Mechanism Underlying the Regulation of Mucin Secretion in the Uterus during Pregnancy
Abstract
:1. Introduction
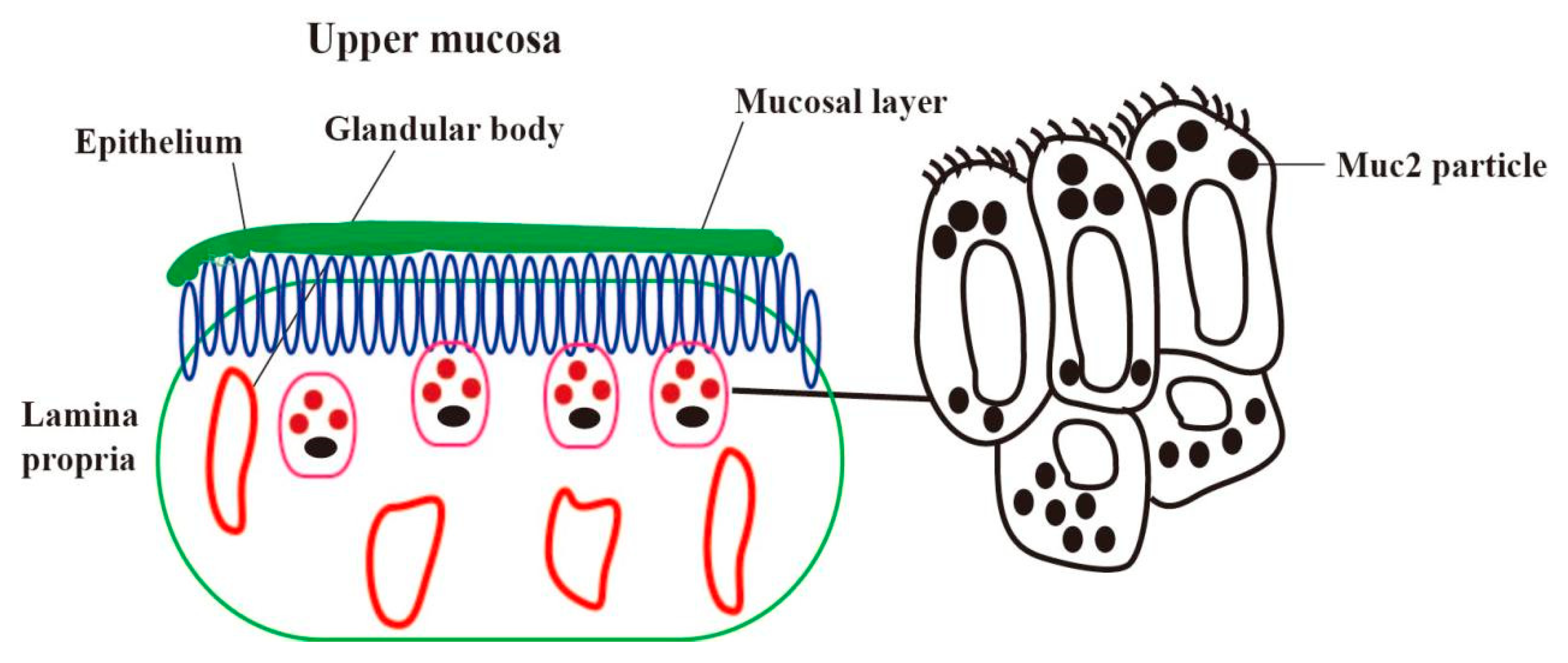
2. Location of Mucin in the Endometrium
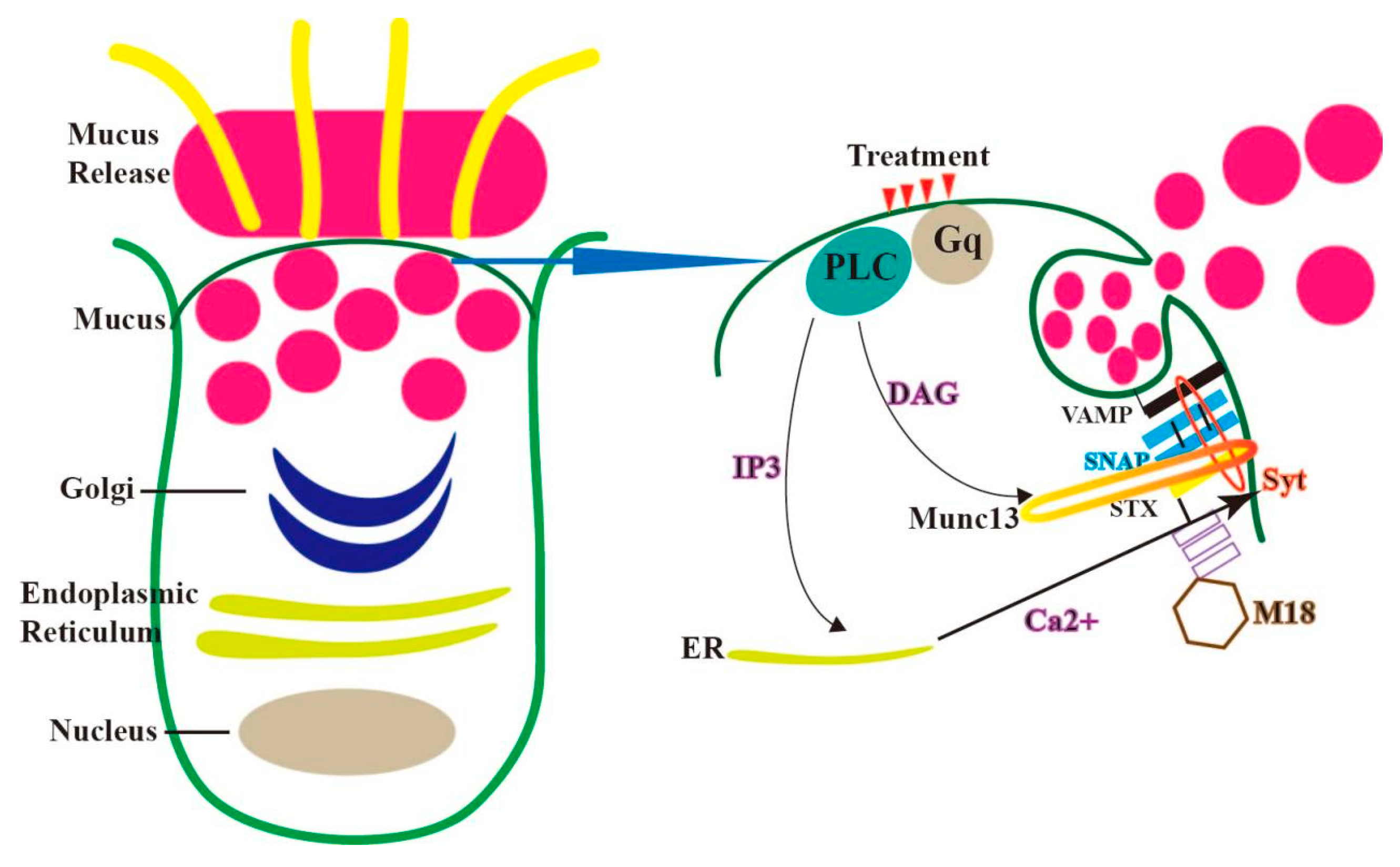
3. Mechanism of the Secretion of Mucin in Endometrial Epithelial Cells
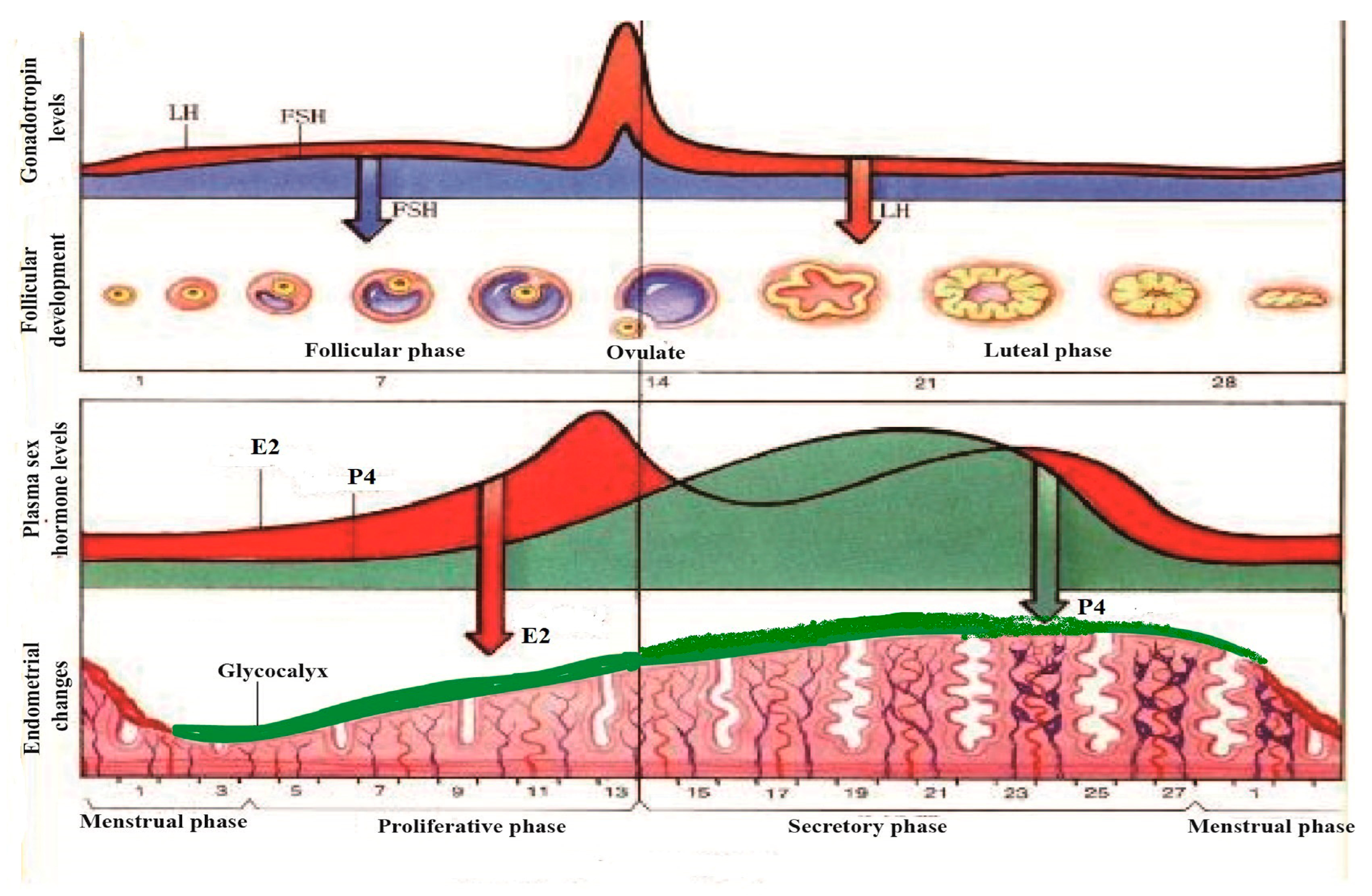
4. The Regulation of Mucin in Uterine Epithelial Cells by Reproductive Hormones during the Pregnancy Cycle
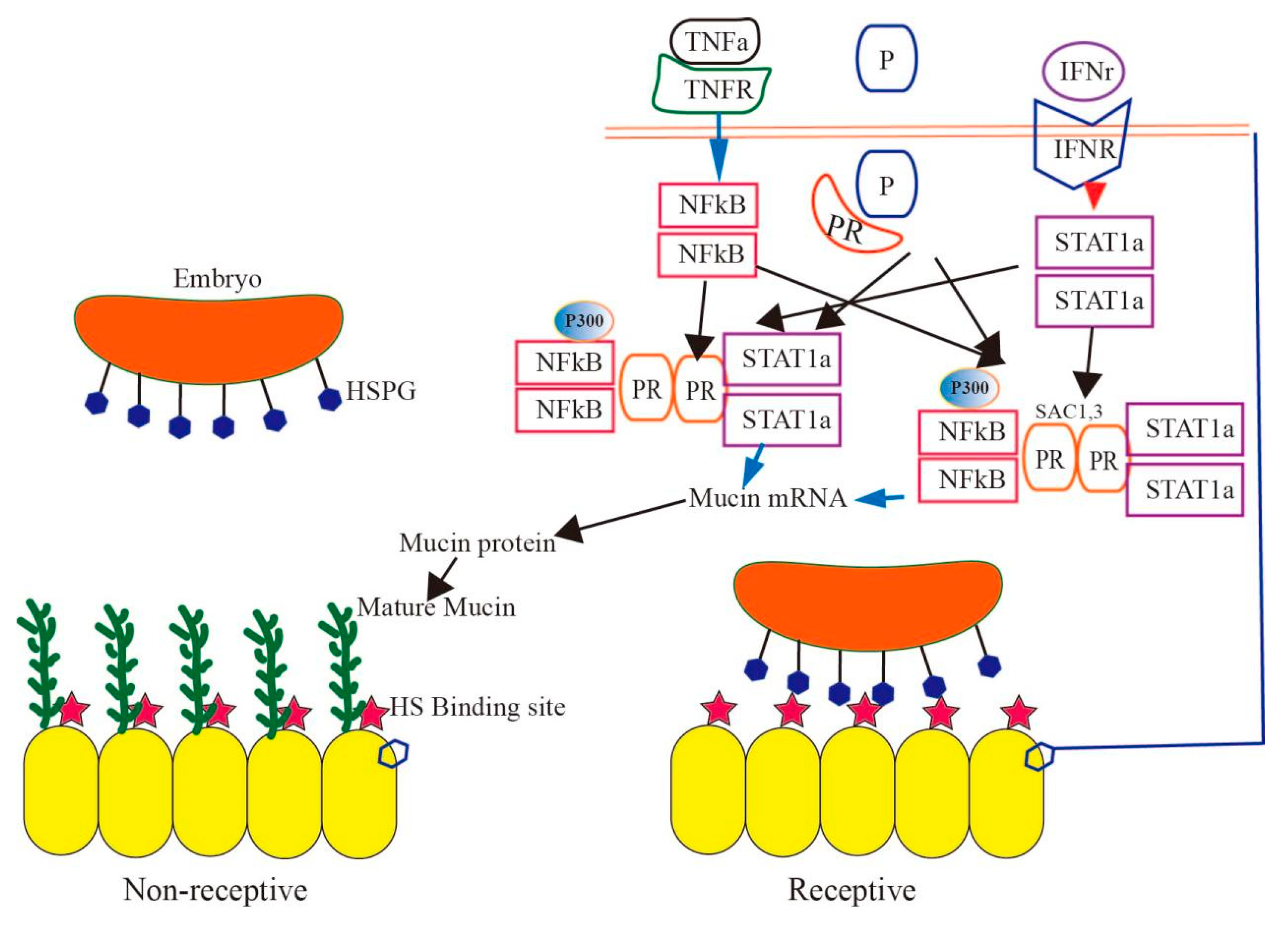
5. Effect of Mucin on Implantation during the Pregnancy Cycle
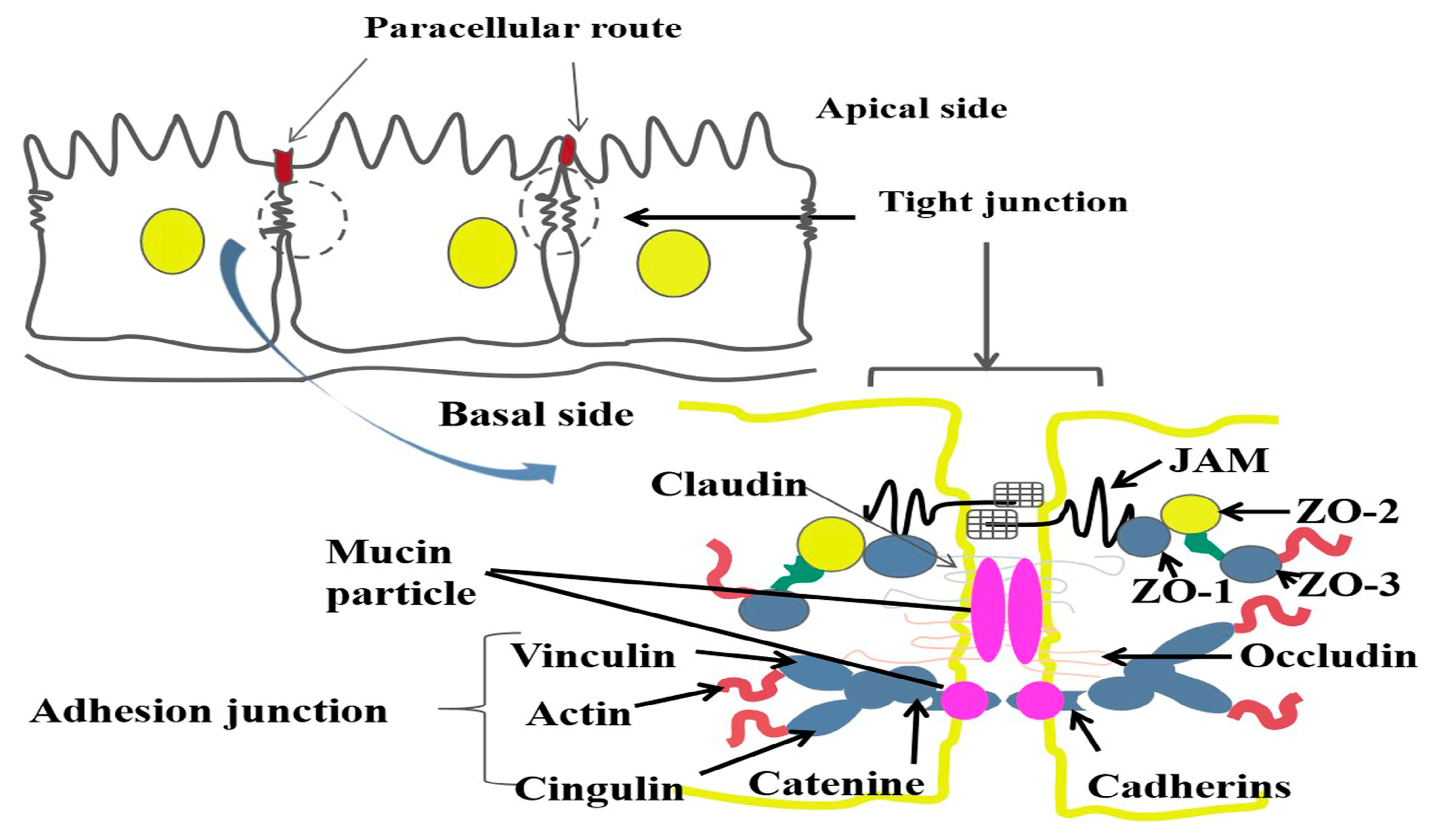
6. The Effect of Mucin on the Tight Structure of Uterine Epithelial Cells during Pregnancy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bogacki, M.; Wasielak, M.; Ziecik, A. The role of cytokines and adhesion molecules in maternal recognition and establishment of pregnancy in pig. J. Anim. Feed. Sci. 2005, 14, 581–594. [Google Scholar] [CrossRef]
- Hoffman, L.H.; Olson, G.E.; Carson, D.D.; Chilton, B.S. Progesterone and implanting blastocysts regulate Muc1 expression in rabbit uterine epithelium. Endocrinology 1998, 139, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Thathiah, A.; Carson, D.D. Mucins and blastocyst attachment. Rev. Endocr. Metab. Disord. 2002, 3, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.W.; Dickey, B.F. Regulated airway goblet cell mucin secretion. Annu. Rev. Physiol. 2008, 70, 487–512. [Google Scholar] [CrossRef]
- Zhu, Y.; Ehre, C.; Abdullah, L.H.; Sheehan, J.K.; Roy, M.; Evans, C.M.; Dickey, B.F.; Davis, C.W. Munc13-2−/− baseline secretion defect reveals source of oligomeric mucins in mouse airways. J. Physiol. 2008, 586, 1977–1992. [Google Scholar] [CrossRef]
- Ehre, C.; Rossi, A.H.; Abdullah, L.H.; De Pestel, K.; Hill, S.; Olsen, J.C.; Davis, C.W. Barrier role of actin filaments in regulated mucin secretion from airway goblet cells. Am. J. Physiol. Cell Physiol. 2005, 288, C46–C56. [Google Scholar]
- Thornton, D.J.; Rousseau, K.; McGuckin, M.A. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 2008, 70, 459–486. [Google Scholar] [CrossRef]
- McNiven, M.A.; Thompson, H.M. Vesicle formation at the plasma membrane and trans-Golgi network: The same but different. Science 2006, 313, 1591–1594. [Google Scholar]
- Abdullah, L.H.; Davis, C.W. Regulation of airway goblet cell mucin secretion by tyrosine phosphorylation signaling pathways. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L591–L599. [Google Scholar] [CrossRef]
- Yu, W.; Niwa, T.; Fukasawa, T.; Hidaka, H.; Senda, T.; Sasaki, Y.; Niki, I. Synergism of protein kinase A, protein kinase C, and myosin light-chain kinase in the secretory cascade of the pancreatic beta-cell. Diabetes 2000, 49, 945–952. [Google Scholar] [CrossRef]
- Zhang, M.M.; An, L.Y.; Hu, W.X.; Li, Z.Y.; Qiang, Y.Y.; Zhao, B.Y.; Han, T.S.; Wu, C.C. Mechanism of endometrial MUC2 in reproductive performance in mice through PI3K/AKT signaling pathway after lipopolysaccharide treatment. Ecotoxicol. Environ. Saf. 2022, 231, 113177. [Google Scholar] [CrossRef] [PubMed]
- Trifaró, J.M.; Rosé, S.D.; Marcu, M.G. Scinderin, a Ca2+-dependent actin filament severing protein that controls cortical actin network dynamics during secretion. Neurochem. Res. 2000, 25, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, A.M.; Azzegagh, Z.; Tuvim, M.J.; Dickey, B.F. Airway Mucin Secretion. Ann. Am. Thorac. Soc. 2018, 15 (Suppl. S3), S164–S170. [Google Scholar] [CrossRef]
- Suri, K.; Bubier, J.A.; Wiles, M.V.; Shultz, L.D.; Amiji, M.M.; Hosur, V. Role of MicroRNA in Inflammatory Bowel Disease: Clinical Evidence and the Development of Preclinical Animal Models. Cells 2021, 10, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.G.; Livraghi-Butrico, A.; Fletcher, A.A.; McElwee, M.M.; Evans, S.E.; Boerner, R.M.; Alexander, S.N.; Bellinghausen, L.K.; Song, A.S.; Petrova, Y.M.; et al. Muc5b is required for airway defence. Nature 2014, 505, 412–416. [Google Scholar] [CrossRef]
- Kaul, S.; Mittal, S.K.; Feigenbaum, L.; Kruhlak, M.J.; Roche, P.A. Expression of the SNARE protein SNAP-23 is essential for cell survival. PLoS ONE 2015, 10, e0118311. [Google Scholar] [CrossRef]
- Zhu, Y.; Abdullah, L.H.; Doyle, S.P.; Nguyen, K.; Ribeiro, C.M.; Vasquez, P.A.; Forest, M.G.; Lethem, M.I.; Dickey, B.F.; Davis, C.W. Baseline Goblet Cell Mucin Secretion in the Airways Exceeds Stimulated Secretion over Extended Time Periods, and Is Sensitive to Shear Stress and Intracellular Mucin Stores. PLoS ONE 2015, 10, e0127267. [Google Scholar] [CrossRef]
- Shumilov, D.; Popov, A.; Fudala, R.; Akopova, I.; Gryczynski, I.; Borejdo, J.; Gryczynski, Z.; Grygorczyk, R. Real-time imaging of exocytotic mucin release and swelling in Calu-3 cells using acridine orange. Methods 2014, 66, 312–324. [Google Scholar] [CrossRef]
- Kim, K.; Petrova, Y.M.; Scott, B.L.; Nigam, R.; Agrawal, A.; Evans, C.M.; Azzegagh, Z.; Gomez, A.; Rodarte, E.M.; Olkkonen, V.M.; et al. Munc18b is an essential gene in mice whose expression is limiting for secretion by airway epithelial and mast cells. Biochem. J. 2012, 446, 383–394. [Google Scholar] [CrossRef]
- Evans, C.M.; Raclawska, D.S.; Ttofali, F.; Liptzin, D.R.; Fletcher, A.A.; Harper, D.N.; McGing, M.A.; McElwee, M.M.; Williams, O.W.; Sanchez, E.; et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat. Commun. 2015, 6, 6281. [Google Scholar] [CrossRef]
- Raheem, K.A.; Marei, W.F.A.; Campbell, B.K.; Fouladi-Nashta, A.A. In vivo and in vitro studies of MUC1 regulation in sheep endometrium. Theriogenology 2016, 85, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Brayman, M.; Thathiah, A.; Carson, D.D. MUC1: A multifunctional cell surface component of reproductive tissue epithelia. Reprod. Biol. Endocrinol. 2004, 2, 4. [Google Scholar] [CrossRef]
- Perry, K.; Haresign, W.; Wathes, D.C.; Khalid, M. Hyaluronan (HA) content, the ratio of HA fragments and the expression of CD44 in the ovine cervix vary with the stage of the oestrous cycle. Reproduction 2010, 140, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Abayasekara, D.R.; Ward, F.; Preece, D.M.; Raheem, K.A.; Wathes, D.C. Altering n-3 to n-6 polyunsaturated fatty acid ratios affects prostaglandin production by ovine uterine endometrium. Anim. Reprod. Sci. 2013, 143, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Stiegeler, S.; Mercurio, K.; Iancu, M.A.; Corr, S.C. The Impact of MicroRNAs during Inflammatory Bowel Disease: Effects on the Mucus Layer and Intercellular Junctions for Gut Permeability. Cells 2021, 10, 3358–3368. [Google Scholar] [CrossRef] [PubMed]
- Van Der Kraak, L.; Gros, P.; Beauchemin, N. Colitis-associated colon cancer: Is it in your genes? World J. Gastroenterol. 2015, 21, 11688–11699. [Google Scholar] [CrossRef]
- Choy, M.C.; Visvanathan, K.; De Cruz, P. An Overview of the Innate and Adaptive Immune System in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 2–13. [Google Scholar] [CrossRef]
- Cichon, C.; Sabharwal, H.; Rüter, C.; Schmidt, M.A. MicroRNAs regulate tight junction proteins and modulate epithelial/endothelial barrier functions. Tissue Barriers 2014, 2, e944446. [Google Scholar] [CrossRef]
- Jensen, M.D.; Andersen, R.F.; Christensen, H.; Nathan, T.; Kjeldsen, J.; Madsen, J.S. Circulating microRNAs as biomarkers of adult Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1038–1044. [Google Scholar] [CrossRef]
- Kalla, R.; Adams, A.T.; Ventham, N.T.; Kennedy, N.A.; White, R.; Clarke, C.; Ivens, A.; Bergemalm, D.; Vatn, S.; Lopez-Jimena, B.; et al. Whole Blood Profiling of T-cell-Derived microRNA Allows the Development of Prognostic models in Inflammatory Bowel Disease. J. Crohns Colitis 2020, 14, 1724–1733. [Google Scholar] [CrossRef]
- Feng, S.Y.S.; Hollis, J.H.; Samarasinghe, T.; Phillips, D.J.; Rao, S.; Yu, V.Y.H.; Walker, A.M. Endotoxin-induced cerebral pathophysiology: Differences between fetus and newborn. Physiol. Rep. 2019, 7, e13973. [Google Scholar] [CrossRef]
- Lu, D.; Hu, W.; Tian, T.; Wang, M.; Zhou, M.; Wu, C. The Mechanism of Lipopolysaccharide’s Effect on Secretion of Endometrial Mucins in Female Mice during Pregnancy. Int. J. Mol. Sci. 2022, 23, 9972–9981. [Google Scholar] [CrossRef]
- Hu, W.; Feng, P.; Zhang, M.; Tian, T.; Wang, S.; Zhao, B.; Li, Y.; Wang, S.; Wu, C. Endotoxins Induced ECM-Receptor Interaction Pathway Signal Effect on the Function of MUC2 in Caco2/HT29 Co-Culture Cells. Front. Immunol. 2022, 13, 916933. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, C.; Mangogna, A.; Bossi, F.; Ricci, G.; Kishore, U.; Bulla, R. Uterine Immunity and Microbiota: A Shifting Paradigm. Front. Immunol. 2019, 10, 2387. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Barua, N.; Ip, M. Mucin-degrading gut commensals isolated from healthy faecal donor suppress intestinal epithelial inflammation and regulate tight junction barrier function. Front. Immunol. 2022, 13, 1021094. [Google Scholar] [CrossRef] [PubMed]
- Sitkin, S.; Pokrotnieks, J. Clinical Potential of Anti-inflammatory Effects of Faecalibacterium prausnitzii and Butyrate in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, e40–e41. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Dharmaprakash, V.; Nighot, P.; Guo, S.; Nighot, M.; Do, T.; Ma, T.Y. Bifidobacterium bifidum Enhances the Intestinal Epithelial Tight Junction Barrier and Protects against Intestinal Inflammation by Targeting the Toll-like Receptor-2 Pathway in an NF-κB-Independent Manner. Int. J. Mol. Sci. 2021, 22, 8070–8073. [Google Scholar] [CrossRef] [PubMed]
- Tailford, L.E.; Crost, E.H.; Kavanaugh, D.; Juge, N. Mucin glycan foraging in the human gut microbiome. Front. Genet. 2015, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Lui, R.N.; Wong, S.H.; Lau, L.H.S.; Chan, T.T.; Cheung, K.C.Y.; Li, A.; Chin, M.L.; Tang, W.; Ching, J.Y.L.; Lam, K.L.Y.; et al. Faecal microbiota transplantation for treatment of recurrent or refractory Clostridioides difficile infection in Hong Kong. Hong. Kong Med. J. 2019, 25, 178–182. [Google Scholar]
- Reunanen, J.; Kainulainen, V.; Huuskonen, L.; Ottman, N.; Belzer, C.; Huhtinen, H.; de Vos, W.M.; Satokari, R. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl. Environ. Microbiol. 2015, 81, 3655–3662. [Google Scholar] [CrossRef]
- Barbara, G.; Barbaro, M.R.; Fuschi, D.; Palombo, M.; Falangone, F.; Cremon, C.; Marasco, G.; Stanghellini, V. Inflammatory and Microbiota-Related Regulation of the Intestinal Epithelial Barrier. Front. Nutr. 2021, 8, 718356. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- von Moltke, J.; Ji, M.; Liang, H.E.; Locksley, R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016, 529, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.C. Mucus and mucins in diseases of the intestinal and respiratory tracts. J. Intern. Med. 2019, 285, 479–490. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.C. Mucins and the Microbiome. Annu. Rev. Biochem. 2020, 89, 769–793. [Google Scholar] [CrossRef] [PubMed]
- Huus, K.E.; Petersen, C.; Finlay, B.B. Diversity and dynamism of IgA-microbiota interactions. Nat. Rev. Immunol. 2021, 21, 514–525. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Hansson, G.C. Membrane mucins of the intestine at a glance. J. Cell Sci. 2020, 133, 240929–240935. [Google Scholar] [CrossRef]
- Etienne-Mesmin, L.; Chassaing, B.; Desvaux, M.; De Paepe, K.; Gresse, R.; Sauvaitre, T.; Forano, E.; de Wiele, T.V.; Schüller, S.; Juge, N.; et al. Experimental models to study intestinal microbes-mucus interactions in health and disease. FEMS Microbiol. Rev. 2019, 43, 457–489. [Google Scholar] [CrossRef]
- Johansson, M.E.; Jakobsson, H.E.; Holmén-Larsson, J.; Schütte, A.; Ermund, A.; Rodríguez-Piñeiro, A.M.; Arike, L.; Wising, C.; Svensson, F.; Bäckhed, F.; et al. Normalization of Host Intestinal Mucus Layers Requires Long-Term Microbial Colonization. Cell Host Microbe 2015, 18, 582–592. [Google Scholar] [CrossRef]
- France, M.M.; Turner, J.R. The mucosal barrier at a glance. J. Cell Sci. 2017, 130, 307–314. [Google Scholar] [CrossRef]
- Aihara, E.; Matthis, A.L.; Karns, R.A.; Engevik, K.A.; Jiang, P.; Wang, J.; Yacyshyn, B.R.; Montrose, M.H. Epithelial Regeneration After Gastric Ulceration Causes Prolonged Cell-Type Alterations. Cell Mol. Gastroenterol. Hepatol. 2016, 2, 625–647. [Google Scholar] [CrossRef]
- Baum, B.; Georgiou, M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J. Cell Biol. 2011, 192, 907–917. [Google Scholar] [CrossRef]
- Islam, M.R.; Ikeguchi, Y.; Yamagami, K.; El-Sharawy, M.; Yamauchi, N. Development of an in vitro model to study uterine functions and early implantation using rat uterine explants. Cell Tissue Res. 2017, 370, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Kusama, K.; Yoshie, M.; Tamura, K.; Daikoku, T.; Takarada, T.; Tachikawa, E. Possible roles of the cAMP-mediators EPAC and RAP1 in decidualization of rat uterus. Reproduction 2014, 147, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Yamauchi, N.; Watanabe, R.; Oozono, S.; Kubota, K.; Nishimura, K.; Wood, C.; Soh, T.; Kizaki, K.; Hattori, M.A. In vitro decidualization of rat endometrial stromal cells. Cell Tissue Res. 2009, 335, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Yamauchi, N.; Yamagami, K.; Nakamura, N.; Yamashita, S.; Islam, M.R.; Tabata, S.; Yahiro, K.; Tamura, T.; Hashizume, K.; et al. Development of an in vitro model for the analysis of bovine endometrium using simple techniques. Anim. Sci. J. 2015, 86, 523–531. [Google Scholar] [CrossRef]
- Desseyn, J.L.; Tetaert, D.; Gouyer, V. Architecture of the large membrane-bound mucins. Gene 2008, 410, 215–222. [Google Scholar] [CrossRef]
- Drouet, M.; Vignal, C.; Singer, E.; Djouina, M.; Dubreuil, L.; Cortot, A.; Desreumaux, P.; Neut, C. AIEC colonization and pathogenicity: Influence of previous antibiotic treatment and preexisting inflammation. Inflamm. Bowel Dis. 2012, 18, 1923–1931. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Tian, T.; Wu, C. Mechanism Underlying the Regulation of Mucin Secretion in the Uterus during Pregnancy. Int. J. Mol. Sci. 2023, 24, 15896. https://doi.org/10.3390/ijms242115896
Zhou M, Tian T, Wu C. Mechanism Underlying the Regulation of Mucin Secretion in the Uterus during Pregnancy. International Journal of Molecular Sciences. 2023; 24(21):15896. https://doi.org/10.3390/ijms242115896
Chicago/Turabian StyleZhou, Mengru, Tian Tian, and Chenchen Wu. 2023. "Mechanism Underlying the Regulation of Mucin Secretion in the Uterus during Pregnancy" International Journal of Molecular Sciences 24, no. 21: 15896. https://doi.org/10.3390/ijms242115896
APA StyleZhou, M., Tian, T., & Wu, C. (2023). Mechanism Underlying the Regulation of Mucin Secretion in the Uterus during Pregnancy. International Journal of Molecular Sciences, 24(21), 15896. https://doi.org/10.3390/ijms242115896



