Melatonin Exerts Prominent, Differential Epidermal and Dermal Anti-Aging Properties in Aged Human Eyelid Skin Ex Vivo
Abstract
:1. Introduction
2. Results
2.1. Melatonin Downregulates mTORC1 Activity in Aged Human Skin Ex Vivo
2.2. Melatonin Modulates Expression of MMP-1 and COL17A1 in Skin Tissue
2.3. Melatonin Improves Mitochondrial Marker Expression
2.4. Melatonin Unfolds Differential Anti-Aging Activities in Human Skin: No Effect on Epidermal Lamin B1, Sirtuin-1, and Aging-Associated DNA Damage
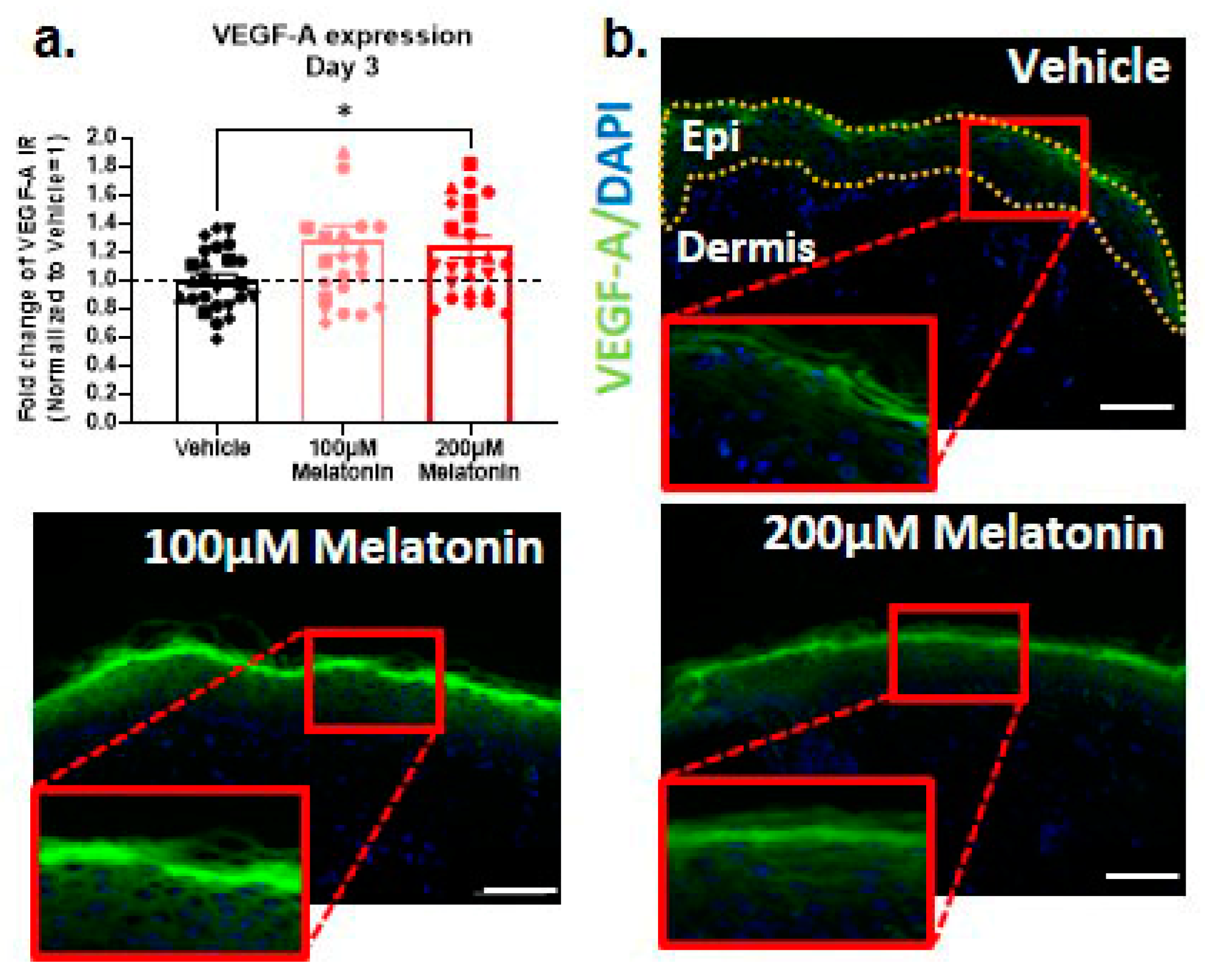
2.5. Melatonin also Promotes Intraepidermal Production of VEGF-A, the Key Driver of Skin Rejuvenation, Ex Vivo
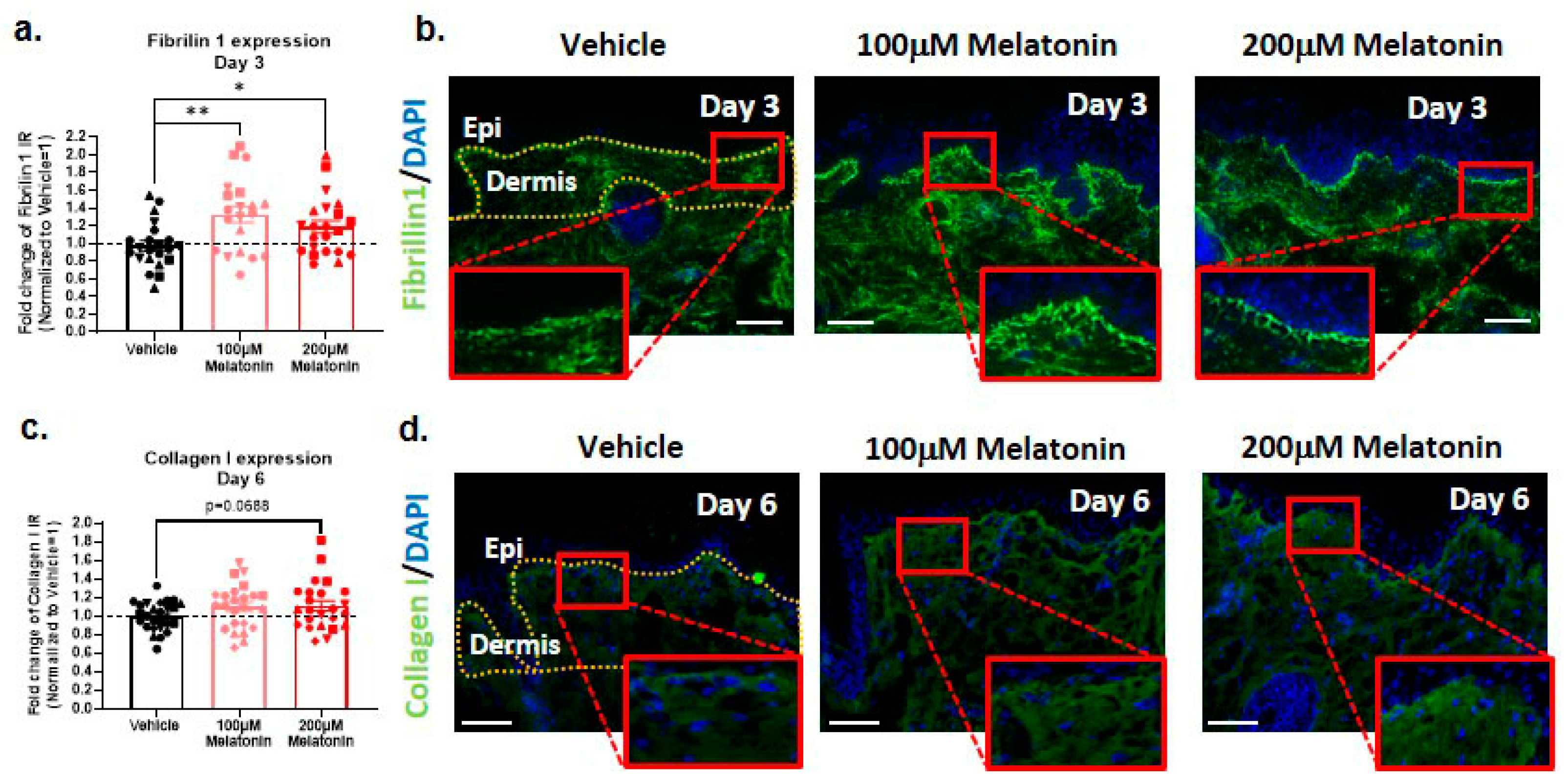
2.6. Melatonin Increases Dermal Fibrillin-1 and Collagen I Content
3. Discussion
4. Materials and Methods
4.1. Eyelid Skin Organ Culture
4.2. Immunohistochemical Staining
4.3. Image Analysis
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gruber, F.; Kremslehner, C.; Eckhart, L.; Tschachler, E. Cell Aging and Cellular Senescence in Skin Aging—Recent Advances in Fibroblast and Keratinocyte Biology. Exp. Gerontol. 2020, 130, 110780. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wan, F.; Su, W.; Xie, W. Research Progress on Skin Aging and Active Ingredients. Molecules 2023, 28, 5556. [Google Scholar] [CrossRef] [PubMed]
- Keren, A.; Bertolini, M.; Keren, Y.; Ullmann, Y.; Paus, R.; Gilhar, A. Human Organ Rejuvenation by VEGF-A: Lessons from the Skin. Sci. Adv. 2022, 8, eabm6756. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Kim, M. Structural and Functional Changes and Possible Molecular Mechanisms in Aged Skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Bulfone-Paus, S.; Griffiths, C.E.M.; Watson, R.E.B. Inflammaging and the Skin. J. Investig. Dermatol. 2021, 141, 1087–1095. [Google Scholar] [CrossRef]
- da Silva, P.F.L.; Schumacher, B. Principles of the Molecular and Cellular Mechanisms of Aging. J. Investig. Dermatol. 2021, 141, 951–960. [Google Scholar] [CrossRef]
- Stone, R.C.; Aviv, A.; Paus, R. Telomere Dynamics and Telomerase in the Biology of Hair Follicles and Their Stem Cells as a Model for Aging Research. J. Investig. Dermatol. 2021, 141, 1031–1040. [Google Scholar] [CrossRef]
- Zou, Z.; Long, X.; Zhao, Q.; Zheng, Y.; Song, M.; Ma, S.; Jing, Y.; Wang, S.; He, Y.; Esteban, C.R.; et al. A Single-Cell Transcriptomic Atlas of Human Skin Aging. Dev. Cell 2021, 56, 383–397.e8. [Google Scholar] [CrossRef]
- Hughes, M.C.B.; Williams, G.M.; Pageon, H.; Fourtanier, A.; Green, A.C. Dietary Antioxidant Capacity and Skin Photoaging: A 15-Year Longitudinal Study. J. Investig. Dermatol. 2021, 141, 1111–1118.e2. [Google Scholar] [CrossRef]
- Goldberg, D.J.; Robinson, D.M.; Granger, C. Clinical Evidence of the Efficacy and Safety of a New 3-in-1 Anti-Aging Topical Night Serum-in-Oil Containing Melatonin, Bakuchiol, and Ascorbyl Tetraisopalmitate: 103 Females Treated from 28 to 84 Days. J. Cosmet. Dermatol. 2019, 18, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Cristel, R.T.; Branham, G.H. Evidence-Based Medicine for Lower Facial Rejuvenation. Facial Plast. Surg. 2023, 39, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.-Y.; Chen, H.; Wang, D.-H.; Huang, C.-X.; Tong, Y.-G.; Chen, Y.-F.; Zhou, R.-Y.; Huang, R.-L. Melatonin Regulates the Ovarian Function and Enhances Follicle Growth in Aging Laying Hens via Activating the Mammalian Target of Rapamycin Pathway. Poult. Sci. 2020, 99, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.S.; Ong, P.F.; Chojnowski, A.; Clavel, C.; Dreesen, O. Loss of Lamin B1 Is a Biomarker to Quantify Cellular Senescence in Photoaged Skin. Sci. Rep. 2017, 7, 15678. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.-K.; Böhm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczyński, K.; Slominski, A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef]
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A Cutaneous Perspective on Its Production, Metabolism, and Functions. J. Investig. Dermatol. 2018, 138, 490–499. [Google Scholar] [CrossRef]
- Grunewald, M.; Kumar, S.; Sharife, H.; Volinsky, E.; Gileles-Hillel, A.; Licht, T.; Permyakova, A.; Hinden, L.; Azar, S.; Friedmann, Y.; et al. Counteracting Age-Related VEGF Signaling Insufficiency Promotes Healthy Aging and Extends Life Span. Science 2021, 373, eabc8479. [Google Scholar] [CrossRef]
- Witte, S.; Boshnakovska, A.; Özdemir, M.; Chowdhury, A.; Rehling, P.; Aich, A. Defective COX1 Expression in Aging Mice Liver. Biol. Open 2023, 12, bio059844. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Adjaye, J.; Akamatsu, H.; Moe-Behrens, G.; Niemann, C. Human Skin Stem Cells and the Ageing Process. Exp. Gerontol. 2008, 43, 986–997. [Google Scholar] [CrossRef]
- Gerber, P.A.; Buhren, B.A.; Schrumpf, H.; Hevezi, P.; Bölke, E.; Sohn, D.; Jänicke, R.U.; Belum, V.R.; Robert, C.; Lacouture, M.E.; et al. Mechanisms of Skin Aging Induced by EGFR Inhibitors. Support. Care Cancer 2016, 24, 4241–4248. [Google Scholar] [CrossRef]
- Baris, O.R.; Klose, A.; Kloepper, J.E.; Weiland, D.; Neuhaus, J.F.G.; Schauen, M.; Wille, A.; Müller, A.; Merkwirth, C.; Langer, T.; et al. The Mitochondrial Electron Transport Chain Is Dispensable for Proliferation and Differentiation of Epidermal Progenitor Cells. Stem Cells 2011, 29, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.T.; Kolesar, J.E.; Kaufman, B.A. Mitochondrial Transcription Factor A Regulates Mitochondrial Transcription Initiation, DNA Packaging, and Genome Copy Number. Biochim. Biophys. Acta BBA—Gene Regul. Mech. 2012, 1819, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.E.; Baris, O.R.; Reuter, K.; Kobayashi, K.; Weiland, D.; Vidali, S.; Tobin, D.J.; Niemann, C.; Wiesner, R.J.; Paus, R. Mitochondrial Function in Murine Skin Epithelium Is Crucial for Hair Follicle Morphogenesis and Epithelial–Mesenchymal Interactions. J. Investig. Dermatol. 2015, 135, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Mah, L.-J.; El-Osta, A.; Karagiannis, T.C. ΓH2AX: A Sensitive Molecular Marker of DNA Damage and Repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef]
- Linowiecka, K.; Slominski, A.T.; Reiter, R.J.; Böhm, M.; Steinbrink, K.; Paus, R.; Kleszczyński, K. Melatonin: A Potential Regulator of DNA Methylation. Antioxidants 2023, 12, 1155. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. MTOR Signaling Pathway and MTOR Inhibitors in Cancer: Progress and Challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Samra, T.; Chéret, J.; Gherardini, J.; Verling, S.; Kassir, R.; Paus, R. Melatonin Protects K15+ Human Hair Follicle Stem Cells and Hair Matrix Keratinocytes against Paclitaxel-Induced Damage Ex Vivo. J. Investig. Dermatol. 2023. ahead of print. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of P16Ink4a-Positive Senescent Cells Delays Ageing-Associated Disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Desdín-Micó, G.; Soto-Heredero, G.; Aranda, J.F.; Oller, J.; Carrasco, E.; Gabandé-Rodríguez, E.; Blanco, E.M.; Alfranca, A.; Cussó, L.; Desco, M.; et al. T Cells with Dysfunctional Mitochondria Induce Multimorbidity and Premature Senescence. Science 2020, 368, 1371–1376. [Google Scholar] [CrossRef]
- Manczak, M.; Jung, Y.; Park, B.S.; Partovi, D.; Reddy, P.H. Time-Course of Mitochondrial Gene Expressions in Mice Brains: Implications for Mitochondrial Dysfunction, Oxidative Damage, and Cytochrome c in Aging. J. Neurochem. 2005, 92, 494–504. [Google Scholar] [CrossRef]
- Krishnamurthy, J.; Torrice, C.; Ramsey, M.R.; Kovalev, G.I.; Al-Regaiey, K.; Su, L.; Sharpless, N.E. Ink4a/Arf Expression Is a Biomarker of Aging. J. Clin. Investig. 2004, 114, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini Denchi, E.; Attwooll, C.; Pasini, D.; Helin, K. Deregulated E2F Activity Induces Hyperplasia and Senescence-like Features in the Mouse Pituitary Gland. Mol. Cell. Biol. 2005, 25, 2660–2672. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, A.; Aguilera-Aguirre, L.; Singh, K.K. Mitochondria in Skin Health, Aging, and Disease. Cell Death Dis. 2020, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.W.; Trüeb, R.M.; Hänggi, G.; Innocenti, M.; Elsner, P. Topical Melatonin for Treatment of Androgenetic Alopecia. Int. J. Trichology 2012, 4, 236–245. [Google Scholar] [CrossRef]
- Fischer, T.W.; Greif, C.; Fluhr, J.W.; Wigger-Alberti, W.; Elsner, P. Percutaneous Penetration of Topically Applied Melatonin in a Cream and an Alcoholic Solution. Ski. Pharmacol. Physiol. 2004, 17, 190–194. [Google Scholar] [CrossRef]
- Golubtsova, N.N.; Filippov, F.N.; Gunin, A.G. Lamin B1 and Lamin B2 in Human Skin in the Process of Aging. Adv. Gerontol. 2016, 6, 275–281. [Google Scholar] [CrossRef]
- Prodromidis, G.; Nikitakis, N.G.; Sklavounou, A. Immunohistochemical Analysis of the Activation Status of the Akt/MTOR/PS6 Signaling Pathway in Oral Lichen Planus. Int. J. Dent. 2013, 2013, 743456. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kromminga, A.; Dunlop, T.W.; Tychsen, B.; Conrad, F.; Suzuki, N.; Memezawa, A.; Bettermann, A.; Aiba, S.; Carlberg, C.; et al. A Role of Melatonin in Neuroectodermal-Mesodermal Interactions: The Hair Follicle Synthesizes Melatonin and Expresses Functional Melatonin Receptors. FASEB J. 2005, 19, 1710–1712. [Google Scholar] [CrossRef]
- Sevilla, A.; Chéret, J.; Slominski, R.M.; Slominski, A.T.; Paus, R. Revisiting the Role of Melatonin in Human Melanocyte Physiology: A Skin Context Perspective. J. Pineal Res. 2022, 72, e12790. [Google Scholar] [CrossRef]
- Del Rey, M.J.; Meroño, C.; Municio, C.; Usategui, A.; Mittelbrunn, M.; García-Consuegra, I.; Criado, G.; Pablos, J.L. TFAM-Deficient Mouse Skin Fibroblasts—An Ex Vivo Model of Mitochondrial Dysfunction. Dis. Model. Mech. 2021, 14, dmm048995. [Google Scholar] [CrossRef]
- Kang, H.T.; Lee, K.B.; Kim, S.Y.; Choi, H.R.; Park, S.C. Autophagy Impairment Induces Premature Senescence in Primary Human Fibroblasts. PLoS ONE 2011, 6, e23367. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.-J.; Xu, D.-P.; Li, H.-B. Melatonin for the Prevention and Treatment of Cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin, Its Metabolites and Their Interference with Reactive Nitrogen Compounds. Molecules 2021, 26, 4105. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Böhm, M.; Paus, R. Translational Neuroendocrinology of Human Skin: Concepts and Perspectives. Trends Mol. Med. 2021, 27, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, A.; Chéret, J.; Lee, W.; Paus, R. Concentration-Dependent Stimulation of Melanin Production as Well as Melanocyte and Keratinocyte Proliferation by Melatonin in Human Eyelid Epidermis. Exp. Dermatol. 2023, 32, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Victorelli, S.; Lagnado, A.; Halim, J.; Moore, W.; Talbot, D.; Barrett, K.; Chapman, J.; Birch, J.; Ogrodnik, M.; Meves, A.; et al. Senescent Human Melanocytes Drive Skin Ageing via Paracrine Telomere Dysfunction. EMBO J. 2019, 38, e101982. [Google Scholar] [CrossRef] [PubMed]
- Vidali, S.; Chéret, J.; Giesen, M.; Haeger, S.; Alam, M.; Watson, R.E.B.; Langton, A.K.; Klinger, M.; Knuever, J.; Funk, W.; et al. Thyroid Hormones Enhance Mitochondrial Function in Human Epidermis. J. Investig. Dermatol. 2016, 136, 2003–2012. [Google Scholar] [CrossRef]
- Paus, R.; Burgoa, I.; Platt, C.I.; Griffiths, T.; Poblet, E.; Izeta, A. Biology of the Eyelash Hair Follicle: An Enigma in Plain Sight. Br. J. Dermatol. 2016, 174, 741–752. [Google Scholar] [CrossRef]
- Neimkin, M.G.; Holds, J.B. Evaluation of Eyelid Function and Aesthetics. Facial Plast. Surg. Clin. N. Am. 2016, 24, 97–106. [Google Scholar] [CrossRef]
- Damasceno, R.W.; Avgitidou, G.; Belfort, R.; Dantas, P.E.C.; Holbach, L.M.; Heindl, L.M. Eyelid Aging: Pathophysiology and Clinical Management. Arq. Bras. Oftalmol. 2015, 78, 328–331. [Google Scholar] [CrossRef]
- Suzuki, T.; Chéret, J.; Scala, F.D.; Akhundlu, A.; Gherardini, J.; Demetrius, D.-L.; O’Sullivan, J.D.B.; Kuka Epstein, G.; Bauman, A.J.; Demetriades, C.; et al. MTORC1 Activity Negatively Regulates Human Hair Follicle Growth and Pigmentation. EMBO Rep. 2023, 24, e56574. [Google Scholar] [CrossRef]
- Matsumura, H.; Mohri, Y.; Binh, N.T.; Morinaga, H.; Fukuda, M.; Ito, M.; Kurata, S.; Hoeijmakers, J.; Nishimura, E.K. Hair Follicle Aging Is Driven by Transepidermal Elimination of Stem Cells via COL17A1 Proteolysis. Science 2016, 351, aad4395. [Google Scholar] [CrossRef]
- Liu, N.; Matsumura, H.; Kato, T.; Ichinose, S.; Takada, A.; Namiki, T.; Asakawa, K.; Morinaga, H.; Mohri, Y.; De Arcangelis, A.; et al. Stem Cell Competition Orchestrates Skin Homeostasis and Ageing. Nature 2019, 568, 344–350. [Google Scholar] [CrossRef]
- Nanba, D.; Toki, F.; Asakawa, K.; Matsumura, H.; Shiraishi, K.; Sayama, K.; Matsuzaki, K.; Toki, H.; Nishimura, E.K. EGFR-Mediated Epidermal Stem Cell Motility Drives Skin Regeneration through COL17A1 Proteolysis. J. Cell Biol. 2021, 220, e202012073. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, L.; Fozouni, P.; Evjen, G.; Chandra, V.; Jiang, J.; Lu, C.; Nicastri, M.; Bretz, C.; Winkler, J.D.; et al. SIRT1 Is Downregulated by Autophagy in Senescence and Ageing. Nat. Cell Biol. 2020, 22, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Matias, I.; Diniz, L.P.; Damico, I.V.; Araujo, A.P.B.; Neves, L.D.S.; Vargas, G.; Leite, R.E.P.; Suemoto, C.K.; Nitrini, R.; Jacob-Filho, W.; et al. Loss of Lamin-B1 and Defective Nuclear Morphology Are Hallmarks of Astrocyte Senescence in Vitro and in the Aging Human Hippocampus. Aging Cell 2022, 21, e13521. [Google Scholar] [CrossRef] [PubMed]
- Bedrosian, T.A.; Houtman, J.; Eguiguren, J.S.; Ghassemzadeh, S.; Rund, N.; Novaresi, N.M.; Hu, L.; Parylak, S.L.; Denli, A.M.; Randolph-Moore, L.; et al. Lamin B1 Decline Underlies Age-Related Loss of Adult Hippocampal Neurogenesis. EMBO J. 2021, 40, e105819. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.-P.; Rodier, F.; Patil, C.K.; Freund, A.; Desprez, P.-Y.; Campisi, J. Tumor Suppressor and Aging Biomarker P16INK4a Induces Cellular Senescence without the Associated Inflammatory Secretory Phenotype. J. Biol. Chem. 2011, 286, 36396–36403. [Google Scholar] [CrossRef]
- Wagner, K.-D.; Wagner, N. The Senescence Markers P16INK4A, P14ARF/P19ARF, and P21 in Organ Development and Homeostasis. Cells 2022, 11, 1966. [Google Scholar] [CrossRef]
- Zorin, V.; Grekhova, A.; Pustovalova, M.; Zorina, A.; Smetanina, N.; Vorobyeva, N.; Kopnin, P.; Gilmutdinova, I.; Moskalev, A.; Osipov, A.N.; et al. Spontaneous ΓH2AX Foci in Human Dermal Fibroblasts in Relation to Proliferation Activity and Aging. Aging 2019, 11, 4536–4546. [Google Scholar] [CrossRef]
- Vidali, S.; Knuever, J.; Lerchner, J.; Giesen, M.; Bíró, T.; Klinger, M.; Kofler, B.; Funk, W.; Poeggeler, B.; Paus, R. Hypothalamic-Pituitary-Thyroid Axis Hormones Stimulate Mitochondrial Function and Biogenesis in Human Hair Follicles. J. Investig. Dermatol. 2014, 134, 33–42. [Google Scholar] [CrossRef]
- Wikramanayake, T.C.; Chéret, J.; Sevilla, A.; Birch-Machin, M.; Paus, R. Targeting Mitochondria in Dermatological Therapy: Beyond Oxidative Damage and Skin Aging. Expert Opin. Ther. Targets 2022, 26, 233–259. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.S.; Zuk, A.V.; Sengle, G. The Fibrillin Microfibril/Elastic Fibre Network: A Critical Extracellular Supramolecular Scaffold to Balance Skin Homoeostasis. Exp. Dermatol. 2021, 30, 25–37. [Google Scholar] [CrossRef]
- Katsuyama, Y.; Yamawaki, Y.; Sato, Y.; Muraoka, S.; Yoshida, M.; Okano, Y.; Masaki, H. Decreased Mitochondrial Function in UVA-Irradiated Dermal Fibroblasts Causes the Insufficient Formation of Type I Collagen and Fibrillin-1 Fibers. J. Dermatol. Sci. 2022, 108, 22–29. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Chen, W.; McDermott, J.; Han, J.-D.J. Molecular and Phenotypic Biomarkers of Aging. F1000Research 2017, 6, 860. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular Matrix Regulation of Fibroblast Function: Redefining Our Perspective on Skin Aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef]
- Ivanova, I.; Bogner, C.; Gronwald, W.; Kreutz, M.; Kurz, B.; Maisch, T.; Kamenisch, Y.; Berneburg, M. UVA-Induced Metabolic Changes in Non-Malignant Skin Cells and the Potential Role of Pyruvate as Antioxidant. Photochem. Photobiol. Sci. 2023, 22, 1889–1899. [Google Scholar] [CrossRef]
- Dong, K.K.; Damaghi, N.; Picart, S.D.; Markova, N.G.; Obayashi, K.; Okano, Y.; Masaki, H.; Grether-Beck, S.; Krutmann, J.; Smiles, K.A.; et al. UV-Induced DNA Damage Initiates Release of MMP-1 in Human Skin. Exp. Dermatol. 2008, 17, 1037–1044. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, Y.; Yang, Y.; Yan, Y.; Kim, A.J.; Guo, C.; Fisher, G.J.; Quan, T. Reduced Expression of Collagen 17A1 in Naturally Aged, Photoaged, and UV-Irradiated Human Skin in Vivo: Potential Links to Epidermal Aging. J. Cell Commun. Signal. 2022, 16, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ho, C.; Wen, D.; Sun, J.; Huang, L.; Gao, Y.; Li, Q.; Zhang, Y. Targeting the Stem Cell Niche: Role of Collagen XVII in Skin Aging and Wound Repair. Theranostics 2022, 12, 6446–6454. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Krishnamurthy, S.; Patel, J.; Kim, E.; Baptiste, B.A.; Croteau, D.L.; Bohr, V.A. Skin Abnormalities in Disorders with DNA Repair Defects, Premature Aging, and Mitochondrial Dysfunction. J. Investig. Dermatol. 2021, 141, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Peñaherrera, S.; Ruiz, C.; Castañeda, V.; Livingston, K.; Barba, D.; Burzio, V.A.; Caicedo, A.; Singh, K.K. Exploring the Role of Mitochondria Transfer/Transplant and Their Long-Non-Coding RNAs in Regenerative Therapies for Skin Aging. Mitochondrion 2023, 70, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Berliocchi, L.; Li, Z.; Rasmussen, L.J. Interactions between Mitochondrial Dysfunction and Other Hallmarks of Aging: Paving a Path toward Interventions That Promote Healthy Old Age. Aging Cell 2023, e13942. [Google Scholar] [CrossRef]
- Hu, H.; Guo, L.; Overholser, J.; Wang, X. Mitochondrial VDAC1: A Potential Therapeutic Target of Inflammation-Related Diseases and Clinical Opportunities. Cells 2022, 11, 3174. [Google Scholar] [CrossRef] [PubMed]
- Safwan-Zaiter, H.; Wagner, N.; Wagner, K.-D. P16INK4A—More Than a Senescence Marker. Life 2022, 12, 1332. [Google Scholar] [CrossRef]
- Wang, M.; Guo, Y.; Wan, M.; Chen, Z.; Zhong, J.L. TAZ Reduces UVA-Mediated Photoaging through Regulates Cell Proliferation in Skin Fibroblasts. Photochem. Photobiol. 2023, 99, 153–159. [Google Scholar] [CrossRef]
- Nosrati, F.; Grillari, J.; Azarnia, M.; Nabiuni, M.; Moghadasali, R.; Karimzadeh, L.; Lämmermann, I. The Expression of Fibrosis-Related Genes Is Elevated in Doxorubicin-Induced Senescent Human Dermal Fibroblasts, but Their Secretome Does Not Trigger a Paracrine Fibrotic Response in Non-Senescent Cells. Biogerontology 2023, 24, 293–301. [Google Scholar] [CrossRef]
- You, Y.; Liang, W. SIRT1 and SIRT6: The Role in Aging-Related Diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166815. [Google Scholar] [CrossRef]
- Hao, L.; Nam, K.-H.; Lee, G.-J.; Kim, D.; Shin, J.-M.; Lee, Y.; Kim, C.-D.; Kim, S.-J.; Yun, S.-K.; Park, B.-H.; et al. SIRT1 Downregulation Provokes Immune-Inflammatory Responses in Hair Follicle Outer Root Sheath Cells and May Contribute to Development of Alopecia Areata. J. Dermatol. Sci. 2023, 111, 2–9. [Google Scholar] [CrossRef]
- Singh, A.K.; Peng, B.-Y.; Chien, S.-T.; Chan, C.-H.; Deng, Y.-H.; Pai, H.-Y.; Wei, H.-J.; Wang, M.-F.; Wang, S.-H.; Wu, C.-Y.; et al. Anti-Aging Biomaterial Sturgeon Chondroitin Sulfate Upregulating Anti-Oxidant and SIRT-1/c-Fos Gene Expression to Reprogram Stem Cell Senescence and Prolong Longevity. Biomater. Sci. 2023, 11, 4522–4536. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Li, J.; Qin, R.; Lu, N.; Goltzman, D.; Miao, D.; Yang, R. 1,25-Dihydroxyvitamin D Deficiency Accelerates Aging-Related Osteoarthritis via Downregulation of Sirt1 in Mice. Int. J. Biol. Sci. 2023, 19, 610–624. [Google Scholar] [CrossRef]
- Purba, T.S.; Ng’andu, K.; Brunken, L.; Smart, E.; Mitchell, E.; Hassan, N.; O’Brien, A.; Mellor, C.; Jackson, J.; Shahmalak, A.; et al. CDK4/6 Inhibition Mitigates Stem Cell Damage in a Novel Model for Taxane-Induced Alopecia. EMBO Mol. Med. 2019, 11, e11031. [Google Scholar] [CrossRef] [PubMed]
- Piccini, I.; Brunken, L.; Chéret, J.; Ghatak, S.; Ramot, Y.; Alam, M.; Purba, T.S.; Hardman, J.; Erdmann, H.; Jimenez, F.; et al. Peroxisome Proliferator-Activated Receptor-γ Signalling Protects Hair Follicle Stem Cells from Chemotherapy-Induced Apoptosis and Epithelial-Mesenchymal Transition. Br. J. Dermatol. 2022, 186, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-X.; He, B.-M.; Wu, Y.; Qiao, J.-F.; Peng, Z.-Y. Melatonin Protects against Sepsis-Induced Cardiac Dysfunction by Regulating Apoptosis and Autophagy via Activation of SIRT1 in Mice. Life Sci. 2019, 217, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Moon, J.-H.; Nazim, U.M.; Lee, Y.-J.; Seol, J.-W.; Eo, S.-K.; Lee, J.-H.; Park, S.-Y. Melatonin Protects Skin Keratinocyte from Hydrogen Peroxide-Mediated Cell Death via the SIRT1 Pathway. Oncotarget 2016, 7, 12075–12088. [Google Scholar] [CrossRef]
- Leem, J.; Bai, G.-Y.; Kim, J.-S.; Oh, J.S. Melatonin Protects Mouse Oocytes from DNA Damage by Enhancing Nonhomologous End-Joining Repair. J. Pineal Res. 2019, 67, e12603. [Google Scholar] [CrossRef]
- Abadie, S.; Leduc, C.; Lintner, K.; Bedos, P. The Alkaloid Centcyamine Increases Expression of Klotho and Lamin B1, Slowing the Onset of Skin Ageing In Vitro and In Vivo. Int. J. Cosmet. Sci. 2021, 43, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, C.T. Pro-Angiogenesis Therapy and Aging: A Mini-Review. Gerontology 2017, 63, 393–400. [Google Scholar] [CrossRef]
- Laborda-Illanes, A.; Sánchez-Alcoholado, L.; Castellano-Castillo, D.; Boutriq, S.; Plaza-Andrades, I.; Aranega-Martín, L.; Peralta-Linero, J.; Alba, E.; González-González, A.; Queipo-Ortuño, M.I. Development of in Vitro and in Vivo Tools to Evaluate the Antiangiogenic Potential of Melatonin to Neutralize the Angiogenic Effects of VEGF and Breast Cancer Cells: CAM Assay and 3D Endothelial Cell Spheroids. Biomed. Pharmacother. 2023, 157, 114041. [Google Scholar] [CrossRef]
- Usta Sofu, G.; Erzurumlu, Y.; Karaca, U.; Candan, I.A.; Savran, M.; Asci, H.; Hasseyid, N. Melatonin Receptor Agonist Ramelteon Alleviates Experimental Acute Ocular Inflammation via HIF-1A/VEGF/E-NOS Signaling. Eur. J. Ophthalmol. 2022, 33, 1018–1025. [Google Scholar] [CrossRef]
- Hwang, S.J.; Jung, Y.; Song, Y.-S.; Park, S.; Park, Y.; Lee, H.-J. Enhanced Anti-Angiogenic Activity of Novel Melatonin-like Agents. J. Pineal Res. 2021, 71, e12739. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, A.; Patel, K.; Hoa, N.; Brzozowska, I.; Jones, M.K.; Tarnawski, A.S. Melatonin Ameliorates Aging-Related Impaired Angiogenesis in Gastric Endothelial Cells via Local Actions on Mitochondria and VEGF-Survivin Signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G682–G689. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, D.; Mazzoccoli, G.; Anderson, G.; Linkova, N.; Dyatlova, A.; Mironova, E.; Polyakova, V.; Kvetnoy, I.; Evsyukova, I.; Carbone, A.; et al. Melatonin, Its Beneficial Effects on Embryogenesis from Mitigating Oxidative Stress to Regulating Gene Expression. Int. J. Mol. Sci. 2021, 22, 5885. [Google Scholar] [CrossRef]
- Li, Y.; Fang, L.; Zhang, R.; Wang, S.; Li, Y.; Yan, Y.; Yu, Y.; Cheng, J.-C.; Sun, Y.-P. Melatonin Stimulates VEGF Expression in Human Granulosa-Lutein Cells: A Potential Mechanism for the Pathogenesis of Ovarian Hyperstimulation Syndrome. Mol. Cell. Endocrinol. 2020, 518, 110981. [Google Scholar] [CrossRef]
- Ashworth, J.L.; Murphy, G.; Rock, M.J.; Sherratt, M.J.; Shapiro, S.D.; Shuttleworth, C.A.; Kielty, C.M. Fibrillin Degradation by Matrix Metalloproteinases: Implications for Connective Tissue Remodelling. Biochem. J. 1999, 340, 171–181. [Google Scholar] [CrossRef]
- Sugahara, Y.; Komorisono, M.; Kuwajima, M.; Yoshikawa, S.; Yoshida, S.; Maeda, K. Anti-Skin-Aging Effects of Human Ceramides via Collagen and Fibrillin Expression in Dermal Fibroblasts. Biosci. Biotechnol. Biochem. 2022, 86, 1240–1246. [Google Scholar] [CrossRef]
- Naylor, E.C.; Watson, R.E.B.; Sherratt, M.J. Molecular Aspects of Skin Ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.E.B.; Gibbs, N.K.; Griffiths, C.E.M.; Sherratt, M.J. Damage to Skin Extracellular Matrix Induced by UV Exposure. Antioxid. Redox Signal. 2014, 21, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.E.B.; Griffiths, C.E.M.; Craven, N.M.; Shuttleworth, C.A.; Kielty, C.M. Fibrillin-Rich Microfibrils Are Reduced in Photoaged Skin. Distribution at the Dermal–Epidermal Junction. J. Investig. Dermatol. 1999, 112, 782–787. [Google Scholar] [CrossRef]
- Attia-Vigneau, J.; Terryn, C.; Lorimier, S.; Sandre, J.; Antonicelli, F.; Hornebeck, W. Regeneration of Human Dermis by a Multi-Headed Peptide. J. Investig. Dermatol. 2014, 134, 58–67. [Google Scholar] [CrossRef]
- do Rocio Valenga Baroni, E.; de Lourdes Pessole Biondo-Simões, M.; Auersvald, A.; Auersvald, L.A.; Montemor Netto, M.R.; Ortolan, M.C.A.B.; Kohler, J.N. Influence of Aging on the Quality of the Skin of White Women: The Role of Collagen. Acta Cir. Bras. 2012, 27, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Guo, Z.; Zhang, Y.; Liu, Y.; Xing, F.; Wang, J.; Luo, X.; Kong, Y.; Zhang, G. Age-Related Changes in the Ratio of Type I/III Collagen and Fibril Diameter in Mouse Skin. Regen. Biomater. 2023, 10, rbac110. [Google Scholar] [CrossRef] [PubMed]
- Nopparat, C.; Sinjanakhom, P.; Govitrapong, P. Melatonin Reverses H2O2-Induced Senescence in SH-SY5Y Cells by Enhancing Autophagy via Sirtuin 1 Deacetylation of the RelA/P65 Subunit of NF-ΚB. J. Pineal Res. 2017, 63, e12407. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-W.; Shin, N.-R.; Jung, T.-Y.; Shin, I.-S.; Moon, C.; Kim, S.-H.; Lee, I.-C.; Kim, S.-H.; Yun, W.-K.; Kim, H.-C.; et al. Melatonin Attenuates Cisplatin-Induced Acute Kidney Injury in Rats via Induction of Anti-Aging Protein, Klotho. Food Chem. Toxicol. 2019, 129, 201–210. [Google Scholar] [CrossRef]
- Kleszczyński, K.; Zillikens, D.; Fischer, T.W. Melatonin Enhances Mitochondrial ATP Synthesis, Reduces Reactive Oxygen Species Formation, and Mediates Translocation of the Nuclear Erythroid 2-Related Factor 2 Resulting in Activation of Phase-2 Antioxidant Enzymes (γ-GCS, HO-1, NQO1) in Ultraviolet Radiation-Treated Normal Human Epidermal Keratinocytes (NHEK). J. Pineal Res. 2016, 61, 187–197. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Poeggeler, B.; Menendez-Pelaez, A.; Chen, L.D.; Saarela, S. Melatonin as a Free Radical Scavenger: Implications for Aging and Age-Related Diseases. Ann. N. Y. Acad. Sci. 1994, 719, 1–12. [Google Scholar] [CrossRef]
- Sayed, R.K.A.; Fernández-Ortiz, M.; Diaz-Casado, M.E.; Aranda-Martínez, P.; Fernández-Martínez, J.; Guerra-Librero, A.; Escames, G.; López, L.C.; Alsaadawy, R.M.; Acuña-Castroviejo, D. Lack of NLRP3 Inflammasome Activation Reduces Age-Dependent Sarcopenia and Mitochondrial Dysfunction, Favoring the Prophylactic Effect of Melatonin. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1699–1708. [Google Scholar] [CrossRef]
- Milani, M.; Sparavigna, A. Antiaging Efficacy of Melatonin-Based Day and Night Creams: A Randomized, Split-Face, Assessor-Blinded Proof-of-Concept Trial. Clin. Cosmet. Investig. Dermatol. 2018, 11, 51–57. [Google Scholar] [CrossRef]
- Park, E.K.; Lee, H.-J.; Lee, H.; Kim, J.-H.; Hwang, J.; Koo, J.I.; Kim, S.-H. The Anti-Wrinkle Mechanism of Melatonin in UVB Treated HaCaT Keratinocytes and Hairless Mice via Inhibition of ROS and Sonic Hedgehog Mediated Inflammatory Proteins. Int. J. Mol. Sci. 2018, 19, 1995. [Google Scholar] [CrossRef] [PubMed]
- Vidali, S.; Feichtinger, R.G.; Emberger, M.; Brunner, S.M.; Gaisbauer, S.; Blatt, T.; Smiles, W.J.; Kreutzer, C.; Weise, J.M.; Kofler, B. Ageing Is Associated with a Reduction in Markers of Mitochondrial Energy Metabolism in the Human Epidermis. Exp. Dermatol. 2023, 32, 900–905. [Google Scholar] [CrossRef]
- Protasoni, M.; Serrano, M. Targeting Mitochondria to Control Ageing and Senescence. Pharmaceutics 2023, 15, 352. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, C.; Pan, X.; Lei, K. Emerging Roles of Mitochondria in Animal Regeneration. Cell Regen. 2023, 12, 14. [Google Scholar] [CrossRef]
- Kleszczyński, K.; Bilska, B.; Stegemann, A.; Flis, D.J.; Ziolkowski, W.; Pyza, E.; Luger, T.A.; Reiter, R.J.; Böhm, M.; Slominski, A.T. Melatonin and Its Metabolites Ameliorate UVR-Induced Mitochondrial Oxidative Stress in Human MNT-1 Melanoma Cells. Int. J. Mol. Sci. 2018, 19, 3786. [Google Scholar] [CrossRef] [PubMed]
- Jauhari, A.; Baranov, S.V.; Suofu, Y.; Kim, J.; Singh, T.; Yablonska, S.; Li, F.; Wang, X.; Oberly, P.; Minnigh, M.B.; et al. Melatonin Inhibits Cytosolic Mitochondrial DNA-Induced Neuroinflammatory Signaling in Accelerated Aging and Neurodegeneration. J. Clin. Investig. 2020, 130, 3124–3136. [Google Scholar] [CrossRef]
- Nasoni, M.G.; Carloni, S.; Canonico, B.; Burattini, S.; Cesarini, E.; Papa, S.; Pagliarini, M.; Ambrogini, P.; Balduini, W.; Luchetti, F. Melatonin Reshapes the Mitochondrial Network and Promotes Intercellular Mitochondrial Transfer via Tunneling Nanotubes after Ischemic-like Injury in Hippocampal HT22 Cells. J. Pineal Res. 2021, 71, e12747. [Google Scholar] [CrossRef]
- Lago, J.C.; Puzzi, M.B. The Effect of Aging in Primary Human Dermal Fibroblasts. PLoS ONE 2019, 14, e0219165. [Google Scholar] [CrossRef]
- Tamura, H.; Kawamoto, M.; Sato, S.; Tamura, I.; Maekawa, R.; Taketani, T.; Aasada, H.; Takaki, E.; Nakai, A.; Reiter, R.J.; et al. Long-Term Melatonin Treatment Delays Ovarian Aging. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef]
- Fitsiou, E.; Pulido, T.; Campisi, J.; Alimirah, F.; Demaria, M. Cellular Senescence and the Senescence-Associated Secretory Phenotype as Drivers of Skin Photoaging. J. Investig. Dermatol. 2021, 141, 1119–1126. [Google Scholar] [CrossRef]
- Haslam, I.S.; Jadkauskaite, L.; Szabó, I.L.; Staege, S.; Hesebeck-Brinckmann, J.; Jenkins, G.; Bhogal, R.K.; Lim, F.-L.; Farjo, N.; Farjo, B.; et al. Oxidative Damage Control in a Human (Mini-) Organ: Nrf2 Activation Protects against Oxidative Stress-Induced Hair Growth Inhibition. J. Investig. Dermatol. 2017, 137, 295–304. [Google Scholar] [CrossRef]
- Fischer, T.W.; Kleszczyński, K.; Hardkop, L.H.; Kruse, N.; Zillikens, D. Melatonin Enhances Antioxidative Enzyme Gene Expression (CAT, GPx, SOD), Prevents Their UVR-Induced Depletion, and Protects against the Formation of DNA Damage (8-Hydroxy-2′-Deoxyguanosine) in Ex Vivo Human Skin. J. Pineal Res. 2013, 54, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.M.; Aliarab, A.; Goodarzi, G.; Shirzad, M.; Jafari, S.M.; Qujeq, D.; Samavarchi Tehrani, S.; Asadi, J. Melatonin: A Smart Molecule in the DNA Repair System. Cell Biochem. Funct. 2022, 40, 4–16. [Google Scholar] [CrossRef]
- Bonté, F.; Girard, D.; Archambault, J.-C.; Desmoulière, A. Skin Changes During Ageing. Subcell. Biochem. 2019, 91, 249–280. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Le Varlet, B.; Chaudagne, C.; Saunois, A.; Barré, P.; Sauvage, C.; Berthouloux, B.; Meybeck, A.; Dumas, M.; Bonté, F. Age-Related Functional and Structural Changes in Human Dermo-Epidermal Junction Components. J. Investig. Dermatol. Symp. Proc. 1998, 3, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding Expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Fuentes-Broto, L. Melatonin: A Multitasking Molecule. Prog. Brain Res. 2010, 181, 127–151. [Google Scholar] [CrossRef]
- Zhang, S.; Yao, X. Mechanism of Action and Promising Clinical Application of Melatonin from a Dermatological Perspective. J. Transl. Autoimmun. 2023, 6, 100192. [Google Scholar] [CrossRef]
- Nikolaev, G.; Robeva, R.; Konakchieva, R. Membrane Melatonin Receptors Activated Cell Signaling in Physiology and Disease. Int. J. Mol. Sci. 2022, 23, 471. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.-K.; Slominski, R.M.; Song, Y.; Qayyum, S.; Placha, W.; Janjetovic, Z.; Kleszczyński, K.; Atigadda, V.; Song, Y.; et al. Melatonin and Its Metabolites Can Serve as Agonists on the Aryl Hydrocarbon Receptor and Peroxisome Proliferator-Activated Receptor Gamma. Int. J. Mol. Sci. 2023, 24, 15496. [Google Scholar] [CrossRef]
- Martín Giménez, V.M.; de Las Heras, N.; Lahera, V.; Tresguerres, J.A.F.; Reiter, R.J.; Manucha, W. Melatonin as an Anti-Aging Therapy for Age-Related Cardiovascular and Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 888292. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin’s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef]
- Paus, R.; van Lessen, M.; Mardaryev, A.; Broadley, D.; Bertolini, M.; Edelkamp, J.; Kuckelhaus, M.; Funk, W.; Biro, T. “Speed-Aging” of Human Skin in Serum-Free Organ Culture Ex Vivo: An Instructive Novel Assay for Preclinical Human Skin Aging Research Demonstrates Senolytic Effects of Caffeine and 2,5-Dimethylpyrazine. Exp. Dermatol. 2023, in press. [Google Scholar]
- Gherardini, J.; Wegner, J.; Chéret, J.; Ghatak, S.; Lehmann, J.; Alam, M.; Jimenez, F.; Funk, W.; Böhm, M.; Botchkareva, N.V.; et al. Transepidermal UV Radiation of Scalp Skin Ex Vivo Induces Hair Follicle Damage That Is Alleviated by the Topical Treatment with Caffeine. Int. J. Cosmet. Sci. 2019, 41, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Chéret, J.; Piccini, I.; Hardman-Smart, J.; Ghatak, S.; Alam, M.; Lehmann, J.; Jimenez, F.; Erdmann, H.; Poblet, E.; Botchkareva, N.; et al. Preclinical Evidence That the PPARγ Modulator, N-Acetyl-GED-0507-34-Levo, May Protect Human Hair Follicle Epithelial Stem Cells against Lichen Planopilaris-Associated Damage. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e195–e197. [Google Scholar] [CrossRef] [PubMed]
- Chéret, J.; Bertolini, M.; Ponce, L.; Lehmann, J.; Tsai, T.; Alam, M.; Hatt, H.; Paus, R. Olfactory Receptor OR2AT4 Regulates Human Hair Growth. Nat. Commun. 2018, 9, 3624. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Chéret, J.; Pinto, D.; Hawkshaw, N.; Ponce, L.; Erdmann, H.; Jimenez, F.; Funk, W.; Paus, R. A Novel Nondrug SFRP1 Antagonist Inhibits Catagen Development in Human Hair Follicles Ex Vivo. Br. J. Dermatol. 2021, 184, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-Y.; Langan, E.A.; Meier, N.T.; Funk, W.; Siemers, F.; Paus, R. Thyroxine (T4) May Promote Re-Epithelialisation and Angiogenesis in Wounded Human Skin Ex Vivo. PLoS ONE 2019, 14, e0212659. [Google Scholar] [CrossRef] [PubMed]
- Hawkshaw, N.J.; Hardman, J.A.; Haslam, I.S.; Shahmalak, A.; Gilhar, A.; Lim, X.; Paus, R. Identifying Novel Strategies for Treating Human Hair Loss Disorders: Cyclosporine A Suppresses the Wnt Inhibitor, SFRP1, in the Dermal Papilla of Human Scalp Hair Follicles. PLoS Biol. 2018, 16, e2003705. [Google Scholar] [CrossRef] [PubMed]
- Nanzadsuren, T.; Myatav, T.; Dorjkhuu, A.; Byamba, K. Association between Serum Melatonin and Skin Aging in an Urban Population of Mongolia. J. Cosmet. Dermatol. 2020, 19, 1501–1507. [Google Scholar] [CrossRef]
- Kleszczyński, K.; Kim, T.-K.; Bilska, B.; Sarna, M.; Mokrzynski, K.; Stegemann, A.; Pyza, E.; Reiter, R.J.; Steinbrink, K.; Böhm, M.; et al. Melatonin Exerts Oncostatic Capacity and Decreases Melanogenesis in Human MNT-1 Melanoma Cells. J. Pineal Res. 2019, 67, e12610. [Google Scholar] [CrossRef]
- Kim, T.-K.; Lin, Z.; Tidwell, W.J.; Li, W.; Slominski, A.T. Melatonin and Its Metabolites Accumulate in the Human Epidermis in Vivo and Inhibit Proliferation and Tyrosinase Activity in Epidermal Melanocytes in Vitro. Mol. Cell. Endocrinol. 2015, 404, 1–8. [Google Scholar] [CrossRef]
- Holtkamp, C.E.; Warmus, D.; Bonowicz, K.; Gagat, M.; Linowiecka, K.; Wolnicka-Glubisz, A.; Reiter, R.J.; Böhm, M.; Slominski, A.T.; Steinbrink, K.; et al. Ultraviolet Radiation-Induced Mitochondrial Disturbances Are Attenuated by Metabolites of Melatonin in Human Epidermal Keratinocytes. Metabolites 2023, 13, 861. [Google Scholar] [CrossRef]
- Slominski, A.; Pisarchik, A.; Semak, I.; Sweatman, T.; Wortsman, J.; Szczesniewski, A.; Slugocki, G.; McNulty, J.; Kauser, S.; Tobin, D.J.; et al. Serotoninergic and Melatoninergic Systems Are Fully Expressed in Human Skin. FASEB J. 2002, 16, 896–898. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Tobin, D.J. The Cutaneous Serotoninergic/Melatoninergic System: Securing a Place under the Sun. FASEB J. 2005, 19, 176–194. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Chen, J.; Gusdon, A.M.; Song, K.; Li, L.; Qu, S. Melatonin Treatment Improves Insulin Resistance and Pigmentation in Obese Patients with Acanthosis Nigricans. Int. J. Endocrinol. 2018, 2018, 2304746. [Google Scholar] [CrossRef] [PubMed]
- Hamadi, S.; Aljaf, A.; Abdulrazak, A.; Mohammed, M. The Role of Topical and Oral Melatonin in Management of Melasma Patients. J. Assoc. Arab Univ. Basic Appl. Sci. 2009, 30–42. [Google Scholar]
- Slominski, A.T.; Kim, T.-K.; Kleszczyński, K.; Semak, I.; Janjetovic, Z.; Sweatman, T.; Skobowiat, C.; Steketee, J.D.; Lin, Z.; Postlethwaite, A.; et al. Characterization of Serotonin and N-Acetylserotonin Systems in the Human Epidermis and Skin Cells. J. Pineal Res. 2020, 68, e12626. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Semak, I.; Fischer, T.W.; Kim, T.-K.; Kleszczynski, K.; Hardeland, R.; Reiter, R.J. Metabolism of Melatonin in the Skin: Why Is It Important? Exp. Dermatol. 2017, 26, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Chéret, J.; Gherardini, J.; Soeberdt, M.; Hundt, J.E.; Abels, C.; Bertolini, M.; Paus, R. Non-Neuronal Kappa-Opioid Receptor Activation Enhances Epidermal Keratinocyte Proliferation, and Modulates Mast Cell Functions in Human Skin Ex Vivo. J. Dermatol. 2020, 47, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Gáspár, E.; Nguyen-Thi, K.T.; Hardenbicker, C.; Tiede, S.; Plate, C.; Bodó, E.; Knuever, J.; Funk, W.; Bíró, T.; Paus, R. Thyrotropin-Releasing Hormone Selectively Stimulates Human Hair Follicle Pigmentation. J. Investig. Dermatol. 2011, 131, 2368–2377. [Google Scholar] [CrossRef] [PubMed]


| Marker | With Melatonin, Day 3 | With Melatonin, Day 6 | ||
|---|---|---|---|---|
| 100 µM | 200 µM | 100 µM | 200 µM | |
| p-S6 |  | ** |  |  |
| MMP-1 |  |  |  | * |
| COL17A1 |  |  | ** | ** |
| TFAM |  |  |  | * |
| VDAC/Porin | * |  | * | * |
| MTCO1 |  |  |  |  |
| VEGF-A | * |  | * |  |
| Fibrillin 1 | ** | * |  |  |
| Collagen I |  |  |  |  |
| p16INK4 |  |  |  |  |
| Lamin B1 |  |  |  |  |
| SIRT1 |  |  |  |  |
| γH2A.x |  |  |  |  |
| Antigen | Fixation | Blocking and/or Permeabilization | Primary Antibody | Secondary Antibody | |
|---|---|---|---|---|---|
| Epidermis | p-S6 | 4% PFA, 10 min at RT | N/A | rabbit monoclonal anti-phospho-S6 ribosomal protein (Ser235/236) (1:200; Cell Signaling, #4858) | goat monoclonal anti-rabbit IgG-Alexa Fluor 488 (1:200; Invitrogen, Waltham, MA, USA, A11034) |
| COL17A1 | Acetone, 10 min at −20 °C | N/A | rabbit monoclonal anti-Collagen 17A1[EPR14758] (1:200; Abcam, #ab186415) | goat monoclonal anti-rabbit IgG-Alexa Fluor 488 (1:400; Invitrogen, A11034) | |
| MMP-1 | 4% PFA, 10 min at RT | 10% goat serum in PBS | mouse monoclonal anti-MMP1 (1:100; Biolegend, #634702) | goat monoclonal anti-mouse IgG-Alexa Fluor 555 (1:200; Invitrogen, A32727) | |
| TFAM | Acetone, 10 min at −20 °C | 0.1% Triton X-100 in PBS | rabbit monoclonal anti-mtFA (TFAM) [EPR12285] (1:500; Abcam, #ab176558) | goat monoclonal anti-rabbit IgG-Alexa Fluor 488 (1:400; Invitrogen, A11034) | |
| VDAC1/Porin | Methanol, 10 min at −20 °C | 10% goat serum and 0.3% Triton X-100 in PBS | rabbit monoclonal anti-VDAC1/Porin (1:100; Abcam, #ab15895) | goat monoclonal anti-rabbit IgG-FITC (1:200; Jackson Immuno Research, West Grove, PA, USA, 111-095-144) followed by an amplification with goat monoclonal anti-FITC-Alexa Fluor® 488 (1:700; Invitrogen, A11096) | |
| MTCO-1 | 4% PFA, 10 min at RT | 10% goat serum and 0.3% Triton X-100 in PBS | rabbit monoclonal anti-MTCO1 [EPR19628] (1:50; Abcam, #ab203912) | goat monoclonal anti-rabbit IgG-FITC (1:200; Jackson Immuno Research, 111-095-144) followed by an amplification with goat monoclonal anti-FITC-Alexa Fluor® 488 (1:700; Invitrogen, A11096) | |
| VEGF-A | 4% PFA, 10 min at RT | N/A | rabbit monoclonal anti-human Alexa Fluor® 488 Anti-VEGFA [EP1176Y] (1:500, Abcam, #ab206886) | N/A | |
| SIRT-1 | Acetone, 10 min at −20 °C | 10% goat serum and 0.3% Triton X-100 in PBS | rabbit monoclonal anti-SIRT1 [E104] (1:200; Abcam, #ab32441) | goat monoclonal anti-rabbit IgG-FITC (1:200; Jackson Immuno Research, 111-095-144) followed by an amplification with goat monoclonal anti-FITC-Alexa Fluor® 488 (1:700; Invitrogen, A11096) | |
| Lamin B1 | 4% PFA, 10 min at RT | N/A | rabbit monoclonal anti-Lamin B1 [EPR8985(B)] (1:100; Abcam, #ab194109) | goat monoclonal anti-rabbit IgG-Alexa Fluor 488 (1:400; Invitrogen, A11034) | |
| γH2A.x | 4% PFA, 10 min at RT | 10% goat serum in PBS | rabbit monoclonal anti-phospho-histone H2A.X (Ser139) (γH2A.X) (1:1500; Cell Signaling, #2577S) | goat monoclonal anti-rabbit IgG-Alexa Fluor 555 (1:400; Invitrogen, A21428) | |
| p16INK4 | 4% PFA, 10 min at RT | 10% goat serum, 0.1% Triton-X100, and 0.2% Saponin in TBS | rabbit monoclonal anti-CDKN2A/p16INK4a [EPR1473] (1:250; Abcam, #ab108349) | goat monoclonal anti-rabbit IgG-FITC (1:200; Jackson Immuno Research, 111-095-144) followed by an amplification with goat monoclonal anti-FITC-Alexa Fluor® 488 (1:700; Invitrogen, A11096) | |
| Dermis | Fibrillin-1 | Acetone, 10 min at −20 °C | 3% H202 in TBS, followed by pre-treatment with Avidin and Biotin and then 10% goat serum in TNT | mouse monoclonal anti-Fibrillin1 biotinylated [11C1.3] (1:800; Abcam, #ab24826) | amplification with FITC-conjugated tyramide |
| Collagen I α-1 | Acetone, 10 min at −20 °C | 10% goat serum in PBS | mouse monoclonal anti-Collagen I alpha-1 (1:500; Novus Bio, #NB600-450) | goat monoclonal anti-mouse IgG-Alexa Fluor 488 (1:400; Invitrogen, A11001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samra, T.; Gomez-Gomez, T.; Linowiecka, K.; Akhundlu, A.; Lopez de Mendoza, G.; Gompels, M.; Lee, W.W.; Gherardini, J.; Chéret, J.; Paus, R. Melatonin Exerts Prominent, Differential Epidermal and Dermal Anti-Aging Properties in Aged Human Eyelid Skin Ex Vivo. Int. J. Mol. Sci. 2023, 24, 15963. https://doi.org/10.3390/ijms242115963
Samra T, Gomez-Gomez T, Linowiecka K, Akhundlu A, Lopez de Mendoza G, Gompels M, Lee WW, Gherardini J, Chéret J, Paus R. Melatonin Exerts Prominent, Differential Epidermal and Dermal Anti-Aging Properties in Aged Human Eyelid Skin Ex Vivo. International Journal of Molecular Sciences. 2023; 24(21):15963. https://doi.org/10.3390/ijms242115963
Chicago/Turabian StyleSamra, Tara, Tatiana Gomez-Gomez, Kinga Linowiecka, Aysun Akhundlu, Gabriella Lopez de Mendoza, Matthew Gompels, Wendy W. Lee, Jennifer Gherardini, Jérémy Chéret, and Ralf Paus. 2023. "Melatonin Exerts Prominent, Differential Epidermal and Dermal Anti-Aging Properties in Aged Human Eyelid Skin Ex Vivo" International Journal of Molecular Sciences 24, no. 21: 15963. https://doi.org/10.3390/ijms242115963





