Alpha-Induced Endoplasmic Reticulum Stress in Human Airway Smooth Muscle. International Journal of Molecular Sciences. 2023; 24(21):15816. https://doi.org/10.3390/ijms242115816
Chicago/Turabian StyleDelmotte, Philippe, Jane Q. Yap, Debanjali Dasgupta, and Gary C. Sieck. 2023. "Chemical Chaperone 4-PBA Mitigates Tumor Necrosis Factor Alpha-Induced Endoplasmic Reticulum Stress in Human Airway Smooth Muscle" International Journal of Molecular Sciences 24, no. 21: 15816. https://doi.org/10.3390/ijms242115816
APA StyleDelmotte, P., Yap, J. Q., Dasgupta, D., & Sieck, G. C. (2023). Chemical Chaperone 4-PBA Mitigates Tumor Necrosis Factor Alpha-Induced Endoplasmic Reticulum Stress in Human Airway Smooth Muscle. International Journal of Molecular Sciences, 24(21), 15816. https://doi.org/10.3390/ijms242115816






