Ginseng Stem-and-Leaf Saponins Mitigate Chlorpyrifos-Evoked Intestinal Toxicity In Vivo and In Vitro: Oxidative Stress, Inflammatory Response and Apoptosis
Abstract
:1. Introduction
2. Results
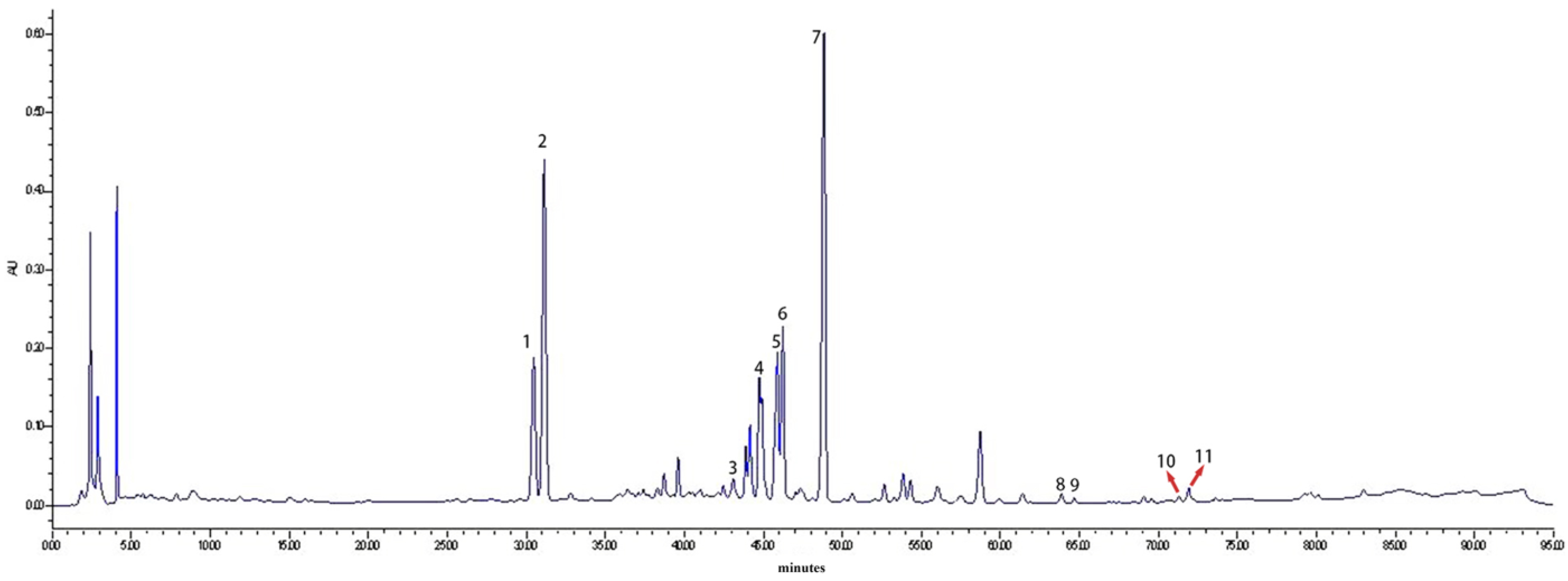
2.1. HPLC Analysis of Saponin Monomers in GSLS
2.2. GSLSs Can Improve CPF-Induced Oxidative Stress and Enhance Antioxidant Activity
2.3. Effects of GSLSs on Serum Inflammatory Factors
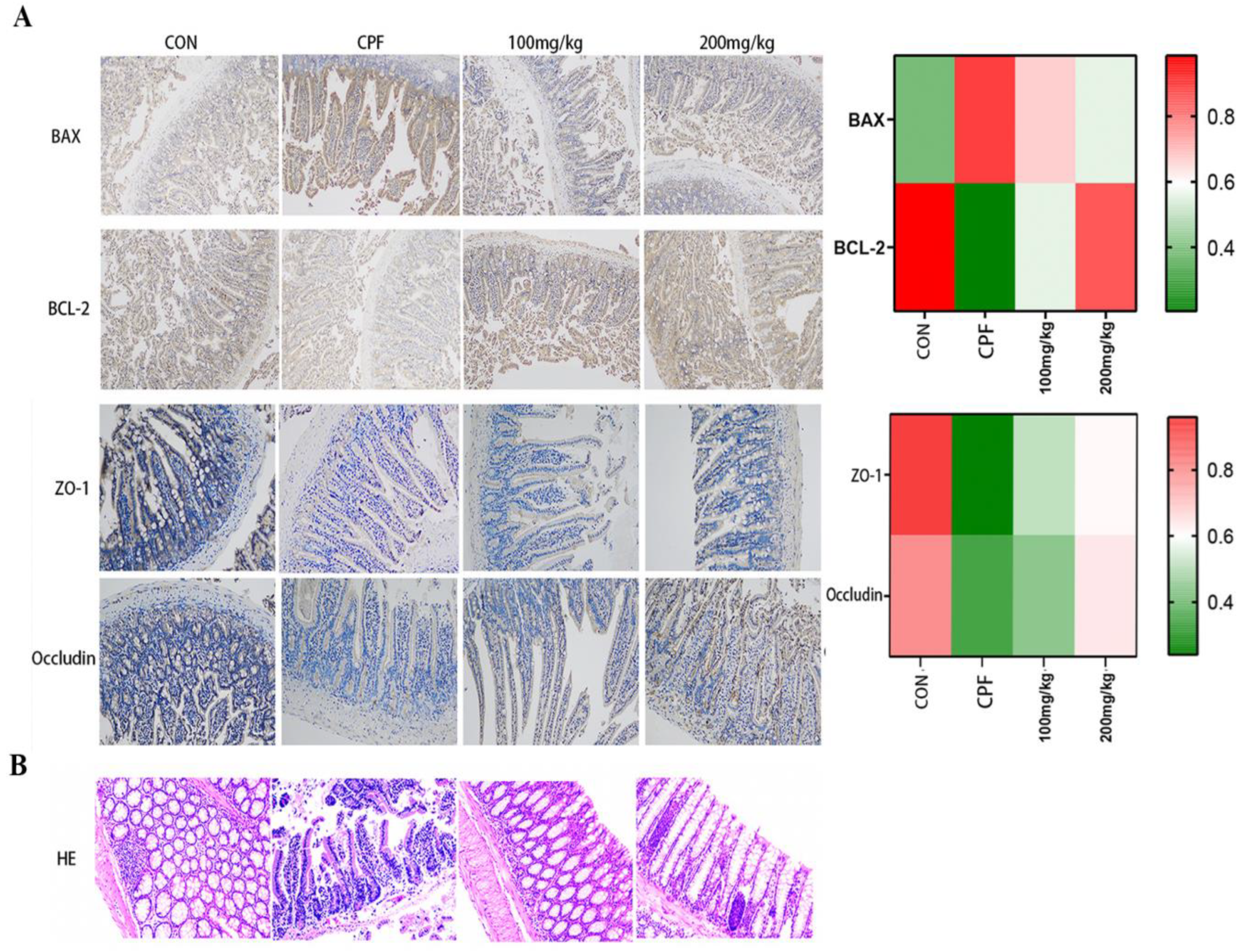
2.4. Immunohistochemical Staining Analysis
2.5. Intestinal Histopathological Analysis
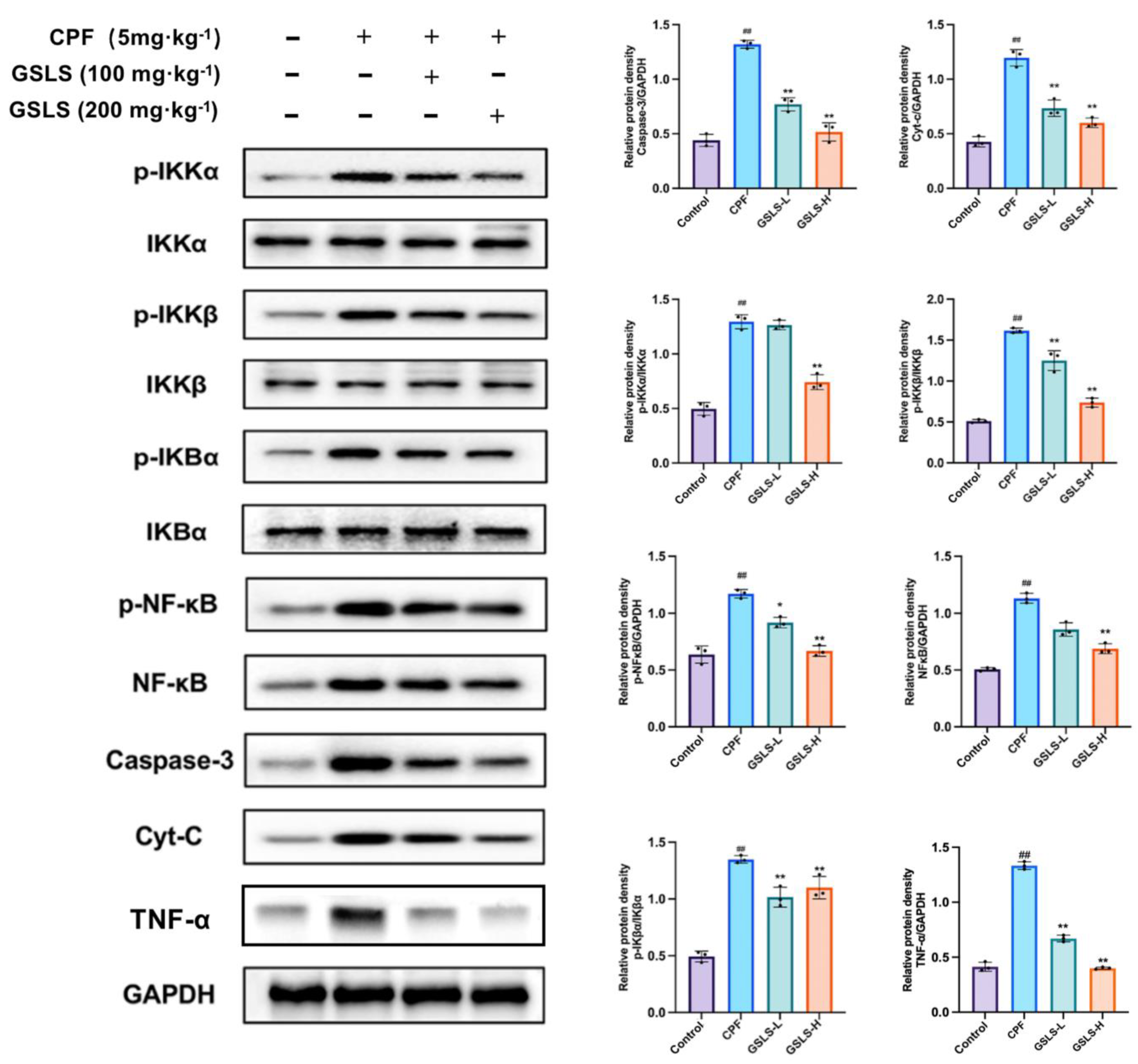
2.6. GSLSs Reduced Phosphorylation, Inflammation and Apoptosis via IKKα/β and pIκBα in Intestinal Tissues
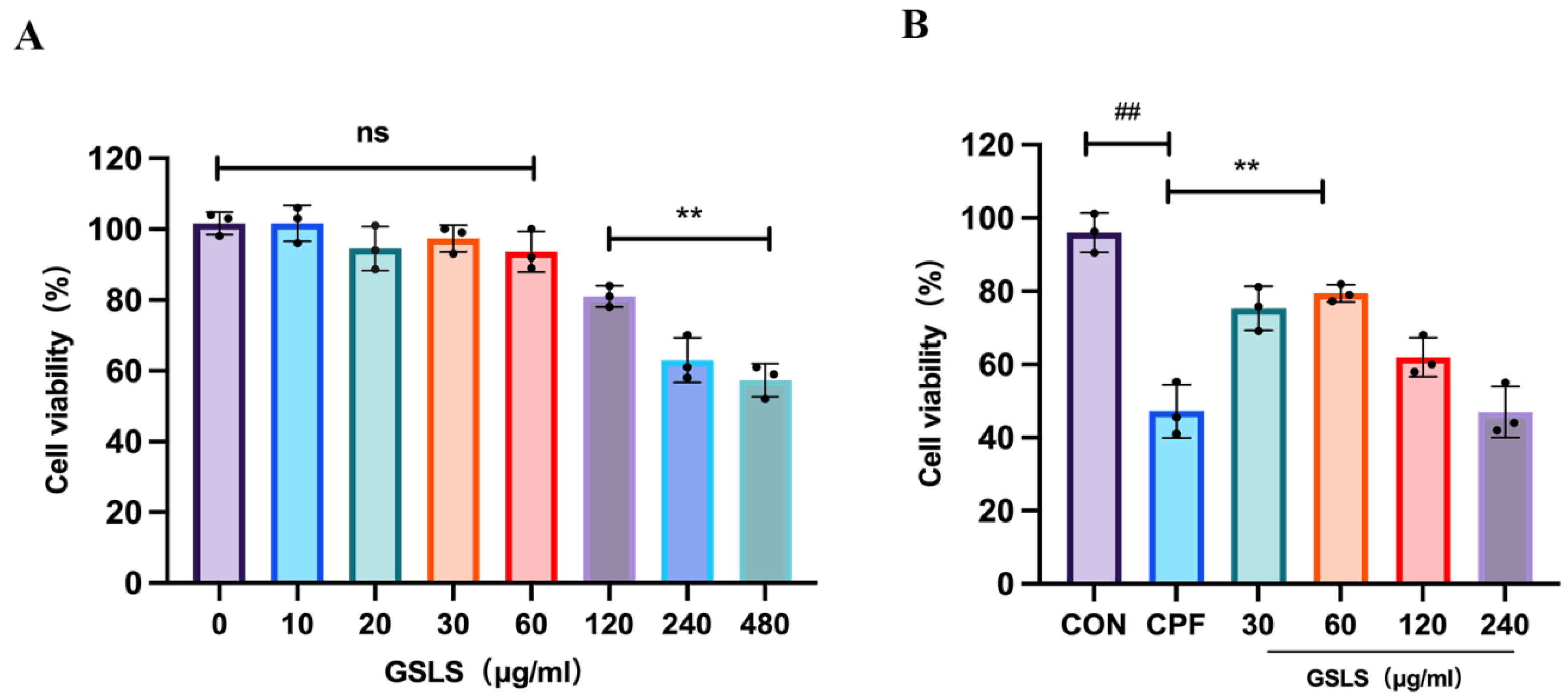
2.7. The Effect of GSLSs on CPF-Induced RAW264.7 Cell Model Using CCK-8
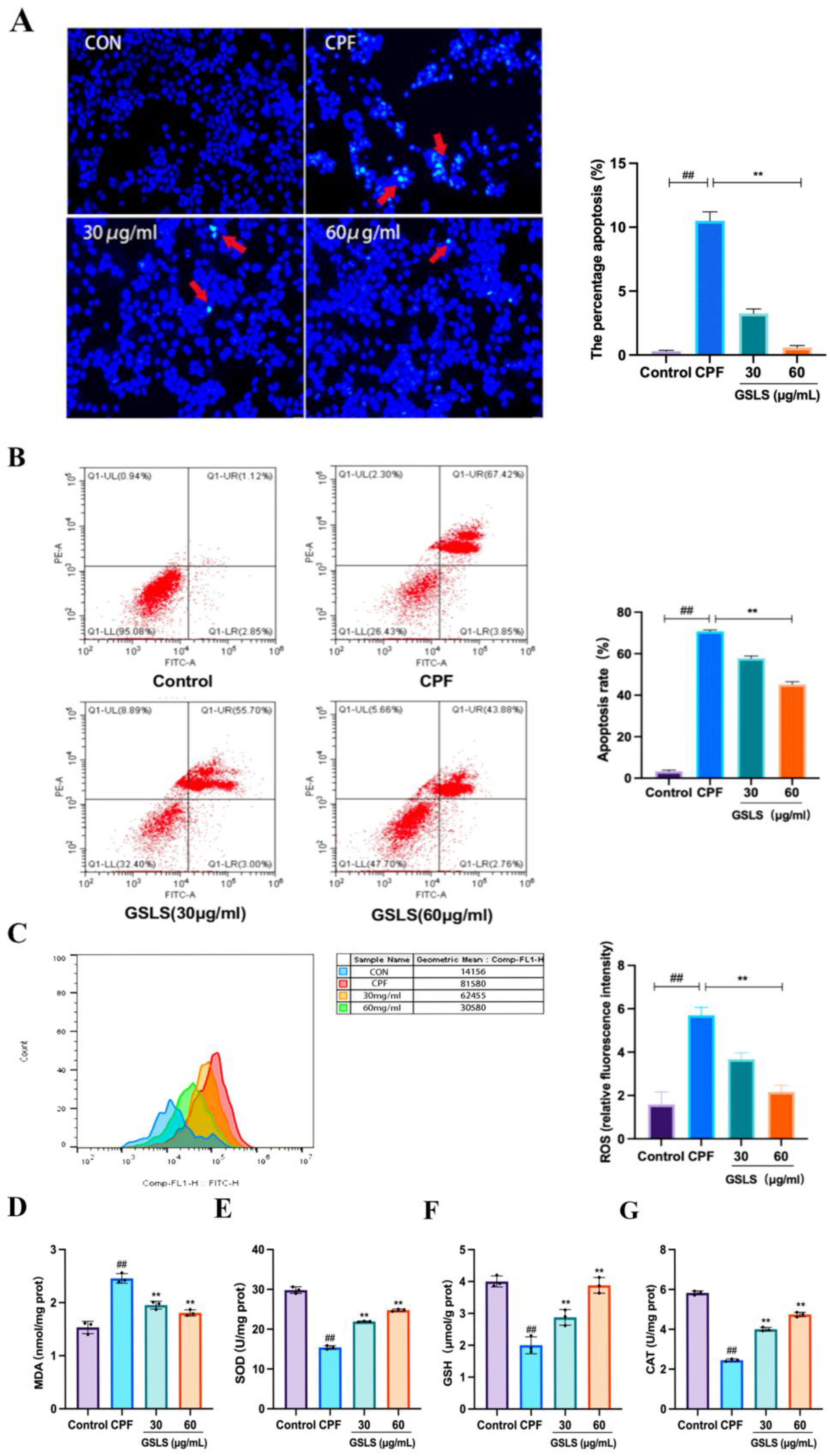
2.8. Analysis of Hoechst Staining Results
2.9. Inhibitory Effect of GSLSs on CPF-Induced Apoptosis in RAW264.7 Cells
2.10. Effects of GSLSs on CPF-Induced Oxidative Stress in RAW264.7 Cells
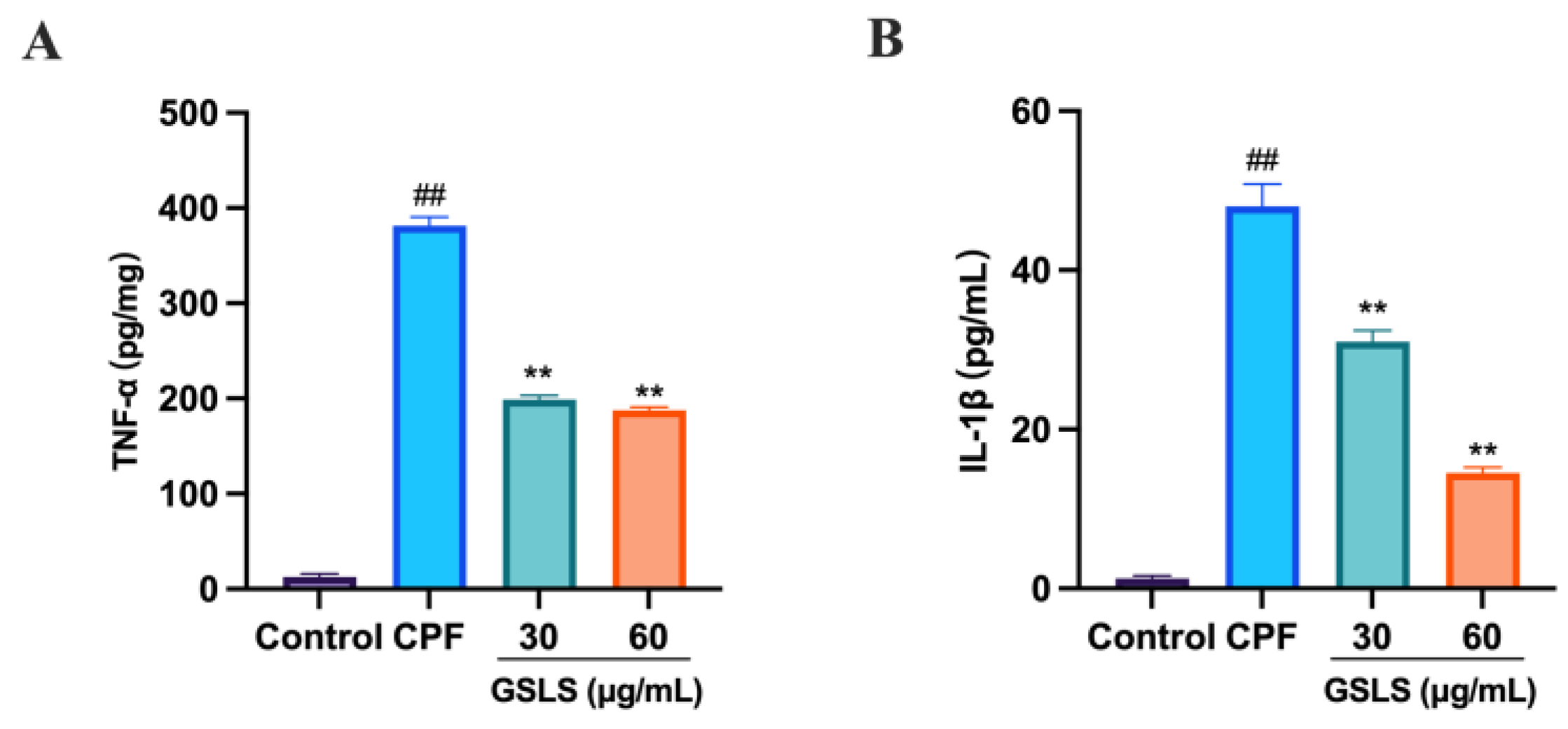
2.11. GSLSs Ameliorated CPF-Induced Inflammatory Factors in RAW264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Design
4.2.1. HPLC Analysis
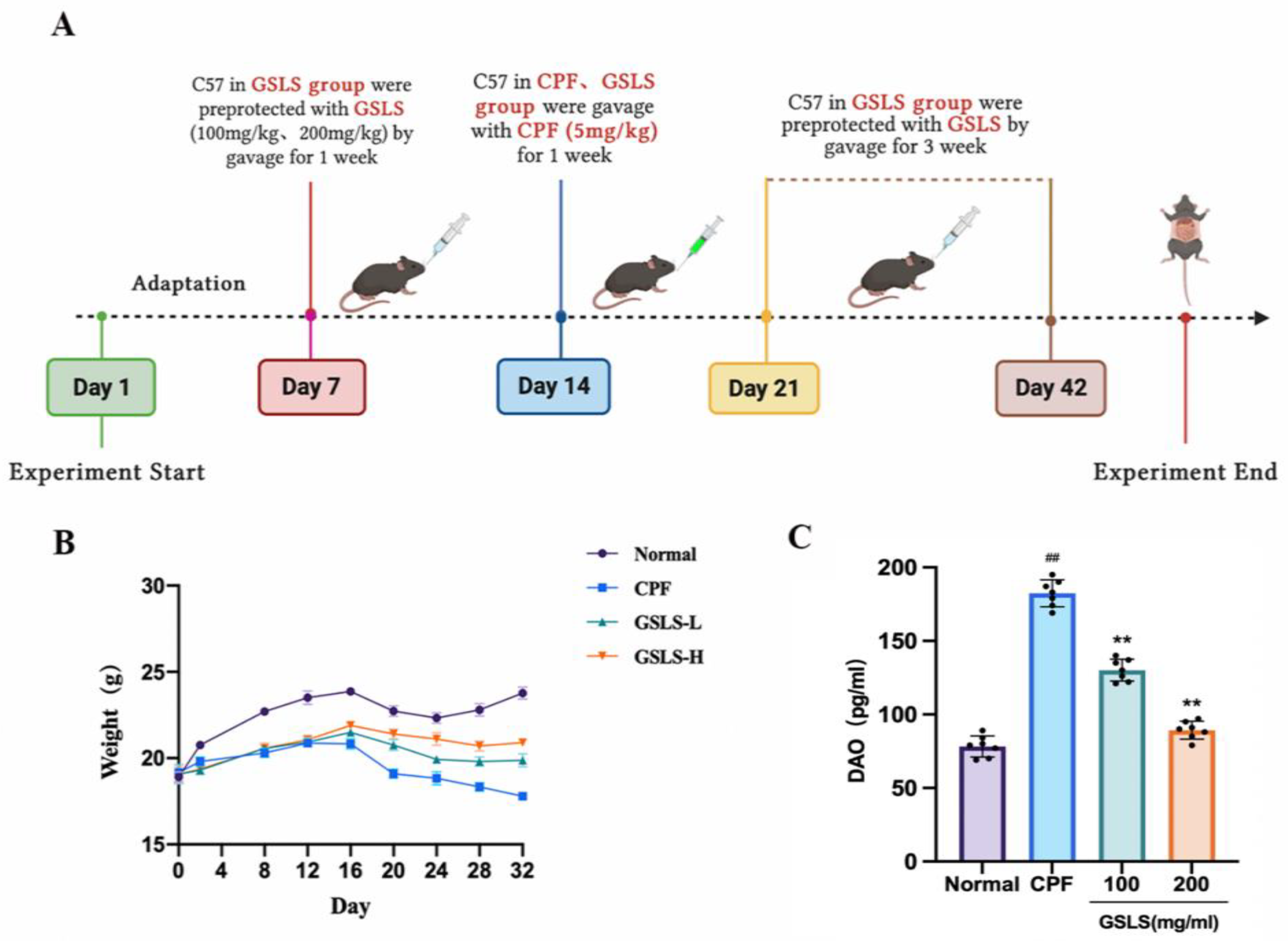
4.2.2. In Vivo Animal Experiments and Design
4.2.3. Analysis of Serum Oxidative Stress Index
4.2.4. DAO and Biochemical Analysis of Intestinal Tissue
4.2.5. The Influence of Proinflammatory Cytokines Was Determined by ELISA
4.2.6. Immunohistochemical
4.2.7. HISTOPATHOLOGICAL Examination
4.2.8. Protein Imprinting Detection
4.3. In Vitro Cell Culture and Treatment
4.3.1. CCK-8 Detection of Cell Viability
4.3.2. Assessment of Biochemical Parameters
4.3.3. Hoechst 33258 Staining
4.3.4. Apoptosis Was Detected by Flow Cytometry
4.3.5. ROS Detection
4.3.6. Statistics and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paidi, M.K.; Satapute, P.; Haider, M.S.; Udikeri, S.S.; Ramachandra, Y.L.; Vo, D.-V.N.; Govarthanan, M.; Jogaiah, S. Mitigation of organophosphorus insecticides from environment: Residual detoxification by bioweapon catalytic scavengers. Environ. Res. 2021, 200, 111368. [Google Scholar] [CrossRef]
- Silva, J.; Marques-Da-Silva, D.; Lagoa, R. Reassessment of the experimental skin permeability coefficients of polycyclic aromatic hydrocarbons and organophosphorus pesticides. Environ. Toxicol. Pharmacol. 2021, 86, 103671. [Google Scholar] [CrossRef]
- Sarlak, Z.; Khosravi-Darani, K.; Rouhi, M.; Garavand, F.; Mohammadi, R.; Sobhiyeh, M.R. Bioremediation of organophosphorus pesticides in contaminated foodstuffs using probiotics. Food Control 2021, 126, 108006. [Google Scholar] [CrossRef]
- Millis, R.M.; Archer, P.W.; Whittaker, J.A.; Trouth, C.O. The role of hypoxia in organophosphorus nerve agent intoxication. Neurotoxicology 1988, 9, 273–285. [Google Scholar]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Singh, J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Rosenbaum, C.; Bird, S.B. Non-muscarinic Therapeutic Targets for Acute Organophosphorus Poisoning. J. Med. Toxicol. 2010, 6, 408–412. [Google Scholar] [CrossRef]
- Guignet, M.; Dhakal, K.; Flannery, B.M.; Hobson, B.A.; Zolkowska, D.; Dhir, A.; Bruun, D.A.; Li, S.; Wahab, A.; Harvey, D.J.; et al. Persistent behavior deficits, neuroinflammation, and oxidative stress in a rat model of acute organophosphate intoxication. Neurobiol. Dis. 2020, 133, 104431. [Google Scholar] [CrossRef]
- Raszewski, G.; Filip, R. Use of oximes in the therapy of acute intoxication by organophosphorus compounds. Prz. Lek. 2004, 61, 181–184. [Google Scholar]
- Abdollahi, M.; Karami-Mohajeri, S. A comprehensive review on experimental and clinical findings in intermediate syndrome caused by organophosphate poisoning. Toxicol. Appl. Pharmacol. 2012, 258, 309–314. [Google Scholar] [CrossRef]
- Wang, G.; Li, J.; Xue, N.; Al-Huqail, A.A.; Majdi, H.S.; Darvishmoghaddam, E.; Assilzadeh, H.; Khadimallah, M.A.; Ali, H.E. Risk assessment of organophosphorus pesticide residues in drinking water resources: Statistical and Monte-Carlo approach. Chemosphere 2022, 307, 135632. [Google Scholar] [CrossRef]
- Jin, S.; Zhu, T.; Deng, S.; Li, D.; Li, J.; Liu, X.; Liu, Y. Dioscin ameliorates cisplatin-induced intestinal toxicity by mitigating oxidative stress and inflammation. Int. Immunopharmacol. 2022, 111, 109111. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Huang, Q.; Lu, M.; Zhang, L.; Yang, Z.; Zong, M.; Tao, L. The organophosphate insecticide chlorpyrifos confers its genotoxic effects by inducing DNA damage and cell apoptosis. Chemosphere 2015, 135, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-M.; Pai, M.-H.; Liu, J.-J.; Yeh, S.-L.; Hou, Y.-C. Effects of dietary exposure to chlorpyrifos on immune cell populations and inflammatory responses in mice with dextran sulfate sodium-induced colitis. Food Chem. Toxicol. 2019, 131, 110596. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Xu, W.; Zhang, Y.; Sun, Q.; Li, Z.; Geng, L.; Teng, X. Transcriptome analysis revealed the mechanism of Luciobarbus capito (L. capito) adapting high salinity: Antioxidant capacity, heat shock proteins, immunity. Mar. Pollut. Bull. 2023, 192, 115017. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, D.; Xiao, H. Methods of Cellular Senescence Induction using Oxidative Stress. In Biological Aging; Methods in Molecular Biology; Springer: Cham, Switzerland, 2013; Volume 1048, pp. 135–144. [Google Scholar] [CrossRef]
- Zhao, C.; Teng, X.; Yue, W.; Suo, A.; Zhou, W.; Ding, D. The effect of acute toxicity from tributyltin on Liza haematocheila liver: Energy metabolic disturbance, oxidative stress, and apoptosis. Aquat. Toxicol. 2023, 258, 106506. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, P.; Gohel, D.; Shinde, A.; Roy, M.; Singh, K.; Singh, R. TRIM32 regulates mitochondrial mediated ROS levels and sensitizes the oxidative stress induced cell death. Cell. Signal. 2020, 76, 109777. [Google Scholar] [CrossRef] [PubMed]
- Scherz-Shouval, R.; Elazar, Z. Regulation of autophagy by ROS: Physiology and pathology. Trends Biochem. Sci. 2011, 36, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Cui, J.; Liu, Y.; Gu, L.; Teng, X.; Tang, Y. EGCG alleviated Mn exposure-caused carp kidney damage via trpm2-NLRP3-TNF-α-JNK pathway: Oxidative stress, inflammation, and tight junction dysfunction. Fish Shellfish Immunol. 2023, 134, 108582. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Han, Q.; Xu, Y.; Jiang, H.; Xing, H.; Teng, X. Impaired immune function and structural integrity in the gills of common carp (Cyprinus carpio L.) caused by chlorpyrifos exposure: Through Oxidative Stress Apoptosis. Fish Shellfish Immunol. 2019, 86, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, Y.; Bi, Y.; Wu, Q.; Xu, S. Baicalin suppressed necroptosis and inflammation against chlorpyrifos toxicity; involving in ER stress and oxidative stress in carp gills. Fish Shellfish Immunol. 2023, 139, 108883. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ma, M.; Chen, C.; Yang, X.; Qian, Y. Three widely used pesticides and their mixtures induced cytotoxicity and apoptosis through the ROS-related caspase pathway in HepG2 cells. Food Chem. Toxicol. 2021, 152, 112162. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kamada, N.; Moon, J.J. Oral nanomedicine for modulating immunity, intestinal barrier functions, and gut microbiome. Adv. Drug Deliv. Rev. 2021, 179, 114021. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xu, W.; Che, X.; Cui, J.; Shang, X.; Teng, X.; Jia, Z. Effect of arsenic stress on the intestinal structural integrity and intestinal flora abundance of Cyprinus carpio. Front. Microbiol. 2023, 14, 1179397. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Benderman, L.J.; Sabu, S.; Parker, J.; Yang, J.; Lu, Q.; Ding, L.; Chen, Y.-H. Tight Junction Protein Claudin-7 Is Essential for Intestinal Epithelial Stem Cell Self-Renewal and Differentiation. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 641–659. [Google Scholar] [CrossRef]
- Miao, Z.; Miao, Z.; Teng, X.; Xu, S. Melatonin alleviates lead-induced fatty liver in the common carps (Cyprinus carpio) via gut-liver axis. Environ. Pollut. 2023, 317, 120730. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Pizzoferrato, M.; Gerardi, V.; Lopetuso, L.; Gasbarrini, A. The gut barrier: New acquisitions and therapeutic approaches. J. Clin. Gastroenterol. 2012, 46, S12–S17. [Google Scholar] [CrossRef]
- Qin, W.; Xu, B.; Chen, Y.; Yang, W.; Xu, Y.; Huang, J.; Duo, T.; Mao, Y.; Zhou, G.; Yan, X.; et al. Dietary ellagic acid supplementation attenuates intestinal damage and oxidative stress by regulating gut microbiota in weanling piglets. Anim. Nutr. 2022, 11, 322–333. [Google Scholar] [CrossRef]
- Yan, M.; Zhu, L.; Wu, S.; Cao, Y.; Mou, N.; Chi, Q.; Wang, G.; Zhong, Y.; Wu, W. ROS responsive polydopamine nanoparticles to relieve oxidative stress and inflammation for ameliorating acute inflammatory bowel. Biomater. Adv. 2022, 142, 213126. [Google Scholar] [CrossRef]
- Cui, J.; Hao, Z.; Zhou, Q.; Qiu, M.; Liu, Y.; Liu, Y.; Teng, X.; Kang, L. Chlorpyrifos induced autophagy and mitophagy in common carp livers through AMPK pathway activated by energy metabolism disorder. Ecotoxicol. Environ. Saf. 2023, 258, 114983. [Google Scholar] [CrossRef]
- Thiermann, H.; Steinritz, D.; Worek, F.; Radtke, M.; Eyer, P.; Eyer, F.; Felgenhauer, N.; Zilker, T. Atropine maintenance dosage in patients with severe organophosphate pesticide poisoning. Toxicol. Lett. 2011, 206, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Mitra, J.K.; Hansda, U.; Bandyopadhyay, D.; Sarkar, S.; Sahoo, J. The role of a combination of N-acetylcysteine and magnesium sulfate as adjuvants to standard therapy in acute organophosphate poisoning: A randomized controlled trial. Heliyon 2023, 9, e15376. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Patwa, J.; Pant, S.; Flora, S.J.S.; Sharma, A. Synthesis and evaluation of small organic molecule as reactivator of organophosphorus inhibited acetylcholinesterase. Drug Chem. Toxicol. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wolthuis, O.L.; Philippens, I.H.C.H.M.; Vanwersch, R.A.P. Side effects of therapeutic drugs against organophosphate poisoning. Neurotoxicol. Teratol. 1989, 11, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.A.; Montagnani, M.; Santacroce, L.; Charitos, I.A.; Bottalico, L. Ancient herbal therapy: A brief history of Panax ginseng. J. Ginseng Res. 2022, 47, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Pei, H.; Chen, W.; Zhu, X.; Wang, Y.; Li, J.; He, Z.; Du, R. Evaluating the effect of ginsenoside Rg1 on CPF-induced brain injury in mice via PI3k/AKT pathway. J. Biochem. Mol. Toxicol. 2023, 37, e23319. [Google Scholar] [CrossRef] [PubMed]
- Aravinthan, A.; Kim, J.H.; Antonisamy, P.; Kang, C.-W.; Choi, J.; Kim, N.S.; Kim, J.-H. Ginseng total saponin attenuates myocardial injury via anti-oxidative and anti-inflammatory properties. J. Ginseng Res. 2015, 39, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Siddiqi, M.H.; Noh, H.-Y.; Kim, Y.-J.; Kim, Y.-J.; Jin, C.-G.; Yang, D.-C. Anti-inflammatory activity of ginsenosides in LPS-stimulated RAW 264.7 cells. Sci. Bull. 2015, 60, 773–784. [Google Scholar] [CrossRef]
- de Oliveira Zanuso, B.; de Oliveira dos Santos, A.R.; Miola, V.F.B.; Campos, L.M.G.; Spilla, C.S.G.; Barbalho, S.M. Panax ginseng and aging related disorders: A systematic review. Exp. Gerontol. 2022, 161, 111731. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Wu, C.F.; Duan, L.; Yang, J.Y. Protective effects of total saponins from stem and leaf of Panax ginseng against cyclophosphamide-induced genotoxicity and apoptosis in mouse bone marrow cells and peripheral lymphocyte cells. Food Chem. Toxicol. 2008, 46, 293–302. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, S.; Zhao, L.; Yang, X.; Yao, Y.; Hou, Z.; Xue, P. Stem-leaves of Panax as a rich and sustainable source of less-polar ginsenosides: Comparison of ginsenosides from Panax ginseng, American ginseng and Panax notoginseng prepared by heating and acid treatment. J. Ginseng Res. 2020, 45, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.; Buckley, N.A.; Eyer, P.; Dawson, A.H. Management of acute organophosphorus pesticide poisoning. Lancet 2008, 371, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-Z.; Ma, B.-E.; Liu, C.; Wang, R. Clinical efficacy of intravenous infusion of atropine with micropump in combination with hemoperfusion on organophosphorus poisoning. Saudi J. Biol. Sci. 2019, 26, 2018–2021. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Nie, H.; Yao, W.; Yang, X.; Zhang, G. A sensitive and selective fluorescent probe for acetylcholinesterase: Synthesis, performance, mechanism and application. Arab. J. Chem. 2022, 15, 103929. [Google Scholar] [CrossRef]
- Rathod, A.L.; Garg, R. Chlorpyrifos poisoning and its implications in human fatal cases: A forensic perspective with reference to Indian scenario. J. Forensic Leg. Med. 2017, 47, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, Y.; Hao, Z.; Liu, Y.; Qiu, M.; Kang, L.; Teng, X.; Tang, Y. Cadmium induced time-dependent kidney injury in common carp via mitochondrial pathway: Impaired mitochondrial energy metabolism and mitochondrion-dependent apoptosis. Aquat. Toxicol. 2023, 261, 106570. [Google Scholar] [CrossRef]
- Vismaya; Rajini, P. Oral exposure to the organophosphorus insecticide, Monocrotophos induces intestinal dysfunction in rats. Food Chem. Toxicol. 2014, 71, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Alruhaimi, R.S. Betulinic acid protects against cardiotoxicity of the organophosphorus pesticide chlorpyrifos by suppressing oxidative stress, inflammation, and apoptosis in rats. Environ. Sci. Pollut. Res. 2023, 30, 51180–51190. [Google Scholar] [CrossRef]
- Abolhassani, M.; Asadikaram, G.; Paydar, P.; Fallah, H.; Aghaee-Afshar, M.; Moazed, V.; Akbari, H.; Moghaddam, S.D.; Moradi, A. Organochlorine and organophosphorous pesticides may induce colorectal cancer; A case-control study. Ecotoxicol. Environ. Saf. 2019, 178, 168–177. [Google Scholar] [CrossRef]
- Zhao, G.-P.; Wang, X.-Y.; Li, J.-W.; Wang, R.; Ren, F.-Z.; Pang, G.-F.; Li, Y.-X. Imidacloprid increases intestinal permeability by disrupting tight junctions. Ecotoxicol. Environ. Saf. 2021, 222, 112476. [Google Scholar] [CrossRef]
- He, L.-X.; Wang, J.-B.; Sun, B.; Zhao, J.; Li, L.; Xu, T.; Li, H.; Sun, J.-Q.; Ren, J.; Liu, R.; et al. Suppression of TNF-α and free radicals reduces systematic inflammatory and metabolic disorders: Radioprotective effects of ginseng oligopeptides on intestinal barrier function and antioxidant defense. J. Nutr. Biochem. 2017, 40, 53–61. [Google Scholar] [CrossRef]
- Lätzer, J.; Papoian, G.A.; Prentiss, M.C.; Komives, E.A.; Wolynes, P.G. Induced Fit, Folding, and Recognition of the NF-κB-Nuclear Localization Signals by IκBα and IκBβ. J. Mol. Biol. 2007, 367, 262–274. [Google Scholar] [CrossRef]
- Malek, S.; Chen, Y.; Huxford, T.; Ghosh, G. IκBβ, but Not IκBα, Functions as a Classical Cytoplasmic Inhibitor of NF-κB Dimers by Masking Both NF-κB Nuclear Localization Sequences in Resting Cells. J. Biol. Chem. 2001, 276, 45225–45235. [Google Scholar] [CrossRef]
- Lindgren, H.; Olsson, A.R.; Pero, R.W.; Leanderson, T. Differential usage of IκBα and IκBβ in regulation of apoptosis versus gene expression. Biochem. Biophys. Res. Commun. 2003, 301, 204–211. [Google Scholar] [CrossRef]
- Wang, W.; Wu, H.; Yu, H.; Zhang, X.; Cui, G.; Wang, K.; Mao, S.; Pan, Y. Typhonium giganteum Lectin Exerts A Pro-Inflammatory Effect on RAW 264.7 via ROS and The NF-κB Signaling Pathway. Toxins 2017, 9, 275. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Hua, M.; Duan, Y.; Gao, Y.; Shao, X.; Wang, H.; Tao, T.; Shen, A.; Cheng, C. TNF-α expression in Schwann cells is induced by LPS and NF-κB-dependent pathways. Neurochem. Res. 2012, 37, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, X.; Hao, Z.; Yu, M.; Tang, Y.; Teng, X.; Sun, W.; Kang, L. Cadmium exposure caused cardiotoxicity in common carps (Cyprinus carpio L.): miR-9-5p, oxidative stress, energetic impairment, mitochondrial division/fusion imbalance, inflammation, and autophagy. Fish Shellfish Immunol. 2023, 138, 108853. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Huang, W.; Cai, S.; Chen, H.; Fu, W.; Chen, Z.; Liu, Y. NF-κB/IκBα signaling pathways are essential for resistance to heat stress-induced ROS production in pulmonary microvascular endothelial cells. Mol. Med. Rep. 2021, 24, 814. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Qiu, M.; Liu, Y.; Liu, Y.; Tang, Y.; Teng, X.; Li, S. Nano-selenium protects grass carp hepatocytes against 4-tert-butylphenol-induced mitochondrial apoptosis and necroptosis via suppressing ROS-PARP1 axis. Fish Shellfish Immunol. 2023, 135, 108682. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Ortiz, C.; Peralta, T.; Ramos, V.; Durán, M.; Behrens, C.; Maureira, D.; Guzmán, M.A.; Bastias, C.; Ferrer, P. Standardization of a colorimetric technique for determination of enzymatic activity of diamine oxidase (DAO) and its application in patients with clinical diagnosis of histamine intolerance. World Allergy Organ. J. 2020, 13, 100457. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Miao, Z.; Teng, X.; Xu, S. Melatonin alleviates lead-induced intestinal epithelial cell pyroptosis in the common carps (Cyprinus carpio) via miR-17-5p/TXNIP axis. Fish Shellfish Immunol. 2022, 131, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Chopyk, D.M.; Stuart, J.D.; Zimmerman, M.G.; Wen, J.; Gumber, S.; Suthar, M.S.; Thapa, M.; Czaja, M.J.; Grakoui, A. Acetaminophen Intoxication Rapidly Induces Apoptosis of Intestinal Crypt Stem Cells and Enhances Intestinal Permeability. Hepatol. Commun. 2019, 3, 1435–1449. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, K.; Shrivastava, A.; Agrawala, P.K.; Prasad, A.K.; Devi, S.C.; Manda, K.; Parmar, V.S.; Dwarakanath, B.S. Mitigation of radiation-induced gastro-intestinal injury by the polyphenolic acetate 7,8-diacetoxy-4-methylthiocoumarin in mice. Sci. Rep. 2019, 9, 14134. [Google Scholar] [CrossRef] [PubMed]
- Woznicki, J.A.; Flood, P.; Bustamante-Garrido, M.; Stamou, P.; Moloney, G.; Fanning, A.; Zulquernain, S.A.; McCarthy, J.; Shanahan, F.; Melgar, S.; et al. Human BCL-G regulates secretion of inflammatory chemokines but is dispensable for induction of apoptosis by IFN-γ and TNF-α in intestinal epithelial cells. Cell Death Dis. 2020, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mo, S.; Jin, Y.; Zheng, Z.; Wang, H.; Wu, S.; Ren, Z.; Wu, J. Ammonia-induced excess ROS causes impairment and apoptosis in porcine IPEC-J2 intestinal epithelial cells. Ecotoxicol. Environ. Saf. 2022, 243, 114006. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Shi, C.; Cheng, C.; Liao, D.; Liu, J.; Chen, G.-T. Ginger polysaccharide UGP1 suppressed human colon cancer growth via p53, Bax/Bcl-2, caspase-3 pathways and immunomodulation. Food Sci. Hum. Wellness 2023, 12, 467–476. [Google Scholar] [CrossRef]
- Ke, N.; Godzik, A.; Reed, J.C. Bcl-B, a Novel Bcl-2 Family Member That Differentially Binds and Regulates Bax and Bak. J. Biol. Chem. 2001, 276, 12481–12484. [Google Scholar] [CrossRef]
- Fu, Y.-P.; Yuan, H.; Xu, Y.; Liu, R.-M.; Luo, Y.; Xiao, J.-H. Protective effects of Ligularia fischeri root extracts against ulcerative colitis in mice through activation of Bcl-2/Bax signalings. Phytomedicine 2022, 99, 154006. [Google Scholar] [CrossRef]
- Pessoa, J. Live-cell visualization of cytochrome c: A tool to explore apoptosis. Biochem. Soc. Trans. 2021, 49, 2903–2915. [Google Scholar] [CrossRef]
- Gómez-Crisóstomo, N.P.; López-Marure, R.; Zapata, E.; Zazueta, C.; Martínez-Abundis, E. Bax induces cytochrome c release by multiple mechanisms in mitochondria from MCF7 cells. J. Bioenerg. Biomembr. 2013, 45, 441–448. [Google Scholar] [CrossRef]
- Jürgensmeier, J.M.; Xie, Z.; Deveraux, Q.; Ellerby, L.; Bredesen, D.; Reed, J.C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 1998, 95, 4997–5002. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhu, X.; Pei, H.; Zhao, Y.; Zong, Y.; Chen, W.; He, Z.; Du, R. Ginseng Stem-and-Leaf Saponins Mitigate Chlorpyrifos-Evoked Intestinal Toxicity In Vivo and In Vitro: Oxidative Stress, Inflammatory Response and Apoptosis. Int. J. Mol. Sci. 2023, 24, 15968. https://doi.org/10.3390/ijms242115968
Liu S, Zhu X, Pei H, Zhao Y, Zong Y, Chen W, He Z, Du R. Ginseng Stem-and-Leaf Saponins Mitigate Chlorpyrifos-Evoked Intestinal Toxicity In Vivo and In Vitro: Oxidative Stress, Inflammatory Response and Apoptosis. International Journal of Molecular Sciences. 2023; 24(21):15968. https://doi.org/10.3390/ijms242115968
Chicago/Turabian StyleLiu, Silu, Xiaoying Zhu, Hongyan Pei, Yan Zhao, Ying Zong, Weijia Chen, Zhongmei He, and Rui Du. 2023. "Ginseng Stem-and-Leaf Saponins Mitigate Chlorpyrifos-Evoked Intestinal Toxicity In Vivo and In Vitro: Oxidative Stress, Inflammatory Response and Apoptosis" International Journal of Molecular Sciences 24, no. 21: 15968. https://doi.org/10.3390/ijms242115968




