Guijing2501 (Citrus unshiu) Has Stronger Cold Tolerance Due to Higher Photoprotective Capacity as Revealed by Comparative Transcriptomic and Physiological Analysis and Overexpression of Early Light-Induced Protein
Abstract
:1. Introduction
2. Results
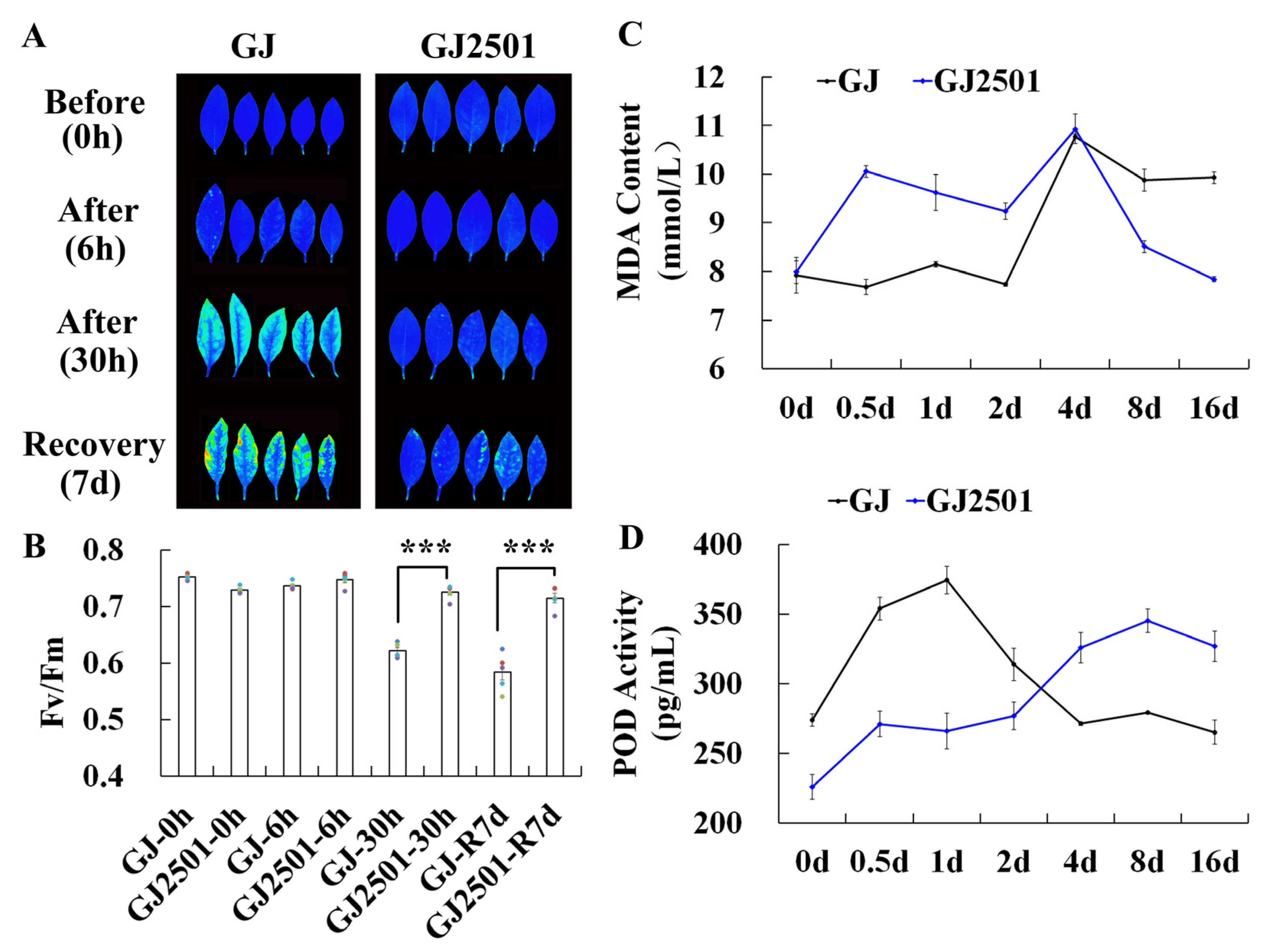
2.1. Differences in Cold Stress Tolerance between GJ and GJ2501
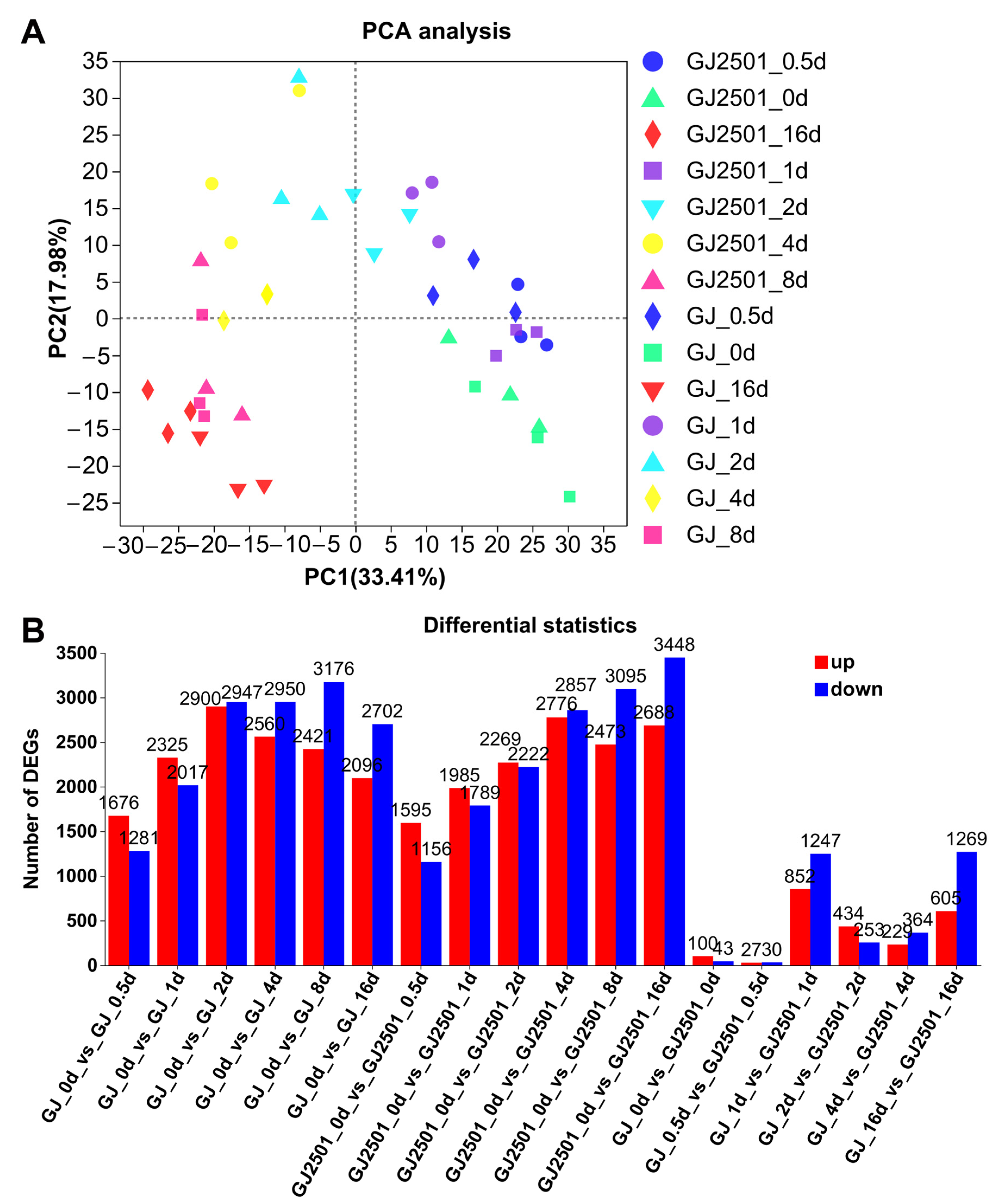
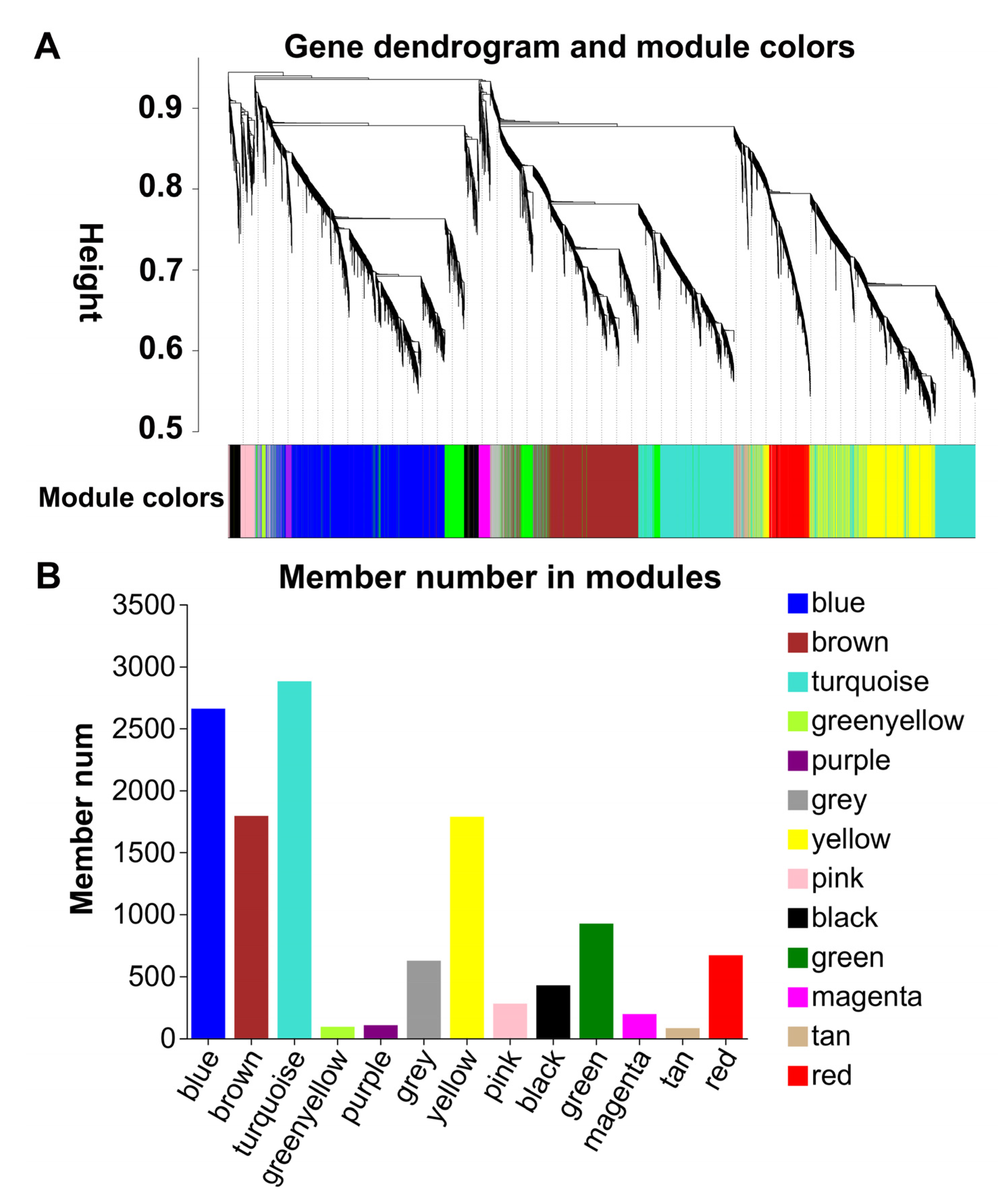
2.2. Transcriptome Profiles of GJ and GJ2501 during Time-Course Cold Stress
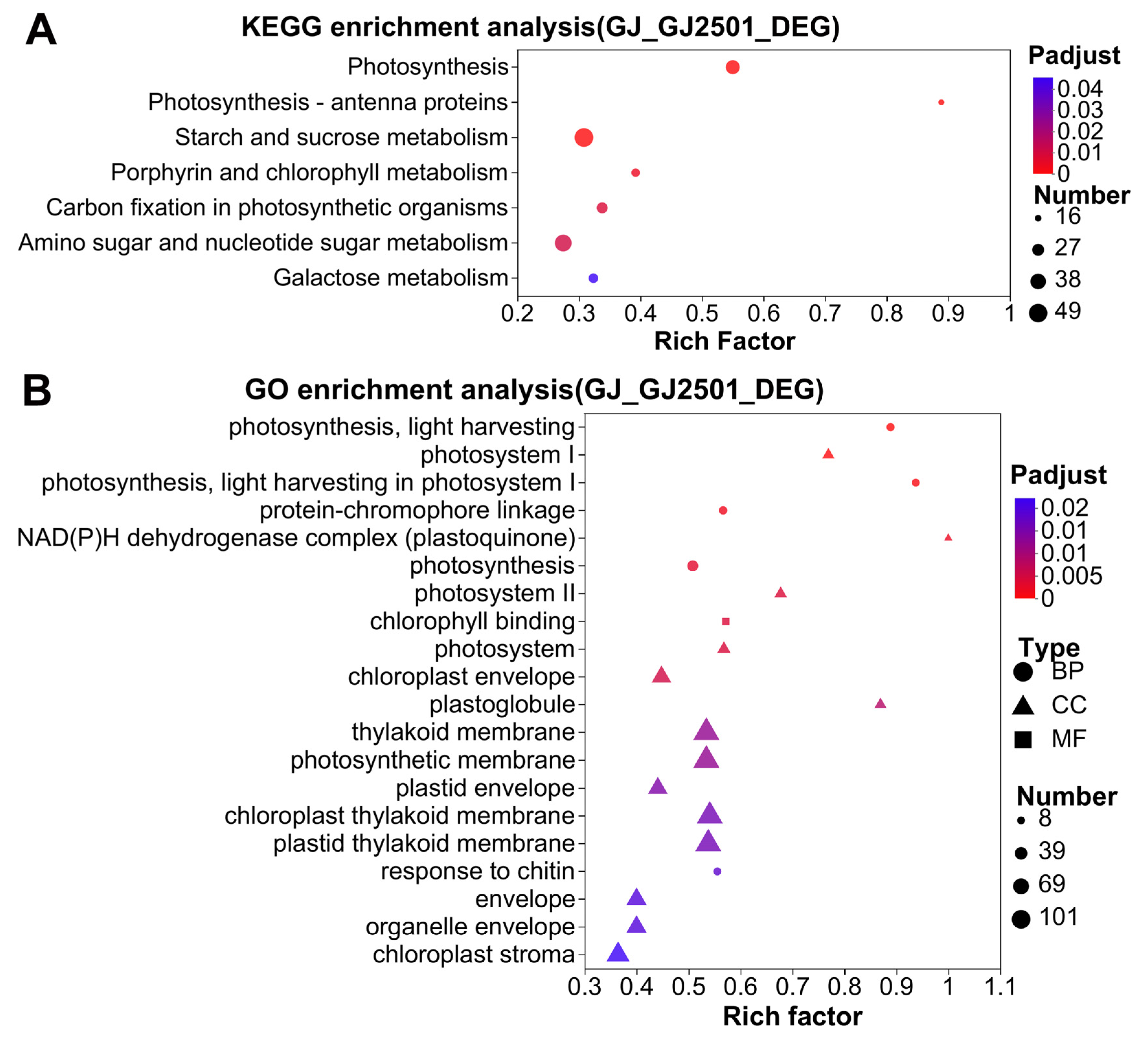
2.3. KEGG and GO Enrichment Analysis of DEGs between GJ and GJ2501
2.4. KEGG and GO Enrichment Analysis of Up-Regulated DEGs in GJ and GJ2501 during Cold Stress
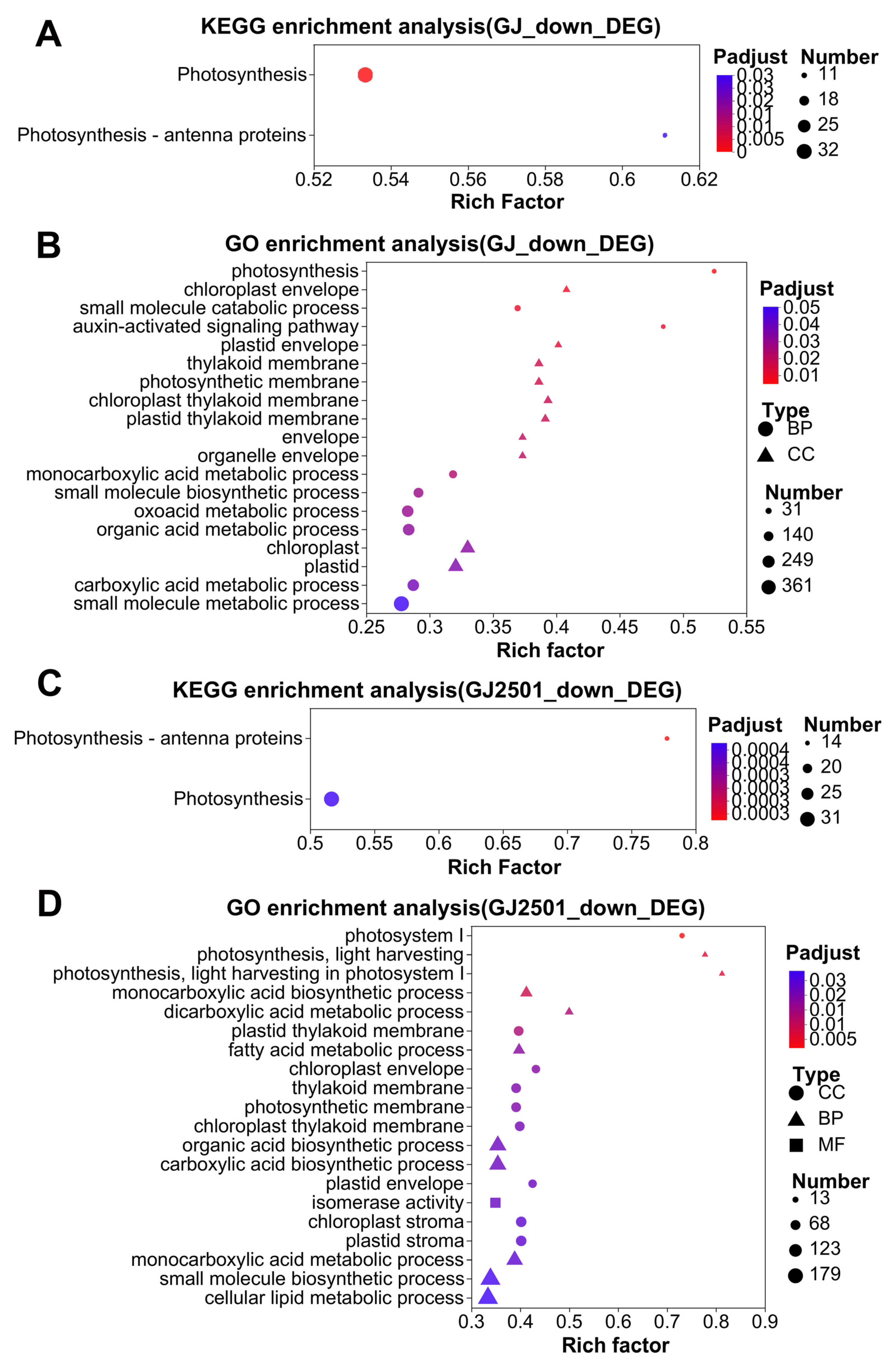
2.5. KEGG and GO Enrichment Analysis of Down-Regulated DEGs in GJ and GJ2501 during Cold Stress
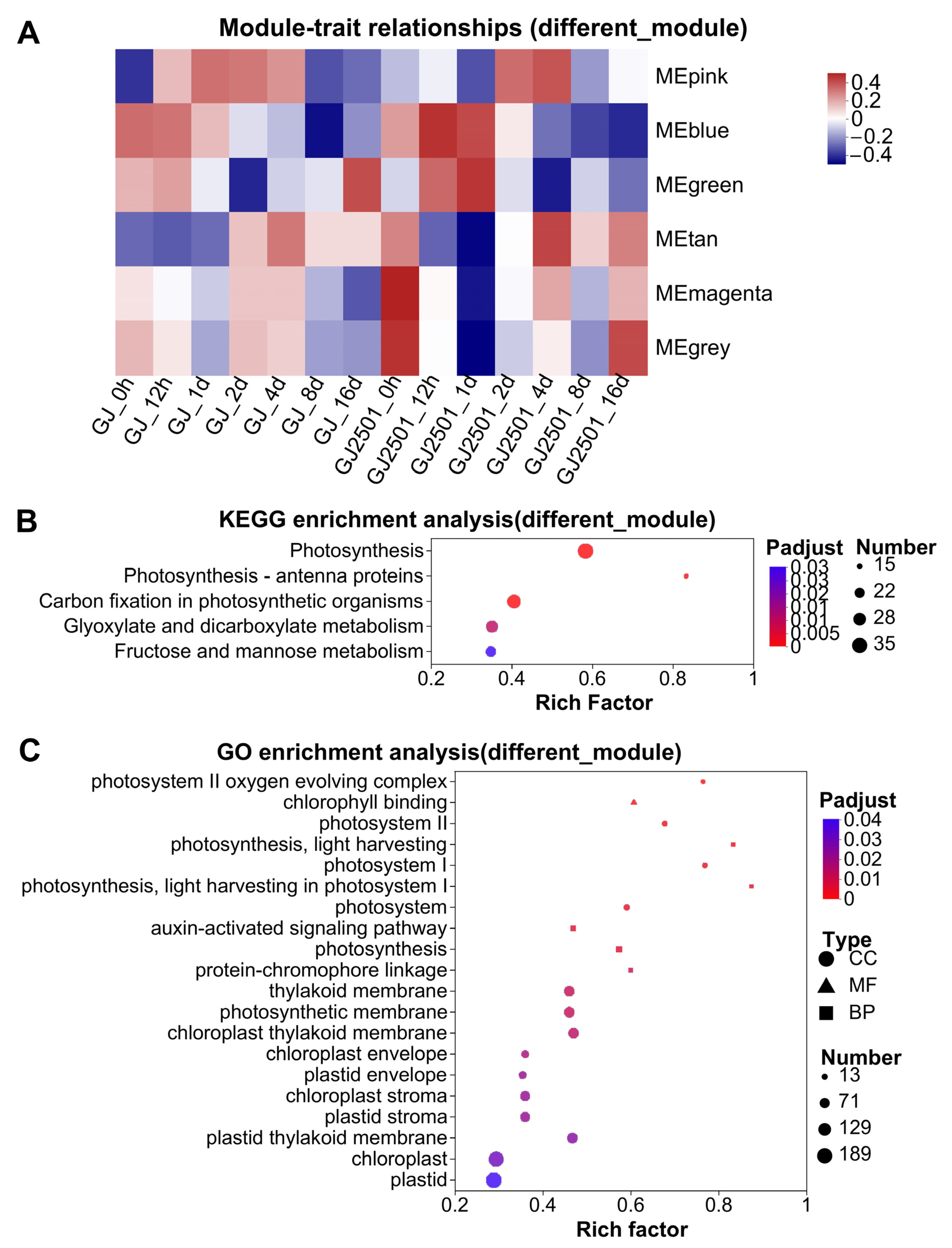
2.6. KEGG and GO Enrichment Analysis of WGCNA Modules with Different Module-Trait Correlation Patterns between GJ and GJ2501
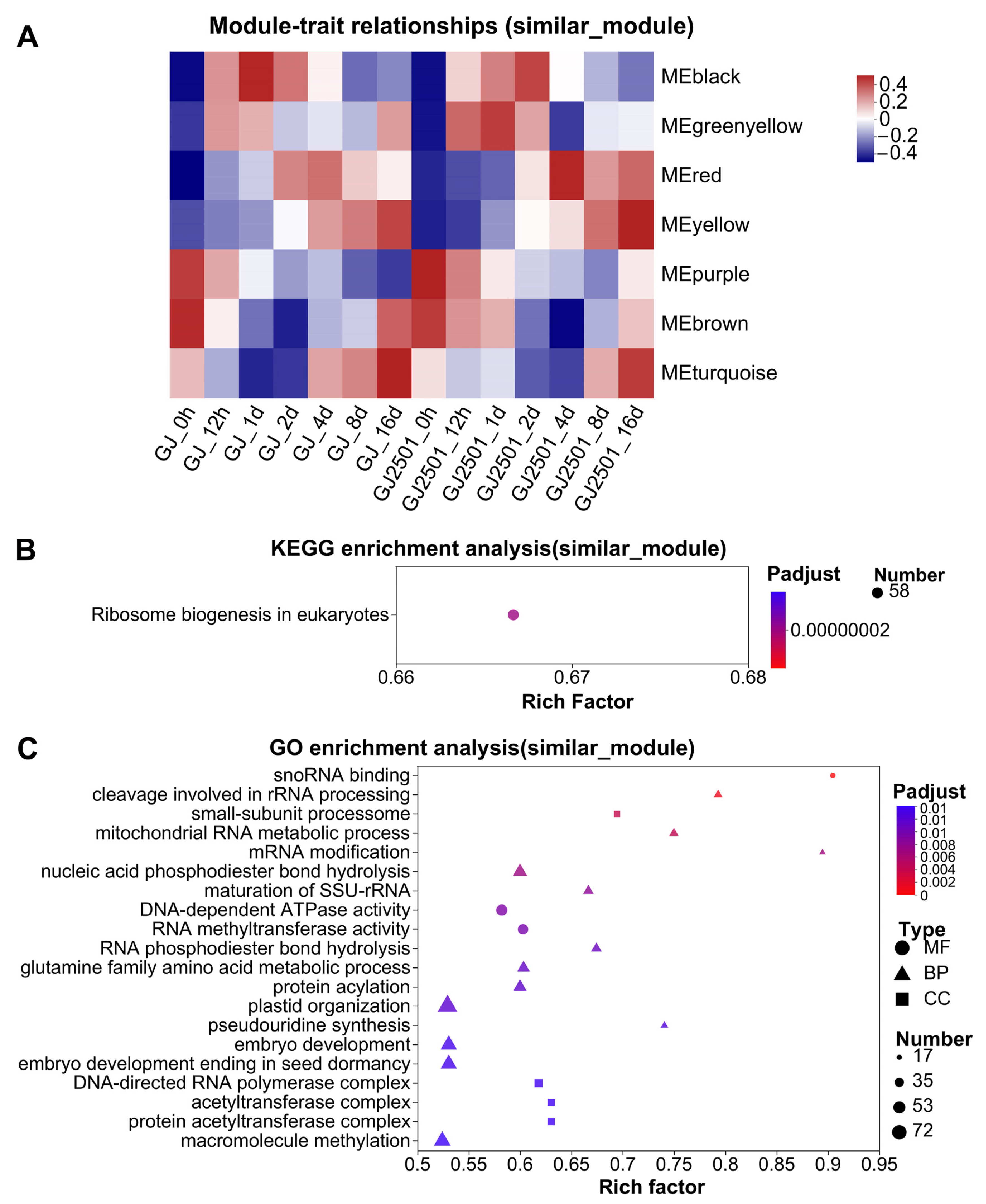
2.7. KEGG and GO Enrichment Analysis of WGCNA Modules with Similar Module-Trait Correlation Patterns between GJ and GJ2501
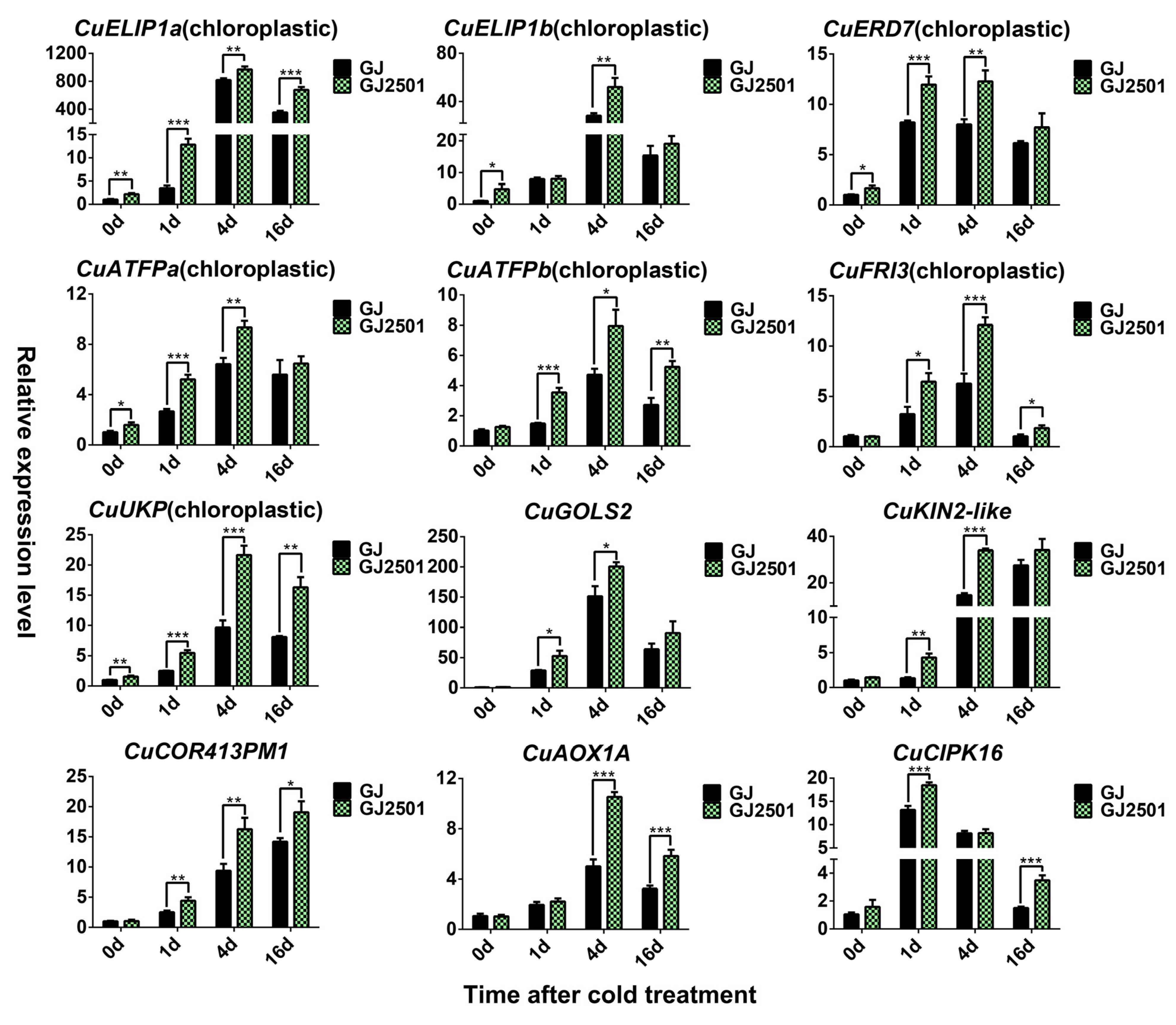
2.8. Verification of Candidate Up-Regulated Cold-Responsive DEGs in GJ and GJ2501 by RT-qPCR Analysis
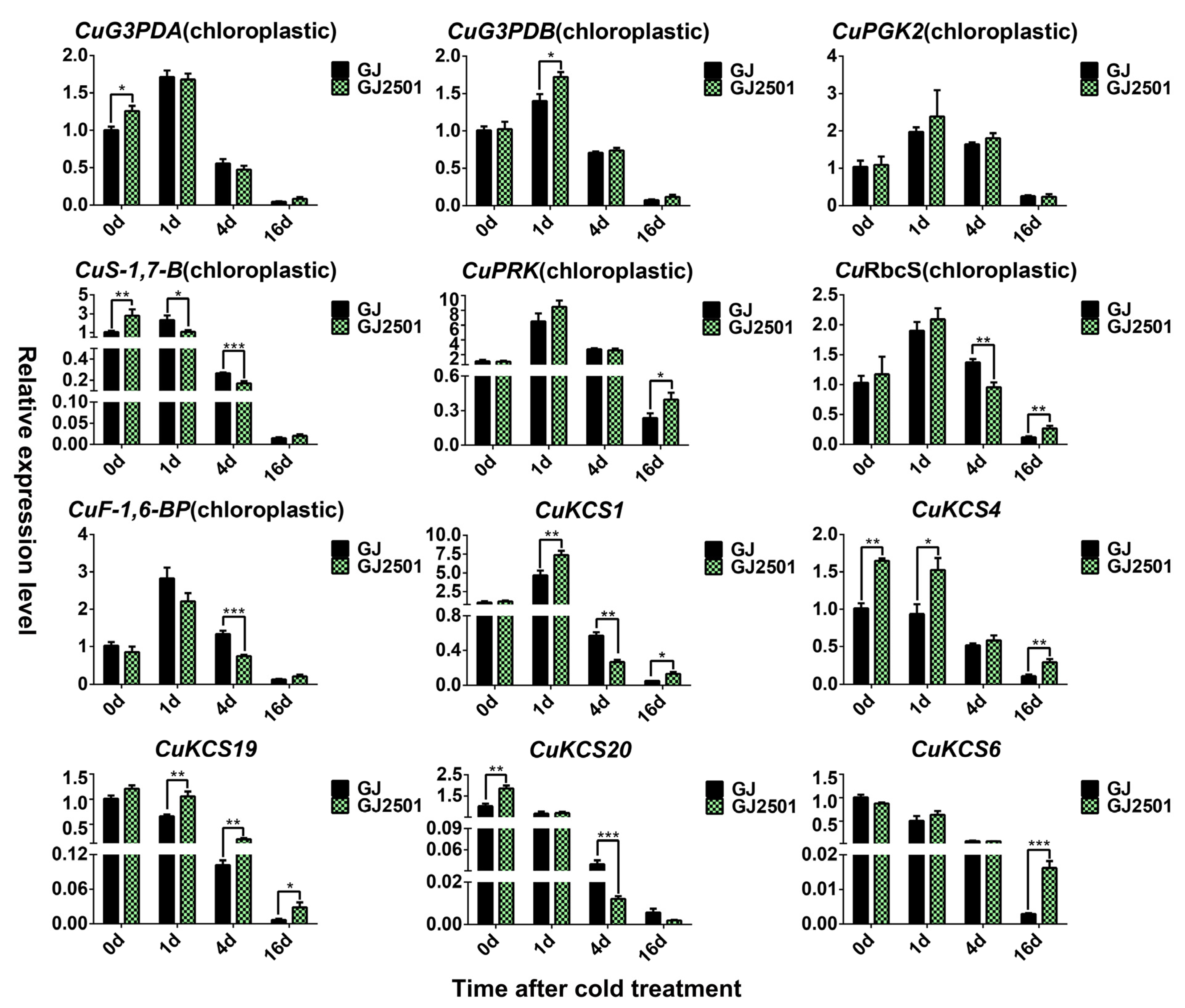
2.9. Verification of Candidate Down-Regulated Cold-Responsive DEGs in GJ and GJ2501 by RT-qPCR Analysis
2.10. Overexpression of CuELIP1a Enhances the Cold Tolerance of Transgenic Arabidopsis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Cold Tolerance Assay
4.2. RNA-Sequencing
4.3. KEGG and GO Enrichment Analysis of Specific Gene Sets
4.4. RT-qPCR Verification of Candidate DEGs
4.5. Cold Tolerance Verification of Arabidopsis Overexpressing CuELIP1a
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Primo-Capella, A.; Martínez-Cuenca, M.R.; Forner-Giner, M.Á. Cold stress in Citrus: A molecular, physiological and biochemical perspective. Horticulturae 2021, 7, 340. [Google Scholar] [CrossRef]
- Deng, X. A review and perspective for citrus breeding in China during the last six decades. Acta Hortic. Sinica 2022, 49, 2063–2074. [Google Scholar]
- Jiang, J.; Hou, R.; Yang, N.; Li, L.; Deng, J.; Qin, G.; Ding, D. Physiological and TMT-labeled proteomic analyses reveal important roles of sugar and secondary metabolism in Citrus junos under cold stress. J. Proteom. 2021, 237, 104145. [Google Scholar] [CrossRef] [PubMed]
- Ferrarezi, R.S.; Rodriguez, K.; Sharp, D. How historical trends in Florida all-citrus production correlate with devastating hurricane and freeze events. Weather 2020, 75, 77–83. [Google Scholar] [CrossRef]
- Attaway, J.A. A History of Florida Citrus Freezes; Florida Science Source: Ocala, FL, USA, 1997; ISBN 978-0-944961-03-2. [Google Scholar]
- Peng, T.; Zhu, X.; Fan, Q.; Sun, P.; Liu, J. Identification and characterization of low temperature stress responsive genes in Poncirus trifoliata by suppression subtractive hybridization. Gene 2012, 492, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, X.; Liu, J.H. Deep sequencing-based characterization of transcriptome of trifoliate orange (Poncirus trifoliata (L.) Raf.) in response to cold stress. BMC Genom. 2015, 16, 555. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.S.; Wang, W.; Zhang, Q.; Liu, J.H. A basic helix-loop-helix transcription factor, PtrbHLH, of Poncirus trifoliata confers cold tolerance and modulates peroxidase-mediated scavenging of hydrogen peroxide. Plant Physiol. 2013, 162, 1178–1194. [Google Scholar] [CrossRef]
- Geng, J.; Wei, T.; Wang, Y.; Huang, X.; Liu, J.H. Overexpression of PtrbHLH, a basic helix-loop-helix transcription factor from Poncirus trifoliata, confers enhanced cold tolerance in pummelo (Citrus grandis) by modulation of H2O2 level via regulating a CAT gene. Tree Physiol. 2019, 39, 2045–2054. [Google Scholar] [CrossRef]
- Wang, M.; Dai, W.; Du, J.; Ming, R.; Dahro, B.; Liu, J.H. ERF 109 of trifoliate orange (Poncirus trifoliata (L.) Raf.) contributes to cold tolerance by directly regulating expression of Prx1 involved in antioxidative process. Plant Biotechnol. J. 2019, 17, 1316–1332. [Google Scholar] [CrossRef]
- Huang, X.S.; Zhang, Q.; Zhu, D.; Fu, X.; Wang, M.; Zhang, Q.; Moriguchi, T.; Liu, J.H. ICE1 of Poncirus trifoliata functions in cold tolerance by modulating polyamine levels through interacting with arginine decarboxylase. J. Exp. Bot. 2015, 66, 3259–3274. [Google Scholar] [CrossRef]
- Zhang, Y.; Ming, R.; Khan, M.; Wang, Y.; Dahro, B.; Xiao, W.; Li, C.; Liu, J.H. ERF9 of Poncirus trifoliata (L.) Raf. undergoes feedback regulation by ethylene and modulates cold tolerance via regulating a glutathione S-transferase U17 gene. Plant Biotechnol. J. 2022, 20, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Ming, R.; Zhang, Y.; Wang, Y.; Khan, M.; Dahro, B.; Liu, J.H. The JA-responsive MYC2-BADH-like transcriptional regulatory module in Poncirus trifoliata contributes to cold tolerance by modulation of glycine betaine biosynthesis. New Phytol. 2021, 229, 2730–2750. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Zhu, X.; Duan, N.; Liu, J.H. Ptr BAM 1, a β-amylase-coding gene of Poncirus trifoliata, is a CBF regulon member with function in cold tolerance by modulating soluble sugar levels. Plant Cell Environ. 2014, 37, 2754–2767. [Google Scholar] [CrossRef]
- Khan, M.; Hu, J.; Dahro, B.; Ming, R.; Zhang, Y.; Wang, Y.; Alhag, A.; Li, C.; Liu, J.H. ERF108 from Poncirus trifoliata (L.) Raf. functions in cold tolerance by modulating raffinose synthesis through transcriptional regulation of PtrRafS. Plant J. 2021, 108, 705–724. [Google Scholar] [CrossRef] [PubMed]
- Dahro, B.; Wang, Y.; Khan, M.; Zhang, Y.; Fang, T.; Ming, R.; Li, C.; Liu, J.H. Two AT-Hook proteins regulate A/NINV7 expression to modulate sucrose catabolism for cold tolerance in Poncirus trifoliata. New Phytol. 2022, 235, 2331–2349. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Bredeson, J.V.; Wu, G.A.; Shu, S.; Rawat, N.; Du, D.; Parajuli, S.; Yu, Q.; You, Q.; Rokhsar, D.S. A chromosome-scale reference genome of trifoliate orange (Poncirus trifoliata) provides insights into disease resistance, cold tolerance and genome evolution in Citrus. Plant J. 2020, 104, 1215–1232. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; You, X.S.; Guo, L.; Zhong, B.L.; Mi, L.F.; Chen, J.M.; Xiao, X. Transcriptome analysis of Chongyi wild mandarin, a wild species more cold-tolerant than Poncirus trifoliata, reveals key pathways in response to cold. Environ. Exp. Bot. 2021, 184, 104371. [Google Scholar] [CrossRef]
- Primo-Capella, A.; Martínez-Cuenca, M.R.; Gil-Muñoz, F.; Forner-Giner, M.A. Physiological characterization and proline route genes quantification under long-term cold stress in Carrizo citrange. Sci. Hortic. 2021, 276, 109744. [Google Scholar] [CrossRef]
- Primo-Capella, A.; Forner-Giner, M.Á.; Martínez-Cuenca, M.R.; Terol, J. Comparative transcriptomic analyses of citrus cold-resistant vs. sensitive rootstocks might suggest a relevant role of ABA signaling in triggering cold scion adaption. BMC Plant Biol. 2022, 22, 209. [Google Scholar] [CrossRef]
- Oustric, J.; Morillon, R.; Luro, F.; Herbette, S.; Lourkisti, R.; Giannettini, J.; Berti, L.; Santini, J. Tetraploid Carrizo citrange rootstock (Citrus sinensis Osb.× Poncirus trifoliata L. Raf.) enhances natural chilling stress tolerance of common clementine (Citrus clementina Hort. ex Tan). J. Plant Physiol. 2017, 214, 108–115. [Google Scholar] [CrossRef]
- Huang, Y.; Si, Y.; Dane, F. Impact of grafting on cold responsive gene expression in Satsuma mandarin (Citrus unshiu). Euphytica 2011, 177, 25–32. [Google Scholar] [CrossRef]
- Kim, M.; Yun, S.K.; Kim, S.S.; Park, Y.; Joa, J.; Han, S.; Shin, K.; Song, K.J. Response of citrus to freezing tolerance differs depending on genotypes and growing conditions. Hortic. Environ. Biotechnol. 2021, 62, 181–189. [Google Scholar] [CrossRef]
- Yelenosky, G. Cold hardiness in citrus. Hortic. Rev. 1985, 7, 201–238. [Google Scholar]
- Martini, X.; Andersen, P. Cold hardy citrus for north Florida. Citrus Ind. 2020, 14, 16–19. Available online: http://citrusindustry.net (accessed on 21 June 2018).
- Sardoei, A.S.; Sharifani, M.; Sarmast, M.K.; Ghasemnejhad, M. Stepwise regression analysis of citrus genotype under cold stress. Gene Cell Tissue. 2023, 10, e126518. [Google Scholar] [CrossRef]
- Salehi Sardoei, A.; Sharifani, M.M.; Khoshhal Sarmast, M.; Ghasemnejad, M. Screening Citrus Cultivars for Freezing Tolerance by Reliable Methods. Int. J. Hortic. Sci. Technol. 2023, 11, 25–34. [Google Scholar]
- He, L.; Wang, Z.; Song, F.; Wu, L.; Jiang, Y.; Peng, J.; Huang, Y.; Liu, T. A Novel Citrus Cultivar ‘Guijing 2501′ with Cold Tolerance. Acta Hortic. Sinica 2021, 48, 1–2. [Google Scholar]
- Xiao, C.; Zhang, H.; Xie, F.; Pan, Z.Y.; Qiu, W.M.; Tong, Z.; Wang, Z.Q.; He, X.J.; Xu, Y.H.; Sun, Z.H. Evolution, gene expression, and protein-protein interaction analyses identify candidate CBL-CIPK signalling networks implicated in stress responses to cold and bacterial infection in citrus. BMC Plant Biol. 2022, 22, 420. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Bba-bioenergetics 2007, 1767, 414–421. [Google Scholar] [CrossRef]
- Dahro, B.; Wang, F.; Peng, T.; Liu, J.H. PtrA/NINV, an alkaline/neutral invertase gene of Poncirus trifoliata, confers enhanced tolerance to multiple abiotic stresses by modulating ROS levels and maintaining photosynthetic efficiency. BMC Plant Biol. 2016, 16, 76. [Google Scholar] [CrossRef]
- Lourkisti, R.; Froelicher, Y.; Herbette, S.; Morillon, R.; Tomi, F.; Gibernau, M.; Giannettini, J.; Berti, L.; Santini, J. Triploid citrus genotypes have a better tolerance to natural chilling conditions of photosynthetic capacities and specific leaf volatile organic compounds. Front. Plant Sci. 2020, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Oustric, J.; Morillon, R.; Ollitrault, P.; Herbette, S.; Luro, F.; Froelicher, Y.; Tur, I.; Dambier, D.; Giannettini, J.; Berti, L. Somatic hybridization between diploid Poncirus and Citrus improves natural chilling and light stress tolerances compared with equivalent doubled-diploid genotypes. Trees 2018, 32, 883–895. [Google Scholar] [CrossRef]
- Santini, J.; Giannettini, J.; Pailly, O.; Herbette, S.; Ollitrault, P.; Berti, L.; Luro, F. Comparison of photosynthesis and antioxidant performance of several Citrus and Fortunella species (Rutaceae) under natural chilling stress. Trees 2013, 27, 71–83. [Google Scholar] [CrossRef]
- Hutin, C.; Nussaume, L.; Moise, N.; Moya, I.; Kloppstech, K.; Havaux, M. Early light-induced proteins protect Arabidopsis from photooxidative stress. Proc. Natl. Acad. Sci. USA 2003, 100, 4921–4926. [Google Scholar] [CrossRef] [PubMed]
- Hayami, N.; Sakai, Y.; Kimura, M.; Saito, T.; Tokizawa, M.; Iuchi, S.; Kurihara, Y.; Matsui, M.; Nomoto, M.; Tada, Y. The responses of Arabidopsis early light-induced protein 2 to ultraviolet B, high light, and cold stress are regulated by a transcriptional regulatory unit composed of two elements. Plant Physiol. 2015, 169, 840–855. [Google Scholar] [CrossRef] [PubMed]
- Heddad, M.; Norén, H.; Reiser, V.; Dunaeva, M.; Andersson, B.; Adamska, I. Differential expression and localization of early light-induced proteins in Arabidopsis. Plant Physiol. 2006, 142, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Yang, H.; Liang, Y.; Li, X.; Oliver, M.J.; Zhang, D. Functional aspects of early light-induced protein (ELIP) genes from the desiccation-tolerant moss Syntrichia caninervis. Int. J. Mol. Sci. 2020, 21, 1411. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lin, W.; Wei, H.; Krebs, S.L.; Arora, R. Phylogenetic analysis and seasonal cold acclimation-associated expression of early light-induced protein genes of Rhododendron catawbiense. Physiol. Plant. 2008, 132, 44–52. [Google Scholar] [CrossRef]
- Zhuo, C.; Cai, J.; Guo, Z. Overexpression of Early Light-Induced Protein (ELIP) Gene from Medicago sativa ssp. falcata Increases Tolerance to Abiotic Stresses. Agron. J. 2013, 105, 1433–1440. [Google Scholar]
- Lippold, F.; vom Dorp, K.; Abraham, M.; Hölzl, G.; Wewer, V.; Yilmaz, J.L.; Lager, I.; Montandon, C.; Besagni, C.; Kessler, F. Fatty acid phytyl ester synthesis in chloroplasts of Arabidopsis. Plant Cell 2012, 24, 2001–2014. [Google Scholar] [CrossRef]
- Barajas-Lopez, J.d.D.; Tiwari, A.; Zarza, X.; Shaw, M.W.; Pascual, J.; Punkkinen, M.; Bakowska, J.C.; Munnik, T.; Fujii, H. EARLY RESPONSE TO DEHYDRATION 7 remodels cell membrane lipid composition during cold stress in Arabidopsis. Plant Cell Physiol. 2021, 62, 80–91. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Xu, J.; Jian, Y.; Duan, S.; Hu, J.; Jin, L.; Li, G. Overexpression of galactinol synthase 1 from Solanum commersonii (ScGolS1) confers freezing tolerance in transgenic potato. Hortic. Plant J. 2023, 9, 541–552. [Google Scholar] [CrossRef]
- Dai, H.; Zhu, Z.; Wang, Z.; Zhang, Z.; Kong, W.; Miao, M. Galactinol synthase 1 improves cucumber performance under cold stress by enhancing assimilate translocation. Hortic. Res. 2022, 9, uhab063. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Chen, C.; Su, N.; Lyu, W.; Yang, J.; Hu, Z.; Zhang, M. Identification and characterization of a natural SNP variant in ALTERNATIVE OXIDASE gene associated with cold stress tolerance in watermelon. Plant Sci. 2021, 304, 110735. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.D.; Campos, C.; Nogales, A.; Cardoso, H. Carrot AOX2a transcript profile responds to growth and chilling exposure. Plants 2021, 10, 2369. [Google Scholar] [CrossRef] [PubMed]
- Primo-Capella, A.; Martínez-Cuenca, M.R.; Forner-Giner, M.Á. Gene expression under short-term low temperatures: Preliminary screening method to obtain tolerant citrus rootstocks. Horticulturae 2021, 7, 447. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 1–16. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Klopfenstein, D.; Zhang, L.; Pedersen, B.S.; Ramírez, F.; Warwick Vesztrocy, A.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M. GOATOOLS: A Python library for Gene Ontology analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef]
- Lalitha, S. Primer premier 5. Biotech Softw. Internet Rep. 2000, 1, 270–272. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Zhang, X.; Henriques, R.; Lin, S.S.; Niu, Q.W.; Chua, N.H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, C.; He, L.; Qiu, W.; Wang, Z.; He, X.; Xiao, Y.; Sun, Z.; Tong, Z.; Jiang, Y. Guijing2501 (Citrus unshiu) Has Stronger Cold Tolerance Due to Higher Photoprotective Capacity as Revealed by Comparative Transcriptomic and Physiological Analysis and Overexpression of Early Light-Induced Protein. Int. J. Mol. Sci. 2023, 24, 15956. https://doi.org/10.3390/ijms242115956
Xiao C, He L, Qiu W, Wang Z, He X, Xiao Y, Sun Z, Tong Z, Jiang Y. Guijing2501 (Citrus unshiu) Has Stronger Cold Tolerance Due to Higher Photoprotective Capacity as Revealed by Comparative Transcriptomic and Physiological Analysis and Overexpression of Early Light-Induced Protein. International Journal of Molecular Sciences. 2023; 24(21):15956. https://doi.org/10.3390/ijms242115956
Chicago/Turabian StyleXiao, Cui, Ligang He, Wenming Qiu, Zeqiong Wang, Xiujuan He, Yuxiong Xiao, Zhonghai Sun, Zhu Tong, and Yingchun Jiang. 2023. "Guijing2501 (Citrus unshiu) Has Stronger Cold Tolerance Due to Higher Photoprotective Capacity as Revealed by Comparative Transcriptomic and Physiological Analysis and Overexpression of Early Light-Induced Protein" International Journal of Molecular Sciences 24, no. 21: 15956. https://doi.org/10.3390/ijms242115956
APA StyleXiao, C., He, L., Qiu, W., Wang, Z., He, X., Xiao, Y., Sun, Z., Tong, Z., & Jiang, Y. (2023). Guijing2501 (Citrus unshiu) Has Stronger Cold Tolerance Due to Higher Photoprotective Capacity as Revealed by Comparative Transcriptomic and Physiological Analysis and Overexpression of Early Light-Induced Protein. International Journal of Molecular Sciences, 24(21), 15956. https://doi.org/10.3390/ijms242115956






