Transcriptomic (DNA Microarray) and Metabolome (LC-TOF-MS) Analyses of the Liver in High-Fat Diet Mice after Intranasal Administration of GALP (Galanin-like Peptide)
Abstract
:1. Introduction
2. Results
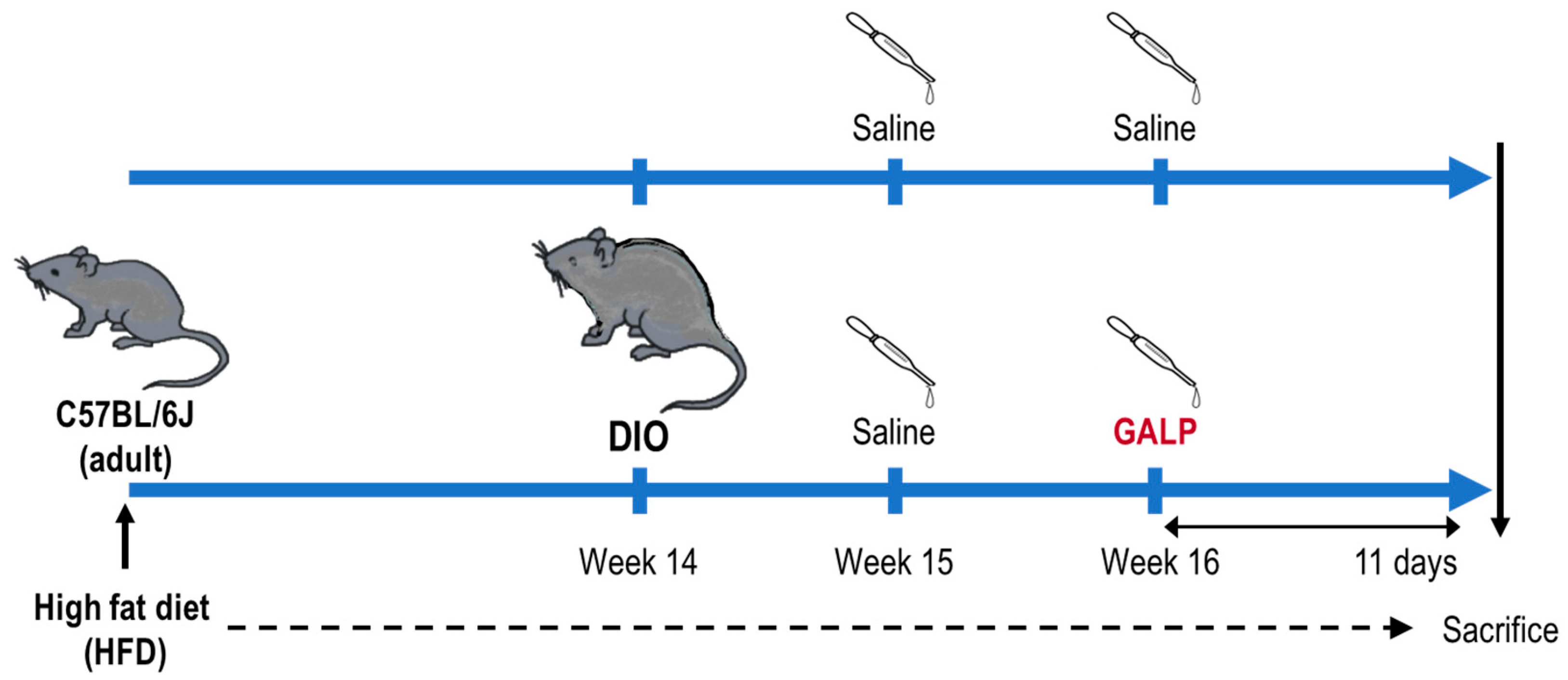
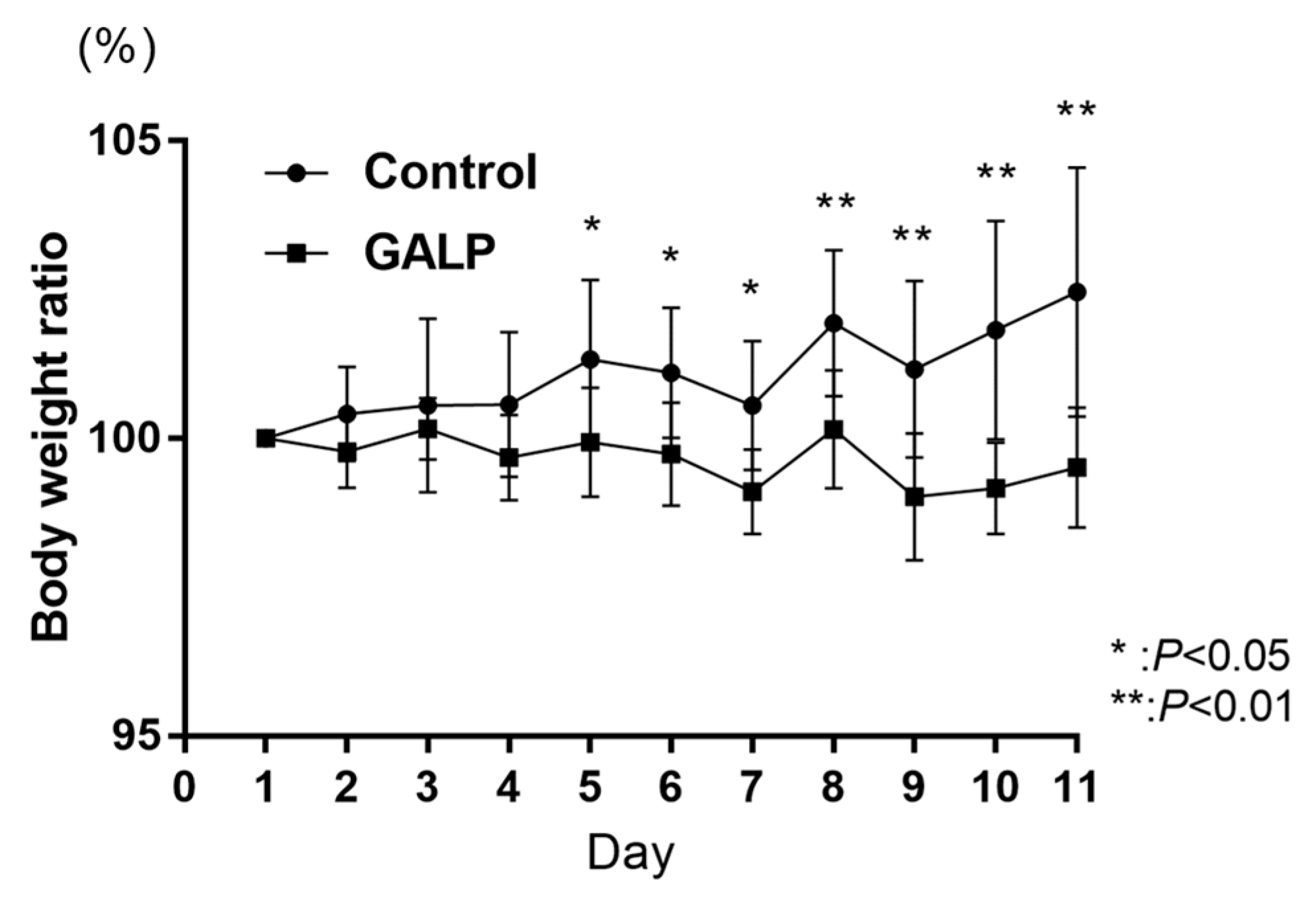
2.1. Body Weight Change in DIO Mice through Intranasal GALP
2.2. Liver DNA Microarray Analysis
2.3. Liver Metabolite Analysis
2.4. Biological Functional Enrichment Analysis
3. Discussion
3.1. DNA Microarray Analysis
3.1.1. Key Genes Whose Expression Was Increased by GALP
Fmo3
Lyve1
Mt1, Mt2
Aldh1a3
Fgfr1
3.1.2. Key Genes Whose Expression Was Decreased by GALP
Antimicrobial Peptides: Defa3, Defa20, Defa6, Defa1, and Defb19
Anti-Inflammatory Proteins: Gkn2, Tff1, and Clca1
Steroid Biosynthesis Pathway: Hsd3b4, Hsd3b5, and Hsd3b1
Bile Acid Secretion and Transport: Slco1a1/Oatp1
Lipid and Cholesterol Metabolism: Cyp26a1, Pnpla5, Prap1, and Fdps
3.2. Metabolite Analysis
3.2.1. Metabolites Increased by GALP
Coenzyme Q10
Hydroxyprogesterone Caproate
Oleoylethanolamide
Erucic Acid
Linolenic Acid (Alpha-Linolenic Acid)
Bile Acids: Deoxycholic Acid, Taurocholic Acid, and Taurochenodeoxycholic Acid
5α-Cholestan-3-One-2 and 5α-Cholestanone
3.2.2. Metabolites Reduced by GALP
Flavanone
Riboflavin
Palmitoylethanolamide-2
Myristic Acid and Arachidic Acid
Ursodeoxycholic Acid (UDCA)
3.3. Pathway Analysis of Expression Dissimilarity Genes
3.3.1. KEGG Upregulated
Fatty Acid Elongation (mmu00062) and the Biosynthesis of Unsaturated Fatty Acids (mmu01040)
Retinol Metabolism (mmu00830)
Melanoma (mmu05218)
3.3.2. KEGG Downregulated
Staphylococcus aureus Infection (mmu05150) and Salmonella Infection (mmu05132)
Cortisol Synthesis and Secretion (mmu04927)
Cushing’s Syndrome (mmu04934)
Cholesterol Metabolism (mmu04979)
Bile Secretion (mmu04976): Slco1a1, Sult2a8, and Slc22a7
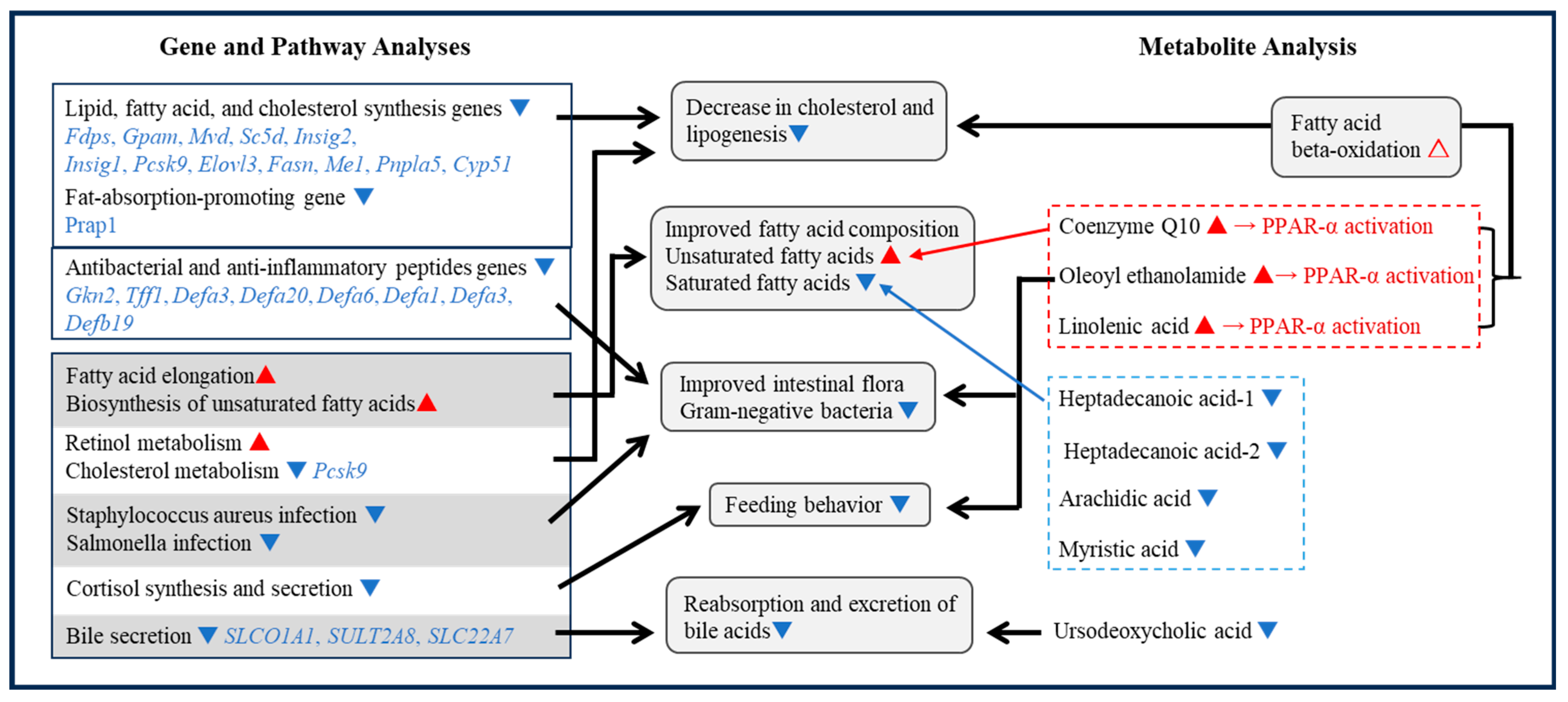
3.4. Integrated Data Analysis
3.4.1. Feeding Inhibition Mechanism
OEA
Cortisol Synthesis-Related Genes
3.4.2. Weight Loss Mechanism
Decreased Expression of Lipid Synthesis-Related Genes
Improved Fatty Acid Composition
Antibacterial and Anti-Inflammatory Peptides and Intestinal Microflora
Bile Uptake and Excretion
β-Oxidation of Fatty Acids
4. Materials and Methods
4.1. Preparation of the DIO Mice
4.2. Intranasal Administration of GALP to the DIO Mice
4.3. Dissection and Sampling
4.4. DNA Microarray Analysis
4.5. Mouse Liver Metabolome Analysis by LC-TOFMS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohtaki, T.; Kumano, S.; Ishibashi, Y.; Ogi, K.; Matsui, H.; Harada, M.; Kitada, C.; Kurokawa, T.; Onda, H.; Fujino, M. Isolation and cDNA cloning of a novel galanin-like peptide (GALP) from porcine hypothalamus. J. Biol. Chem. 1999, 274, 37041–37045. [Google Scholar] [CrossRef] [PubMed]
- Takenoya, F.; Hirako, S.; Wada, N.; Nonaka, N.; Hirabayashi, T.; Kageyama, H.; Shioda, S. Regulation of Feeding Behavior and Energy Metabolism by Galanin-like Peptide (GALP): A Novel Strategy to Fight Against Obesity. Curr. Pharm. Des. 2018, 24, 926–3933. [Google Scholar] [CrossRef] [PubMed]
- Shioda, S.; Kageyama, H.; Takenoya, F.; Shiba, K. Galanin-like peptide: A key player in the homeostatic regulation of feeding and energy metabolism? Int. J. Obes. 2011, 35, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, H.; Shiba, K.; Hirako, S.; Wada, N.; Yamanaka, S.; Nogi, Y.; Takenoya, F.; Nonaka, N.; Hirano, T.; Inoue, S.; et al. Anti-obesity effect of intranasal administration of galanin-like peptide (GALP) in obese mice. Sci. Rep. 2016, 6, 28200. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Kageyama, H.; Hirako, S.; Wang, L.; Takenoya, F.; Ogawa, T.; Shioda, S. Interactive effect of galanin-like peptide (GALP) and spontaneous exercise on energy metabolism. Peptides 2013, 49, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, H.; Endo, K.; Osaka, T.; Watanabe, J.; Wang, L.H.; Ito, K.; Suzuki, M.; Sakagami, J.; Takenoya, F.; Shioda, S. Galanin-like peptide (GALP) facilitates thermogenesis via synthesis of prostaglandin E2 by astrocytes in the periventricular zone of the third ventricle. J. Mol. Neurosci. 2013, 50, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Krasnow, S.M.; Fraley, G.S.; Schuh, S.M.; Baumgartner, J.W.; Clifton, D.K.; Steiner, R.A. A role for galanin-like peptide in the integration of feeding, body weight regulation, and reproduction in the mouse. Endocrinology 2003, 144, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, N.; Farr, S.A.; Kageyama, H.; Shioda, S.; Banks, W.A. Delivery of Galanin-Like Peptide to the Brain: Targeting with Intranasal Delivery and Cyclodextrins. J. Pharmacol. Exp. Ther. 2008, 32, 513–519. [Google Scholar] [CrossRef]
- Hirako, S.; Wada, N.; Kageyama, H.; Takenoya, F.; Izumida, Y.; Kim, H.; Iizuka, Y.; Matsumoto, A.; Okabe, M.; Kimura, A.; et al. Autonomic nervous system-mediated effects of galanin-like peptide on lipid metabolism in liver and adipose tissue. Sci. Rep. 2016, 6, 21481. [Google Scholar] [CrossRef]
- Hirako, S.; Wada, N.; Kageyama, H.; Takenoya, F.; Kim, H.; Iizuka, Y.; Matsumoto, A.; Okabe, M.; Shioda, S. Effect of intranasal administration of Galanin-like Peptide (GALP) on body weight and Hhpatic lipids accumulation in mice with diet-induced obesity. Curr. Pharm. Des. 2017, 23, 3751–3756. [Google Scholar] [CrossRef]
- Hori, M.; Nakamachi, T.; Rakwal, R.; Shibato, J.; Nakamura, K.; Wada, Y.; Tsuchiwaka, D.; Yoshikawa, A.; Tamaki, R.; Shioda, S. Unraveling the ischemic brain transcriptome in a permanent middle cerebral artery occlusion mouse model by DNA microarray analysis. Dis. Model. Mech. 2012, 5, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Rakwal, R.; Shibato, J.; Sawa, C.; Saito, T.; Murayama, A.; Kuwagata, M.; Kageyama, H.; Yagi, M.; Satoh, K.; et al. Seeking gene candidates responsible for developmental origins of health and disease. Congenit. Anom. 2011, 51, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Rudraiah, S.; Rohrer, P.R.; Gurevich, I.; Goedken, M.J.; Rasmussen, T.; Hines, R.N.; Manautou, J.E. Tolerance to acetaminophen hepatotoxicity in the mouse model of autoprotection is associated with induction of flavin-containing monooxygenase-3 (FMO3) in hepatocytes. Toxicol. Sci. 2014, 141, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Stemmer, P.M.; Petriello, M.C. Proteomics-based identification of interaction partners of the Xenobiotic Detoxification Enzyme FMO3 reveals involvement in urea cycle. Toxics 2022, 10, 60. [Google Scholar] [CrossRef]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R. Genes and gene expression modules associated with caloric restriction and aging in the laboratory mouse. BMC Genom. 2009, 10, 585. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Shen, Y.; Li, W.; Li, Q.; Miao, Y.; Zhong, Y. Upregulation of flavin-containing monooxygenase 3 mimics calorie restriction to retard liver aging by inducing autophagy. Aging 2020, 12, 931–944. [Google Scholar] [CrossRef]
- Leiser, S.F.; Miller, H.; Rossner, R.; Fletcher, M.; Leonard, A.; Primitivo, M.; Rintala, N.; Ramos, F.J.; Miller, D.L.; Kaeberlein, M. Cell nonautonomous activation of flavin-containing monooxygenase promotes longevity and health span. Science 2015, 350, 1375–1378. [Google Scholar] [CrossRef]
- Savetsky, I.L.; Albano, N.J.; Cuzzone, D.A.; Gardenier, J.C.; Torrisi, J.S.; García Nores, G.D.; Nitti, M.D.; Hespe, G.E.; Nelson, T.S.; Kataru, R.P.; et al. Lymphatic Function regulates contact hypersensitivity dermatitis in obesity. J. Investig. Dermatol. 2015, 135, 2742–2752. [Google Scholar] [CrossRef]
- Escobedo, N.; Proulx, S.T.; Karaman, S.; Dillard, M.E.; Johnson, N.; Detmar, M.; Oliver, G. Restoration of lymphatic function rescues obesity in prox1-haploinsufficient mice. JCI Insight 2016, 1, 85096. [Google Scholar] [CrossRef]
- Sato, M.; Kawakami, T.; Kondoh, M.; Takiguchi, M.; Kadota, Y.; Himeno, S.; Suzuki, S. Development of high-fat-diet-induced obesity in female metallothionein-null mice. FASEB J. 2010, 24, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Beattie, J.H.; Wood, A.M.; Newman, A.M.; Bremner, I.; Choo, K.H.; Michalska, A.E.; Duncan, J.S.; Trayhurn, P. Obesity and hyperleptinemia in metallothionein (-I and -II) null mice. Proc. Natl. Acad. Sci. USA 1998, 95, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Kadota, Y.; Toriuchi, Y.; Aki, Y.; Mizuno, Y.; Kawakami, T.; Nakaya, T.; Sato, M.; Suzuki, S. Metallothioneins regulate the adipogenic differentiation of 3T3-L1 cells via the insulin signaling pathway. PLoS ONE 2017, 12, e0176070. [Google Scholar] [CrossRef] [PubMed]
- Higashimoto, M.; Sano, M.; Kondoh, M.; Sato, M. Different responses of metallothionein and leptin induced in the mouse by fasting stress. Biol. Trace Elem. Res. 2002, 89, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, M.; Kamada, K.; Kuronaga, M.; Higashimoto, M.; Takiguchi, M.; Watanabe, Y.; Sato, M. Antioxidant property of metallothionein in fasted mice. Toxicol. Lett. 2003, 143, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, J.; Zhu, D.; Jiang, X.; Wei, L.; Wang, W.; Chen, Y.Q. Adipose tissue plays a major role in retinoic acid-mediated metabolic homoeostasis. Adipocyte 2022, 11, 47–55. [Google Scholar] [CrossRef]
- Kennedy, L.; Meadows, V.; Sybenga, A.; Demieville, J.; Chen, L.; Hargrove, L.; Ekser, B.; Dar, W.; Ceci, L.; Kundu, D.; et al. Mast Cells promote nonalcoholic fatty liver disease phenotypes and microvesicular steatosis in mice fed a Western diet. Hepatology 2021, 74, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Liu, D.; Gao, Q.; Ni, J.; Qian, L.; Ni, Y.; Fang, Q.; Jia, W.; Li, H. Bifidobacterium adolescentis alleviates liver steatosis and steatohepatitis by increasing fibroblast growth factor 21 sensitivity. Front. Endocrinol. 2021, 12, 773340. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M.J. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Chen, J.; Yi, K.; Peng, L.; Xie, J.; Gou, X.; Peng, T.; Tang, L. Phlorizin ameliorates obesity-associated endotoxemia and insulin resistance in high-fat diet-fed mice by targeting the gut microbiota and intestinal barrier integrity. Gut Microbes 2020, 12, 1842990. [Google Scholar] [CrossRef]
- Wertenbruch, S.; Drescher, H.; Grossarth, V.; Kroy, D.; Giebeler, A.; Erschfeld, S.; Heinrichs, D.; Soehnlein, O.; Trautwein, C.; Brandenburg, L.O.; et al. The anti-microbial peptide LL-37/CRAMP is elevated in patients with liver diseases and acts as a protective factor during mouse liver injury. Digestion 2015, 91, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xue, X.; Zhang, J.; Liang, S.; Xu, C.; Wang, Y.; Zhu, J. Liver expressed antimicrobial peptide 2 is associated with steatosis in mice and humans. Exp. Clin. Endocrinol. Diabetes 2021, 129, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Heuer, J.; Heuer, F.; Stürmer, R.; Harder, S.; Schlüter, H.; Braga Emidio, N.; Muttenthaler, M.; Jechorek, D.; Meyer, F.; Hoffmann, W. The tumor suppressor TFF1 occurs in different forms and interacts with multiple partners in the human gastric mucus barrier: Indications for diverse protective functions. Int. J. Mol. Sci. 2020, 21, 2508. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.; Yoon, J.H.; Choi, W.S.; Ashktorab, H.; Smoot, D.T.; Nam, S.W.; Lee, J.Y.; Park, W.S. Heterodimeric interaction between GKN2 and TFF1 entails synergistic antiproliferative and pro-apoptotic effects on gastric cancer cells. Gastric Cancer 2017, 20, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Shi, G.P. Calcium-activated chloride channel regulator 1 (CLCA1): More than a regulator of chloride transport and mucus production. World Allergy Organ. J. 2019, 12, 100077. [Google Scholar] [CrossRef] [PubMed]
- Hagenbuch, B.; Meier, P.J. Organic anion transporting polypeptides of the OATP/ SLC21 family: Phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflug. Arch. 2004, 447, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Cockrum, M.A.; Napoli, J.L. Cyp26a1 supports post-natal retinoic acid homeostasis and glucoregulatory control. J. Biol. Chem. 2023, 299, 104669. [Google Scholar] [CrossRef]
- Dupont, N.; Chauhan, S.; Arko-Mensah, J.; Castillo, E.F.; Masedunskas, A.; Weigert, R.; Robenek, H.; Proikas-Cezanne, T.; Deretic, V. Neutral lipid stores and lipase PNPLA5 contribute to autophagosome biogenesis. Curr. Biol. 2014, 24, 609–620. [Google Scholar] [CrossRef]
- Peng, H.; Chiu, T.Y.; Liang, Y.J.; Lee, C.J.; Liu, C.S.; Suen, C.S.; Yen, J.J.; Chen, H.T.; Hwang, M.J.; Hussain, M.M.; et al. PRAP1 is a novel lipid-binding protein that promotes lipid absorption by facilitating MTTP-mediated lipid transport. J. Biol. Chem. 2021, 296, 100052. [Google Scholar] [CrossRef]
- Sohet, F.M.; Neyrinck, A.M.; Pachikian, B.D.; de Backer, F.C.; Bindels, L.B.; Niklowitz, P.; Menke, T.; Cani, P.D.; Delzenne, N.M. Coenzyme Q10 supplementation lowers hepatic oxidative stress and inflammation associated with diet-induced obesity in mice. Biochem. Pharmacol. 2009, 78, 1391–1400. [Google Scholar] [CrossRef]
- Martinefski, M.R.; Rodriguez, M.R.; Buontempo, F.; Lucangioli, S.E.; Bianciotti, L.G.; Tripodi, V.P. Coenzyme Q 10 supplementation: A potential therapeutic option for the treatment of intrahepatic cholestasis of pregnancy. Eur. J. Pharmacol. 2020, 882, 173270. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Huo, J.; Ding, X.; Yang, M.; Li, L.; Dai, J.; Hosoe, K.; Kubo, H.; Mori, M.; Higuchi, K.; et al. Coenzyme Q10 improves lipid metabolism and ameliorates obesity by regulating CaMKII-mediated PDE4 inhibition. Sci. Rep. 2017, 7, 8253. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.M.; Cottrell, J.N.; Comley, K.M.; Cunningham, M.W., Jr.; Witcher, A.; Vaka, V.R.; Ibrahim, T.; LaMarca, B. 17-Hydroxyprogesterone caproate improves hypertension and renal endothelin-1 in response to sFlt-1 induced hypertension in pregnant rats. Pregnancy Hypertens. 2020, 22, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Gaetani, S.; Oveisi, F.; Lo Verme, J.; Serrano, A.; Rodríguez de Fonseca, F.; Rosengarth, A.; Luecke, H.; Di Giacomo, B.; Tarzia, G.; et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature 2003, 425, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Pavón, F.J.; Serrano, A.; Romero-Cuevas, M.; Alonso, M.; de Fonseca, F.R. Oleoylethanolamide: A new player in peripheral control of energy metabolism. Therapeutic implications. Drug Discov. Today Dis. Mech. 2010, 7, e175–e183. [Google Scholar] [CrossRef]
- Guzman, M.; Lo Verme, J.; Fu, J.; Oveisi, F.; Blazquez, C.; Piomelli, D. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha (ppar-alpha). J. Biol. Chem. 2004, 279, 27849–27854. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, L.; Chen, L.; Lin, X.; Xu, Y.; Ren, J.; Fu, J.; Qiu, Y. Effect of oleoylethanolamide on diet-induced nonalcoholic fatty liver in rats. J. Pharmacol. Sci. 2015, 127, 244–250. [Google Scholar] [CrossRef]
- Proulx, K.; Cota, D.; Castañeda, T.R.; Tschöp, M.H.; D’Alessio, D.A.; Tso, P.; Woods, S.C.; Seeley, R.J. Mechanisms of oleoylethanolamide-induced changes in feeding behavior and motor activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R729–R737. [Google Scholar] [CrossRef]
- Piomelli, D. A fatty gut feeling. Trends Endocrinol. Metab. 2013, 24, 332–341. [Google Scholar] [CrossRef]
- Giudetti, A.M.; Vergara, D.; Longo, S.; Friuli, M.; Eramo, B.; Tacconi, S.; Fidaleo, M.; Dini, L.; Romano, A.; Gaetani, S. Oleoylethanolamide reduces hepatic oxidative dtress and endoplasmic reticulum stress in high-fat diet-fed rats. Antioxidants 2021, 10, 1289. [Google Scholar] [CrossRef]
- Tutunchi, H.; Zolrahim, F.; Nikbaf-Shandiz, M.; Naeini, F.; Ostadrahimi, A.; Naghshi, S.; Salek, R.; Najafipour, F. Effects of oleoylethanolamide supplementation on inflammatory biomarkers, oxidative stress and antioxidant parameters of obese patients with NAFLD on a calorie-restricted diet: A randomized controlled trial. Front. Pharmacol. 2023, 14, 1144550. [Google Scholar] [CrossRef]
- Lin, L.; Mabou Tagne, A.; Squire, E.N.; Lee, H.L.; Fotio, Y.; Ramirez, J.; Zheng, M.; Torrens, A.; Ahmed, F.; Ramos, R.; et al. Diet-Induced obesity disrupts histamine-dependent oleoylethanolamide dignaling in the mouse liver. Pharmacology 2022, 107, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Dohi, H.; Egashira, Y.; Hirai, S. Erucic acid derived from rosemary regulates differentiation of mesenchymal stem cells into osteoblasts/adipocytes via suppression of peroxisome proliferator-activated receptor γ transcriptional activity. Phytother. Res. 2020, 34, 1358–1366. [Google Scholar] [CrossRef]
- Takahashi, A.; Ishizaki, M.; Kimira, Y.; Egashira, Y.; Hirai, S. Erucic acid-rich yellow mustard oil improves insulin resistance in KK-Ay mice. Molecules 2021, 26, 546. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Cervera, M.Á.; Valenzuela, R.; Hernandez-Rodas, M.C.; Barrera, C.; Espinosa, A.; Marambio, M.; Valenzuela, A. Vegetable oils rich in alpha linolenic acid increment hepatic n-3 LCPUFA, modulating the fatty acid metabolism and antioxidant response in rats. Prostaglandins Leukot. Essent. Fat. Acids 2016, 111, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Ma, C.; Liu, C.; Gao, C.; Nie, R.; Wang, H. Hypolipidemic activity of peony seed oil rich in α-Linolenic, is mediated through inhibition of lipogenesis and upregulation of fatty acid β-oxidation. J. Food Sci. 2016, 81, H1001–H1009. [Google Scholar] [CrossRef] [PubMed]
- González-Mañán, D.; D’Espessailles, A.; Dossi, C.G.; San Martín, M.; Mancilla, R.A.; Tapia, G.S. Rosa Mosqueta Oil prevents oxidative stress and inflammation through the upregulation of PPAR-α and NRF2 in C57BL/6J mice fed a high-fat diet. J. Nutr. 2017, 147, 579–588. [Google Scholar] [CrossRef]
- Chanda, D.; Oligschlaeger, Y.; Geraets, I.; Liu, Y.; Zhu, X.; Li, J.; Nabben, M.; Coumans, W.; Luiken, J.J.F.P.; Glatz, J.F.C.; et al. 2-Arachidonoylglycerol ameliorates inflammatory stress-induced insulin resistance in cardiomyocytes. J. Biol. Chem. 2017, 292, 7105–7114. [Google Scholar] [CrossRef]
- Aguilera Vasquez, N.; Nielsen, D.E. The endocannabinoid system and eating behaviours: A review of the current state of the evidence. Curr. Nutr. Rep. 2022, 11, 665–674. [Google Scholar] [CrossRef]
- Castellanos-Jankiewicz, A.; Guzmán-Quevedo, O.; Fénelon, V.S.; Zizzari, P.; Quarta, C.; Bellocchio, L.; Tailleux, A.; Charton, J.; Fernandois, D.; Henricsson, M.; et al. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab. 2021, 33, 1483–1492. [Google Scholar] [CrossRef]
- Wu, Q.; Liang, X.; Wang, K.; Lin, J.; Wang, X.; Wang, P.; Zhang, Y.; Nie, Q.; Liu, H.; Zhang, Z.; et al. Intestinal hypoxia-inducible factor 2α regulates lactate levels to shape the gut microbiome and alter thermogenesis. Cell Metab. 2021, 33, 1988–2003.e7. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.C.; Duan, G.Z.; Mao, W.; Liu, Q.; Zhang, Y.L.; Li, P.F. Taurochenodeoxycholic acid mediates cAMP-PKA-CREB signaling pathway. Chin. J. Nat. Med. 2020, 18, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Shi, L.; Duan, G.; Ma, Y.; Li, P. Taurochenodeoxycholic acid Increases cAMP content via specially interacting with bile acid Receptor TGR5. Molecules 2021, 26, 66. [Google Scholar] [CrossRef] [PubMed]
- Huong, D.T.; Takahashi, Y.; Ide, T. Activity and mRNA levels of enzymes involved in hepatic fatty acid oxidation in mice fed citrus flavonoids. Nutrition 2006, 22, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Clayton, P.; Hill, M.; Bogoda, N.; Subah, S.; Venkatesh, R. Palmitoylethanolamide: A natural compound for health management. Int. J. Mol. Sci. 2021, 22, 5305. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zou, X.; Gao, Z.; Mao, C.; Su, H.; Li, C.; Chen, N. Improved fatty acid profile reduces body fat and arterial stiffness in obese adolescents upon combinatorial intervention with exercise and dietary restriction. J. Exerc. Sci. Fit. 2021, 19, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, X.; Huang, F.; Zhao, A.; Ge, K.; Zhao, Q.; Jia, W. Ursodeoxycholic acid alters bile acid and fatty acid profiles in a mouse model of diet-induced obesity. Front. Pharmacol. 2019, 10, 842. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.R.; Liebermann, D.A. Gadd45 in senescence. Adv. Exp. Med. Biol. 2022, 1360, 109–116. [Google Scholar]
- Wu, Y.K.; Ren, Z.N.; Zhu, S.L.; Wu, Y.Z.; Wang, G.; Zhang, H.; Chen, W.; He, Z.; Ye, X.L.; Zhai, Q.X. Sulforaphane ameliorates non-alcoholic fatty liver disease in mice by promoting FGF21/FGFR1 signaling pathway. Acta Pharmacol. Sin. 2022, 43, 1473–1483. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Collado, M.C.; Salminen, S.; Isolauri, E. Early differences in fecal microbiota composition in children may predict overweight. Am. J. Clin. Nutr. 2008, 87, 534–538. [Google Scholar] [CrossRef]
- Teeple, K.; Rajput, P.; Gonzalez, M.; Han-Hallett, Y.; Fernández-Juricic, E.; Casey, T. High fat diet induces obesity, alters eating pattern and disrupts corticosterone circadian rhythms in female ICR mice. PLoS ONE 2023, 18, e0279209. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Matye, D.J.; Chavan, H.; Krishnamurthy, P.; Li, F.; Li, T. Sortilin 1 modulates hepatic cholesterol lipotoxicity in mice via functional interaction with liver carboxylesterase 1. J. Biol. Chem. 2017, 292, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Pasta, A.; Cremonini, A.L.; Pisciotta, L.; Buscaglia, A.; Porto, I.; Barra, F.; Ferrero, S.; Brunelli, C.; Rosa, G.M. PCSK9 inhibitors for treating hypercholesterolemia. Expert. Opin. Pharmacother. 2020, 21, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Fork, C.; Bauer, T.; Golz, S.; Geerts, A.; Weiland, J.; Del Turco, D.; Schömig, E.; Gründemann, D. OAT2 catalyses efflux of glutamate and uptake of orotic acid. Biochem. J. 2011, 436, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Setchell, K.D.R. Will the real bile acid sulfotransferase please stand up? Identification of Sult2a8 as a major hepatic bile acid sulfonating enzyme in mice. J. Lipid Res. 2017, 58, 1033–1035. [Google Scholar] [CrossRef] [PubMed]
- Takatsu, Y.; Matsumoto, H.; Ohtaki, T.; Kumano, S.; Kitada, C.; Onda, H.; Nishimura, O.; Fujino, M. Distribution of galanin-like peptide in the rat brain. Endocrinology 2001, 142, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.M.; Gundlach, A.L.; Morris, M.J. Exaggerated feeding response to central galanin-like peptide administration in diet-induced obese rats. Neuropeptides 2005, 39, 333–336. [Google Scholar] [CrossRef]
- Lawrence, C.B.; Baudoin, F.M.; Luckman, S.M. Centrally administered galanin-like peptide modifies food intake in the rat: A comparison with galanin. J. Neuroendocrinol. 2002, 14, 853–860. [Google Scholar] [CrossRef]
- Kauffman, A.S.; Buenzle, J.; Fraley, G.S.; Rissman, E.F. Effects of galanin-like peptide (GALP) on locomotion, reproduction, and body weight in female and male mice. Horm. Behav. 2005, 48, 141–151. [Google Scholar] [CrossRef]
- Krasnow, S.M.; Hohmann, J.G.; Gragerov, A.; Clifton, D.K.; Steiner, R.A. Analysis of the contribution of galanin receptors 1 and 2 to the central actions of galanin-like peptide. Neuroendocrinology 2004, 79, 268–277. [Google Scholar] [CrossRef]
- Man, P.S.; Lawrence, C.B. Interleukin-1 mediates the anorexic and febrile actions of galanin-like Peptide. Endocrinology 2008, 149, 5791–5802. [Google Scholar] [CrossRef]
- Hansen, K.R.; Krasnow, S.M.; Nolan, M.A.; Fraley, G.S.; Baumgartner, J.W.; Clifton, D.K.; Steiner, R.A. Activation of the sympathetic nervous system by galanin-like peptide--a possible link between leptin and metabolism. Endocrinology 2003, 144, 4709–4717. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Friuli, M.; Eramo, B.; Gallelli, C.A.; Koczwara, J.B.; Azari, E.K.; Paquot, A.; Arnold, M.; Langhans, W.; Muccioli, G.G.; et al. “To brain or not to brain”: Evaluating the possible direct effects of the satiety factor oleoylethanolamide in the central nervous system. Front. Endocrinol. 2023, 14, 1158287. [Google Scholar] [CrossRef] [PubMed]
- Tutunchi, H.; Saghafi-Asl, M.; Ostadrahimi, A. A systematic review of the effects of oleoylethanolamide, a high-affinity endogenous ligand of PPAR-α, on the management and prevention of obesity. Clin. Exp. Pharmacol. Physiol. 2020, 47, 543–552. [Google Scholar] [CrossRef]
- Rodríguez de Fonseca, F.; Navarro, M.; Gómez, R.; Escuredo, L.; Nava, F.; Fu, J.; Murillo-Rodríguez, E.; Giuffrida, A.; LoVerme, J.; Gaetani, S.; et al. An anorexic lipid mediator regulated by feeding. Nature 2001, 414, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Azari, E.K.; Ramachandran, D.; Weibel, S.; Arnold, M.; Romano, A.; Gaetani, S.; Langhans, W.; Mansouri, A. Vagal afferents are not necessary for the satiety effect of the gut lipid messenger oleoylethanolamide. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R167–R178. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.J.; Tilbrook, A. The glucocorticoid contribution to obesity. Stress 2011, 14, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Black, P.H. The inflammatory consequences of psychologic stress: Relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med. Hypotheses 2006, 67, 879–891. [Google Scholar] [CrossRef]
- Nieuwenhuizen, A.G.; Rutters, F. The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol. Behav. 2008, 94, 169–177. [Google Scholar] [CrossRef]
- Rahimi, L.; Rajpal, A.; Ismail-Beigi, F. Glucocorticoid-Induced Fatty Liver Disease. Diabetes Metab. Syndr. Obes. 2020, 13, 1133–1145. [Google Scholar] [CrossRef]
- Kadmiel, M.; Cidlowski, J.A. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol. Sci. 2013, 34, 518–530. [Google Scholar] [CrossRef]
- de La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G440–G448. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.X.; Tao, R.; Li, S.C.; Shen, B.Z.; Meng, L.X.; Zhu, Z.Y. Role of defensins in diabetic wound healing. World J. Diabetes 2022, 13, 962–971. [Google Scholar] [CrossRef]
- Yele, V.; Sanapalli, B.K. Therapeutic modalities of human β defensin-2 with prospective significance in diabetic wound treatment. Wound Manag. Prev. 2023, 69, 9–45. [Google Scholar] [CrossRef]
- Maraga, E.; Safadi, R.; Amer, J.; Higazi, A.A.; Fanne, R.A. Alleviation of hepatic steatosis by alpha-defensin is associated with enhanced lipolysis. Medicina 2023, 59, 983. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Birmann, B.M.; Epstein, M.M.; Martínez-Maza, O.; Breen, E.C.; Wu, K.; Giovannucci, E.L. Influence of dietary patterns on plasma soluble CD14, a surrogate marker of gut barrier dysfunction. Curr. Dev. Nutr. 2017, 1, e001396. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Costa, A.; Becagli, M.V.; Monroy, M.M.; Provensi, G.; Passani, M.B. Gut microbiota and oleoylethanolamide in the regulation of intestinal homeostasis. Front. Endocrinol. 2023, 14, 1135157. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, M.; Bonechi, E.; Provensi, G.; Costa, A.; Clarke, G.; Ballerini, C.; De Filippo, C.; Passani, M.B. Oleoylethanolamide treatment affects gut microbiota composition and the expression of intestinal cytokines in Peyer’s patches of mice. Sci. Rep. 2018, 8, 14881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, R.; Zheng, X.; Lei, S.; Huang, F.; Xie, G.; Kwee, S.; Yu, H.; Farrar, C.; Sun, B.; et al. Ursodeoxycholic acid accelerates bile acid enterohepatic circulation. Br. J. Pharmacol. 2019, 176, 2848–2863. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: A review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef]
- Tiefenbach, J.; Magomedova, L.; Liu, J.; Reunov, A.A.; Tsai, R.; Eappen, N.S.; Jockusch, R.A.; Nislow, C.; Cummins, C.L.; Krause, H.M. Idebenone and coenzyme Q10 are novel PPARα/γ ligands, with potential for treatment of fatty liver diseases. Dis. Model. Mech. 2018, 11, dmm034801. [Google Scholar] [CrossRef]
- Murru, E.; Muntoni, A.L.; Manca, C.; Aroni, S.; Pistis, M.; Banni, S.; Carta, G. Profound modification of fatty acid profile and endocannabinoid-related mediators in PPARα agonist fenofibrate-treated mice. Int. J. Mol. Sci. 2022, 24, 709. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, W.; Chen, J.; Wang, X.; Wang, Y. AMP-activated protein kinase is required for the anti-adipogenic effects of alpha-linolenic acid. Nutr. Metab. 2015, 12, 10. [Google Scholar] [CrossRef]
- Shih, D.M.; Wang, Z.; Lee, R.; Meng, Y.; Che, N.; Charugundla, S.; Qi, H.; Wu, J.; Pan, C.; Brown, J.M.; et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J. Lipid Res. 2015, 56, 22–37. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Fold-Change | Gene Name | Fold-Change |
|---|---|---|---|
| Fmo3 | 10.94 | Gkn2 | 0.04 |
| Wfdc3 | 3.36 | Tff1 | 0.08 |
| Lyve1 | 3.09 | Defa3 | 0.13 |
| Mt2 | 3.05 | Hsd3b4 | 0.17 |
| Olfr1342 | 2.94 | Hsd3b5 | 0.17 |
| Aldh1a3 | 2.72 | Defa20 | 0.18 |
| Mt1 | 2.59 | Clca1 | 0.23 |
| Mt1 | 2.58 | Defa6 | 0.24 |
| Slc22a26 | 2.57 | Moxd1 | 0.25 |
| Mt1 | 2.55 | Defa1 | 0.27 |
| Mt1 | 2.55 | Cyp26a1 | 0.27 |
| Mt1 | 2.52 | Pnpla5 | 0.28 |
| Mt1 | 2.51 | Pdia6 | 0.30 |
| Mt1 | 2.50 | Slco1a1 | 0.31 |
| Mt1 | 2.49 | Prap1 | 0.31 |
| Mt1 | 2.48 | Fdps | 0.32 |
| Lrtm2 | 2.48 | Pik3c2g | 0.38 |
| Mt1 | 2.46 | Defb19 | 0.39 |
| Fgfr1 | 2.39 | Krt20 | 0.39 |
| Krt23 | 2.37 | Hsd3b1 | 0.40 |
| ID | Compound Name | Relative Area | Comparative Analysis | |
|---|---|---|---|---|
| Vehicle | GALP | Ratio | ||
| GALP vs Vehicle | ||||
| P_0057 | Coenzyme Q10 | N.D. | 0.0001 | 1< |
| P_0043 | Hydroxyprogesterone caproate | N.D. | 0.0001 | 1< |
| P_0010 | Oleoyl ethanolamide AEA(18:1) | 0.0002 | 0.0006 | 3.38 |
| P_0052 | 1,2-Dipalmitoyl-glycero-3-phosphoethanolamine | 0.0003 | 0.0006 | 2.22 |
| P_0023 | AC(14:0)-1 | 0.0001 | 0.0001 | 2.18 |
| N_0031 | Erucic acid | 0.0000 | 0.0001 | 1.99 |
| N_0037 | Deoxycholic acid | 0.0000 | 0.0000 | 1.84 |
| P_0028 | 5α-Cholestan-3-one-2 | 0.0001 | 0.0002 | 1.72 |
| N_0047 | Taurocholic acid | 0.0350 | 0.0596 | 1.70 |
| N_0046 | Taurochenodeoxycholic acid | 0.0047 | 0.0078 | 1.65 |
| N_0012 | Linolenic acid | 0.0000 | 0.0000 | 1.57 |
| P_0042 | AC(18:0)-2 | 0.0002 | 0.0003 | 1.52 |
| P_0025 | 2-Arachidonoylglycerol | 0.0013 | 0.0019 | 1.51 |
| N_0030 | FA(22:3) | 0.0001 | 0.0000 | 0.74 |
| P_0049 | α-Tocopherol acetate | 0.0006 | 0.0004 | 0.73 |
| N_0011 | Heptadecanoic acid-2 FA(17:0)-2 | 0.0001 | 0.0001 | 0.67 |
| P_0038 | γ-Tocopherol | 0.0003 | 0.0002 | 0.67 |
| P_0050 | Zeaxanthin | 0.0001 | 0.0000 | 0.67 |
| N_0023 | Arachidic acid | 0.0001 | 0.0000 | 0.66 |
| P_0006 | Palmitoylethanolamide-2 | 0.0004 | 0.0003 | 0.62 |
| P_0048 | AC(22:0) | 0.0003 | 0.0002 | 0.61 |
| P_0016 | AC(13:1) | 0.0003 | 0.0002 | 0.59 |
| N_0003 | Myristic acid | 0.0001 | 0.0001 | 0.59 |
| P_0008 | AC(10:0) | 0.0019 | 0.0011 | 0.56 |
| N_0001 | FA(12:0) | 0.0001 | 0.0000 | 0.50 |
| N_0010 | Heptadecanoic acid-1 FA(17:0)-1 | 0.0001 | 0.0000 | 0.48 |
| N_0004 | FA(15:0) | 0.0000 | 0.0000 | 0.45 |
| N_0008 | FA(17:1)-1 | 0.0000 | 0.0000 | 0.39 |
| P_0024 | Riboflavin | 0.0001 | 0.0000 | 0.23 |
| P_0047 | AC(20:0)-1 | 0.0001 | N.D. | <1 |
| N_0009 | FA(17:1)-2 | 0.0000 | N.D. | <1 |
| P_0001 | Flavanone | 0.0004 | N.D. | <1 |
| N_0038 | Ursodeoxycholic acid | 0.0000 | N.D. | <1 |
| Mono (Poly) unsaturated fatty acid | ||||
| Saturated fatty acid | ||||
| Endocannabinoids | ||||
| Bile acid | ||||
| Category KEGG_PATHWAY | ||
| UP | ||
| mmu00062 | Fatty acid elongation | ACOT1, ELOVL7, ACOT3 |
| mmu01040 | Biosynthesis of unsaturated fatty acids | ACOT1, ELOVL7, ACOT3 |
| mmu00140 | Steroid hormone biosynthesis | CYP2B13, SULT1E1, CYP2B10 |
| mmu00830 | Retinol metabolism | ALDH1A3, CYP2B13, CYP2B10 |
| mmu04010 | MAPK signaling pathway | GADD45B, MAP3K6, FGFR1 |
| mmu04978 | Mineral absorption | MT2, MT1 |
| mmu04913 | Ovarian steroidogenesis | ACOT1, ACOT3 |
| mmu05218 | Melanoma | GADD45B, FGFR1 |
| Down | ||
| mmu05150 | Staphylococcus aureus infection | CFD, DEFA6, DEFA3, DEFA1, DEFA20, KRT20 |
| mmu04927 | Cortisol synthesis and secretion | NR4A1, HSD3B4, HSD3B5, STAR, HSD3B1 |
| mmu04925 | Aldosterone synthesis and secretion | NR4A1, HSD3B4, HSD3B5, STAR, HSD3B1 |
| mmu04913 | Ovarian steroidogenesis | HSD3B4, HSD3B5, STAR, HSD3B1 |
| mmu04934 | Cushing syndrome | NR4A1, HSD3B4, HSD3B5, STAR, HSD3B1 |
| mmu00900 | Terpenoid backbone biosynthesis | FDPS, IDI1, MVD |
| mmu00140 | Steroid hormone biosynthesis | HSD3B4, HSD3B5, CYP2C66, HSD3B1 |
| mmu04915 | Estrogen signaling pathway | TFF1, FOS, KRT20, HSPA1A |
| mmu04979 | Cholesterol metabolism | STAR, SORT1, PCSK9 |
| mmu04621 | NOD-like receptor signaling pathway | DEFA6, DEFA3, DEFA1, DEFA20 |
| mmu04024 | cAMP signaling pathway | HCN4, SUCNR1, PDE4B, FOS |
| mmu05202 | Transcriptional misregulation in cancer | DEFA6, DEFA3, DEFA1, DEFA20 |
| mmu04976 | Bile secretion | SLCO1A1, SULT2A8, SLC22A7 |
| mmu05132 | Salmonella infection | MYO6, PIK3C2G, FOS, TUBA4A |
| mmu00100 | Steroid biosynthesis | SC5D, CYP51 |
| mmu04010 | MAPK signaling pathway | EFNA1, NR4A1, FOS, HSPA1A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takenoya, F.; Shibato, J.; Yamashita, M.; Kimura, A.; Hirako, S.; Chiba, Y.; Nonaka, N.; Shioda, S.; Rakwal, R. Transcriptomic (DNA Microarray) and Metabolome (LC-TOF-MS) Analyses of the Liver in High-Fat Diet Mice after Intranasal Administration of GALP (Galanin-like Peptide). Int. J. Mol. Sci. 2023, 24, 15825. https://doi.org/10.3390/ijms242115825
Takenoya F, Shibato J, Yamashita M, Kimura A, Hirako S, Chiba Y, Nonaka N, Shioda S, Rakwal R. Transcriptomic (DNA Microarray) and Metabolome (LC-TOF-MS) Analyses of the Liver in High-Fat Diet Mice after Intranasal Administration of GALP (Galanin-like Peptide). International Journal of Molecular Sciences. 2023; 24(21):15825. https://doi.org/10.3390/ijms242115825
Chicago/Turabian StyleTakenoya, Fumiko, Junko Shibato, Michio Yamashita, Ai Kimura, Satoshi Hirako, Yoshihiko Chiba, Naoko Nonaka, Seiji Shioda, and Randeep Rakwal. 2023. "Transcriptomic (DNA Microarray) and Metabolome (LC-TOF-MS) Analyses of the Liver in High-Fat Diet Mice after Intranasal Administration of GALP (Galanin-like Peptide)" International Journal of Molecular Sciences 24, no. 21: 15825. https://doi.org/10.3390/ijms242115825
APA StyleTakenoya, F., Shibato, J., Yamashita, M., Kimura, A., Hirako, S., Chiba, Y., Nonaka, N., Shioda, S., & Rakwal, R. (2023). Transcriptomic (DNA Microarray) and Metabolome (LC-TOF-MS) Analyses of the Liver in High-Fat Diet Mice after Intranasal Administration of GALP (Galanin-like Peptide). International Journal of Molecular Sciences, 24(21), 15825. https://doi.org/10.3390/ijms242115825






