Effect of a Composite Alginate/Grape Pomace Extract Packaging Material for Improving Meat Storage
Abstract
:1. Introduction
2. Results and Discussions
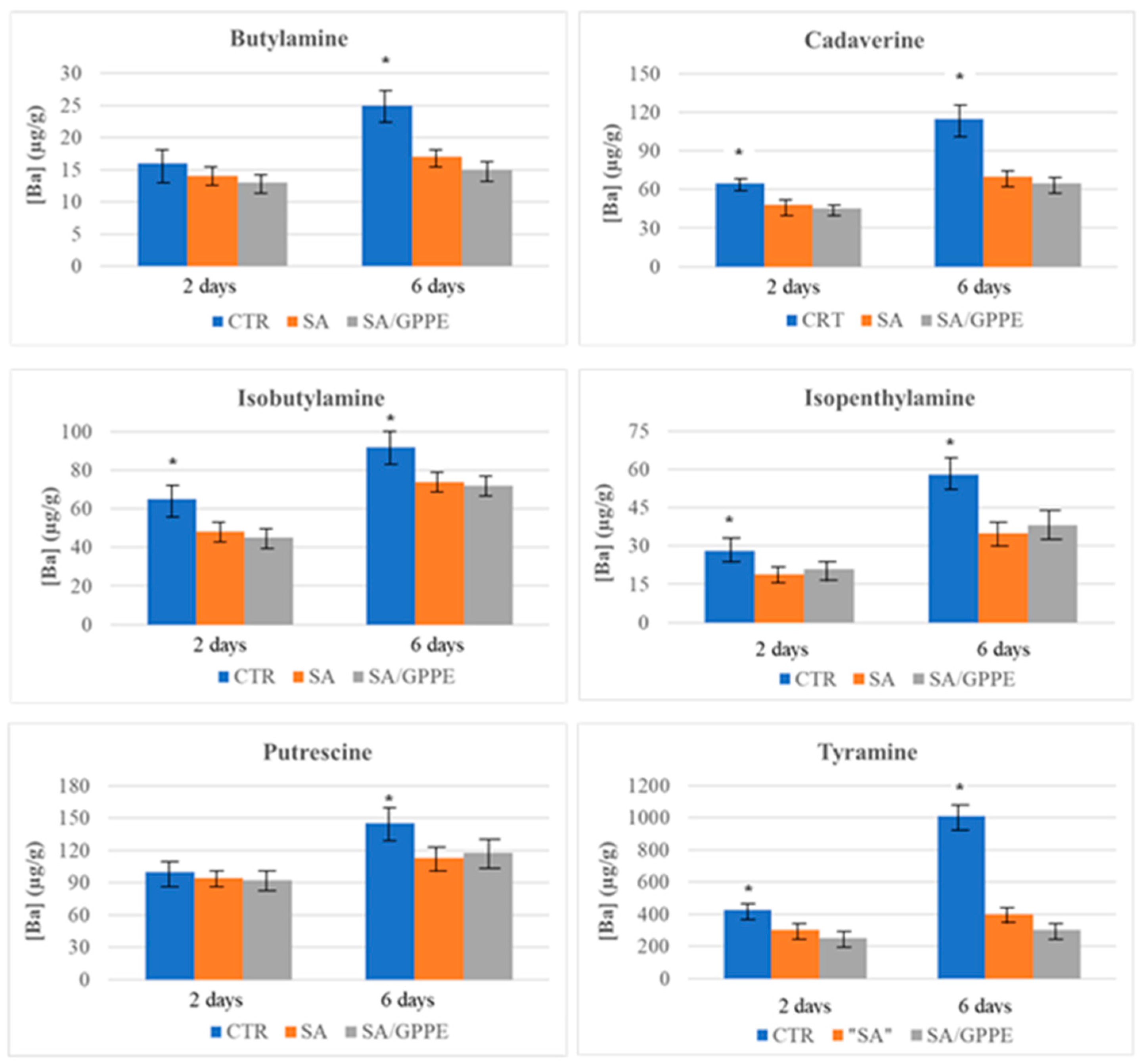
2.1. SPME/GC–MS Analysis for BAs Detection
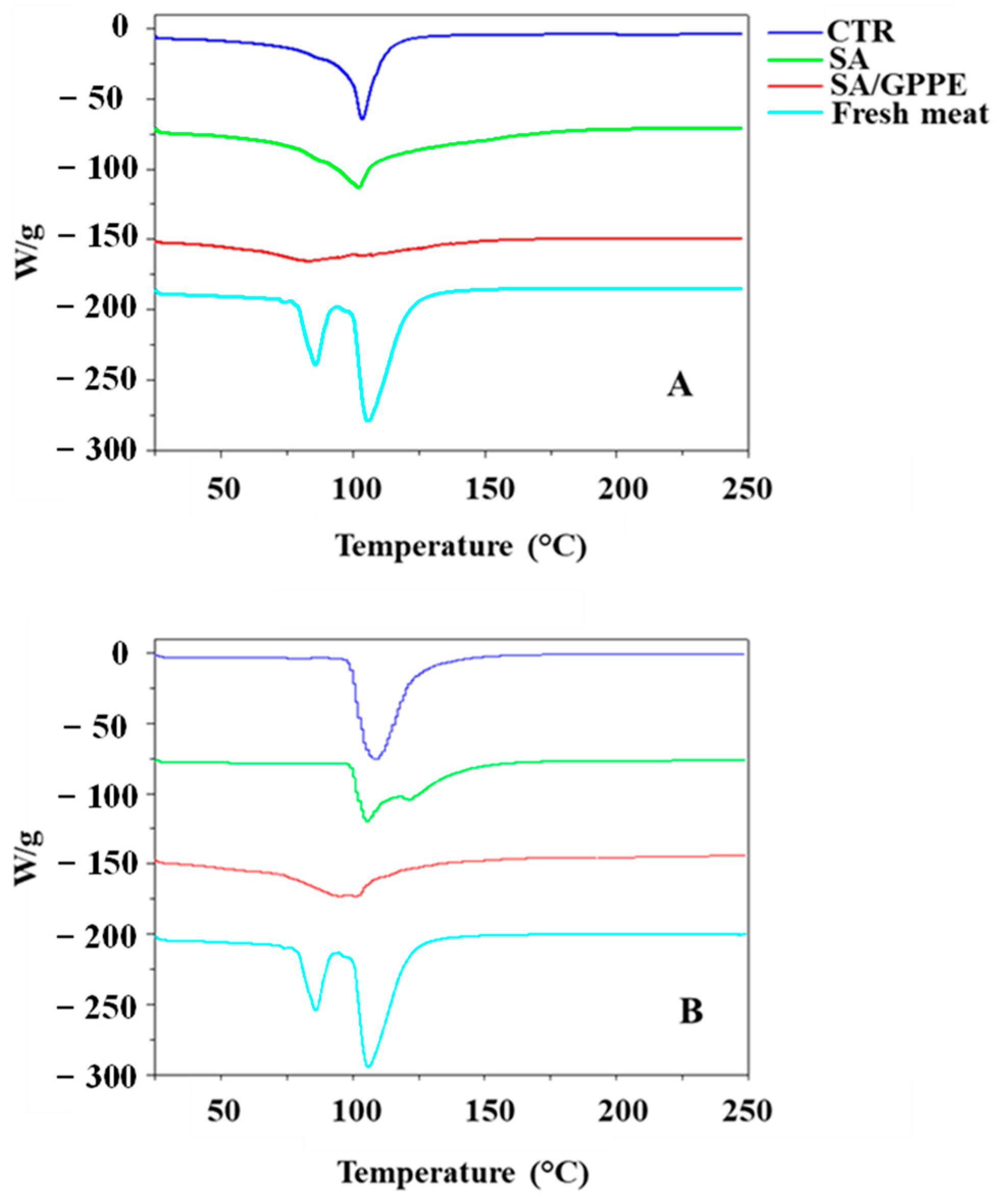
2.2. DSC Analysis: Assessment of the Beef Meat Samples Aging
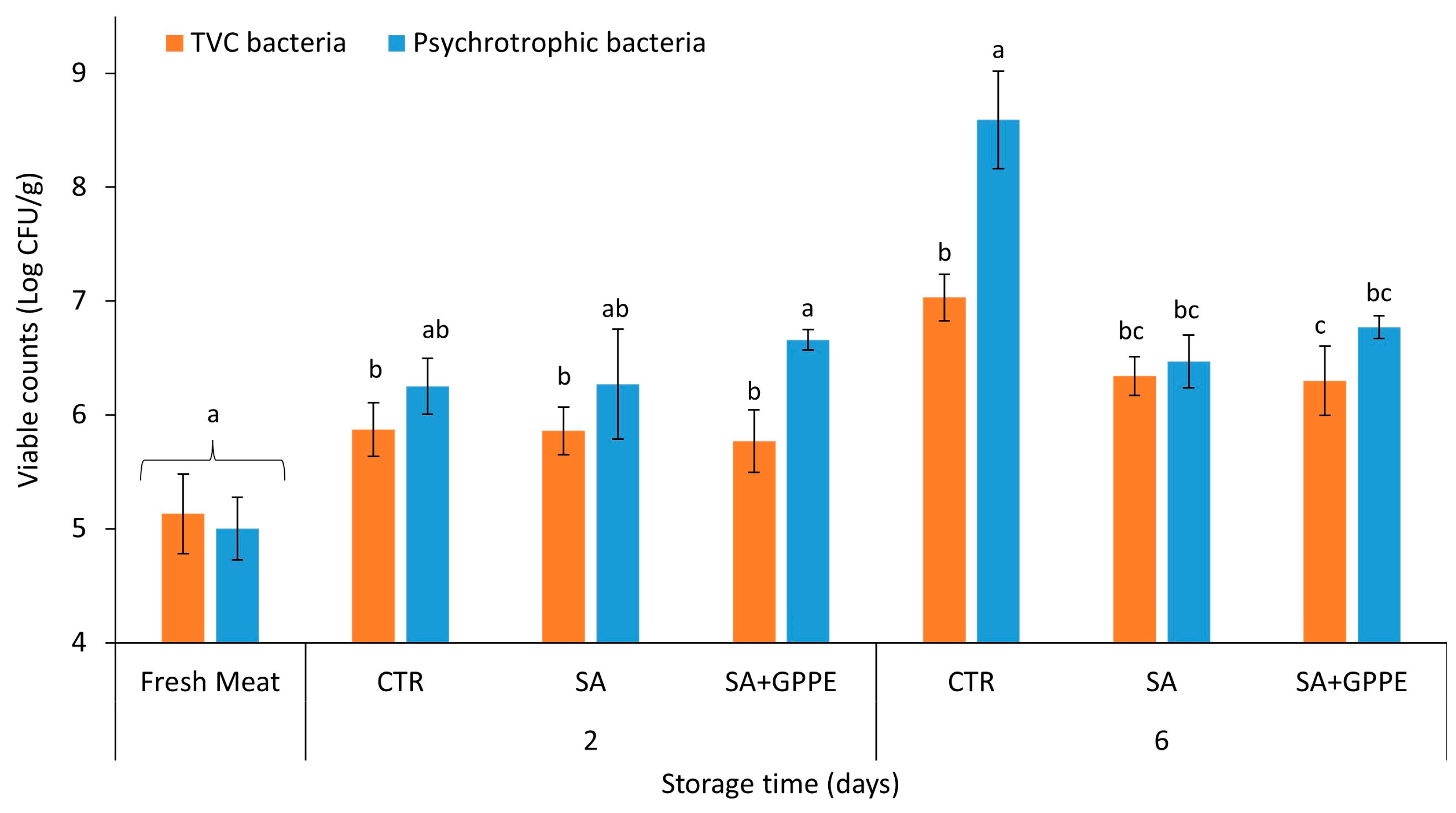
2.3. Microbiological Evaluation of Beef Meat Samples
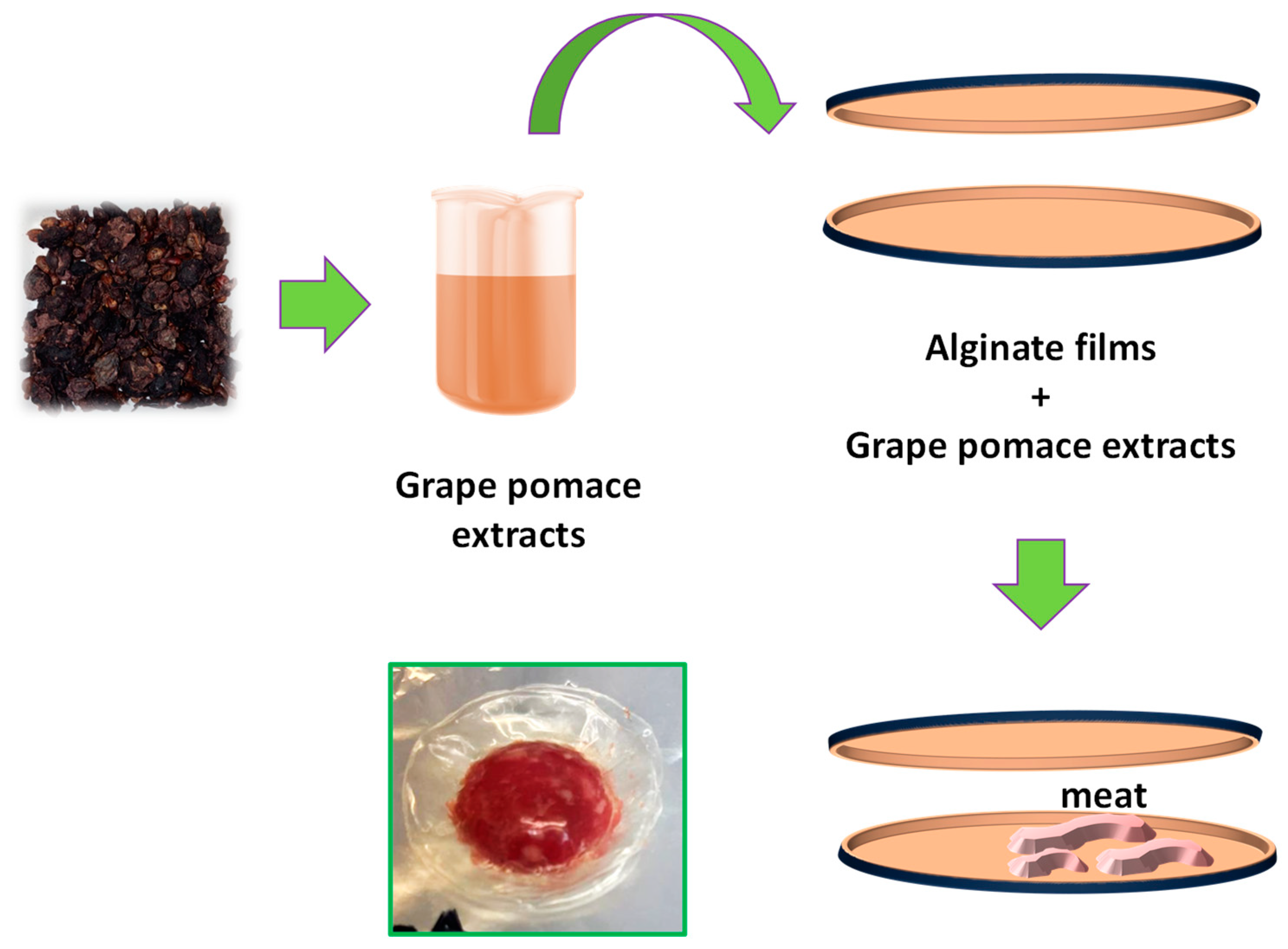
3. Materials and Methods
3.1. Active Packaging Coatings
3.1.1. Chemicals
3.1.2. GPPE Extraction
3.1.3. SA and SA/GPPE Film Preparation
3.2. BAs Determination
3.2.1. Chemicals
3.2.2. SPME/GC–MS Protocol
3.3. Real Samples
- -
- SPME/GC–MS sample: 0.25 g of beef meat was weighed directly in 15 mL vials (Sigma-Aldrich) and spiked with 0.15 mL of IS solution (0.2 mg/mL). Then, 5 mL of 2,4,6-trichloroanisole (TCA) 0.3 N was added in each vial, and a homogenized was readily obtained using Homogenizer Lab Gen 7 (Cole-Parmer Instrument Company, Vernon Hills, IL, USA) operated to a medium speed for 5 min in ice. The homogenized was centrifuged at 2.370 g for 5 min at 22 °C (Hermele Z216MK, Labor Technik, Wasserburg, Bodensee, Germany). The supernatant was transferred in a hermetically sealed sterile vial. Afterward, 0.05 mL was transferred into the sealed vial for the quantitative evolution study, following the SPME/GC–MS protocol (Section 3.2.2).
- -
- pH measurements: 0.25 g of beef meat were homogenized in 10 mL of distilled water under constant stirring. Measurements were performed at each storage time (0, 2, and 6 days) for all samples wrapped in the three different types of selected protective films using a calibrated pH meter (Hanna Instruments, Padova, Italy).
- -
- DSC analysis: experiments were performed with a Q200 instrument (TA Instruments, New Castle, DE, USA) in the range from 25 °C to 300 °C, according to experimental conditions described in [30].
3.4. Microbiological Analysis
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bardòcz, S. Polyamine and their consequences for food quality and human health. Trends Food Sci. Technol. 1995, 6, 341–346. [Google Scholar] [CrossRef]
- Rice, S.L.; Eitenmiller, R.R.; Koehler, P.E. Biologically active amines in food: A review. J. Milk Food Technol. 1976, 39, 353–358. [Google Scholar] [CrossRef]
- Ten Brink, B.; Damink, C.; Joosten, H.M.L.J.; Huisin’t Veld, J.H.J. Occurance and formation of biologically amines in food. Internat. J. Food Microbiol. 1976, 11, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, A.R. Significance of biogenic amines to food safety and human health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- Nevijo, Z.; Bogdanović, T.; Severin, K.; Dobranić, V.; Kazazić, S.; Grbavac, J.; Pleadin, J.; Petričević, S.; Kiš, M. Biogenic amine content in retailed cheese varieties produced with commercial bacterial or mold cultures. Processes 2022, 10, 10. [Google Scholar] [CrossRef]
- Schirone, M.; Esposito, L.; D’Onofrio, F.; Visciano, P.; Martuscelli, M.; Mastrocola, D.; Paparella, A. Biogenic amines in meat and meat products: A review of the science and future perspectives. Foods 2022, 11, 788. [Google Scholar] [CrossRef]
- Gale, E.F. The bacterial amino acid decarboxylase. Adv. Enzymol. 1946, 6, 1–32. [Google Scholar] [CrossRef]
- Ekici, K.; Omer, A.K. Biogenic amines formation and their importance in fermented foods. BIO Web Conf. 2020, 17, 00232. [Google Scholar] [CrossRef]
- Halàsz, A.; Baràth, A.; Simon-Sarkadi, L.; Holzapfel, W. Biogenic amines and their production by micro-organisms in food. Trends Food Sci. Technol. 1994, 5, 42–48. [Google Scholar] [CrossRef]
- Silla Santos, M.H. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- McKee, J.M. Butylamines. In Reference Module in Biomedical Sciences, Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 589–594. [Google Scholar] [CrossRef]
- Kopacic, S.; Walzl, A.; Zankel, A.; Leitner, E.; Bauer, W. Alginate and chitosan as a functional barrier for paper-based packaging materials. Coatings 2018, 8, 235. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Marasciulo, C.; Maggi, F.; Caprioli, G.; Mustafa, A.M.; Fini, P.; De Vietro, N.; Aresta, A.M.; Cosma, P. Realizing eco-friendly water-resistant sodium-alginate-based films blended with a polyphenolic aqueous extract from grape pomace waste for potential food packaging applications. Int. J. Mol. Sci. 2023, 24, 11462. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Laurenzana, A.; Scavone, F.; Frediani, E.; Fibbi, G.; Fanelli, F.; Sibillano, T.; Giannini, C.; Fini, P.; et al. The “end life” of the grape pomace waste become the new beginning: The development of a virtuous cycle for the green synthesis of gold nanoparticles and removal of emerging contaminants from water. Antioxidants 2022, 11, 994. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Sameen, D.E.; Zeng, J.; Li, S.; Qin, W.; Liu, Y. An overview of tea polyphenols as bioactive agents for food packaging applications. LWT 2022, 167, 113845. [Google Scholar] [CrossRef]
- Hesham, M.; Ahmed, M.Y.; Nabila, A.D.; Ahmed, I.A.-K. Eco-friendly polymer composites for green packaging: Future vision and challenges. Compos. Part B Eng. 2019, 172, 16–25. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2021, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Önal, A. A review: Current analytical methods for the determination of biogenic amines in foods. Food Chem. 2007, 103, 1475–1486. [Google Scholar] [CrossRef]
- Aresta, A.; Cotugno, P.; De Vietro, N.; Longo, C.; Mercurio, M.; Ferriol, P.; Zambonin, C.; Nonnis Marzano, C. Volatile organic compounds, indole, and biogenic amines assessment in two mediterranean irciniidae (Porifera, Demospongiae). Mar. Drugs 2021, 19, 711. [Google Scholar] [CrossRef]
- Jastrzębska, A. A comparative study for determination of biogenic amines in meat samples by capillary isotachophoresis with two electrolyte systems. Eur. Food Res. Technol. 2012, 235, 563–572. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, W.; Yang, K.; Li, C. Effects of Sodium Alginate Edible Coating with Cinnamon Essential Oil Nanocapsules and Nisin on Quality and Shelf Life of Beef Slices during Refrigeration. J. Food Prot. 2022, 85, 896–905. [Google Scholar] [CrossRef]
- Tadeusz, K.; Krzysztoforski, K.; Palka, K. The effect of post-mortem ageing and heating on water retention in bovine muscles. Meat Sci. 2007, 75, 655–660. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 338, 1–29. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32005R2073&from=EN (accessed on 25 October 2023).
- Brooks, J.C.; Alvarado, M.; Stephens, T.P.; Kellermeier, J.D.; Tittor, A.W.; Miller, M.F.; Brashears, M.M. Spoilage and safety characteristics of ground beef packaged in traditional and modified atmosphere packages. J. Food Prot. 2008, 71, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.S.; Ju, T.; Yang, X.; Gänzle, M. A Meta-Analysis of Bacterial Communities in Food Processing Facilities: Driving Forces for Assembly of Core and Accessory Microbiomes across Different Food Commodities. Microorganisms 2023, 11, 1575. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Tabatabaei Yazdi, F.; Shahidi, F.; Ali Mortazavi, S.; Mohebbi, M. Principle component analysis (PCA) for investigation of relationship between population dynamics of microbial pathogenesis, chemical and sensory characteristics in beef slices containing Tarragon essential oil. Microb. Pathog. 2017, 105, 37–50. [Google Scholar] [CrossRef]
- Wickramasinghe, N.N.; Ravensdale, J.; Coorey, R.; Chandry, S.P.; Dykes, G.A. The Predominance of Psychrotrophic Pseudomonads on Aerobically Stored Chilled Red Meat. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1622–1635. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Namieśnik, J.; Płotka-Wasylka, J. Direct solid phase microextraction combined with gas chromatography–Mass spectrometry for the determination of biogenic amines in wine. Talanta 2018, 183, 276–282. [Google Scholar] [CrossRef]
- Rizzi, V.; Fiorini, F.; Lamanna, G.; Gubitosa, J.; Prasetyanto, E.A.; Fini, P.; Fanelli, F.; Nacci, A.; De Cola, L.; Cosma, P. Polyamidoamine-based hydrogel for removal of blue and red dyes from wastewater. Adv. Sustain. Syst. 2018, 2, 1700146. [Google Scholar] [CrossRef]
- Dilara Konuk, T.; Figen, K. Active packaging films as a carrier of black cumin essential oil: Development and effect on quality and shelf-life of chicken breast meat. Food Packag. Shelf Life 2019, 19, 210–217. [Google Scholar] [CrossRef]
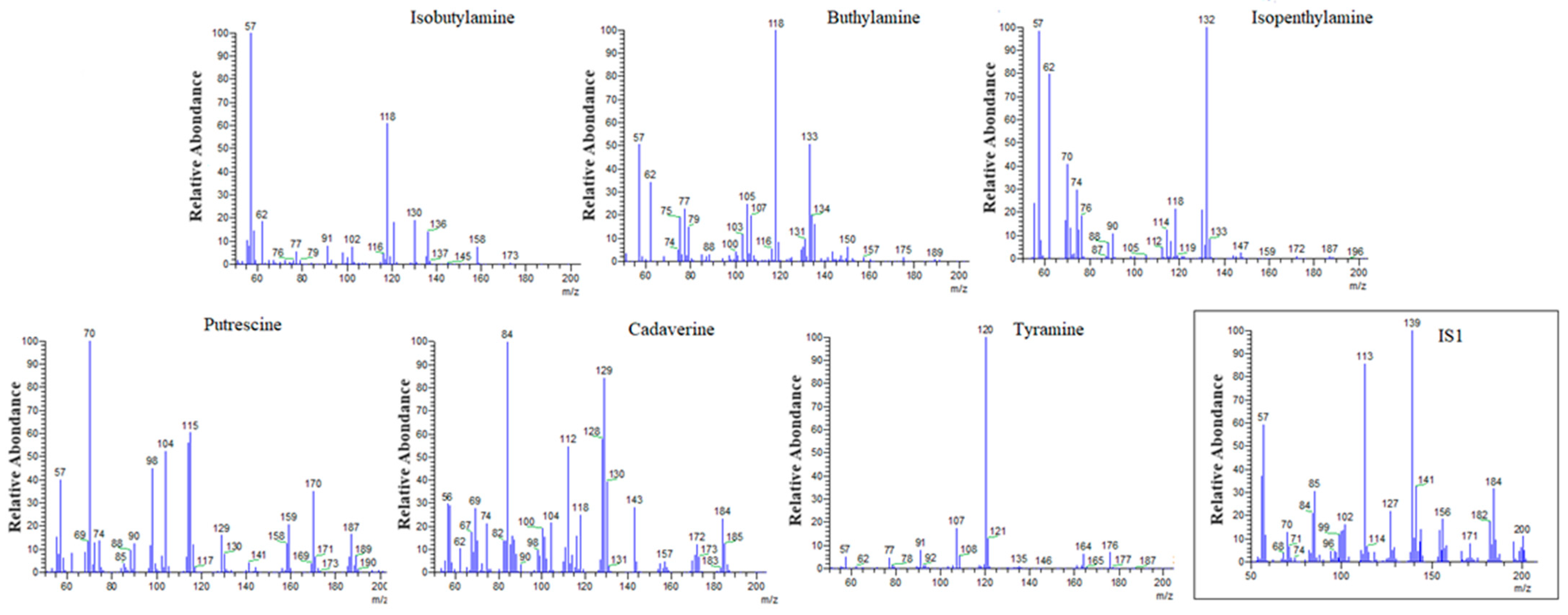

| BAs | RT | Ion (m/z) |
|---|---|---|
| Butylamine | 6.71 ± 0.05 | 118 + 130 |
| Cadaverine | 12.92 ± 0.04 | 84 + 130 |
| Isobutylamine | 6.02 ± 0.07 | 118 + 130 |
| Isopenthylamine | 7.44 ± 0.08 | 118 + 132 |
| Putrescine | 12.58 ± 0.09 | 130 + 170 |
| Tyramine | 14.18 ± 0.05 | 107 + 120 |
| 1,7-diaminoheptane (IS) | 14.01 ± 0.05 | 112 + 130 |
| BAs | Slope | Corr. Coefficient | LOD (μg/mL) | LOQ (μg/mL) | Concentration Levels (μg/mL) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Within-Day | Between-Days | |||||||||
| 0.050 | 0.25 | 2.5 | 0.05 | 0.25 | 2.5 | |||||
| Butylamine | 1 × 106 | 0.9998 | 0.006 | 0.019 | 12.4% | 3.8% | 3.5% | 15.3% | 4.7% | 5.0% |
| Cadaverine | 5 × 106 | 0.9996 | 0.020 | 0.067 | 2.2% | 3.7% | 4.2% | 2.9% | 4.3% | 5.0% |
| Isobutylamine | 1 × 106 | 0.9981 | 0.023 | 0.076 | 18.3% | 5.2% | 5.8% | 18.2% | 6.0% | 8.1% |
| Isopenthylamine | 1 × 106 | 0.9994 | 0.009 | 0.030 | 15.1% | 3.9% | 4.0% | 16.1% | 4.0% | 4.2% |
| Putrescine | 3 × 105 | 0.9940 | 0.044 | 0.146 | 5.0% | 3.5% | 3.7% | 5.5% | 3.4% | 3.8% |
| Tyramine | 4 × 106 | 0.9998 | 0.003 | 0.010 | nd | nd | 14.1% | nd | nd | 15.3% |
| BAs | Estimated Concentration (μg/g ± RSD) | LOD (μg/g) | LOQ (μg/g) | Concentration Levels (μg/g) | |||
|---|---|---|---|---|---|---|---|
| Twice Times LOQ or Estimated Concentration | Five Times LOQ or Estimated Concentration | ||||||
| Rec. % | RSD % | Rec. % | RSD % | ||||
| Butylamine | nd | 3.7 | 12.5 | 90.1 ± 2.2 | 10.0 | 94.3 ± 3.5 | 6.0 |
| Cadaverine | nd | 12.5 | 41.6 | 81.9 ± 3.5 | 18.0 | 86.8 ± 2.7 | 14.0 |
| Isobutylamine | LOD | 14.3 | 47.8 | 90.4 ± 2.7 | 10.0 | 99.0 ± 5.5 | 0.7 |
| Isopenthylamine | nd | 5.6 | 18.7 | 90.8 ± 3.2 | 9.3 | 93.1 ± 2.7 | 6.8 |
| Putrescine | nd | 27.5 | 91.6 | 82.2 ± 4.8 | 17.8 | 83.4 ± 4.2 | 16.6 |
| Tyramine | 31.25 ± 1.8 | 1.8 | 6.2 | 82.3 ± 4.9 | 19.0 | 88.9 ± 3.8 | 11.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aresta, A.M.; De Vietro, N.; Gubitosa, J.; Rizzi, V.; De Pasquale, I.; Fini, P.; Cosma, P.; Curri, M.L.; Zambonin, C. Effect of a Composite Alginate/Grape Pomace Extract Packaging Material for Improving Meat Storage. Int. J. Mol. Sci. 2023, 24, 15958. https://doi.org/10.3390/ijms242115958
Aresta AM, De Vietro N, Gubitosa J, Rizzi V, De Pasquale I, Fini P, Cosma P, Curri ML, Zambonin C. Effect of a Composite Alginate/Grape Pomace Extract Packaging Material for Improving Meat Storage. International Journal of Molecular Sciences. 2023; 24(21):15958. https://doi.org/10.3390/ijms242115958
Chicago/Turabian StyleAresta, Antonella Maria, Nicoletta De Vietro, Jennifer Gubitosa, Vito Rizzi, Ilaria De Pasquale, Paola Fini, Pinalysa Cosma, Maria Lucia Curri, and Carlo Zambonin. 2023. "Effect of a Composite Alginate/Grape Pomace Extract Packaging Material for Improving Meat Storage" International Journal of Molecular Sciences 24, no. 21: 15958. https://doi.org/10.3390/ijms242115958
APA StyleAresta, A. M., De Vietro, N., Gubitosa, J., Rizzi, V., De Pasquale, I., Fini, P., Cosma, P., Curri, M. L., & Zambonin, C. (2023). Effect of a Composite Alginate/Grape Pomace Extract Packaging Material for Improving Meat Storage. International Journal of Molecular Sciences, 24(21), 15958. https://doi.org/10.3390/ijms242115958













