Research Progress in Skin Aging, Metabolism, and Related Products
Abstract
:1. Introduction
2. The Impact of Skin Aging on Metabolism
2.1. Skin Metabolism
2.1.1. Glucose Metabolism
2.1.2. Protein Metabolism
2.1.3. Lipid Metabolism
3. The Impact of Metabolism on Skin Aging
3.1. The Effect of Glucose Metabolism on Skin Aging
3.2. The Impact of Protein Metabolism on Skin Aging
3.3. The Effect of Lipid Metabolism on Skin Aging
4. Metabolism and Aging in Specific Skin Cells
4.1. Keratinocytes
4.2. Fibroblasts
4.3. Melanocytes
5. Membrane or Cytoskeleton Proteins in Skin Metabolism and Aging
5.1. Connexins and Intercellular Communication
5.2. Desmin and Cytoskeletal Integrity
5.3. Occludins and Barrier Function
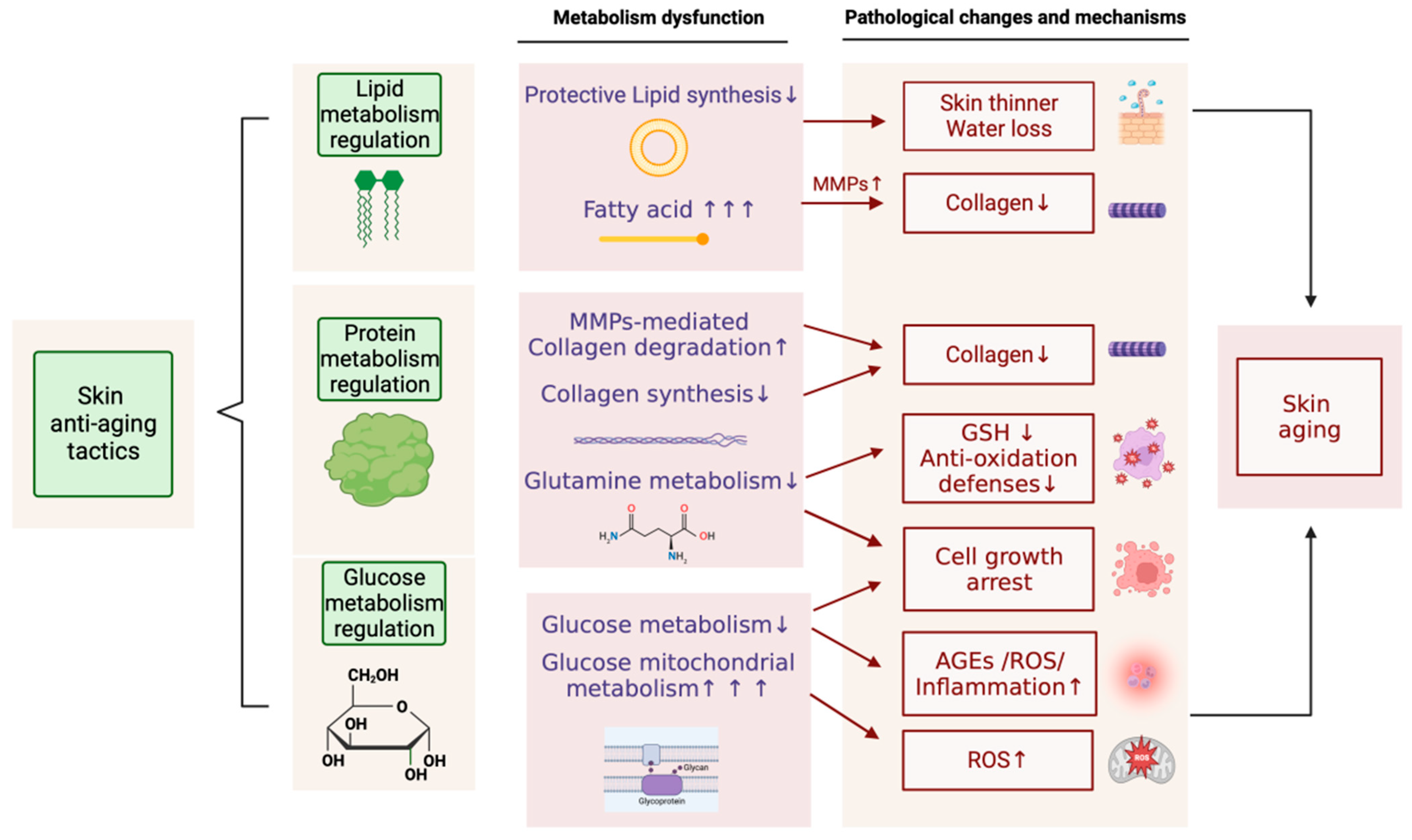
6. Anti-Aging Strategies Based on Metabolic Regulation
6.1. Inhibiting Skin Glycation
6.2. Increasing Skin Protein Levels
6.2.1. Inhibiting Collagen Degradation
6.2.2. Promoting Collagen Synthesis
6.2.3. Simultaneously Inhibiting Collagen Degradation and Promoting Collagen Synthesis
6.3. Regulating Skin Lipid Metabolism
6.4. Regulating Mitochondrial Energy Metabolism in the Skin
7. Discussions and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin senescence: Mechanisms and impact on whole-body aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar] [CrossRef] [PubMed]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Zhao, M.M.; He, Y.F.; Meng, H.; Meng, Q.Y.; Shi, Q.Y.; Yi, F. Facial Skin Aging Stages in Chinese Females. Front. Med. 2022, 9, 870926. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.H. Aging of the skin barrier. Clin. Dermatol. 2019, 37, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Park, T.J.; Kang, H.Y. Skin-Aging Pigmentation: Who Is the Real Enemy? Cells 2022, 11, 2541. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y. Skin Pigmentation Abnormalities and Their Possible Relationship with Skin Aging. Int. J. Mol. Sci. 2021, 22, 3727. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Elewa, R.; Makrantonaki, E. Aesthetic aspects of skin aging, prevention, and local treatment. Clin. Dermatol. 2019, 37, 365–372. [Google Scholar] [CrossRef]
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Wang, Y.; Deng, Z.; Xu, S.; Xie, H.; Zhang, Y.; Li, J. Exploring metformin as a candidate drug for rosacea through network pharmacology and experimental validation. Pharmacol. Res. 2021, 174, 105971. [Google Scholar] [CrossRef]
- Ho, C.Y.; Dreesen, O. Faces of cellular senescence in skin aging. Mech. Ageing Dev. 2021, 198, 111525. [Google Scholar] [CrossRef]
- Khavkin, J.; Ellis, D.A. Aging skin: Histology, physiology, and pathology. Facial Plast. Surg. Clin. 2011, 19, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Danielyan, L.; Zellmer, S.; Sickinger, S.; Tolstonog, G.V.; Salvetter, J.; Lourhmati, A.; Reissig, D.D.; Gleiter, C.H.; Gebhardt, R.; Buniatian, G.H. Keratinocytes as depository of ammonium-inducible glutamine synthetase: Age- and anatomy-dependent distribution in human and rat skin. PLoS ONE 2009, 4, e4416. [Google Scholar] [CrossRef]
- Xiao-hui, H.; Feng-hou, G.; Yong, F. Senescence of human skin fibroblasts induced by ultraviolet B and its mechanism. J. Shanghai Jiaotong Univ. 2010, 30, 807–811. (In Chinese) [Google Scholar]
- Brito, S.; Baek, J.M.; Cha, B.; Heo, H.; Lee, S.H.; Lei, L.; Jung, S.Y.; Lee, S.M.; Lee, S.H.; Kwak, B.M.; et al. Nicotinamide mononucleotide reduces melanin production in aged melanocytes by inhibiting cAMP/Wnt signaling. J. Dermatol. Sci. 2022, 106, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Rognoni, E.; Watt, F.M. Skin Cell Heterogeneity in Development, Wound Healing, and Cancer. Trends Cell Biol. 2018, 28, 709–722. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.W.; Huang, X.; Song, B.L.; Wang, Y.; Wang, Y. Regulation of glucose and lipid metabolism in health and disease. Sci. China Life Sci. 2019, 62, 1420–1458. [Google Scholar] [CrossRef]
- Umbayev, B.; Askarova, S.; Almabayeva, A.; Saliev, T.; Masoud, A.R.; Bulanin, D. Galactose-Induced Skin Aging: The Role of Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 7145656. [Google Scholar] [CrossRef]
- Solano, F. Metabolism and Functions of Amino Acids in the Skin. Adv. Exp. Med. Biol. 2020, 1265, 187–199. [Google Scholar] [CrossRef]
- Laing, S.; Bielfeldt, S.; Ehrenberg, C.; Wilhelm, K.P. A Dermonutrient Containing Special Collagen Peptides Improves Skin Structure and Function: A Randomized, Placebo-Controlled, Triple-Blind Trial Using Confocal Laser Scanning Microscopy on the Cosmetic Effects and Tolerance of a Drinkable Collagen Supplement. J. Med. Food 2020, 23, 147–152. [Google Scholar] [CrossRef]
- Rigal, A.; Michael-Jubeli, R.; Bigouret, A.; Nkengne, A.; Bertrand, D.; Baillet-Guffroy, A.; Tfayli, A. Skin surface lipid composition in women: Increased 2,3-oxidosqualene correlates with older age. Eur. J. Dermatol. 2020, 30, 103–110. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Pérez-Felpete, N.; Fernández-Fernández, C.; Donapetry-García, C.; Pazos-García, C. Liver glucose metabolism in humans. Biosci. Rep. 2016, 36, e00416. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Cho, S.H.; Park, D.; Jung, E. Anti-skin aging properties of protocatechuic acid in vitro and in vivo. J. Cosmet. Dermatol. 2020, 19, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Keast, D. Energy metabolism and the skin. Int. J. Biochem. 1991, 23, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Lin, X.; Bu, C.; Zhang, X. Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies. Nutr. Metab. 2018, 15, 72. [Google Scholar] [CrossRef]
- Anisimova, A.S.; Alexandrov, A.I.; Makarova, N.E.; Gladyshev, V.N.; Dmitriev, S.E. Protein synthesis and quality control in aging. Aging 2018, 10, 4269–4288. [Google Scholar] [CrossRef]
- Tuvdendorj, D.; Børsheim, E.; Sharp, C.P.; Zhang, X.; Barone, C.M.; Chinkes, D.L.; Wolfe, R.R. Amino Acid Availability Regulates the Effect of Hyperinsulinemia on Skin Protein Metabolism in Pigs. J. Biol. Chem. 2015, 290, 17776–17783. [Google Scholar] [CrossRef]
- Schönborn, K.; Willenborg, S.; Schulz, J.N.; Imhof, T.; Eming, S.A.; Quondamatteo, F.; Brinckmann, J.; Niehoff, A.; Paulsson, M.; Koch, M.; et al. Role of collagen XII in skin homeostasis and repair. Matrix. Biol. 2020, 94, 57–76. [Google Scholar] [CrossRef]
- Choi, H.J.; Alam, M.B.; Baek, M.E.; Kwon, Y.G.; Lim, J.Y.; Lee, S.H. Protection against UVB-Induced Photoaging by Nypa fruticans via Inhibition of MAPK/AP-1/MMP-1 Signaling. Oxid. Med. Cell. Longev. 2020, 2020, 2905362. [Google Scholar] [CrossRef]
- Schmelzer, C.E.H.; Hedtke, T.; Heinz, A. Unique molecular networks: Formation and role of elastin cross-links. IUBMB Life 2020, 72, 842–854. [Google Scholar] [CrossRef]
- Ali, S.M. In vivo confocal Raman spectroscopic imaging of the human skin extracellular matrix degradation due to accumulated intrinsic and extrinsic aging. Photodermatol. Photoimmunol. Photomed 2021, 37, 140–152. [Google Scholar] [CrossRef]
- Ji, S.; Xiong, M.; Chen, H.; Liu, Y.; Zhou, L.; Hong, Y.; Wang, M.; Wang, C.; Fu, X.; Sun, X. Cellular rejuvenation: Molecular mechanisms and potential therapeutic interventions for diseases. Signal. Transduct. Target Ther. 2023, 8, 116. [Google Scholar] [CrossRef]
- Nagarajan, S.R.; Paul-Heng, M.; Krycer, J.R.; Fazakerley, D.J.; Sharland, A.F.; Hoy, A.J. Lipid and glucose metabolism in hepatocyte cell lines and primary mouse hepatocytes: A comprehensive resource for in vitro studies of hepatic metabolism. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E578–E589. [Google Scholar] [CrossRef]
- Muresan, X.M.; Narzt, M.S.; Woodby, B.; Ferrara, F.; Gruber, F.; Valacchi, G. Involvement of cutaneous SR-B1 in skin lipid homeostasis. Arch Biochem. Biophys. 2019, 666, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Shan, D.; Li, J.; Swallah, M.S.; Yang, X.; Ji, L.; Wang, S.; Gong, H.; Lyu, B.; Yu, H. Potential functionality of β-conglycinin with subunit deficiencies: Soy protein may regulate glucose and lipid metabolism. Food Funct. 2022, 13, 12291–12302. [Google Scholar] [CrossRef] [PubMed]
- Gruber, F.; Marchetti-Deschmann, M.; Kremslehner, C.; Schosserer, M. The Skin Epilipidome in Stress, Aging, and Inflammation. Front. Endocrinol. 2020, 11, 607076. [Google Scholar] [CrossRef]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.J. Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef]
- He, X.; Wan, F.; Su, W.; Xie, W. Research Progress on Skin Aging and Active Ingredients. Molecules 2023, 28, 5556. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, M.; Shin, S.; Woo, J.; Son, D.; Ryu, D.; Yoo, J.; Park, D.; Jung, E. Effect of Cirsium japonicum Flower Extract on Skin Aging Induced by Glycation. Molecules 2022, 27, 2093. [Google Scholar] [CrossRef]
- Danby, F.W. Nutrition and aging skin: Sugar and glycation. Clin. Dermatol. 2010, 28, 409–411. [Google Scholar] [CrossRef]
- Lee, Y.I.; Lee, S.G.; Jung, I.; Suk, J.; Lee, M.H.; Kim, D.U.; Lee, J.H. Effect of a Topical Collagen Tripeptide on Antiaging and Inhibition of Glycation of the Skin: A Pilot Study. Int. J. Mol. Sci. 2022, 23, 1101. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, F.; Voorhees, J.J.; Fisher, G.J. Rejuvenation of Aged Human Skin by Injection of Cross-linked Hyaluronic Acid. Plast. Reconstr. Surg. 2021, 147, 43s–49s. [Google Scholar] [CrossRef] [PubMed]
- Miskevich, D.; Chaban, A.; Dronina, M.; Abramovich, I.; Gottlieb, E.; Shams, I. Glutamine Homeostasis and Its Role in the Adaptive Strategies of the Blind Mole Rat, Spalax. Metabolites 2021, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.H.; Fan, L.; Zhang, Y.; Shen, Y.; Su, Z.T.; Peng, G.N.; Deng, J.L.; Zhong, Z.J.; Wu, X.F.; Yu, S.M.; et al. Antioxidant Capacity and Protective Effect of Cow Placenta Extract on D-Galactose-Induced Skin Aging in Mice. Nutrients 2022, 14, 4659. [Google Scholar] [CrossRef] [PubMed]
- Zoanni, B.; Aiello, G.; Negre-Salvayre, A.; Aldini, G.; Carini, M.; D’Amato, A. Lipidome Investigation of Carnosine Effect on Nude Mice Skin to Prevent UV-A Damage. Int. J. Mol. Sci. 2023, 24, 10009. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, Y.K.; Kim, S.; Kim, J.E.; Tian, Y.D.; Doh, E.J.; Lee, D.H.; Chung, J.H. Adipochemokines induced by ultraviolet irradiation contribute to impaired fat metabolism in subcutaneous fat cells. Br. J. Dermatol. 2018, 178, 492–501. [Google Scholar] [CrossRef]
- Gruber, F.; Kremslehner, C.; Eckhart, L.; Tschachler, E. Cell aging and cellular senescence in skin aging—Recent advances in fibroblast and keratinocyte biology. Exp. Gerontol. 2020, 130, 110780. [Google Scholar] [CrossRef]
- Feng, Z.; Qin, Y.; Huo, F.; Jian, Z.; Li, X.; Geng, J.; Li, Y.; Wu, J. NMN recruits GSH to enhance GPX4-mediated ferroptosis defense in UV irradiation induced skin injury. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166287. [Google Scholar] [CrossRef]
- Ying, T.H.; Chen, C.W.; Hsiao, Y.P.; Hung, S.J.; Chung, J.G.; Yang, J.H. Citric acid induces cell-cycle arrest and apoptosis of human immortalized keratinocyte cell line (HaCaT) via caspase- and mitochondrial-dependent signaling pathways. Anticancer. Res. 2013, 33, 4411–4420. [Google Scholar]
- Tan, C.Y.R.; Tan, C.L.; Chin, T.; Morenc, M.; Ho, C.Y.; Rovito, H.A.; Quek, L.S.; Soon, A.L.; Lim, J.S.Y.; Dreesen, O.; et al. Nicotinamide Prevents UVB- and Oxidative Stress-Induced Photoaging in Human Primary Keratinocytes. J. Investig. Dermatol. 2022, 142, 1670–1681.e1612. [Google Scholar] [CrossRef]
- Verdier-Sévrain, S.; Bonté, F. Skin hydration: A review on its molecular mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Fuchs, E. Building and Maintaining the Skin. Cold Spring Harb. Perspect. Biol. 2022, 14, a040840. [Google Scholar] [CrossRef] [PubMed]
- Soydas, T.; Sayitoglu, M.; Sarac, E.Y.; Cınar, S.; Solakoglu, S.; Tiryaki, T.; Sultuybek, G.K. Metformin reverses the effects of high glucose on human dermal fibroblasts of aged skin via downregulating RELA/p65 expression. J. Physiol. Biochem. 2021, 77, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Wang, W.; Wang, C.; Lao, G.; Liu, D.; Mai, L.; Yan, L.; Yang, C.; Ren, M. AGEs trigger autophagy in diabetic skin tissues and fibroblasts. Biochem. Biophys. Res. Commun. 2016, 471, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Shin, K.O.; Muraoka, S.; Choi, Y.; Park, J.H.; Park, S.H.; Hwang, J.T.; Park, K.; Uchida, Y. The Epidermal Environment’s Influence on the Dermal Environment in Response to External Stress. Skin. Pharmacol. Physiol. 2023, 36, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Mort, R.L.; Jackson, I.J.; Patton, E.E. The melanocyte lineage in development and disease. Development 2015, 142, 620–632. [Google Scholar] [CrossRef]
- Lee, Y.I.; Choi, S.; Roh, W.S.; Lee, J.H.; Kim, T.G. Cellular Senescence and Inflammaging in the Skin Microenvironment. Int. J. Mol. Sci. 2021, 22, 3849. [Google Scholar] [CrossRef]
- Boo, Y.C. Arbutin as a Skin Depigmenting Agent with Antimelanogenic and Antioxidant Properties. Antioxidants 2021, 10, 1129. [Google Scholar] [CrossRef]
- Lee, S.H.; Bae, I.H.; Lee, E.S.; Kim, H.J.; Lee, J.; Lee, C.S. Glucose Exerts an Anti-Melanogenic Effect by Indirect Inactivation of Tyrosinase in Melanocytes and a Human Skin Equivalent. Int. J. Mol. Sci. 2020, 21, 1736. [Google Scholar] [CrossRef]
- Kerns, M.L.; Chien, A.L.; Kang, S. A Role for NRF2-Signaling in the Treatment and Prevention of Solar Lentigines. Plast. Reconstr. Surg. 2021, 148, 27s–31s. [Google Scholar] [CrossRef]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef]
- Qiu, Y.; Zheng, J.; Chen, S.; Sun, Y. Connexin Mutations and Hereditary Diseases. Int. J. Mol. Sci. 2022, 23, 4255. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.M.; Proksch, E. The skin’s barrier. G Ital. Dermatol. Venereol. 2009, 144, 689–700. [Google Scholar] [PubMed]
- Totland, M.Z.; Rasmussen, N.L.; Knudsen, L.M.; Leithe, E. Regulation of gap junction intercellular communication by connexin ubiquitination: Physiological and pathophysiological implications. Cell Mol. Life Sci. 2020, 77, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Perez, A.; Pacheco-Costa, R.; Atkinson, E.G.; Deosthale, P.; Davis, H.M.; Essex, A.L.; Dilley, J.E.; Gomez, L.; Rupert, J.E.; Zimmers, T.A.; et al. Age- and sex-dependent role of osteocytic pannexin1 on bone and muscle mass and strength. Sci. Rep. 2019, 9, 13903. [Google Scholar] [CrossRef]
- Caruso, G.; Di Pietro, L.; Caraci, F. Gap Junctions and Connexins in Microglia-Related Oxidative Stress and Neuroinflammation: Perspectives for Drug Discovery. Biomolecules 2023, 13, 505. [Google Scholar] [CrossRef]
- Elsnicova, B.; Hornikova, D.; Tibenska, V.; Kolar, D.; Tlapakova, T.; Schmid, B.; Mallek, M.; Eggers, B.; Schlötzer-Schrehardt, U.; Peeva, V.; et al. Desmin Knock-Out Cardiomyopathy: A Heart on the Verge of Metabolic Crisis. Int. J. Mol. Sci. 2022, 23, 12020. [Google Scholar] [CrossRef]
- Piao, L.; Huang, Z.; Inoue, A.; Kuzuya, M.; Cheng, X.W. Human umbilical cord-derived mesenchymal stromal cells ameliorate aging-associated skeletal muscle atrophy and dysfunction by modulating apoptosis and mitochondrial damage in SAMP10 mice. Stem Cell Res. Ther. 2022, 13, 226. [Google Scholar] [CrossRef]
- Winter, L.; Unger, A.; Berwanger, C.; Spörrer, M.; Türk, M.; Chevessier, F.; Strucksberg, K.H.; Schlötzer-Schrehardt, U.; Wittig, I.; Goldmann, W.H.; et al. Imbalances in protein homeostasis caused by mutant desmin. Neuropathol. Appl. Neurobiol. 2019, 45, 476–494. [Google Scholar] [CrossRef]
- Bai, J.; Liu, T.; Tu, B.; Yuan, M.; Shu, Z.; Fan, M.; Huo, S.; Guo, Y.; Wang, L.; Wang, H.; et al. Autophagy loss impedes cancer-associated fibroblast activation via downregulating proline biosynthesis. Autophagy 2023, 19, 632–643. [Google Scholar] [CrossRef]
- Miyasaka, Y.; Murakami, K.; Ito, K.; Kumaki, J.; Makabe, K.; Hatori, K. Condensed desmin and actin cytoskeletal communication in lipid droplets. Cytoskeleton 2019, 76, 477–490. [Google Scholar] [CrossRef]
- Hnia, K.; Ramspacher, C.; Vermot, J.; Laporte, J. Desmin in muscle and associated diseases: Beyond the structural function. Cell Tissue. Res. 2015, 360, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Paulin, D.; Li, Z. Desmin: A major intermediate filament protein essential for the structural integrity and function of muscle. Exp. Cell Res. 2004, 301, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mavroidis, M.; Athanasiadis, N.C.; Rigas, P.; Kostavasili, I.; Kloukina, I.; Te Rijdt, W.P.; Kavantzas, N.; Chaniotis, D.; van Tintelen, J.P.; Skaliora, I.; et al. Desmin is essential for the structure and function of the sinoatrial node: Implications for increased arrhythmogenesis. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H557–H570. [Google Scholar] [CrossRef] [PubMed]
- Bäsler, K.; Brandner, J.M. Tight junctions in skin inflammation. Pflugers. Arch. 2017, 469, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Itoh, M.; Saitou, M.; Ando-Akatsuka, Y.; Furuse, M.; Yoneda, K.; Imamura, S.; Fujimoto, K.; Tsukita, S. Subcellular distribution of tight junction-associated proteins (occludin, ZO-1, ZO-2) in rodent skin. J. Investig. Dermatol. 1998, 110, 862–866. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Qiao, S.; Yang, D.; Li, Z.; Xu, J.; Li, W.; Su, L.; Liu, W. Occludin degradation makes brain microvascular endothelial cells more vulnerable to reperfusion injury in vitro. J. Neurochem. 2021, 156, 352–366. [Google Scholar] [CrossRef]
- Li, M.; Wang, S.; Wang, S.; Zhang, L.; Wu, D.; Yang, R.; Ji, A.; Li, Y.; Wang, J. Occludin downregulation in high glucose is regulated by SSTR(2) via the VEGF/NRP1/Akt signaling pathway in RF/6A cells. Exp. Ther. Med. 2017, 14, 1732–1738. [Google Scholar] [CrossRef]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef]
- Liu, B.Y.; Zhang, B.L.; Gao, D.Y.; Li, Q.; Xu, X.Y.; Shum, W. Epididymal epithelial degeneration and lipid metabolism impairment account for male infertility in occludin knockout mice. Front. Endocrinol. 2022, 13, 1069319. [Google Scholar] [CrossRef]
- Bäsler, K.; Bergmann, S.; Heisig, M.; Naegel, A.; Zorn-Kruppa, M.; Brandner, J.M. The role of tight junctions in skin barrier function and dermal absorption. J. Control. Release 2016, 242, 105–118. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, X.M.; Yang, L.J.; Wu, P.L. Tranexamic acid accelerates skin barrier recovery and upregulates occludin in damaged skin. Int. J. Dermatol. 2014, 53, 959–965. [Google Scholar] [CrossRef]
- Torices, S.; Daire, L.; Simon, S.; Naranjo, O.; Mendoza, L.; Teglas, T.; Fattakhov, N.; Adesse, D.; Toborek, M. Occludin: A gatekeeper of brain Infection by HIV-1. Fluids Barriers CNS 2023, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Malik, A.; Guy, C.S.; Karki, R.; Vogel, P.; Kanneganti, T.D. Pyrin Inflammasome Regulates Tight Junction Integrity to Restrict Colitis and Tumorigenesis. Gastroenterology 2018, 154, 948–964.e948. [Google Scholar] [CrossRef] [PubMed]
- Brunner, J.; Ragupathy, S.; Borchard, G. Target specific tight junction modulators. Adv. Drug. Deliv. Rev. 2021, 171, 266–288. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Son, D.; Kim, M.; Lee, S.; Roh, K.B.; Ryu, D.; Lee, J.; Jung, E.; Park, D. Ameliorating Effect of Akebia quinata Fruit Extracts on Skin Aging Induced by Advanced Glycation End Products. Nutrients 2015, 7, 9337–9352. [Google Scholar] [CrossRef] [PubMed]
- Sell, D.R.; Nelson, J.F.; Monnier, V.M. Effect of chronic aminoguanidine treatment on age-related glycation, glycoxidation, and collagen cross-linking in the Fischer 344 rat. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B405–B411. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Wang, Y.; Jin, J.; Yang, Z.; Guo, R.; Li, X.; Yang, L.; Li, Z. Resveratrol Treats UVB-Induced Photoaging by Anti-MMP Expression, through Anti-Inflammatory, Antioxidant, and Antiapoptotic Properties, and Treats Photoaging by Upregulating VEGF-B Expression. Oxid. Med. Cell. Longev. 2022, 2022, 6037303. [Google Scholar] [CrossRef]
- Augustyniak, A.; McMahon, H. Effect of Marine-Derived Saccharides on Human Skin Fibroblasts and Dermal Papilla Cells. Mar. Drugs 2023, 21, 330. [Google Scholar] [CrossRef]
- Kumar, A.; Archo, S.; Singh, C.P.; Naikoo, S.H.; Singh, B.; Kaur, S.; Tasduq, S.A. Photoprotective effect of 18β-glycyrrhetinic acid derivatives against ultra violet (UV)-B-Induced skin aging. Bioorg. Med. Chem. Lett. 2022, 76, 128984. [Google Scholar] [CrossRef]
- He, Y.L.; Lin, L.; Zheng, H.; Mo, Y.; Zhou, C.; Sun, S.; Hong, P.; Qian, Z.J. Potential anti-skin aging effect of a peptide AYAPE isolated from Isochrysis zhanjiangensis on UVB-induced HaCaT cells and H(2)O(2)-induced BJ cells. J. Photochem. Photobiol. B 2022, 233, 112481. [Google Scholar] [CrossRef]
- Li, W.; Mu, X.; Wu, X.; He, W.; Liu, Y.; Liu, Y.; Deng, J.; Nie, X. Dendrobium nobile Lindl. Polysaccharides protect fibroblasts against UVA-induced photoaging via JNK/c-Jun/MMPs pathway. J. Ethnopharmacol. 2022, 298, 115590. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, N.; Yan, Y.Q.; Liu, Y.; Xiong, K.; Liu, Y.; Xia, Q.M.; Zhang, H.; Liu, Z.D. Recent advances in the anti-aging effects of phytoestrogens on collagen, water content, and oxidative stress. Phytother. Res. 2020, 34, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants 2022, 11, 1663. [Google Scholar] [CrossRef] [PubMed]
- Min, D.; Park, S.; Kim, H.; Lee, S.H.; Ahn, Y.; Jung, W.; Kim, H.J.; Cho, Y.W. Potential anti-ageing effect of chondroitin sulphate through skin regeneration. Int. J. Cosmet. Sci. 2020, 42, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.A.; Yu, P.J.; Yang, D.Q.; Chen, W. The Antisenescence Effect of Exosomes from Human Adipose-Derived Stem Cells on Skin Fibroblasts. Biomed. Res. Int. 2022, 2022, 1034316. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Xiao, Z.; Tong, H.; Liu, Y.; Wu, Y.; Ge, C. Oral Intake of Chicken Bone Collagen Peptides Anti-Skin Aging in Mice by Regulating Collagen Degradation and Synthesis, Inhibiting Inflammation and Activating Lysosomes. Nutrients 2022, 14, 1622. [Google Scholar] [CrossRef]
- Liu, J.; Chen, T.; Zhao, Y.; Ding, Z.; Ge, W.; Zhang, J. Blood donation improves skin aging through the reduction of iron deposits and the increase of TGF-β1 in elderly skin. Mech. Ageing. Dev. 2022, 205, 111687. [Google Scholar] [CrossRef]
- Hiebert, P.; Martyts, A.; Schwestermann, J.; Janke, K.; Hafner, J.; Boukamp, P.; Mazza, E.; Werner, S. Activation of Nrf2 in fibroblasts promotes a skin aging phenotype via an Nrf2-miRNA-collagen axis. Matrix. Biol. 2022, 113, 39–60. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Lei, X.; Lei, L.; Zhao, J.; Zeng, K.; Ming, J. Premna microphylla Turcz pectin protected UVB-induced skin aging in BALB/c-nu mice via Nrf2 pathway. Int. J. Biol. Macromol. 2022, 215, 12–22. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Liu, X.; Wu, J.; Liu, C.; Gong, W.; Zhao, Z.; Hong, J.; Lin, D.; Wang, Y.; et al. Antioxidant peptidomics reveals novel skin antioxidant system. Mol. Cell Proteom. 2009, 8, 571–583. [Google Scholar] [CrossRef]
- Lin, P.; Hua, N.; Hsu, Y.C.; Kan, K.W.; Chen, J.H.; Lin, Y.H.; Lin, Y.H.; Kuan, C.M. Oral Collagen Drink for Antiaging: Antioxidation, Facilitation of the Increase of Collagen Synthesis, and Improvement of Protein Folding and DNA Repair in Human Skin Fibroblasts. Oxid. Med. Cell. Longev. 2020, 2020, 8031795. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.E.; Song, M.J.; Hara, M.; Imanaka-Yoshida, K.; Lee, D.H.; Chung, J.H.; Lee, S.T. Effects of Tenascin C on the Integrity of Extracellular Matrix and Skin Aging. Int. J. Mol. Sci. 2020, 21, 8693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, H.; Zheng, Y.; Zhang, L.; Wang, X.; Luo, Z.; Tang, J.; Lin, L.; Du, Z.; Dong, C. The effects and mechanism of collagen peptide and elastin peptide on skin aging induced by D-galactose combined with ultraviolet radiation. J. Photochem. Photobiol. B 2020, 210, 111964. [Google Scholar] [CrossRef]
- Nadra, K.; André, M.; Marchaud, E.; Kestemont, P.; Braccini, F.; Cartier, H.; Kéophiphath, M.; Fanian, F. A hyaluronic acid-based filler reduces lipolysis in human mature adipocytes and maintains adherence and lipid accumulation of long-term differentiated human preadipocytes. J. Cosmet. Dermatol. 2021, 20, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, A.; Bartnicka, E.; Namieciński, P.; Rotsztejn, H. Influence of the complex of retinol-vitamin C on skin surface lipids. J. Cosmet. Dermatol. 2015, 14, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Park, P.J.; Rha, C.S.; Kim, S.T. Theaflavin-Enriched Fraction Stimulates Adipogenesis in Human Subcutaneous Fat Cells. Int. J. Mol. Sci. 2019, 20, 2034. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Han, Y.; Ahn, S.; An, S.; Shin, J.C.; Choi, H.; Kim, H.J.; Park, N.H.; Kim, Y.J.; Jin, S.H.; et al. Kojyl cinnamate esters are peroxisome proliferator-activated receptor α/γ dual agonists. Bioorg. Med. Chem. 2018, 26, 5654–5663. [Google Scholar] [CrossRef]
- Liu, C.S.; Nam, T.G.; Han, M.W.; Ahn, S.M.; Choi, H.S.; Kim, T.Y.; Chun, O.K.; Koo, S.I.; Kim, D.O. Protective effect of detoxified Rhus verniciflua stokes on human keratinocytes and dermal fibroblasts against oxidative stress and identification of the bioactive phenolics. Biosci. Biotechnol. Biochem. 2013, 77, 1682–1688. [Google Scholar] [CrossRef]
- Birch-Machin, M.A. The role of mitochondria in ageing and carcinogenesis. Clin. Exp. Dermatol. 2006, 31, 548–552. [Google Scholar] [CrossRef]
- Li, L.; Sawashita, J.; Ding, X.; Yang, M.; Xu, Z.; Miyahara, H.; Mori, M.; Higuchi, K. Caloric restriction reduces the systemic progression of mouse AApoAII amyloidosis. PLoS ONE 2017, 12, e0172402. [Google Scholar] [CrossRef]
- Soukas, A.A.; Hao, H.; Wu, L. Metformin as Anti-Aging Therapy: Is It for Everyone? Trends. Endocrinol. Metab. 2019, 30, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Bharath, L.P.; Agrawal, M.; McCambridge, G.; Nicholas, D.A.; Hasturk, H.; Liu, J.; Jiang, K.; Liu, R.; Guo, Z.; Deeney, J.; et al. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab. 2020, 32, 44–55.e46. [Google Scholar] [CrossRef] [PubMed]
- Le Pelletier, L.; Mantecon, M.; Gorwood, J.; Auclair, M.; Foresti, R.; Motterlini, R.; Laforge, M.; Atlan, M.; Fève, B.; Capeau, J.; et al. Metformin alleviates stress-induced cellular senescence of aging human adipose stromal cells and the ensuing adipocyte dysfunction. eLife 2021, 10, e62635. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Mechanistic Basis and Clinical Evidence for the Applications of Nicotinamide (Niacinamide) to Control Skin Aging and Pigmentation. Antioxidants 2021, 10, 1315. [Google Scholar] [CrossRef]
- Massudi, H.; Grant, R.; Braidy, N.; Guest, J.; Farnsworth, B.; Guillemin, G.J. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS ONE 2012, 7, e42357. [Google Scholar] [CrossRef]
- Sosa-Díaz, E.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. The role of vitamin D on redox regulation and cellular senescence. Free Radic. Biol. Med. 2022, 193, 253–273. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Gao, X.; Xie, W. Research Progress in Skin Aging, Metabolism, and Related Products. Int. J. Mol. Sci. 2023, 24, 15930. https://doi.org/10.3390/ijms242115930
He X, Gao X, Xie W. Research Progress in Skin Aging, Metabolism, and Related Products. International Journal of Molecular Sciences. 2023; 24(21):15930. https://doi.org/10.3390/ijms242115930
Chicago/Turabian StyleHe, Xin, Xinyu Gao, and Weidong Xie. 2023. "Research Progress in Skin Aging, Metabolism, and Related Products" International Journal of Molecular Sciences 24, no. 21: 15930. https://doi.org/10.3390/ijms242115930
APA StyleHe, X., Gao, X., & Xie, W. (2023). Research Progress in Skin Aging, Metabolism, and Related Products. International Journal of Molecular Sciences, 24(21), 15930. https://doi.org/10.3390/ijms242115930





