Neuromodulators as Interdomain Signaling Molecules Capable of Occupying Effector Binding Sites in Bacterial Transcription Factors
Abstract
1. Introduction
2. Results
2.1. Structural Models of Neuromodulators and Transcription Factors Selected for Analysis
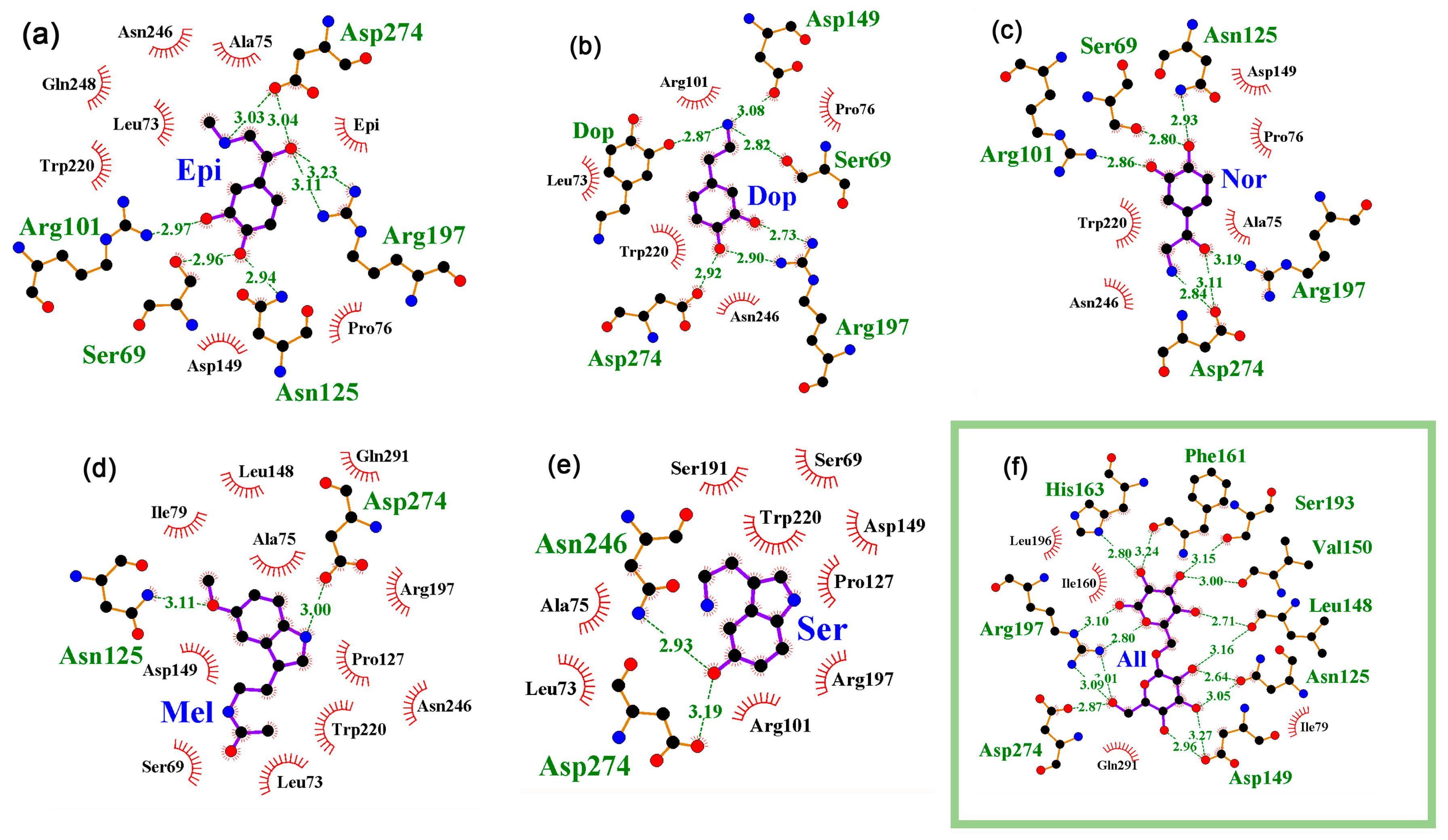
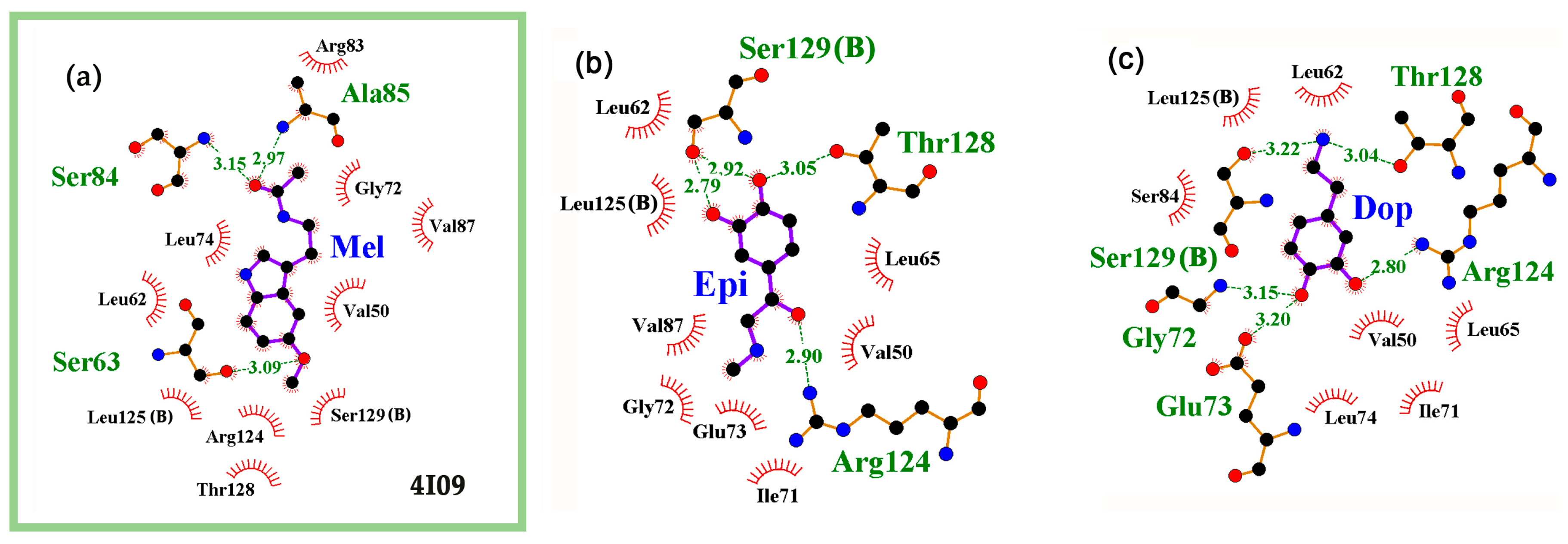
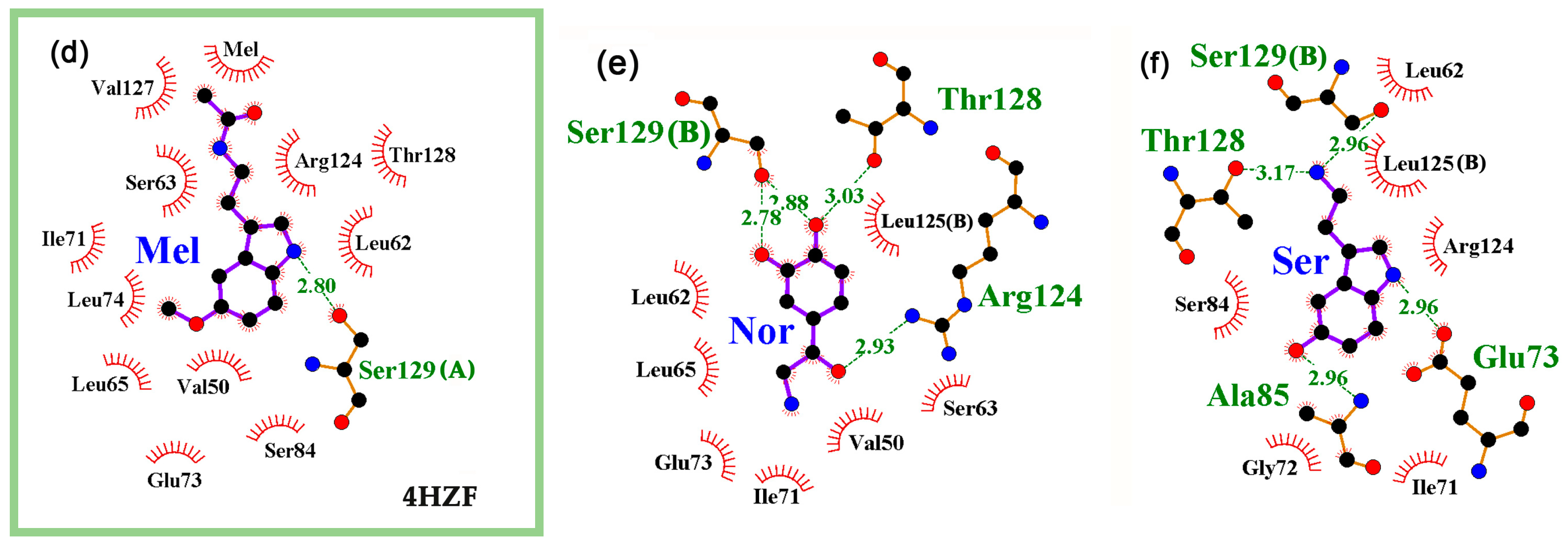
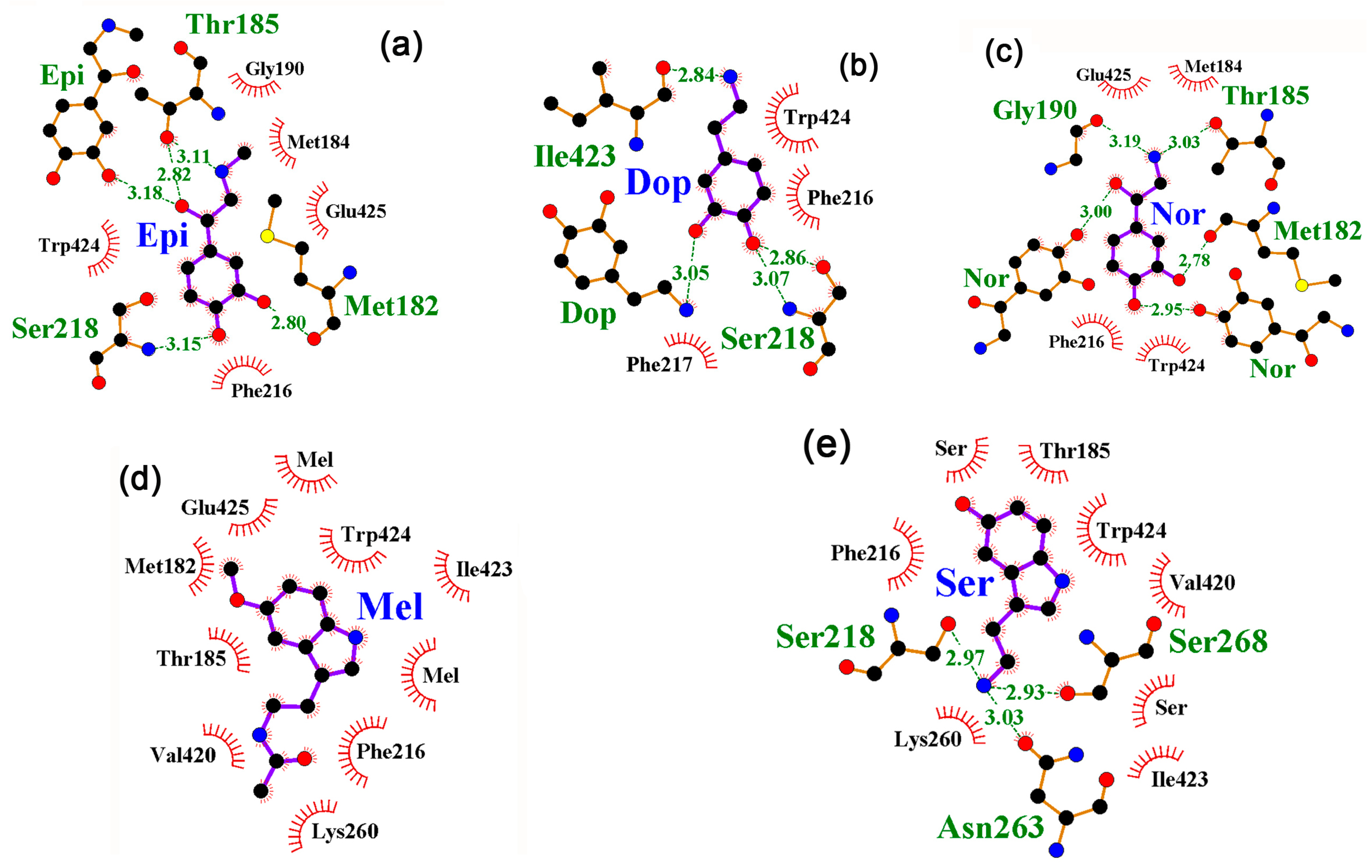
2.2. The Interaction of Neuromodulators with the Local Transcription Factor LacI Was Predicted at the Site of IPTG Binding for All Studied Protein Models
2.3. A Specific Interaction of at Least one Neuromodulator with the cAMP Binding Site Is Predicted for All Thirteen 3D Models of CRP
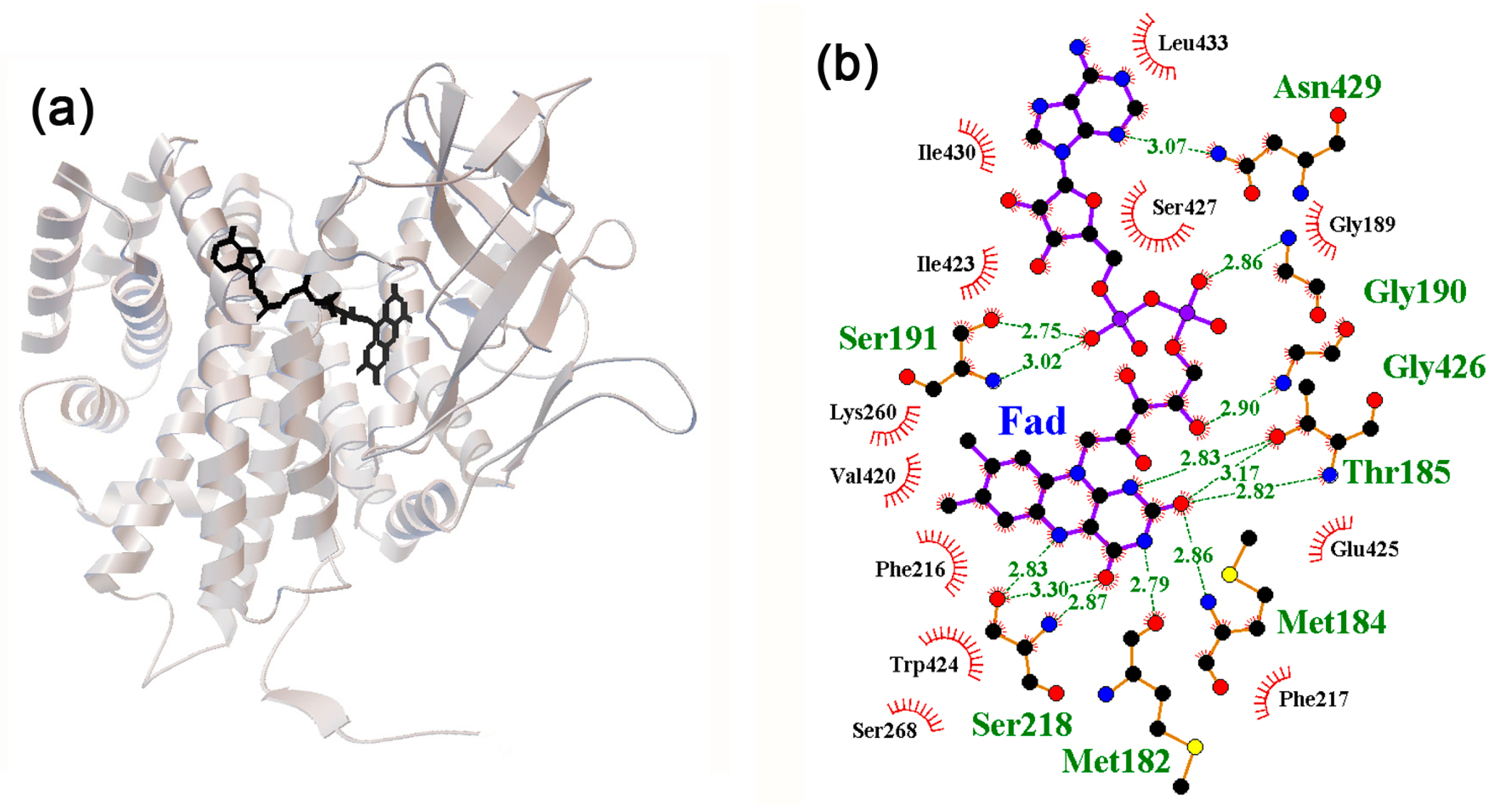
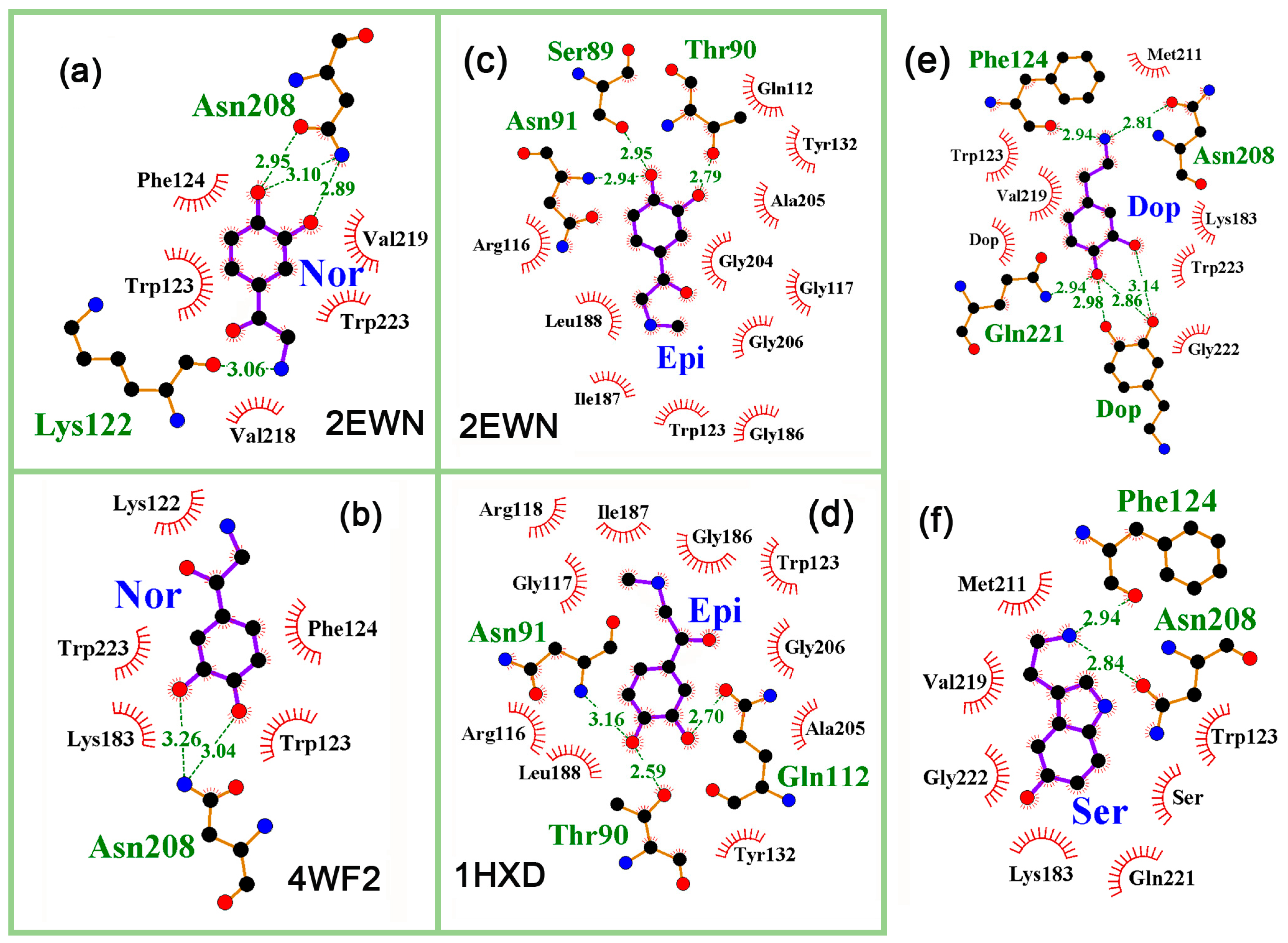
2.4. Interaction of Neuromodulators with Effector Binding Sites of Bifunctional Proteins
2.5. Overall Assessment of the “Propensity” of Bacterial Transcription Factors to Bind NM
3. Discussion
4. Materials and Methods
4.1. Structural Models of Neuromodulators Used in This Work
4.2. Structural Models of Transcription Factors Used in This Work
4.3. Flexible Protein–Ligand Docking
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyte, M. The role of microbial endocrinology in infectious disease. J. Endocrinol. 1993, 137, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M. Microbial endocrinology and infectious disease in the 21st century. Trends Microbiol. 2004, 12, 14–20. [Google Scholar] [CrossRef]
- Freestone, P.P.E.; Sandrini, S.M.; Haigh, R.D.; Lyte, M. Microbial endocrinology: How stress influences susceptibility to infection. Trends Microbiol. 2008, 16, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Freestone, P. Microbial endocrinology comes of age. Microbe 2009, 4, 169–175. [Google Scholar] [CrossRef]
- Freestone, P. Communication between bacteria and their hosts. Scientifica 2013, 2013, 361073. [Google Scholar] [CrossRef]
- Boukerb, A.M.; Cambronel, M.; Rodrigues, S.; Mesguida, O.; Knowlton, R.; Feuilloley, M.G.J.; Zommiti, M.; Connil, N. Inter-kingdom signaling of stress hormones: Sensing, transport and modulation of bacterial physiology. Front. Microbiol. 2021, 12, 690942. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Eisenhofer, G.; Kopin, I.J. Sources and significance of plasma levels of catechols and their metabolites in humans. J. Pharmacol. Exp. Ther. 2003, 305, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Flierl, M.A.; Rittirsch, D.; Nadeau, B.A.; Chen, A.J.; Sarma, J.V.; Zetoune, F.S.; McGuire, S.R.; List, R.P.; Day, D.E.; Hoesel, L.M.; et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature 2007, 449, 721–725. [Google Scholar] [CrossRef]
- Bubenik, G.A. Gastrointestinal melatonin: Localization, function, and clinical relevance. Dig. Dis. Sci. 2002, 47, 2336–2348. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Martin, A.M.; Sun, E.W.; Rogers, G.B.; Keating, D.J. The influence of the gut microbiome on host metabolism through the regulation of gut hormone release. Front. Physiol. 2019, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, S.; Ghafoor, A.; Mehri, S.; Barazi, A.; Dziura, M.; Trant, J.F.; Dieni, C.A. Catechin and other catechol-containing secondary metabolites: Bacterial biotransformation and regulation of carbohydrate metabolism. Pharma Nutr. 2021, 17, 100273. [Google Scholar] [CrossRef]
- Gonçalves, S.; Nunes-Costa, D.; Cardoso, S.M.; Empadinhas, N.; Marugg, J.D. Enzyme promiscuity in serotonin biosynthesis, from bacteria to plants and humans. Front. Microbiol. 2022, 13, 873555. [Google Scholar] [CrossRef]
- Woods, D.E.; Jones, A.L.; Hill, P.J. Interaction of insulin with Pseudomonas pseudomallei. Infect. Immun. 1993, 61, 4045–4050. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Han, H.; Ma, J.; Liu, G.; Wu, X.; Huang, X.; Fang, R.; Baba, K.; Bin, P.; et al. Administration of exogenous melatonin improves the diurnal rhythms of the gut microbiota in mice fed a high-fat diet. mSystems 2020, 5, e00002-20. [Google Scholar] [CrossRef]
- Zaborina, O.; Lepine, F.; Xiao, G.; Valuckaite, V.; Chen, Y.; Li, T.; Ciancio, M.; Zaborin, A.; Petrof, E.O.; Turner, J.R.; et al. Dynorphin activates quorum sensing quinolone signaling in Pseudomonas aeruginosa. PLoS Pathog. 2007, 3, e35. [Google Scholar] [CrossRef]
- Chojnacki, C.; Popławski, T.; Blasiak, J.; Chojnacki, J.; Reiter, R.J.; Klupinska, G. Expression of melatonin synthesizing enzymes in Helicobacter pylori infected gastric mucosa. BioMed Res. Int. 2013, 2013, 845032. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.R.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Chen, H.; Nwe, P.-K.; Yang, Y.; Rosen, C.E.; Bielecka, A.A.; Kuchroo, M.; Cline, G.W.; Kruse, A.C.; Ring, A.M.; Crawford, J.M.; et al. A forward chemical genetic screen reveals gut microbiota metabolites that modulate host physiology. Cell 2019, 177, 1217–1231. [Google Scholar] [CrossRef]
- Lyte, M.; Ernst, S. Catecholamine induced growth of gram negative bacteria. Life Sci. 1992, 50, 203–212. [Google Scholar] [CrossRef]
- Coulanges, V.; Andre, P.; Vidon, D.J.-M. Effect of siderophores, catecholamines, and catechol compounds on Listeria spp. growth in iron-complexed medium. Biochem. Biophys. Res. Commun. 1998, 249, 526–530. [Google Scholar] [CrossRef]
- Freestone, P.P.E.; Haigh, R.D.; Lyte, M. Specificity of catecholamine-induced growth in Escherichia coli O157:H7, Salmonella enterica and Yersinia enterocolitica. FEMS Microbiol. Lett. 2007, 269, 221–228. [Google Scholar] [CrossRef]
- Doherty, N.C.; Tobias, A.; Watson, S.; Atherton, J.C. The effect of the human gut-signalling hormone, norepinephrine, on the growth of the gastric pathogen Helicobacter pylori. Helicobacter 2009, 14, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Freestone, P.P.E.; Neal, C.P.; Olson, B.A.; Haigh, R.D.; Bayston, R.; Williams, P.H. Stimulation of Staphylococcus epidermidis growth and biofilm formation by catecholamine inotropes. Lancet 2003, 361, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Arulanandam, B.; Nguyen, K.; Frank, C.; Erickson, A.; Francis, D. Norepinephrine induced growth and expression of virulence associated factors in enterotoxigenic and enterohemorrhagic strains of Escherichia coli. Adv. Exp. Med. Biol. 1997, 412, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Arulanandam, B.P.; Frank, C.D. Production of Shiga-like toxins by Escherichia coli O157:H7 can be influenced by the neuroendocrine hormone norepinephrine. J. Lab. Clin. Med. 1996, 128, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Green, B.T.; Lyte, M.; Chen, C.; Xie, Y.; Casey, M.A.; Kulkarni-Narla, A.; Vulchanova, L.; Brown, D.R. Adrenergic modulation of Escherichia coli O157:H7 adherence to the colonic mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1238–G1246. [Google Scholar] [CrossRef] [PubMed]
- Bansal, T.; Engler, T.D.; Lee, J.; Hegde, M.; Wood, T.K.; Jayaraman, A. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect. Immun. 2007, 75, 4597–4607. [Google Scholar] [CrossRef]
- Nakano, M.; Takahashi, A.; Sakai, Y.; Nakaya, Y. Modulation of pathogenicity with norepinephrine related to the type III secretion system of Vibrio parahaemolyticus. J. Infect. Dis. 2007, 195, 1353–1360. [Google Scholar] [CrossRef]
- Methner, U.; Rabsch, W.; Reissbrodt, R.; Williams, P.H. Effect of norepinephrine on colonisation and systemic spread of Salmonella enterica in infected animals: Role of catecholatesiderophore precursors and degradation products. Int. J. Med. Microbiol. 2008, 298, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.A.; Keenan, C.M.; Wallace, L.E.; Woods, C.; Cavin, J.-B.; Flockton, A.R.; Macklin, W.B.; Belkind-Gerson, J.; Hirota, S.A.; Sharkey, K.A. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome 2021, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Graniczkowska, K.B.; Shaffer, C.L.; Cassone, V.M. Transcriptional effects of melatonin on the gut commensal bacterium Klebsiella aerogenes. Genomics 2022, 114, 110321. [Google Scholar] [CrossRef] [PubMed]
- Karavolos, M.H.; Spencer, H.; Bulmer, D.M.; Thompson, A.; Winzer, K.; Williams, P.; Hinton, J.C.D.; Anjam Khan, C.M. Adrenaline modulates the global transcriptional profile of Salmonella revealing a role in the antimicrobial peptide and oxidative stress resistance responses. BMC Genom. 2008, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Dowd, S.E. Escherichia coli O157:H7 gene expression in the presence of catecholamine norepinephrine. FEMS Microbiol. Lett. 2007, 273, 214–223. [Google Scholar] [CrossRef]
- O’Neal, M.J.; Schafer, E.R.; Madsen, M.L.; Minion, F.C. Global transcriptional analysis of Mycoplasma hyopneumoniae following exposure to norepinephrine. Microbiology 2008, 154, 2581–2588. [Google Scholar] [CrossRef][Green Version]
- Xu, F.; Wu, C.; Guo, F.; Cui, G.; Zeng, X.; Yang, B.; Lin, J. Transcriptomic analysis of Campylobacter jejuni NCTC 11168 in response to epinephrine and norepinephrine. Front. Microbiol. 2015, 6, 452. [Google Scholar] [CrossRef][Green Version]
- Sperandio, V.; Torres, A.G.; Jarvis, B.; Nataro, J.P.; Kaper, J.B. Bacteria-host communication: The language of hormones. Proc. Natl. Acad. Sci. USA 2003, 100, 8951–8956. [Google Scholar] [CrossRef]
- Franzin, F.M.; Sircili, M.P. Locus of enterocyte effacement: A pathogenicity island involved in the virulence of enteropathogenic and enterohemorragic Escherichia coli subjected to a complex network of gene regulation. BioMed Res. Int. 2015, 2015, 534738. [Google Scholar] [CrossRef]
- Yang, Q.; Anh, N.D.; Bossier, P.; Defoirdt, T. Norepinephrine and dopamine increase motility, biofilm formation, and virulence of Vibrio harveyi. Front. Microbiol. 2014, 5, 584. [Google Scholar] [CrossRef]
- Hegde, M.; Wood, T.K.; Jayaraman, A. The neuroendocrine hormone norepinephrine increases Pseudomonas aeruginosa PA14 virulence through the las quorum-sensing pathway. Appl. Microbiol. Biotechnol. 2009, 84, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Toulouse, C.; Schmucker, S.; Metesch, K.; Pfannstiel, J.; Michel, B.; Starke, I.; Möller, H.M.; Stefanski, V.; Steuber, J. Mechanism and impact of catecholamine conversion by Vibrio cholerae. Biochim. Et Biophys. (BBA) Bioenerg. 2019, 1860, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Scardaci, R.; Varese, F.; Manfredi, M.; Marengo, E.; Mazzoli, R.; Pessione, E. Enterococcus faecium NCIMB10415 responds to norepinephrine by altering protein profiles and phenotypic characters. J. Proteom. 2021, 231, 104003. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Frank, C.D.; Green, B.T. Production of an autoinducer of growth by norepinephrine cultured Escherichia coli O157:H7. FEMS Microbiol. Lett. 1996, 139, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Freestone, P.P.E.; Haigh, R.D.; Williams, P.H.; Lyte, M. Stimulation of bacterial growth by heat-stable, norepinephrine-induced autoinducers. FEMS Microbiol. Lett. 1999, 172, 53–60. [Google Scholar] [CrossRef]
- Rodrigues, M.V.; Kis, P.; Xavier, K.B.; Ventura, M.R. Synthesis and Potential of Autoinducer-2 and Analogs to Manipulate Inter-Species Quorum Sensing. Isr. J. Chem. 2023, 63, e202200091. [Google Scholar] [CrossRef]
- Clarke, M.B.; Hughes, D.T.; Zhu, C.; Boedeker, E.C.; Sperandio, V. The QseC sensor kinase: A bacterial adrenergic receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 10420–10425. [Google Scholar] [CrossRef]
- Reading, N.C.; Torres, A.G.; Kendall, M.M.; Hughes, D.T.; Yamamoto, K.; Sperandio, V. A novel two-component signaling system that activates transcription of an enterohemorrhagic Escherichia coli effector involved in remodeling of host actin. J. Bacteriol. 2007, 189, 2468–2476. [Google Scholar] [CrossRef]
- Reading, N.C.; Rasko, D.A.; Torres, A.G.; Sperandio, V. The two-component system QseEF and the membrane protein QseG link adrenergic and stress sensing to bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 5889–5894. [Google Scholar] [CrossRef]
- Karavolos, M.H.; Bulmer, D.M.; Spencer, H.; Rampioni, G.; Schmalen, I.; Baker, S.; Pickard, D.; Gray, J.; Fookes, M.; Winzer, K.; et al. Salmonella Typhi sense host neuroendocrine stress hormones and release the toxin haemolysin E. EMBO Rep. 2011, 12, 252–258. [Google Scholar] [CrossRef]
- Hamed, A.; Pullinger, G.; Stevens, M.; Farveen, F.; Freestone, P. Characterisation of the E. coli and Salmonella qseC and qseE mutants reveals a metabolic rather than adrenergic receptor role. FEMS Microbiol. Lett. 2022, 369, fnac012. [Google Scholar] [CrossRef] [PubMed]
- Pullinger, G.D.; Carnell, S.C.; Sharaff, F.F.; van Diemen, P.M.; Dziva, F.; Morgan, E.; Lyte, M.; Freestone, P.P.E.; Stevens, M.P. Norepinephrine augments Salmonella enterica-induced enteritis in a manner associated with increased net replication but independent of the putative adrenergic sensor kinases QseC and QseE. Infect. Immun. 2010, 78, 372–380. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freestone, P.P.E.; Haigh, R.D.; Lyte, M. Blockade of catecholamine-induced growth by adrenergic and dopaminergic receptor antagonists in Escherichia coli O157:H7, Salmonella enterica and Yersinia enterocolitica. BMC Microbiol. 2007, 7, 8. [Google Scholar] [CrossRef]
- Luqman, A.; Kharisma, V.D.; Ruiz, R.A.; Götz, F. In silico and in vitro study of trace amines (TA) and dopamine (DOP) interaction with human alpha 1-adrenergic receptor and the bacterial adrenergic receptor QseC. Cell. Physiol. Biochem. 2020, 54, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Freestone, P.P.E.; Haigh, R.D.; Lyte, M. Catecholamine inotrope resuscitation of antibiotic-damaged staphylococci and its blockade by specific receptor antagonists. J. Infect. Dis. 2008, 197, 1044–1052. [Google Scholar] [CrossRef]
- Freestone, P.P.E.; Lyte, M.; Neal, C.P.; Maggs, A.F.; Haigh, R.D.; Williams, P.H. The mammalian neuroendocrine hormone norepinephrine supplies iron for bacterial growth in the presence of transferrin or lactoferrin. J. Bacteriol. 2000, 182, 6091–6098. [Google Scholar] [CrossRef] [PubMed]
- Burton, C.L.; Chhabra, S.R.; Swift, S.; Baldwin, T.J.; Withers, H.; Hill, S.J.; Williams, P. The growth response of Escherichia coli to neurotransmitters and related catecholamine drugs requires a functional enterobactin biosynthesis and uptake system. Infect. Immun. 2002, 70, 5913–5923. [Google Scholar] [CrossRef]
- Karavolos, M.H.; Winzer, K.; Williams, P.; Anjam Khan, C.M. Pathogen espionage: Multiple bacterial adrenergic sensors eavesdrop on host communication systems. Mol. Microbiol. 2013, 87, 455–465. [Google Scholar] [CrossRef]
- Freestone, P.P.E.; Haigh, R.D.; Williams, P.H.; Lyte, M. Involvement of enterobactin in norepinephrine-mediated iron supply from transferrin to enterohaemorrhagic Escherichia coli. FEMS Microbiol. Lett. 2003, 222, 39–43. [Google Scholar] [CrossRef]
- Anderson, M.T.; Armstrong, S.K. Norepinephrine mediates acquisition of transferrin-iron in Bordetella bronchiseptica. J. Bacteriol. 2008, 190, 3940–3947. [Google Scholar] [CrossRef]
- Neal, C.P.; Freestone, P.P.E.; Maggs, A.F.; Haigh, R.D.; Williams, P.H.; Lyte, M. Catecholamine inotropes as growth factors for Staphylococcus epidermidis and other coagulase-negative staphylococci. FEMS Microbiol. Lett. 2001, 194, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, S.M.; Shergill, R.; Woodward, J.; Muralikuttan, R.; Haigh, R.D.; Lyte, M.; Freestone, P.P. Elucidation of the mechanism by which catecholamine stress hormones liberate iron from the innate immune defense proteins transferrin and lactoferrin. J. Bacteriol. 2010, 192, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, S.; Masania, R.; Zia, F.; Haigh, R.; Freestone, P. Role of porin proteins in acquisition of transferrin iron by enteropathogens. Microbiology 2013, 159, 2639–2650. [Google Scholar] [CrossRef]
- Perraud, Q.; Cantero, P.; Roche, B.; Gasser, V.; Normant, V.P.; Kuhn, L.; Hammann, P.; Mislin, G.L.A.; Ehret-Sabatier, L.; Schalk, I.J. Phenotypic adaption of Pseudomonas aeruginosa by hacking siderophores produced by other microorganisms. Mol. Cell. Proteom. 2020, 19, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Brown, D.R. Evidence for PMAT- and OCT-like biogenic amine transporters in a probiotic strain of Lactobacillus: Implications for interkingdom communication within the microbiota-gut-brain axis. PLoS ONE 2018, 13, e0191037. [Google Scholar] [CrossRef]
- Tierrafría, V.H.; Rioualen, C.; Salgado, H.; Lara, P.; Gama-Castro, S.; Lally, P.; Gómez-Romero, L.; Peña-Loredo, P.; López-Almazo, A.G.; Alarcón-Carranza, G.; et al. RegulonDB 11.0: Comprehensive high-throughput datasets on transcriptional regulation in Escherichia coli K-12. Microb. Genom. 2022, 8, mgen000833. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The Universal Protein Knowledge base in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- Misra, R.V.; Horler, R.S.; Reindl, W.; Goryanin, I.I.; Thomas, G.H. Echo BASE: An integrated post-genomic database for Escherichia coli. Nucleic Acids Res. 2005, 33, D329–D333. [Google Scholar] [CrossRef]
- Bekker, G.-J.; Yokochi, M.; Suzuki, H.; Ikegawa, Y.; Iwata, T.; Kudou, T.; Yura, K.; Fujiwara, T.; Kawabata, T.; Kurisu, G. Protein Data Bank Japan: Celebrating our 20th anniversary during a global pandemic as the Asian hub of three dimensional macromolecular structural data. Protein Sci. 2022, 31, 173–186. [Google Scholar] [CrossRef]
- Bell, C.E.; Barry, J.; Matthews, K.S.; Lewis, M. Structure of a variant of lac repressor with increased thermostability and decreased affinity for operator. J. Mol. Biol. 2001, 313, 99–109. [Google Scholar] [CrossRef]
- Friedman, A.M.; Fischmann, T.O.; Steitz, T.A. Crystal structure of lac repressor core tetramer and its implications for DNA looping. Science 1995, 268, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, K.A.E.; Vihinen, M. Crystal structure of a 1.6-hexanediol bound tetrameric form of Escherichia coli lac-repressor refined to 2.1 A resolution. Proteins 2009, 75, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.D.; Garruss, A.S.; Moretti, R.; Chan, S.; Arbing, M.A.; Cascio, D.; Rogers, J.K.; Isaacs, F.J.; Kosuri, S.; Baker, D.; et al. Engineering an allosteric transcription factor to respond to new ligands. Nat. Methods 2016, 13, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein-ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- Ishizuka, H.; Hanamura, A.; Inada, T.; Aiba, H. Mechanism of the down-regulation of cAMP receptor protein by glucose in Escherichia coli: Role of autoregulation of the crp gene. EMBO J. 1994, 13, 3077–3082. [Google Scholar] [CrossRef]
- Shimada, T.; Fujita, N.; Yamamoto, K.; Ishihama, A. Novel roles of cAMP receptor protein (CRP) in regulation of transport and metabolism of carbon sources. PLoS ONE 2011, 6, e20081. [Google Scholar] [CrossRef]
- Schultz, S.C.; Shields, G.C.; Steitz, T.A. Crystal structure of a CAP-DNA complex: The DNA is bent by 90 degrees. Science 1991, 253, 1001–1007. [Google Scholar] [CrossRef]
- Passner, J.M.; Schultz, S.C.; Steitz, T.A. Modeling the cAMP-induced allosteric transition using the crystal structure of CAP-cAMP at 2.1 A resolution. J. Mol. Biol. 2000, 304, 847–859. [Google Scholar] [CrossRef]
- Chu, S.Y.; Tordova, M.; Gilliland, G.L.; Gorshkova, I.; Shi, Y.; Wang, S.; Schwarz, F.P. The structure of the T127L/S128A mutant of cAMP receptor protein facilitates promoter site binding. J. Biol. Chem. 2001, 276, 11230–11236. [Google Scholar] [CrossRef]
- Napoli, A.A.; Lawson, C.L.; Ebright, R.H.; Berman, H.M. Indirect readout of DNA sequence at the primary-kink site in the CAP-DNA complex: Recognition of pyrimidine-purine and purine-purine steps. J. Mol. Biol. 2006, 357, 173–183. [Google Scholar] [CrossRef]
- Tao, W.; Gao, Z.; Gao, Z.; Zhou, J.; Huang, Z.; Dong, Y.; Yu, S. The 1.6A resolution structure of activated D138L mutant of catabolite gene activator protein with two cAMP bound in each monomer. Int. J. Biol. Macromol. 2011, 48, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.R.; Lawson, C.L. Structure of catabolite activator protein with cobalt(II) and sulfate. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2014, 70, 560–563. [Google Scholar] [CrossRef]
- Rodgers, T.L.; Townsend, P.D.; Burnell, D.; Jones, M.L.; Richards, S.A.; McLeish, T.C.B.; Pohl, E.; Wilson, M.R.; Cann, M.J.; Wilson, M.R. Modulation of global low-frequency motions underlies allosteric regulation: Demonstration in CRP/FNR family transcription factors. PLoS Biol. 2013, 11, e1001651. [Google Scholar] [CrossRef] [PubMed]
- Townsend, P.D.; Rodgers, T.L.; Glover, L.C.; Korhonen, H.J.; Richards, S.A.; Colwell, L.J.; Pohl, E.; Wilson, M.R.; Hodgson, D.R.W.; McLeish, T.C.B.; et al. The role of protein-ligand contacts in allosteric regulation of the Escherichia coli catabolite activator protein. J. Biol. Chem. 2015, 290, 22225–22235. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.; Kerby, R.L.; Conrad, M.; Roberts, G.P. Study of highly constitutively active mutants suggests how cAMP activates cAMP receptor protein. J. Biol. Chem. 2006, 281, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Bowles, T.; Metz, A.H.; O’Quin, J.; Wawrzak, Z.; Eichman, B.F. Structure and DNA binding of alkylation response protein AidB. Proc. Natl. Acad. Sci. USA 2008, 105, 15299–15304. [Google Scholar] [CrossRef]
- Hamill, M.J.; Jost, M.; Wong, C.; Eliot, S.J.; Drennan, S.L. Flavin-induced oligomerization in Escherichia coli adaptive response protein AidB. Biochemistry 2011, 50, 10159–10169. [Google Scholar] [CrossRef]
- Weaver, L.H.; Kwon, K.; Beckett, D.; Matthews, B.W. Corepressor-induced organization and assembly of the biotin repressor: A model for allosteric activation of a transcriptional regulator. Proc. Natl. Acad. Sci. USA 2001, 98, 6045–6050. [Google Scholar] [CrossRef]
- Wilson, K.P.; Shewchuk, L.M.; Brennan, R.G.; Otsuka, A.J.; Matthews, B.W. Escherichia coli biotin holoenzyme synthetase/bio repressor crystal structure delineates the biotin- and DNA-binding domains. Proc. Natl. Acad. Sci. USA 1992, 89, 9257–9261. [Google Scholar] [CrossRef]
- Wood, Z.A.; Weaver, L.H.; Brown, P.H.; Beckett, D.; Matthews, B.W. Co-repressor Induced Order and Biotin Repressor Dimerization: A Case for Divergent Followed by Convergent Evolution. J. Mol. Biol. 2006, 357, 509–523. [Google Scholar] [CrossRef]
- Eginton, C.; Cressman, W.J.; Bachas, S.; Wade, H.; Beckett, D. Allosteric Coupling via Distant Disorder-to-Order Transitions. J. Mol. Biol. 2015, 427, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Manav, M.C.; Turnbull, K.J.; Jurėnas, D.; Garcia-Pino, A.; Gerdes, K.; Brodersen, D.E. The E. coli HicB antitoxin contains a structurally stable helix-turn-helix DNA binding domain. Structure 2019, 27, 1675–1685.e3. [Google Scholar] [CrossRef] [PubMed]
- Ostrander, E.L.; Larson, J.D.; Schuermann, J.P.; Tanner, J.J. A conserved active site tyrosine residue of proline dehydrogenase helps enforce the preference for proline over hydroxyproline as the substrate. Biochemistry 2009, 48, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Haile, F.M.; Singh, R.K.; Larson, J.D.; Smithen, D.; Chan, J.Y.; Tanner, J.J.; Becker, D.F. Involvement of the beta3-alpha3 loop of the proline dehydrogenase domain in allosteric regulation of membrane association of proline utilization A. Biochemistry 2013, 52, 4482–4491. [Google Scholar] [CrossRef] [PubMed]
- Rippa, V.; Amoresano, A.; Esposito, C.; Landini, P.; Volkert, M.; Duilio, A. Specific DNA binding and regulation of its own expression by the AidB protein in Escherichia coli. J. Bacteriol. 2010, 192, 6136–6142. [Google Scholar] [CrossRef]
- Rohankhedkar, M.S.; Mulrooney, S.B.; Wedemeyer, W.J.; Hausinger, R.P. The AidB component of the Escherichia coli adaptive response to alkylating agents is a flavin-containing, DNA-binding protein. J. Bacteriol. 2006, 188, 223–230. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, M.; Zhu, W.; Zhou, Y.; Wanduragala, S.; Rewinkel, D.; Tanner, J.J.; Becker, D.F. Redox-induced changes in flavin structure and roles of flavin N(5) and the ribityl 2′-OH group in regulating PutA-membrane binding. Biochemistry 2007, 46, 483–491. [Google Scholar] [CrossRef]
- Zhu, W.; Becker, D.F. Flavin redox state triggers conformational changes in the PutA protein from Escherichia coli. Biochemistry 2003, 42, 5469–5477. [Google Scholar] [CrossRef]
- LiCata, V.J.; Ackers, G.K. Long-range, small magnitude nonadditivity of mutational effects in proteins. Biochemistry 1995, 34, 3133–3139. [Google Scholar] [CrossRef]
- Van Duyne, G.D.; Ghosh, G.; Maas, W.K.; Sigler, P.B. Structure of the oligomerization and L-arginine binding domain of the arginine repressor of Escherichia coli. J. Mol. Biol. 1996, 256, 377–391. [Google Scholar] [CrossRef]
- Thaw, P.; Sedelnikova, S.E.; Muranova, T.; Wiese, S.; Ayora, S.; Alonso, J.C.; Brinkman, A.B.; Akerboom, J.; Van Der Oost, J.; Rafferty, J.B. Structural insight into gene transcriptional regulation and effector binding by the Lrp/Asnc family. Nucleic Acids Res. 2006, 34, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Mechaly, A.E.; Diaz, S.S.; Sassoon, N.; Buschiazzo, A.; Betton, J.M.; Alzari, P.M. Structural coupling between autokinase and phosphotransferase reactions in a bacterial histidine kinase. Structure 2017, 25, 939–944.e3. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; McDonald, L.; Cygler, M.; Ekiel, I. Coiled-coil helix rotation selects repressing or activating state of transcriptional regulator DhaR. Structure 2014, 22, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Van Aalten, D.M.F.; DiRusso, C.C.; Knudsen, J. The structural basis of acyl coenzyme A-dependent regulation of the transcription factor FadR. EMBO J. 2001, 20, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Lorca, G.L.; Ezersky, A.; Lunin, V.V.; Walker, J.R.; Altamentova, S.; Evdokimova, E.; Vedadi, M.; Bochkarev, A.; Savchenko, A. Glyoxylate and pyruvate are antagonistic effectors of the Escherichia coli IclR transcriptional regulator. J. Biol. Chem. 2007, 282, 16476–16491. [Google Scholar] [CrossRef]
- Ha, J.H.; Eo, Y.; Grishaev, A.; Guo, M.; Smith, J.A.I.; Sintim, H.O.; Kim, E.H.; Cheong, H.K.; Bentley, W.E.; Ryu, K.S. Crystal structures of the LsrR proteins complexed with phospho-AI-2 and two signal-interrupting analogues reveal distinct mechanisms for ligand recognition. J. Am. Chem. Soc. 2013, 135, 15526–15535. [Google Scholar] [CrossRef]
- Rafferty, J.B.; Somers, W.S.; Saint-Girons, I.; Phillips, S.E.V. Three-dimensional crystal structures of Escherichia coli Met repressor with and without corepressor. Nature 1989, 341, 705–710. [Google Scholar] [CrossRef]
- Garvie, C.W.; Phillips, S.E. Direct and indirect readout in mutant Met repressor-operator complexes. Structure 2000, 8, 905–914. [Google Scholar] [CrossRef]
- Kalivoda, K.A.; Steenbergen, S.M.; Vimr, E.R. Control of the Escherichia coli sialoregulon by transcriptional repressor NanR. J. Bacteriol. 2013, 195, 4689–4701. [Google Scholar] [CrossRef]
- Rappas, M.; Schumacher, J.; Niwa, H.; Buck, M.; Zhang, X. Structural basis of the nucleotide driven conformational changes in the Aaa(+) domain of transcription activator Pspf. J. Mol. Biol. 2006, 357, 481–492. [Google Scholar] [CrossRef]
- Darbari, V.C.; Lawton, E.; Lu, D.; Burrows, P.C.; Wiesler, S.; Joly, N.; Zhang, N.; Zhang, X.; Buck, M. Molecular basis of nucleotide-dependent substrate engagement and remodeling by an AAA+ activator. Nucleic Acids Res. 2014, 42, 9249–9261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huffman, J.L.; Lu, F.; Zalkin, H.; Brennan, R.G. Role of residue 147 in the gene regulatory function of the Escherichia coli purine repressor. Biochemistry 2002, 41, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Arvidson, D.N.; Lu, F.; Faber, C.; Zalkin, H.; Brennan, R.G. The structure of PurR mutant L54M shows an alternative route to DNA kinking. Nat. Struct. Mol. Biol. 1998, 5, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Choi, K.Y.; Zalkin, H.; Brennan, R.G. Crystal structure of LacI member, PurR, bound to DNA: Minor groove binding by alpha helices. Science 1994, 266, 763–770. [Google Scholar] [CrossRef]
- Hars, U.; Horlacher, R.; Boos, W.; Welte, W.; Diederichs, K. Crystal structure of the effector-binding domain of the trehalose-repressor of Escherichia coli, a member of the LacI family, in its complexes with inducer trehalose-6-phosphate and noninducer trehalose. Protein Sci. 1998, 7, 2511–2521. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Schevitz, R.W.; Zhang, R.G.; Lawson, C.L.; Joachimiak, A.; Marmorstein, R.Q.; Luisi, B.F.; Sigler, P.B. Crystal structure of trp repressor/operator complex at atomic resolution. Nature 1988, 335, 321–329. [Google Scholar] [CrossRef]
- Ponnu, J.; Wah, V.; Schmid, M. Trehalose-6-phosphate: Connecting plant metabolism and development. Front. Plant Sci. 2011, 2, 70. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Zhang, D.; Qi, S.; Liu, Y. Metabolite interactions between host and microbiota during health and disease: Which feeds the other? Biomed. Pharmacother. 2023, 160, 114295. [Google Scholar] [CrossRef]
- Sathe, R.R.M.; Paerl, R.W.; Hazra, A.B. Exchange of vitamin B1 and its biosynthesis intermediates shapes the composition of synthetic microbial cocultures and reveals complexities of nutrient sharing. J. Bacteriol. 2022, 204, e0050321. [Google Scholar] [CrossRef]
- Dalangin, R.; Kim, A.; Campbell, R.E. The role of amino acids in neurotransmission and fluorescent tools for their detection. Int. J. Mol. Sci. 2020, 21, 6197. [Google Scholar] [CrossRef] [PubMed]
- Walvekar, A.S.; Laxman, S. Methionine at the heart of anabolism and signaling: Perspectives from budding yeast. Front. Microbiol. 2019, 10, 2624. [Google Scholar] [CrossRef] [PubMed]
- Parsons, Y.D.; Persson, B.; Mekhalfia, A.; Blackburn, G.M.; Stockley, P.G. Probing the molecular mechanism of action of co-repressor in the E. coli methionine repressor-operator complex using surface plasmon resonance (SPR). Nucleic Acids Res. 1995, 23, 211–216. [Google Scholar] [CrossRef]
- Phillips, K.; Phillips, S.E.V. Electrostatic activation of Escherichia coli methionine repressor. Structure 1994, 2, 309–316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tripet, B.P.; Goel, A.; Copie, V. Internal dynamics of the tryptophan repressor (TrpR) and two functionally distinct TrpR variants, L75F-TrpR and A77V-TrpR, in their l-Trp-bound forms. Biochemistry 2011, 50, 5140–5153. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.; Oehler, S. Effector overlap between the lac and mel operons of Escherichia coli: Induction of the mel operon with β-galactosides. J. Bacteriol. 2017, 199, e00796-16. [Google Scholar] [CrossRef]
- Gerbault, P.; Liebert, A.; Itan, Y.; Powell, A.; Currat, M.; Burger, J.; Swallow, D.M.; Thomas, M.G. Evolution of lactase persistence: An example of human niche construction. Philos. Trans. R. Soc. B 2011, 366, 863–877. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: Anadvanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDockVina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Tutukina, M.N.; Potapova, A.V.; Vlasov, P.K.; Purtov, Y.A.; Ozoline, O.N. Structural modeling of the ExuR and UxuR transcription factors of E. coli: Search for the ligands affecting their regulatory properties. J. Biomol. Struct. Dyn. 2016, 34, 2296–2304. [Google Scholar] [CrossRef]
- Bessonova, T.A.; Shumeiko, S.A.; Purtov, Y.A.; Antipov, S.S.; Preobrazhenskaya, E.V.; Tutukina, M.N.; Ozoline, O.N. Hexuronates influence the oligomeric form of the Dps structural protein of bacterial nucleoid and its ability to bind to linear DNA fragments. Biophysics 2016, 61, 825–883. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]



| TF | Functional Category | Size of Regulon | N. of Models | |
|---|---|---|---|---|
| Operons | Genes | |||
| Transcription factors | ||||
| ArgR | Biosynthesis of L-arginine (main intestinal metabolite) | 16 | 64 | 1 |
| AscG | Transport and assimilation of β-glucosides | 4 | 5 | 1 |
| AsnC | Asparagine biosynthesis | 2 | 4 | 1 |
| CpxR | Stress response | 40 | 72 | 1 |
| CRP | Global regulator of catabolite repression | 274 | 625 | 13 |
| DhaR | Activator of dihydroxyacetone kinase genes (detoxification) | 2 | 4 | 1 |
| FadR | Global regulator of lipid and fatty acid metabolism | 17 | 23 | 1 |
| LacI | Transport of lactose and its conversion into glucose and galactose | 1 | 3 | 5 |
| IclR | Control of a glyoxylate bypass operon upon acetate accumulation | 2 | 4 | 1 |
| LsrR | AI-2 uptake, stress response, host invasion, and biofilm formation | 4 | 9 | 2 |
| MetJ | Biosynthesis and transport of methionine (essential amino acid) | 10 | 15 | 2 |
| NanR | Sialic acid transport and assimilation (bacterial pathogenesis) | 4 | 11 | 1 |
| NikR | Nickel uptake (host-specific induction) | 1 | 6 | 2 |
| PspF | Stress and phage shock response | 3 | 8 | 2 |
| PurR | Purine biosynthesis | 20 | 32 | 3 |
| RutR | Pyrimidine metabolism | 7 | 17 | 2 |
| TreR | Trehalose transport/degradation (host colonization and virulence) | 1 | 1 | 1 |
| TrpR | Tryptophan and phenylalanine biosynthesis (essential amino acids) | 5 | 10 | 3 |
| Transcription factors with enzymatic activity and enzymes with transcription regulatory function | ||||
| AidB | Isovaleryl-CoA dehydrogenase, resistance against alkylation agents | 1 | 1 | 2 |
| BirA | Biotin ligase (protein biotinilation), repressor of biotin synthesis | 2 | 5 | 4 |
| HicB | Antitoxin of HicA-HicB system, extracytoplasmic stress response | 2 | 2 | 1 |
| PutA | Proline/pyrroline-5-carboxylate dehydrogenase, oxidative stress response | 2 | 2 | 3 |
| Model | Reference | Resolution (Å) | Ligand, Other Compounds | Mutations | Epi | Dop | Nor | Mel | Ser |
|---|---|---|---|---|---|---|---|---|---|
| 1JYE | [70] | 1.7 | Glycerol | K84L A109T | |||||
| 1JYF | [70] | 3.0 | Glycerol | A109T | |||||
| 1TLF | [71] | 2.6 | IPTG, C2H5Hg | ||||||
| 3EDC | [72] | 2.1 | Hexane-1,6-diol | ||||||
| 4RZS | [73] | 2.71 | Glycerol | D152T V153A, I159L, S196D |
| Model | Reference | Resolution (Å) | Ligand, Other Compounds | Mut | Epi | Dop | Nor | Mel | Ser |
|---|---|---|---|---|---|---|---|---|---|
| 1CGP | [77] | 3.0 | DNA, cAMP | ||||||
| 1G6N | [78] | 2.1 | cAMP | ||||||
| 1HW5 | [79] | 1.82 | cAMP | ||||||
| 1I5Z | - | 1.9 | cAMP, triol | ||||||
| 1I6X | - | 2.2 | cAMP, triol | D54H | |||||
| 1ZRF | [80] | 2.1 | DNA, cAMP and 1,4-dioxane | ||||||
| 3KCC | [81] | 1.66 | cAMP, glycerol | D138L | |||||
| 3QOP | - | 1.96 | cAMP, glycerol | ||||||
| 4FT8 | [82] | 1.966 | cAMP, SO4 and Co2+ | ||||||
| 4HZF | [83] | 1.48 | cAMP, glycerol and HPO42− | ||||||
| 4I0B | [83] | 1.50 | cAMP | H160L | |||||
| 4I09 | [83] | 2.05 | cAMP | V132L | |||||
| 4R8H | [84] | 1.46 | RP-adenosine-3′,5′-cyclic-mono-phosphorothioate, glycerol |
| Protein | Model | Reference | Resolu-tion (Å) | Ligand, Other Compounds | Mut | Epi | Dop | Nor | Mel | Ser |
|---|---|---|---|---|---|---|---|---|---|---|
| AidB | 3DJL | [86] | 1.7 | FAD, Ca2+ | ||||||
| 3U33 | [87] | 2.8 | FAD, Cl− | |||||||
| BirA | 1HXD | [88] | 2.4 | Biotin | ||||||
| 1BIB | [89] | 2.8 | Biotin | |||||||
| 2EWN | [90] | 2.8 | Biotinol-5AMP | |||||||
| 4WF2 | [91] | 2.31 | Biotinol-5AMP | G142A | ||||||
| HicB | 6HPC | [92] | 2.26 | - | ||||||
| PutA | 2FZN | - | 2.0 | FAD, proline | ||||||
| 3E2Q | [93] | 1.75 | FAD, 4-hydroxyproline and pentaethyleneglycol | Y540S | ||||||
| 4JNZ | [94] | 1.85 | FAD,4-hydrofuran-2-carboxylicacid and pentaethyleneglycol | D370N |
| Protein | Model | Ref. | Resolu-tion (Å) | Effectors, Other Compounds | Mut | Epi | Dop | Nor | Mel | Ser |
|---|---|---|---|---|---|---|---|---|---|---|
| ArgR | 1XXB | [100] | 2.6 | Arginine | ||||||
| AscG | 3BRQ | - | 2.0 | β-D-fructofuranose, SO42− and Na+ | ||||||
| AsnC | 2CG4 | [101] | 2.4 | Asparagine, Mg2+ | G37E | |||||
| CpxR | 4UHK | [102] | 2.6 | Phosphorylated protein, Mg2+ | ||||||
| DhaR | 4LRZ | [103] | 2.32 | ADP (co-effector), Mg2+ | ||||||
| FadR | 1H9G | [104] | 2.1 | Co-enzyme A, myristic acid | ||||||
| IclR | 2O99 | [105] | 1.7 | 1,2-Ethanediol, Glycolicacid | M* | |||||
| LsrR | 4L4Z | [106] | 2.3 | (2S)-2,3,3-trihydroxy-4-oxopentyl dihydrogen phosphate | ||||||
| 4L51 | [106] | 1.9 | 5-O-phosphono-alpha-D-ribofuranose | |||||||
| MetJ | 1CMC | [107] | 1.8 | S-Adenosylmethionine, Mg2+ | ||||||
| 1MJO | [108] | 2.1 | DNA, S-Adenosylmethionine, Ca2+ | Q44L | ||||||
| NanR | 6ON4 | [109] | 2.1 | N-acetyl-β-neuraminic acid, PEG, Zn2+ | ||||||
| NikR | 2HZA | - | 2.1 | Ni2+ (effector), 3-Cyclohexylpropyl 4-O-α-D-gluco-pyranosyl-β-D-glucopyranoside | M** | |||||
| 3OD2 | - | 2.6 | ||||||||
| PspF | 2C9C | [110] | 2.1 | ATP (effector), Mg2+ | R227A | |||||
| 4QOS | [111] | 1.42 | ADP (effector), glycerol and HEPES | E108Q | ||||||
| PurR | 1JFT | [112] | 2.5 | DNA, hypoxantine and PO43− | W146A | |||||
| 1VPW | [113] | 2.7 | DNA, hypoxantine (effector) | L53M | ||||||
| 2PUC | [114] | 2.6 | DNA, guanine (effector) | R189A | ||||||
| RutR | 3LOC | - | 2.5 | Uracil | ||||||
| 4XK4 | - | 2.27 | Dihydropyrimidine-2,4(1H,3H)-dione | |||||||
| TreR | 4XXH | [115] | 2.4 | Trehalose-6-phosphate | ||||||
| TrpR | 1TRO | [116] | 1.9 | DNA, tryptophan | Q13E | |||||
| 1ZT9 | - | 2.0 | Tryptophan, SO42− | |||||||
| 6F7F | - | 2.13 | Indolylpropionic acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purtov, Y.A.; Ozoline, O.N. Neuromodulators as Interdomain Signaling Molecules Capable of Occupying Effector Binding Sites in Bacterial Transcription Factors. Int. J. Mol. Sci. 2023, 24, 15863. https://doi.org/10.3390/ijms242115863
Purtov YA, Ozoline ON. Neuromodulators as Interdomain Signaling Molecules Capable of Occupying Effector Binding Sites in Bacterial Transcription Factors. International Journal of Molecular Sciences. 2023; 24(21):15863. https://doi.org/10.3390/ijms242115863
Chicago/Turabian StylePurtov, Yuri A., and Olga N. Ozoline. 2023. "Neuromodulators as Interdomain Signaling Molecules Capable of Occupying Effector Binding Sites in Bacterial Transcription Factors" International Journal of Molecular Sciences 24, no. 21: 15863. https://doi.org/10.3390/ijms242115863
APA StylePurtov, Y. A., & Ozoline, O. N. (2023). Neuromodulators as Interdomain Signaling Molecules Capable of Occupying Effector Binding Sites in Bacterial Transcription Factors. International Journal of Molecular Sciences, 24(21), 15863. https://doi.org/10.3390/ijms242115863






